Introduction
Angiopoietin like 4 (ANGPTL4) was first identified
as a novel protein with a similar structure to other
angiopoietin-like proteins. Angiopoietin-like proteins have a
structure composed of an N-terminal coil-coil structure and a
C-terminal fibrinogen-like domain (1). ANGPTL4 is primarily expressed in the
liver, adipose tissues, heart, skeletal muscle, intestine, blood
plasma, ovaries and the placenta in humans (2). ANGPTL4 is involved in many
physiological and pathological conditions including lipid
metabolism, glucose homoeostasis, inflammation, kidney disease,
wound healing, cell differentiation, tumorigenesis, angiogenesis,
vascular permeability and redox regulation (3). Peroxisome proliferator-activated
receptors (PPARs) transcriptionally stimulate ANGPTL4 expression
via the PPAR-response element and transforming growth factor β
(TGF-β), which transcriptionally stimulates ANGPTL4 expression via
the TGF-β responsive enhancer. Furthermore, PPARs and TGF-β can
synergistically or antagonistically regulate ANGPTL4 expression
(4). Hypoxia also elevates the
expression of ANGPTL4 by transcriptional regulation by
hypoxia-inducible factor 1 (HIF-1) (5). PPARs and HIF-1 have a synergistic
interaction in regulating ANGPTL4 transcription by changing the
conformational proximity of corresponding response elements
(6).
Reactive oxygen species (ROS) are a class of
chemically reactive oxygen-containing compounds, including
superoxide, hydroxyl radicals and hydrogen peroxide
(H2O2), which serve important roles in normal
biochemical functions and abnormal pathological processes (7). The accumulation of ROS can cause
protein dysfunction and DNA damage. ROS also function as chemical
messengers to activate signaling pathways that are involved in cell
proliferation, differentiation, and apoptosis (8). Of particular interest is
H2O2, which is mainly produced in biological
systems by the dismutation of the superoxide anion in a reaction
carried out by the enzyme superoxide dismutase.
H2O2 is not only thought to function as a
cellular damaging agent that reacts toward lipids, proteins, and
DNA, but also acts as a crucial mediator of intracellular
signaling. As a crucial second messenger,
H2O2 can activate a myriad of signaling
pathways of which the best known are the mitogen-activated protein
kinase (MAPK) pathways (9). The
MAPKs are highly conserved serine/threonine protein kinases that
function in various fundamental cellular processes, including
proliferation, differentiation, motility, apoptosis and survival
(10). Increasing the
concentration of H2O2 promotes
phosphorylation of apoptosis signal-regulating kinase 1 (ASK1).
ASK1 is a MAPK that activates MAPK8 (commonly known as JNK) and p38
MAPK, but not MAPK1 (commonly known as ERK) (11). Besides, H2O2
may activate MAPKs pathways via other mechanisms such as the
nuclear factor-κB MAPK pathway (12,13).
Furthermore, a previous study demonstrated that increased p38
activity produced a positive feedback to enhance ROS generation by
upregulating nicotinamide adenine dinucleotide phosphate-oxidase,
H2O2 and p38 to develop a positive feedback
loop (14). Accordingly, MAPKs act
on respiratory burst oxidase homologs, a type of NADPH oxidase in
plants, and thus accelerate ROS production (15).
ERK and JNK are both involved in the induction of
ANGPTL4 by para-methoxyamphetamine (PMA) in human airway smooth
muscle cells, and a role for p38 in PMA-induced ANGPTL4 increase
has been excluded (16). In
addition, the ERK inhibitor, U0126, and the JNK inhibitor,
SP600125, greatly inhibit the increase in ANGPTL4 expression
(16). In the human glioblastoma
cell line LN229, ANGPTL4 expression is significantly induced by
epidermal growth factor receptor variant type III (EGFRIII). In
this process, the MAPK pathway, especially ERK is activated with
the transcription factor c-Myc, which regulates ANGPTL4
transcription (17).
Considering the importance of MAPK signaling
pathways with regard to H2O2 and ANGPTL4, it
was hypothesized that there may be a mutual effect of
H2O2 on ANGPTL4. To the best of our
knowledge, the potential role of H2O2 in the
regulation of ANGPTL4 release has not been investigated previously.
The present study was therefore undertaken to determine the effects
of H2O2 treatment on ANGPTL4 release in
macrophage cells.
Materials and methods
Materials
H2O2 (cat. no. 323381) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and
diluted in PBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Fetal bovine serum (FBS; cat. no. F8240) was purchased from
Hangzhou Four Seasons Green Engineering Materials Co., Ltd.,
(Hangzhou, China) and Dulbecco's modified Eagle's medium (DMEM;
cat. no. C11995500BT) from Gibco; Thermo Fisher Scientific, Inc.
Primary antibody against α-tubulin (cat. no. 60031-1-ig) was
purchased from Wuhan SanYing Biotechnology, Inc. (Wuhan, China).
