Introduction
Osteoporosis is a chronic metabolic disease, defined
as a skeletal disorder characterized by low bone mass density and
microarchitectural deterioration, which predisposes patients to
fracture (1). It is generally
believed that osteoporosis is the failure of dynamic balance
between the osteoblastic bone formation and osteoclastic bone
resorption, and the functional activities of osteoblasts serve a
crucial role in the occurrence and development of osteoporosis
(2,3). Runt-related transcription factor 2
(Runx2), a master bone growth regulatory factor, has been thought
to be involved in the proliferation and differentiation of
osteoblasts (4,5). Studies have reported that physiologic
levels of Runx2 may promote osteoblast function, while abnormal
expression of Runx2 cause the occurrence of bone related diseases
(6,7). Puerarin is an important isoflavonoid
phytoestrogen extracted from the root of Radix Puerariae,
which is widely prescribed for patients in China. Studies
demonstrated that puerarin could promote the proliferation,
differentiation and mineralization of osteoblasts in vitro
(8,9). Previous studies of the authors have
also suggested that puerarin may promote the bone formation in
MC3T3-E1 osteoblast-like cells (10,11).
In a recent report, puerarin was demonstrated to ameliorate bone
loss in estrogen-deficient rats, which indicated that puerarin had
good anti-osteoporotic effect and may prevent osteoporosis for
postmenopausal women (12).
Although experiments have indicated that puerarin served an
important role in osteoblasts and osteoporosis in vivo and
in vitro, the exact anti-osteoporotic mechanism of puerarin
remains unclear.
MicroRNAs (miRs) are small single-stranded
non-coding RNAs, 18–22 nucleotides in length, which have emerged as
a class of important gene regulators on cellular functions in
recent years (13). They are known
to suppress protein expression by binding to the 3′-untranslated
region (UTR) of their target mRNAs (14). It has been suggested that miRNAs
may be involved in regulating cell proliferation, differentiation
and apoptosis by targeting their downstream genes (15,16).
However, specific miRNAs in the regulation of osteoblasts
activities have not been well characterized and the mechanisms by
which these miRNAs target gene expression in osteoblasts remain
elucidated.
In the present study, the promoting effects of
puerarin on osteoblasts viability and differentiation were examined
and the authors indicated that the expression of Runx2 was
significantly increased in osteoblasts following puerarin
treatment, while that of miR-204 was downregulated. Following this,
the authors explored the underlying target relationship between
miR-204 and Runx2 using a dual luciferase reporter gene assay.
Furthermore, by modulating miR-204 activity, overexpression of
miR-204 was markedly decreased the protein level of Runx2, whereas
miR-204 inhibition reversed the effect, indicating that Runx2 was a
direct target gene of miR-204. Together, these findings suggested
that puerarin promotes the viability and differentiation of
MC3T3-E1 cells by increasing the expression of Runx2 via miR-204
downregulation.
Materials and methods
Osteoblast culture
MC3T3-E1 cells were purchased from the Shanghai Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in 25 cm2 flasks
in α-modified Eagle's medium (α-MEM; Wisent Inc., St. Bruno, QC,
Canada) supplemented with 10% fetal bovine serum (FBS, Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin, maintained at 37°C in a
humidified 5% CO2 incubator (Sanyo Electric Co., Ltd.,
Moriguchi, Japan).
MTT assay on osteoblasts
viability
Briefly, 5×103 cells/well were seeded in
96-well culture plates (Corning Incorporated, Corning, NY, USA).
The cell culture medium was discarded after 24 h, and 180 µl
different final concentrations (0.01, 0.1 and 1 mg/ml) puerarin
were added to the experimental wells, while an equal volume of
serum-free medium was added to the control wells. Following
culturing for 24, 48 and 72 h, the proliferation of osteoblasts was
measured by adding 20 µl MTT (5 mg/ml, Amresco LLC, Solon, OH, USA)
in each well and incubating for another 4 h. A total of 150 µl
dimethyl sulfoxide was added in each well. Following this, the
supernatant was removed and absorbance was measured at 490 nm after
oscillation for 10 min using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The viability of osteoblasts
after transfection with miR-204 mimics, inhibitor or miR negative
control was also examined using MTT assay.