Primary antibody against ANGPTL4 (cat. no. 40-9800) was obtained
from Invitrogen; Thermo Fisher Scientific, Inc. Primary antibodies
against phosphorylated (p-)p38 MAPK (cat. no. 4511), p38 MAPK (cat.
no. 8690), p-ERK1/2 (cat. no. 4370), ERK1/2 (cat. no. 4695), p-JNK
(cat. no. 4668) and JNK (cat. no. 9252) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA), as were U0126 (cat.
no. 9903) and SB203580 (cat. no. 5633). SP600125 (cat. no. S5567)
was purchased from Sigma-Aldrich; Merck KGaA. Goat anti-rabbit
secondary antibody (cat. no. 2301) and rabbit anti-mouse secondary
antibody (cat. no. ZB2305) were obtained from OriGene Technologies,
Inc. (Beijing, China). The primary anti-ANGPTL4 antibody (cat. no.
251458) used for cell immunofluorescence was obtained from Abbiotec
LLC (San Diego, CA, USA) and the goat anti rabbit fluorescent
secondary antibody (cat. no. A-11034) was from Applied Biosystems;
Thermo Fisher Scientific, Inc. Cell Counting Kit-8 (CCK-8) was
obtained from BestBio (Shanghai, China) and the mouse ANGPTL4 ELISA
kit (cat. no. KB18884) was purchased from Jiang Lai Bio-Technology
(Shanghai, China).
Cell culture and treatment
Murine macrophage RAW264.7 cells were obtained from
Shanghai Type Culture Collection (Shanghai, China). Cells were
cultured in DMEM supplemented with 10% FBS, 1% penicillin and
streptomycin, and incubated at 37°C in 5% CO2. For the
experiments, the cells were stimulated for 24 h with
H2O2 at various concentrations. The specific
inhibitors U0126, SB203580 and SP600125 were dissolved in DMSO to
appropriate concentrations and used at 20, 40 and 10 mM,
respectively. For pretreatments, cells were incubated for 30 min
with SP600125, 90 min with SB203580, or 90 min with U0126, prior to
H2O2 treatment.
Cell viability assay
Cell viability was evaluated by CCK-8 assay. The
cells were cultured at a density of 105 cells/well on
96-well plates in 100 µl culture medium as described above.
Following 24 h of culturing, the cells were stimulated with or
without H2O2 (0.25 or 0.5 mM) for 24 h. Then
the cells of each microwell were incubated with 10 µl CCK8 and 90
µl culture medium for 2 h at 37°C and then the absorption values
were measured at 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Cell viability was
expressed as a percentage of the control.
Western blot analysis
Following pretreatment and
H2O2 treatment, cells and culture media were
separated by centrifugation at 4°C for 20 min at 2,000 × g. Total
protein was extracted from cells for western blotting, while the
culture medium was saved for ELISAs. Total protein was extracted
using radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Beijing, China), which contained 0.1 M
phenylmethylsulfonyl fluoride. Following washing with PBS,
grinding, lysis and centrifugation (at 4°C, 13,000 × g, 15 min),
the supernatant was collected. Then proteins (100 µg per lane) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. Membranes were blocked by incubation for 90
min at room temperature in 5% non-fat milk in TBS + 0.2% Tween-20
(TBST). Membranes were incubated at 4°C overnight with the
following specific primary antibodies: α-tubulin (1:5,000), ANGPTL4
(1:1,000), p-p38 MAPK (1:1,000), p38 MAPK (1:1,000), p-ERK1/2
(1:1,000), ERK (1:1,000), p-JNK (1:1,000) and JNK (1:1,000).
Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000) in 5% non-fat
milk in TBST for 1 h at room temperature. Finally, bands were
detected using chemiluminescence kit (EMD Millipore Billerica, MA,
USA) and quantified with the FluorChem E system (ProteinSimple;
Bio-Techne, Minneapolis, MN, USA).
Immunofluorescence
Cells (~1×105/ml) were cultured on cover
slips and then treated with or without H2O2
for 24 h. Cells were then fixed in 4% paraformaldehyde for 15 min
prior to extraction with 0.5% Triton X-100. Then, nonspecific
antibodies were blocked by incubation with goat serum (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30
min at 37°C and then incubated with anti-ANGPTL4 antibody at 4°C
overnight, followed by incubation with AlexaFluor-488 goat
anti-rabbit IgG (cat. no. A-11034; Thermo Fisher Scientific, Inc.)
for 45 min at 37°C. Slides were then incubated with
4′,6-diamidino-2-phenylindole to counterstain and fluorescent
signals were detected using a light microscope; >3 fields of
view were examined in ~9 slides.