Alkaline phosphatase (ALP) activity
expression of osteoblasts
Osteoblasts were cultured in a 24-well culture
plate. Following treatment with different final concentrations
(0.01, 0.1 and 1 mg/ml) puerarin for 48 h, cells were washed three
times with PBS. The ALP positive cells were stained and ALP
activity was assayed using a commercial kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions. The ALP activities were also examined
in osteoblasts following transfection with miR-204 mimics,
inhibitor or miR negative control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Osteoblasts were seeded in a 24-well plate in α-MEM
(Wisent, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), at the density of 1×105 cells/well.
The concentration of 0.01, 0.1 and 1 mg/ml puerarin was added to
the experimental wells, and cultured in a humidified 5%
CO2 incubator at 37°C for 48 h. Total RNA was prepared
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Purity and concentration of the isolated RNA were detected by a
nucleic acid protein analyzer (BioDrop µLite; Biodrop, Cambridge,
UK). The RNA was then reversed-transcribed to cDNA with a reverse
transcription kit (Takara Bio, Inc., Otsu, Japan), following the
manufacturer's protocol. The final reaction solution (25 µl)
contained 2 µl cDNA product, 12.5 µl 2X Realtime PCR SYBR premix
(Takara Bio, Inc.), 0.5 µl each of forward and reverse primers and
9.5 µl distilled water. The two-step PCR amplification conditions
were: Pre-denaturation of 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec, 60°C for 34 sec. The fluorescence signal detection
was measured by Mx3000P real-time PCR system (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). Target genes included
transforming growth factor (TGF)-β1, Smad2, Smad3 and Runx2, while
β-actin served as an internal control. Primers were designed and
synthesized by GenScript (Piscataway NJ, USA) and are presented in
Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction.
| Gene | Forward sequence
(5′-3′) | Reverse sequence
(5′-3′) |
|---|
| TGF-β1 |
CTCCCGTGGCTTCTAGTGC |
GCCTTAGTTTGGACAGGATCTG |
| Smad2 |
ATGTCGTCCATCTTGCCATTC |
AACCGTCCTGTTTTCTTTAGCTT |
| Smad3 |
CACGCAGAACGTGAACACC |
GGCAGTAGATAACGTGAGGGA |
| Runx2 |
CGGACGAGGCAAGAGTTTCA |
GGATGAGGAATGCGCCCTAA |
| β-actin |
GTGCTATGTTGCTCTAGACTTCG |
ATGCCACAGGATTCCATACC |
Western blot analysis
Protein was extracted from the cells with total
protein extraction kit (cat. no. BC3710; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) after treatment with
0.01, 0.1 and 1 mg/ml puerarin for 48 h, and then the protein
content was measured by the bicinchoninic acid assay method,
according to the manufacturer's instructions (cat. no. PC0020;
Beijing Solarbio Science & Technology Co., Ltd.). For western
blot analysis, 25 µg of samples were subjected to SDS-PAGE gel and
electro-transferred onto a polyvinylidene difluoride membrane. The
membrane was then blocked with 5% skim milk in TBS containing 0.1%
Tween-20 (TBST) for 1 h at room temperature. Following washing
three times in TBST for 5 min, the protein samples were incubated
with primary antibodies (Cell Signaling Technology, Inc., Danvers,
MA, USA) for TGF-β1 (cat. no. 709), Smad2 (cat. no. 3122), Smad3
(cat. no. 9523), Runx2 (cat. no. 12556) and β-actin (cat. no. 4970;
all 1:1,000) at 4°C overnight. Following washing another three
times with TBST, the samples were incubated with horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibodies (cat.
no. 7074; 1:3,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Following a final wash of TBST, the protein bands of
interest were visualized by the enhanced chemiluminescence
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The intensity of the bands was measured using the ImageJ2× v2.1.4.7
software (National Institutes of Health, Bethesda, MD, USA) to
assess the relative protein levels.
Expression profile analysis of
miRNAs
Expression profiles analysis of miRNAs was performed
by KangChen Biology Engineering Co., Ltd. (Shanghai, China)
following treatment of osteoblasts with 0.1 mg/ml puerarin for 48
h.
Network prediction for Runx2-targeting
miRNAs
The Runx2-targeting miRNAs were predicted using
TargetScan (http://genes.mit.edu/targetscan), PicTar (http://pictar.bio.nyu.edu), miRBase (http://mirbase.org/) and miRDB (http://mirdb.org/miRDB/) programs, and then compared
with the results of expression profile analysis to determine the
Runx2-targeting miRNAs.
Validation of Runx2-targeting miRNA by
RT-qPCR
Following incubating osteoblasts with puerarin (0.1
mg/ml) for 48 h, total RNA was extracted using TRIzol reagent
(Takara Bio, Inc.). The reverse-transcription reaction solution (20
µl) contained 10.0 µl 5X Reverse Transcription Mix (Takara Bio,
Inc.), 1.0 µl Stem-loop RT primers (GenScript), 500 ng miRNA, 2.0
µl HiScript Enzyme Mix (cat. no. R223; Vazyme, Piscataway, NJ, USA)
and RNase-free water. The PCR amplification conditions were: 25°C
for 5 min, 45°C for 50 min, 85°C for 50 min. Specific miRNA
stem-loop RT primers for mouse miR-204 and internal control U6 were
designed and synthesized by Shanghai Sangon Pharmaceutical Co. Ltd.
(Shanghai, China). Primers used in the stem-loop RT-qPCR are as
following: RT primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGGCAT-3′; PCR
upstream primer, 5′-GCGGCGGTTCCCTTTGTCATCC-3′; downstream primer
5′-ATCCAGTGCAGGGTCCGAGG-3′ for miR-204; PCR upstream primer,
5′-CTCGCTTCGGCAGCACA-3′; downstream primer,
5′-AACGCTTCACGAATTTGCGT-3′ for U6.
Construction of recombinant
plasmid
Empty vectors were prepared for constructing Runx2
expression vectors, sequences of the wild-type Runx2 3′-UTR
(ttctatgcacgtattgtacaaattgtgct
ttgtgccacaggtcatgatcgtggatgagtttactctgaacttcaaagggactatttgtatt
gtatgttgcaactgtaaattgaattatttggcatttccccctctcatgattgtaatatt) and
mutant Runx2 3′-UTR (ttctatgcacgtattgtacaaattgtgctttgtgcc
acaggtcatgatcgtggatgagtttactctgaacttcaaatccactatttgtattgtatgttg
caactgtaaattgaattatttggcatttccccctctcatgattgtaatatt) were inserted
into the empty vectors. The recombinant plasmids were synthesized
by Shanghai GenePharma Co, Ltd. (Shanghai, China).
Dual luciferase reporter gene
assay
Osteoblasts were seeded in 24-well plates at a
density of 2×104 per well. At 24 h incubation, cells in
each well were transfected with the recombinations of wildtype
Runx2 3′-UTR or mutant Runx2 3′-UTR plasmids (100 ng) and miR-204
mimics (2 µM) or miR control (2 µM) using Lipofectamine 2000 (1 ml;
Invitrogen; Thermo Fisher Scientific, Inc.). Cells were collected
following 48 h transfection, then firefly and Renilla
luciferase activities were analyzed using Dual-Luciferase Reporter
Assay System (Promega Corporation, Madison, WI, USA). The ratio of
firefly to Renilla luciferase activity was used as relative
luciferase activity.
Overexpression and inhibition of
miR-204
The mouse miR-204 mimics, miR-204 inhibitor and
negative control were obtained from Shanghai GenePharma Co., Ltd.
Cells were seeded in six-well plates at a density of
1×105 per well and transfected with 16 µl RNAs using 4
µl Lipofectamine 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) when the cells reached 90% confluence.
Control cells were only transfected with 4 µl transfection reagent.
Then cells were cultured in a humidified 5% CO2
incubator at 37°C for 48 h. Cells were ready for RT-qPCR and
western blot analysis of Runx2. Viability and ALP activity were
also examined.