ELISA
The culture medium in 6-well plates was collected as
described above, and the quantity of ANGPTL4 protein in the culture
medium was determined by ELISA. The absorbance at 450 nm was
detected using a microplate reader and ANGPTL4 levels were
calculated according to a standard curve.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) and
all experimental data are presented as the mean ± standard
deviation of at least three independent experiments. Comparisons
between two groups were performed using two-tailed Student's
t-tests. Multiple comparisons were performed by one way analysis of
variance followed by Bonferroni's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
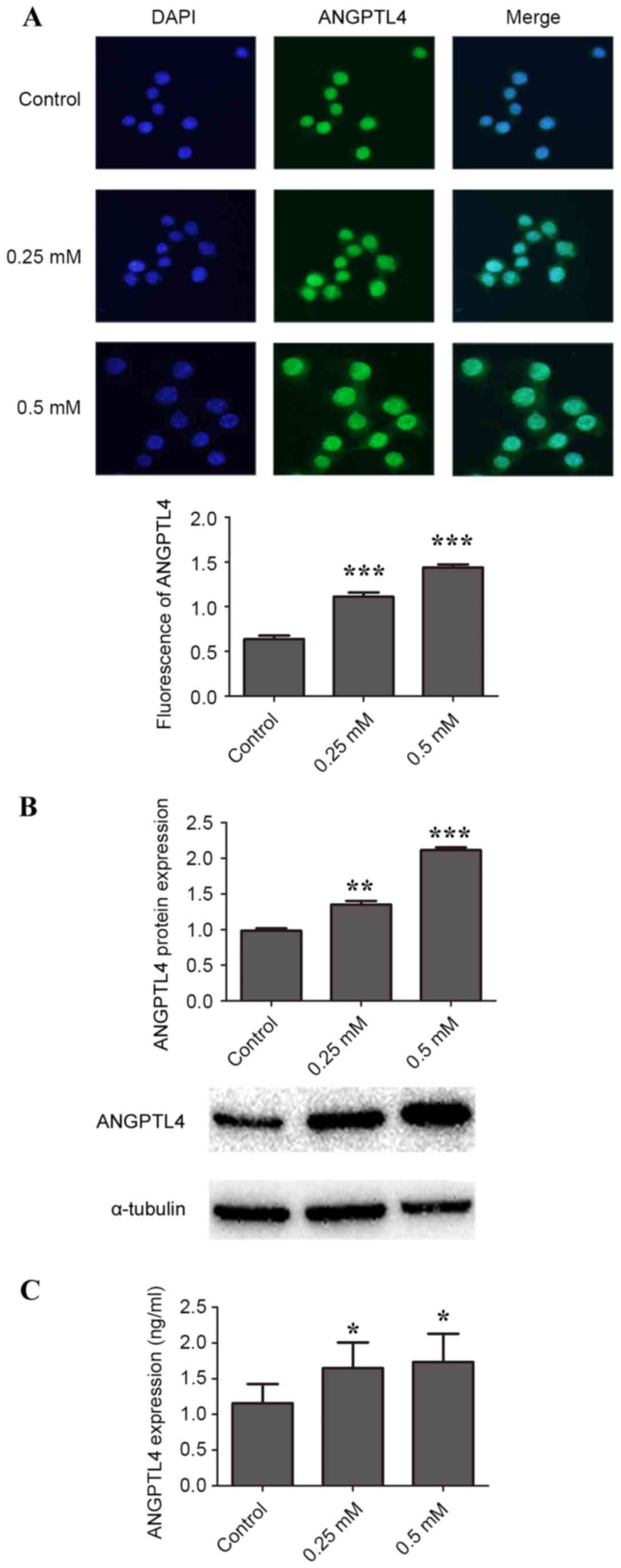
H2O2 induces ANGPTL4 release in
RAW264.7 macrophage cells
To study the effect of H2O2 on
ANGPTL4 protein expression, 0, 0.125, 0.25, 0.375 and 0.5 mM
H2O2 was added to macrophage cells. The
lowest concentration, 0.125 mM, increased ANGPTL4 protein levels
slightly compared with control, but was not statistically
significant (data not shown). As demonstrated by
immunofluorescence, incubation of cells with 0.25 and 0.5 mM
H2O2 for 24 h resulted in significantly more
ANGPTL4 in the cytoplasm and the nucleus compared with control
(P<0.001; Fig. 1A). Significant
upregulation of ANGPTL4 protein expression compared with the
control was also observed by western blotting (Fig. 1B). In addition, significantly more
ANGPTL4 was secreted into the medium in cells treated with 0.25 and
0.5 mM H2O2 compared with control, as
detected by ELISA (P<0.05; Fig.
1C).
Regulation of ANGPTL4 by H2O2 is
mediated by MAPK pathways
To gain insight into the underlying mechanism of
H2O2-induced ANGPTL4 release, the roles of
MAPK signaling pathways were investigated. The level of ERK1/2
(Fig. 2A), p38 MAPK (Fig. 2B) and JNK (Fig. 2C) phosphorylation in cells
stimulated with 0.25 and 0.5 mM H2O2 was
significantly increased compared with control. It was therefore
concluded that H2O2 effectively induced
phosphorylation of ERK1/2, p38 MAPK and JNK proteins in the
macrophage cells; however, this conclusion is insufficient to
determine that H2O2-induced ANGPTL4
expression is dependent on MAPK pathway activation.