Statistical analysis
The results were presented as mean ± standard
deviation. Data comparisons were analyzed by Student's t-test or
one-way analysis of variance with SPSS software (version, 17.0;
SPSS Inc., Chicago, IL, USA). Comparisons between groups were
analyzed using the Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
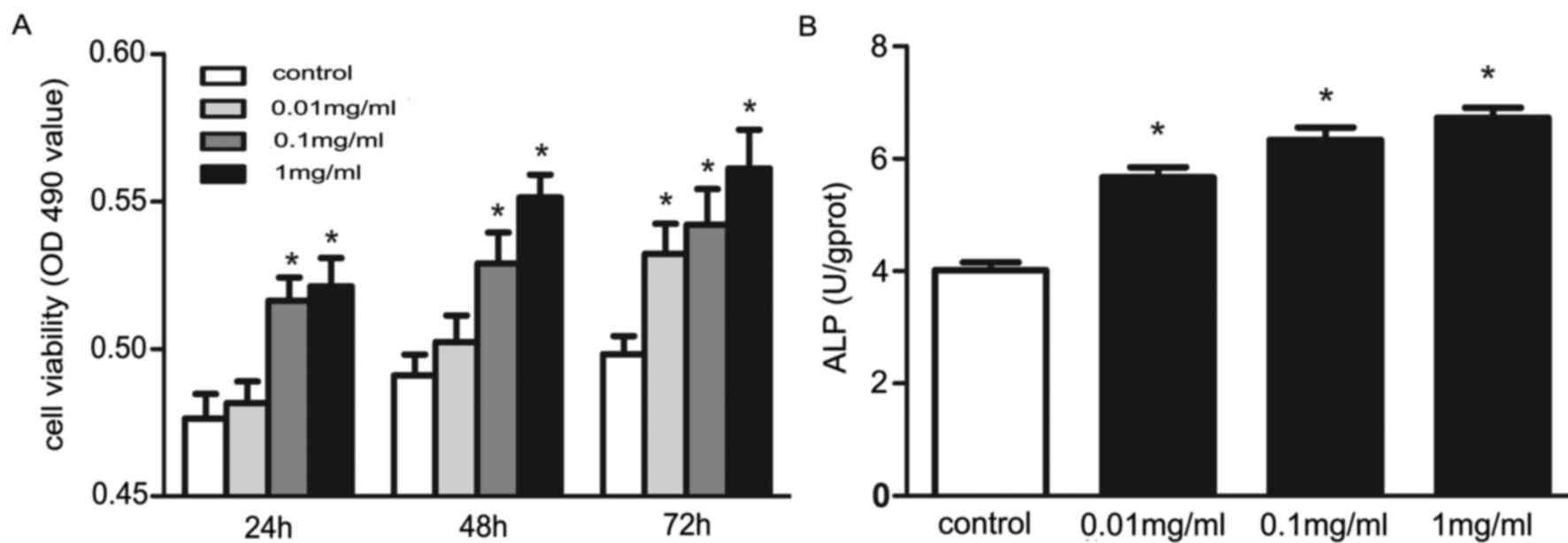
Puerarin promotes osteoblast viability
and differentiation
The authors first examined whether puerarin could
affect osteoblast viability. Osteoblasts were cultured with
puerarin at various concentrations. The cells treated with puerarin
had significantly higher viability compared with that of the
control cells (P<0.05), except those treated with puerarin at a
lower concentration (0.01 mg/ml) for 24 h and 48 h (Fig. 1A). The osteoblasts treated with
puerarin for 48 h at various concentrations (0.01, 0.1 and 1
mg/ml) had significantly higher ALP activity than that of
the control cells (P<0.05; Fig.
1B). These data suggested that puerarin may promote osteoblast
viability and differentiation.