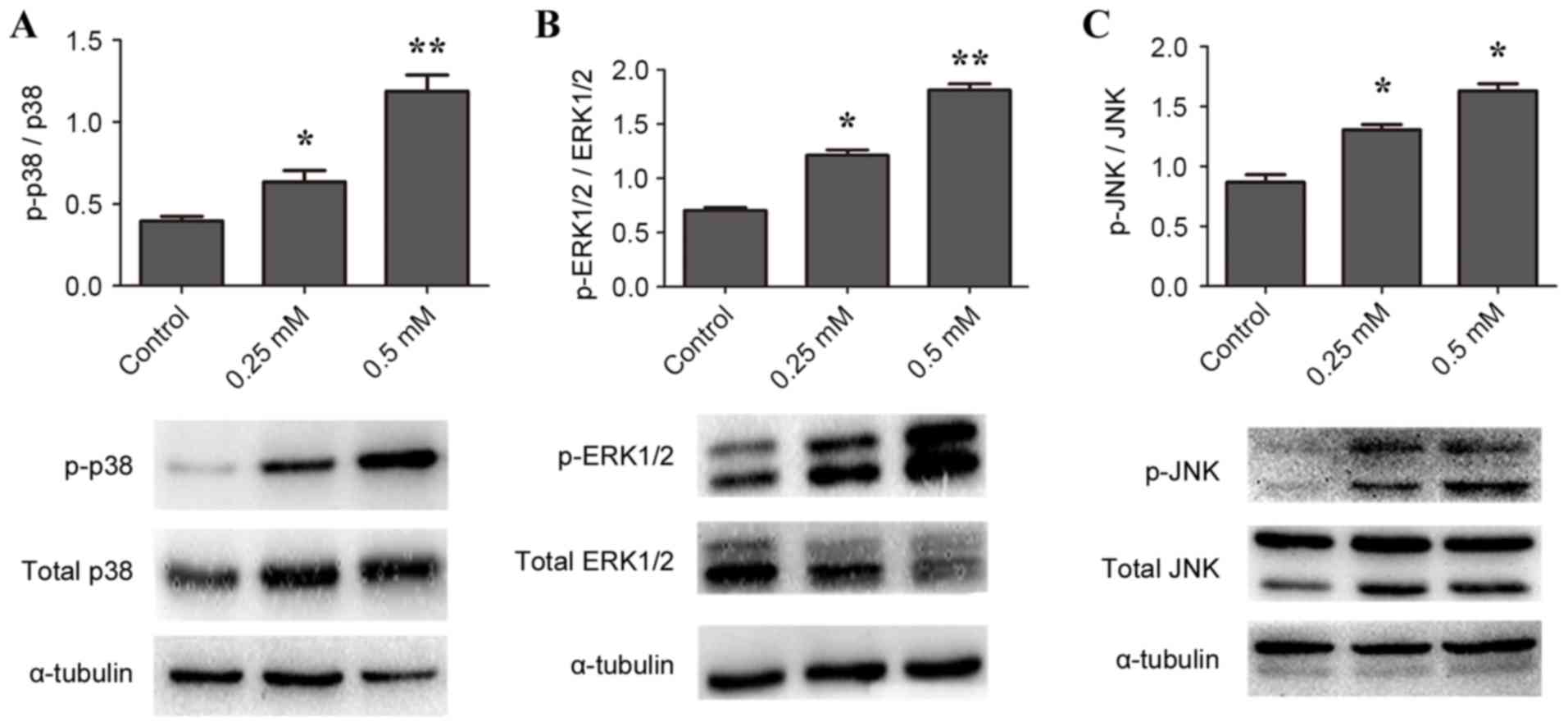 | Figure 2.H2O2 activates
ERK1/2, p38 MAPK and JNK protein phosphorylation expression in
RAW264.7 macrophages. Cells were stimulated with 0 (control), 0.25
or 0.5 mM H2O2 for 24 h, then protein
expression in cell lysates was measured by western blotting. Cell
lysates were probed for (A) p- and total ERK1/2, (B) p- and total
p38 MAPK and (C) p- and total JNK, with α-tubulin as loading
control. *P<0.05, **P<0.01 and ***P<0.001 vs. control.
ERK1/2, MAPK1; MAPK, mitogen-activated protein kinase; JNK, MAPK8;
p-, phosphorylated. |
U0126 and SB203580 inhibit
H2O2-induced ANGPTL4 release in RAW264.7 macrophage cells
To explore this issue further, the effects of
specific inhibitors of p38 MAPK (SB203580), JNK (SP600125) and
ERK1/2 (U0126) on H2O2-induced ANGPTL4
expression were investigated. As illustrated in Fig. 3A, compared with the uninhibited
samples, preincubation of the cells with 20 mM UO126 for 90 min to
block ERK1/2 activation significantly inhibited overexpression of
ANGPTL4 protein induced by H2O2 (P<0.01).