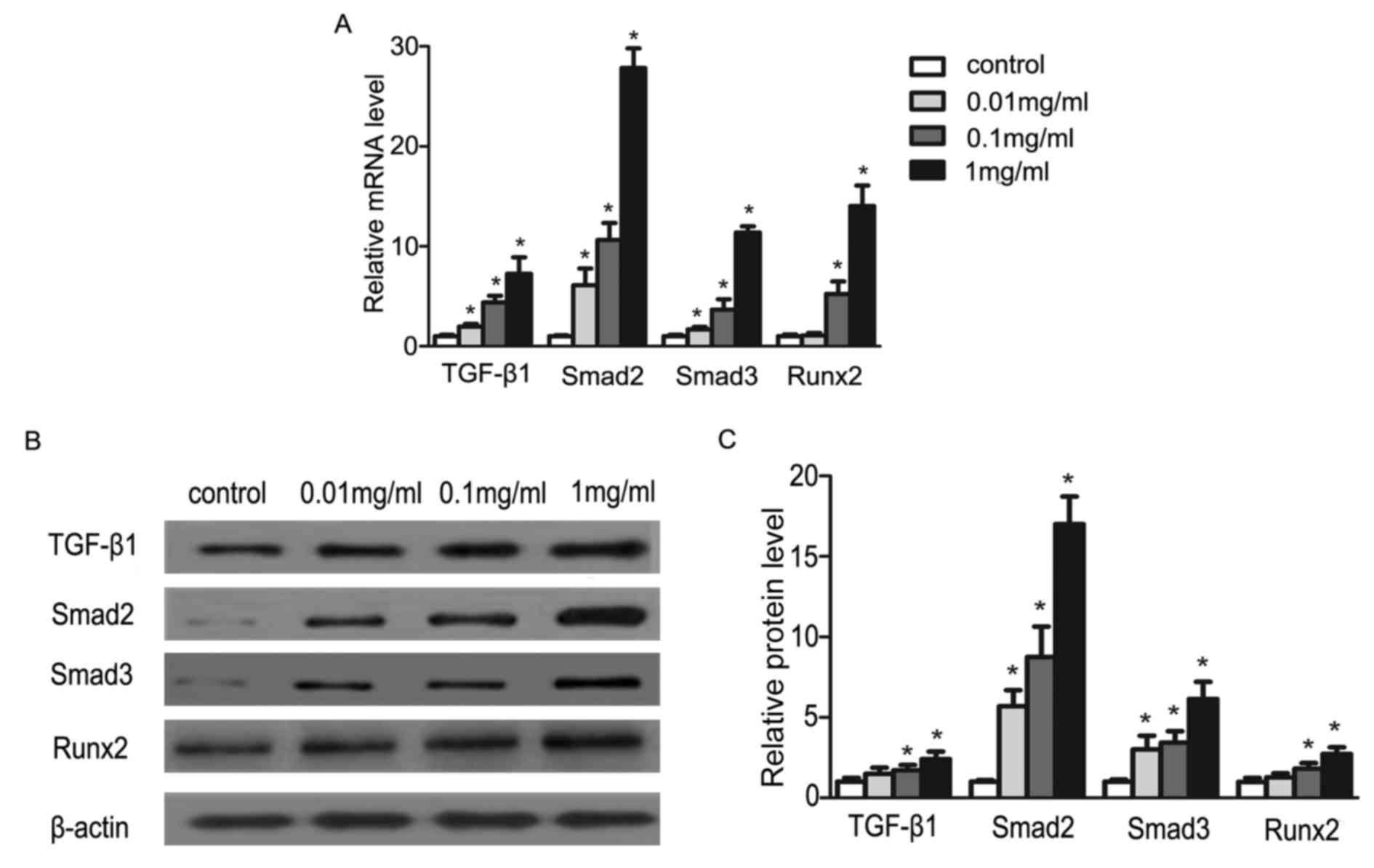
Puerarin increases the expression of
TGF-β1, Smad2, Smad3 and Runx2
In order to explore the proliferation effect of
puerarin on osteoblasts, the authors studied the expression of
osteoblast-related regulatory factors (TGF-β1, Smad2, Smad3 and
Runx2). Following treating cells with puerarin for 48 h, we
measured TGF-β1, Smad2, Smad3 and Runx2 gene expression with
RT-qPCR and also the protein expression with western blotting. The
transcription levels and the translation levels of the genes were
all significantly increased in the cells treated with puerarin
(P<0.05; Fig. 2). The data
imply that these factors may be involved in the promotion effects
of puerarin on osteoblasts. Studies have indicated that Runx2 is a
master regulatory factor that serves a crucial role on the
regulation of transcription in osteoblasts. Afterwards, the authors
focused on the expression of Runx2-targeting miRNAs (17).
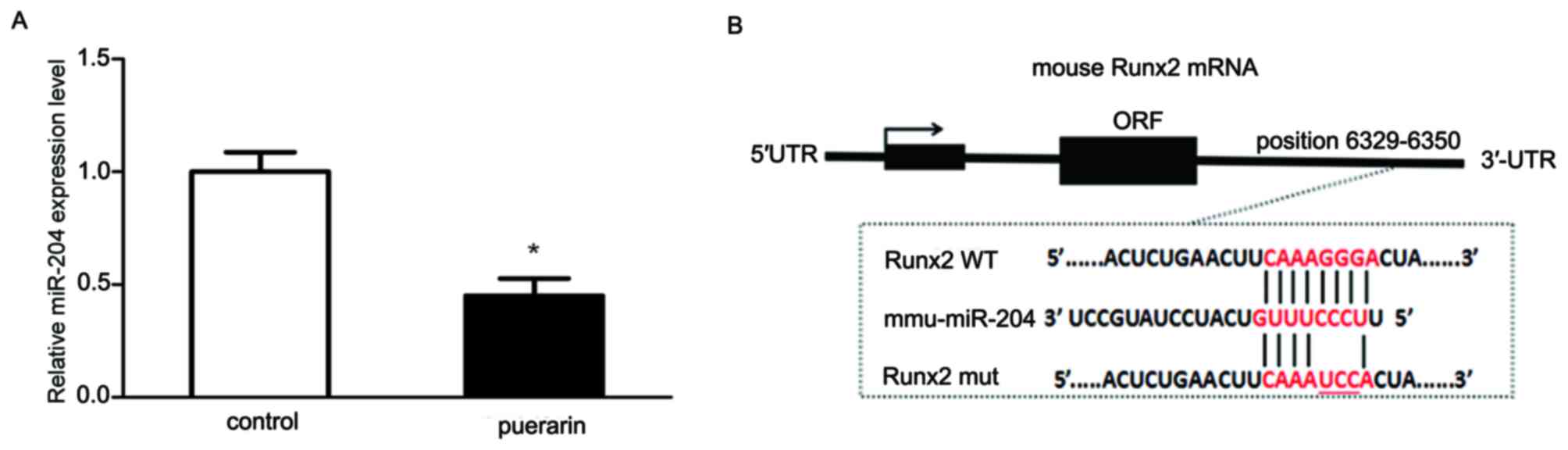
Puerarin downregulates the expression
of miR-204
Analysis of miRNA expression profiles demonstrated
that the expression of miR-204 and other 118 miRNAs changed
following puerarin treatment for 48 h (18). In addition, miRNA target analysis
programs (TargetScan, PicTar, miRBase and miRDB) predicted that
miR-204 may directly target the osteogenic crucial regulator Runx2.
Then, the effect of puerarin on miR-204 expression was examined by
stem-loop RT-qPCR. The expression level of miR-204 was
significantly decreased following culturing the cells with 0.1
mg/ml puerarin for 48 h, compared with that of the cells in absence
of puerarin (P<0.05; Fig. 3A).
The results suggested that puerarin downregulated the expression of
miR-204.
Combined with the previous validation that puerarin
promoted osteoblast viability and differentiation through
upregulation of Runx2, however, the authors demonstrated that
miR-204 was downregulated. Moreover, according to the
bioinformatics analysis, miR-204 was suggested to bind to the
3′-UTR of Runx2 through specific binding sites (Fig. 3B). Taken together, future studies
are prompted to determine whether Runx2 is a direct downstream
target of miR-204.