Similarly, preincubation of cells with 40 mM SB203580 for 90 min to
block p38 MAPK pathway activation inhibited overexpression of
ANGPTL4 protein induced by H2O2 compared with
uninhibited samples (P<0.05; Fig.
3B). However, compared with uninhibited samples,
H2O2-induced ANGPTL4 protein levels were not
affected in cells preincubated with 10 mM SP600125 for 30 min to
block JNK pathway activation (Fig.
3C). Due to the toxicity to the macrophage cells, it was not
possible to increase the concentration of SP600125 further.
Together these results indicated that inhibition of p38 MAPK and
ERK1/2, but not JNK, attenuated H2O2-induced
ANGPTL4 release in macrophage cells.
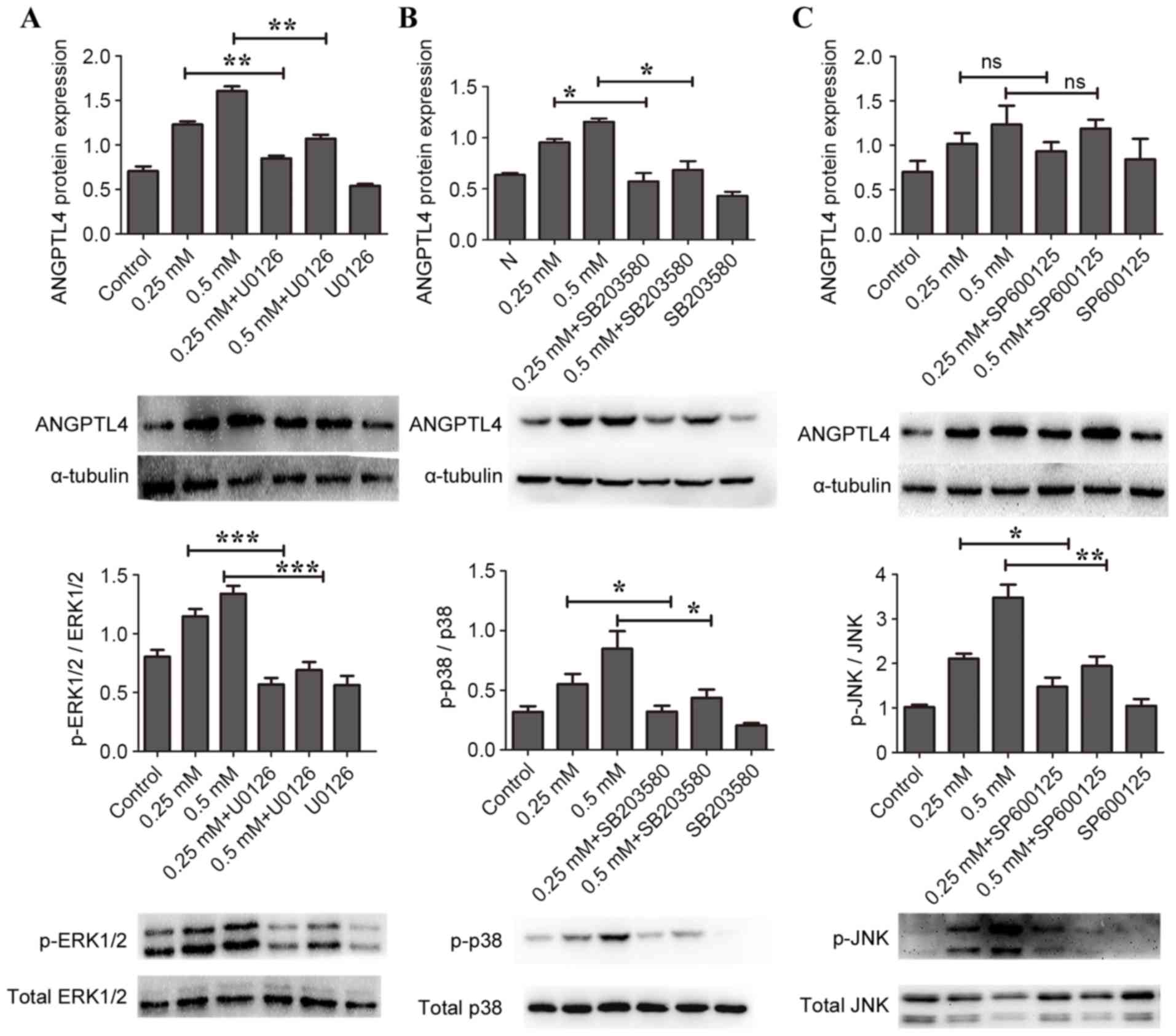 | Figure 3.Effects of MAPK inhibitors on
H2O2-induced ANGPTL4 release in RAW264.7
macrophage cells. Cells were pretreated with specific inhibitors
for ERK1/2 (20 mM U0126), p38 MAPK (40 mM SB203580) or JNK (10 mM
SP600125), followed by stimulation with 0, 0.25 or 0.5 mM
H2O2 for 24 h. Protein expression levels were
then determined by western blotting, with α-tubulin as loading
control. (A) ANGPTL4, p-ERK1/2 and total ERK1/2. (B) ANGPTL4, p-p38
MAPK and total p38 MAPK. (C) ANGPTL4, p-JNK and total JNK.
*P<0.05, **P<0.01 and ***P<0.001, with comparisons
indicated by brackets. MAPK, mitogen-activated protein kinase;
ANGPTL4, angiopoietin like 4; ERK1/2, MAPK1; JNK, MAPK8; p-,
phosphorylated; Control, 0 mM H2O2 and no
inhibitor; ns, not significant. |
Discussion
In the present study, ANGPTL4 was identified as a
protein that was upregulated in Raw264.7 macrophages exposed to
high concentrations of exogenous H2O2.
Macrophages are immune cells that take part in immune response
associated disease, including atherosclerosis. Atherosclerosis
primarily refers to a maladaptive immune response caused by the
accumulation of cholesterol-laden macrophages in the artery wall
(18,19). Macrophages serve a key role via
transition to foam cells that trigger the formation of an
atherosclerotic lesion (20,21).
Furthermore, macrophage cell autophagy participates in
atherosclerosis by exerting a protective influence (22). Macrophage cells secrete various
kinds of pro-inflammatory cytokines, which form an indispensable
part of the inflammation reaction in the genesis of atherosclerosis
(23,24)., ANGPTL4 is highly expressed in
macrophages. A previous study has indicated that ANGPTL4 decreases
the uptake of oxidized low-density lipoprotein (ox-LDL) in
macrophage cells and consequently suppresses foam cell formation
which prevents the pathological development of atherosclerosis
(25). By contrast,
H2O2 has been demonstrated to promote the
oxidative modification of LDL into ox-LDL and accelerate foam cell
formation (26). Therefore,
ANGPTL4 and H2O2 take opposite roles in the
formation of foam cells. The present study linked ANGPTL4 and
H2O2 via MAPK pathways. p38, ERK and JNK are
all activated by high concentrations of H2O2
in macrophages. However, only the ERK inhibitor U0126 and the p38
inhibitor SB203580 were demonstrated to suppress ANGPTL4 expression
induced by H2O2. Although the JNK pathway was
activated, the special inhibitor SP600125 did not significantly
decrease H2O2-induced ANGPTL4 expression. In
different cell types, H2O2 stimulates
different subgroups of MAPKs and the mechanism is not well
understood (27). ANGPTL4
interacts with integrins to stimulate NADPH oxidase-dependent
production of ROS, particularly O2. A high ratio of
O2:H2O2 activates the ERK pathways (28). Interestingly, the C-terminal of
ANGPTL4 can inhibit the phosphorylation of ERK induced by b-FGF in
turn (29).
ANGPTL4 is a lipid metabolism associated factor and
abundant evidence has demonstrated that the hypertriglyceridemic
effect of ANGPTL4 is mainly attributable to inhibition of
lipoprotein lipase (LPL), the enzyme that hydrolyses triglycerides
(30,31). To date, the role of ANGPTL4 in
atherosclerosis remains controversial. For the past few decades,
the emphasis of research of ANGPTL4 on atherosclerosis has been
concentrated on the regulation of lipid metabolism and it is still
unknown whether ANGPTL4 directly affects the genesis and
development of atherosclerosis. Some studies suggest that ANGPTL4
serves a protective role in atherosclerosis, and the mechanism is
independent of the influence of ANGPTL4 on triglyceride levels.
Indeed, one study demonstrated that gene knockout of ANGPTL4 is
sufficient to protect against the development and progression of
atherosclerosis by suppressing formation of foam cells and thereby
reducing the atherosclerotic lesion size (32). In consideration of the diverse
physiological functions of ANGPTL4, intensive experiments are
required to resolve these discrepancies.
Besides promoting foam cell formation,
H2O2 has critical roles in the pathogenesis
of atherosclerosis by regulating the migration of smooth muscle
cells, monocyte infiltration and apoptosis of vascular cells during
advanced atherosclerotic process (33–35).
H2O2 has also been reported as inducer of
autophagy which is involved in lipid homeostasis and dyslipidemias
associated with atherosclerosis. During the development of
atherosclerosis, H2O2 may inhibit smooth
muscle cell migration by mediating the downregulation of myosin
phosphatase target subunit 1 (36,37).
Numerous studies have revealed that oxidative stress caused by
H2O2 is a major factor contributing to the
damage of endothelial cells; the resulting endothelial injury is an
important pathological process of atherosclerosis (38). In addition,
H2O2 is a crucial component of the redox
signaling cascade which is involved in smooth muscle cell
proliferation and migration in atherosclerosis (39). Additionally, the autophagy caused
by H2O2 also contributes to atherosclerosis
(40).
The findings of the present study demonstrate that
H2O2 treatment of macrophage cells results in
significantly increased ANGPTL4 expression and that the p38 MAPK
and ERK1/2 signaling pathways are involved in the process. However,
further studies are required to explain the exact effect of
H2O2 on ANGPTL4 expression in
atherosclerosis. Whether H2O2 acts as a
trigger to activate other inflammatory factors that consequently
promote ANGPTL4 expression is unknown. In addition, the inhibitors
of p38 MAPK and ERK1/2 have not blocked H2O2
induced ANGPTL4 protein release completely, and whether other
signaling pathways such as the PPARs pathways are implicated in
this process requires further research. The present study reveals
the communication between H2O2 and ANGPTL4
for the first time, and their interaction may produce an effect on
atherosclerosis.
Acknowledgements
The authors thank the staff and participants for
their contribution. The present study was supported by a grant from
the National Science Foundation of Shandong Province (grant no.
ZR2013HM003).
References
|
1
|
Kim I, Kim HG, Kim H, Kim HH, Park SK, Uhm
CS, Lee ZH and Koh GY: Hepatic expression, synthesis and secretion
of a novel fibrinogen/angiopoietin-related protein that prevents
endothelial-cell apoptosis. Biochem J. 346:603–610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mandard S, Zandbergen F, van Straten E,
Wahli W, Kuipers F, Müller M and Kersten S: The fasting-induced
adipose factor/angiopoietin-like protein 4 is physically associated
with lipoproteins and governs plasma lipid levels and adiposity. J
Biol Chem. 281:934–944. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu P, Goh YY, Chin HF, Kersten S and Tan
NS: Angiopoietin-like 4: A decade of research. Biosci Rep.
32:211–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stockert J, Adhikary T, Kaddatz K,
Finkernagel F, Meissner W, Müller-Brüsselbach S and Müller R:
Reverse crosstalk of TGFβ and PPARβ/δ signaling identified by
transcriptional profiling. Nucleic Acids Res. 39:119–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drager LF, Yao Q, Hernandez KL, Shin MK,
Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G,
et al: Chronic intermittent hypoxia induces atherosclerosis via
activation of adipose angiopoietin-like 4. Am J Respir Crit Care
Med. 188:240–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue T, Kohro T, Tanaka T, Kanki Y, Li G,
Poh HM, Mimura I, Kobayashi M, Taguchi A, Maejima T, et al:
Cross-enhancement of ANGPTL4 transcription by HIF1 alpha and PPAR
beta/delta is the result of the conformational proximity of two
response elements. Genome Biol. 15:R632014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reczek CR and Chandel NS: ROS-dependent
signal transduction. Curr Opin Cell Biol. 33:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marinho HS, Real C, Cyrne L, Soares H and
Antunes F: Hydrogen peroxide sensing, signaling and regulation of
transcription factors. Redox Biol. 2:535–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee Y, Kim YJ, Kim MH and Kwak JM: MAPK
cascades in guard cell signal transduction. Front Plant Sci.
7:802016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Forman HJ: Use and abuse of exogenous H202
in studies of signal transduction. Free Radic Biol Med. 42:926–932.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zikaki K, Aggeli IK, Gaitanaki C and Beis
I: Curcumin induces the apoptotic intrinsic pathway via
upregulation of reactive oxygen species and JNKs in H9c2 cardiac
myoblasts. Apoptosis. 19:958–974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vilema-Enríquez G, Arroyo A, Grijalva M,
Amador-Zafra RI and Camacho J: Molecular and cellular effects of
hydrogen peroxide on human lung cancer cells: Potential therapeutic
implications. Oxid Med Cell Longev. 2016:19081642016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Deng Y, Lai W, Guan X, Sun X, Han
Q, Wang F, Pan X, Ji Y, Luo H, et al: Maternal inflammation
activated ROS-p38 MAPK predisposes offspring to heart damages
caused by isoproterenol via augmenting ROS generation. Sci Rep.
6:301462016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jalmi SK and Sinha AK: ROS mediated MAPK
signaling in abiotic and biotic stress-striking similarities and
differences. Front Plant Sci. 6:7692015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stapleton CM, Joo JH, Kim YS, Liao G,
Panettieri RA Jr and Jetten AM: Induction of ANGPTL4 expression in
human airway smooth muscle cells by PMA through activation of PKC
and MAPK pathways. Exp Cell Res. 316:507–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katanasaka Y, Kodera Y, Kitamura Y,
Morimoto T, Tamura T and Koizumi F: Epidermal growth factor
receptor variant type III markedly accelerates angiogenesis and
tumor growth via inducing c-myc mediated angiopoietin-like 4
expression in malignant glioma. Mol Cancer. 12:312013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moore KJ, Sheedy FJ and Fisher EA:
Macrophages in atherosclerosis: A dynamic balance. Nat Rev Immunol.
13:709–721. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shirai T, Hilhorst M, Harrison DG, Goronzy
JJ and Weyand CM: Macrophages in vascular inflammation-from
atherosclerosis to vasculitis. Autoimmunity. 48:139–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galkina E and Ley K: Immune and
inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol.
27:165–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel KM, Strong A, Tohyama J, Jin X,
Morales CR, Billheimer J, Millar J, Kruth H and Rader DJ:
Macrophage sortilin promotes LDL uptake, foam cell formation, and
atherosclerosis. Circ Res. 116:789–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao X, Sluimer JC, Wang Y, Subramanian M,
Brown K, Pattison JS, Robbins J, Martinez J and Tabas I: Macrophage
autophagy plays a protective role in advanced atherosclerosis. Cell
Metab. 15:545–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Briot A, Civelek M, Seki A, Hoi K, Mack
JJ, Lee SD, Kim J, Hong C, Yu J, Fishbein GA, et al: Endothelial
NOTCH1 is suppressed by circulating lipids and antagonizes
inflammation during atherosclerosis. J Exp Med. 212:2147–2163.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Youn SW and Park KK:
Small-nucleic-acid-based therapeutic strategy targeting the
transcription factors regulating the vascular inflammation,
remodeling and fibrosis in atherosclerosis. Int J Mol Sci.
16:11804–11833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Georgiadi A, Wang Y, Stienstra R,
Tjeerdema N, Janssen A, Stalenhoef A, Van Der Vliet JA, de Roos A,
Tamsma JT, Smit JW, et al: Overexpression of angiopoietin-like
protein 4 protects against atherosclerosis development.
Arterioscler Thromb Vasc Biol. 33:1529–1537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tavakoli S and Asmis R: Reactive oxygen
species and thiol redox signaling in the macrophage biology of
atherosclerosis. Antioxid Redox Signal. 17:1785–1795. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sies H: Role of metabolic H2O2 generation:
Redox signaling and oxidative stress. J Biol Chem. 289:8735–8741.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC,
Pal M, Lam CR, Boukamp P, Pan JY, Tan SH, et al: Angiopoietin-like
4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio
and confers anoikis resistance to tumors. Cancer Cell. 19:401–415.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK,
Zhang J, Zhu W, Wu D and Xu A: Suppression of the Raf/MEK/ERK
signaling cascade and inhibition of angiogenesis by the carboxyl
terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc
Biol. 28:835–840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yau MH, Wang Y, Lam KS, Zhang J, Wu D and
Xu A: A highly conserved motif within the NH2-terminal coiled-coil
domain of angiopoietin-like protein 4 confers its inhibitory
effects on lipoprotein lipase by disrupting the enzyme
dimerization. J Biol Chem. 284:11942–11952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sukonina V, Lookene A, Olivecrona T and
Olivecrona G: Angiopoietin-like protein 4 converts lipoprotein
lipase to inactive monomers and modulates lipase activity in
adipose tissue. Proc Natl Acad Sci USA. 103:17450–17455. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adachi H, Fujiwara Y, Kondo T, Nishikawa
T, Ogawa R, Matsumura T, Ishii N, Nagai R, Miyata K, Tabata M, et
al: Angptl 4 deficiency improves lipid metabolism, suppresses foam
cell formation and protects against atherosclerosis. Biochem
Biophys Res Commun. 379:806–811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai MH and Jiang MJ: Reactive oxygen
species are involved in regulating alpha1-adrenoceptor-activated
vascular smooth muscle contraction. J Biomed Sci. 17:672010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lassègue B, San Martín A and Griendling
KK: Biochemistry, physiology, and pathophysiology of NADPH oxidases
in the cardiovascular system. Circ Res. 110:1364–1390. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Konior A, Schramm A, Czesnikiewicz-Guzik M
and Guzik TJ: NADPH oxidases in vascular pathology. Antioxid Redox
Signal. 20:2794–2814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng JC, Cheng HP, Tsai IC and Jiang MJ:
ROS-mediated downregulation of MYPT1 in smooth muscle cells: A
potential mechanism for the aberrant contractility in
atherosclerosis. Lab Invest. 93:422–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perrotta I and Aquila S: The role of
oxidative stress and autophagy in atherosclerosis. Oxid Med Cell
Longev. 2015:1303152015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Griendling KK and FitzGerald GA: Oxidative
stress and cardiovascular injury: Part II: Animal and human
studies. Circulation. 108:2034–2040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moon SK, Thompson LJ, Madamanchi N,
Ballinger S, Papaconstantinou J, Horaist C, Runge MS and Patterson
C: Aging, oxidative responses, and proliferative capacity in
cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ
Physiol. 280:H2779–H2788. 2001.PubMed/NCBI
|
|
40
|
Li X, Xu M, Pitzer AL, Xia M, Boini KM, Li
PL and Zhang Y: Control of autophagy maturation by acid
sphingomyelinase in mouse coronary arterial smooth muscle cells:
protective role in atherosclerosis. J Mol Med (Berl). 92:473–485.
2014. View Article : Google Scholar : PubMed/NCBI
|