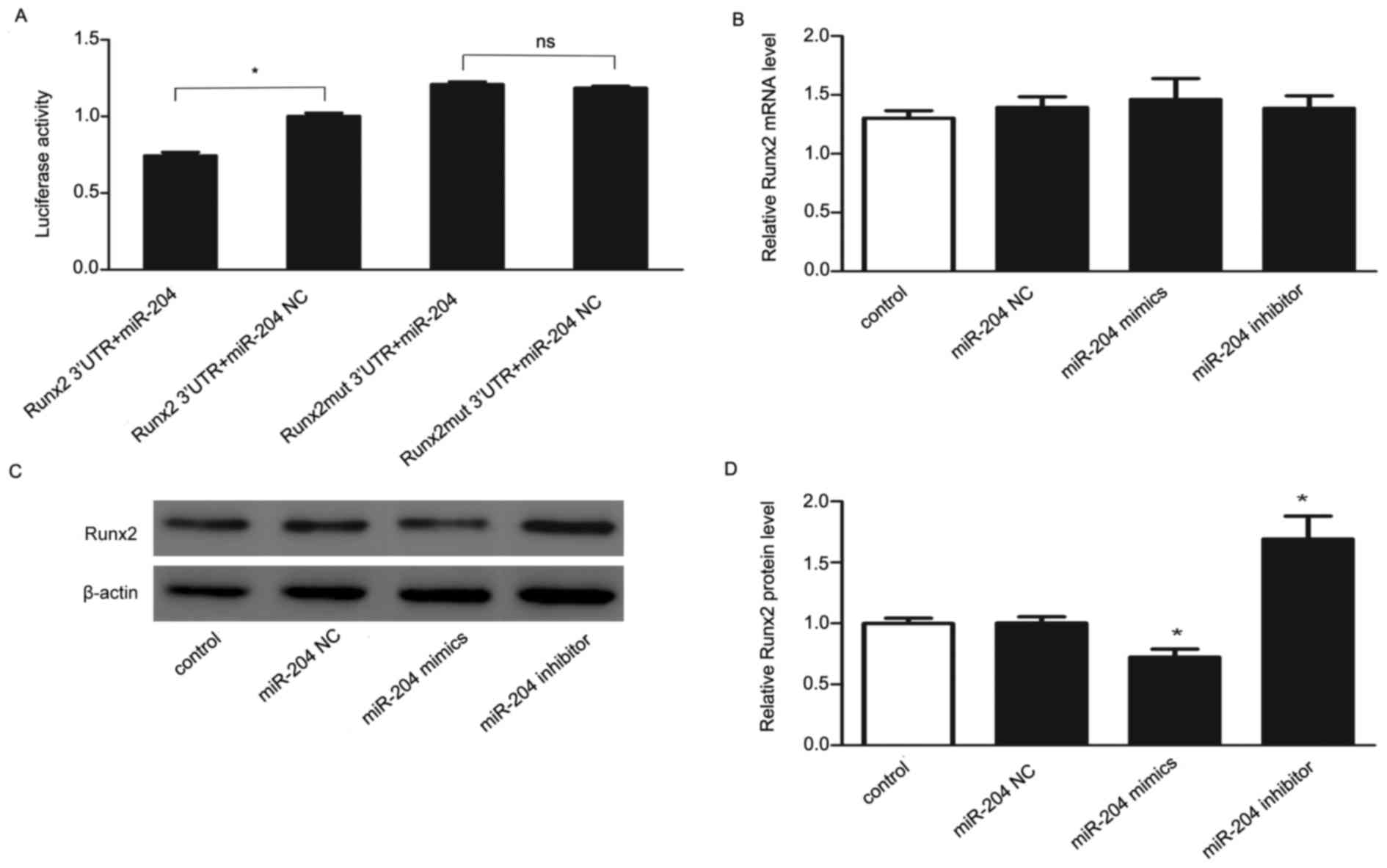
Runx2 is the target of miR-204 in
mouse osteoblasts
To further investigate whether Runx2 can be directly
targeted by miR-204, luciferase reporters containing wildtype or
mutant 3′-UTR of Runx2 were engineering and transfected with
miR-204 mimics or miR negative control. As presented in Fig. 4A, transfection with miR-204 mimics
of wildtype-Runx2-3′-UTR recombinant plasmid had lower luciferase
activity compared with that of the miR negative control, while
transfection with miR-204 mimics of mutant-Runx2-3′-UTR recombinant
plasmid had no statistically significant difference compared with
that of the miR negative control. These data indicated that miR-204
dramatically repressed the luciferase activity of the reporter
containing the wild-type full-length 3′-UTR of Runx2, but not the
mutant full-length 3′-UTR of Runx2, implying that miR-204 may
directly target Runx2.
The effects of overexpression and inhibition of
miR-204 on Runx2 were then investigated. The expression of Runx2
was analyzed using RT-qPCR and western blotting methods in
osteoblasts at 48 h following transfection with miR-204 mimics,
inhibitor or miR negative control. The results of RT-qPCR (Fig. 4B) did not show significant
difference, but western blotting analysis (Figs. 4C and D) indicated that
overexpression of miR-204 in osteoblasts significantly decreased
the expression level of Runx2, while inhibition of miR-204 had the
opposite effect. The effects of alternation on protein level,
rather than on mRNA level, suggesting that a role of miR-204 for
post-transcriptional regulation. Collectively, these results
indicated that Runx2 is a direct target of miR-204.
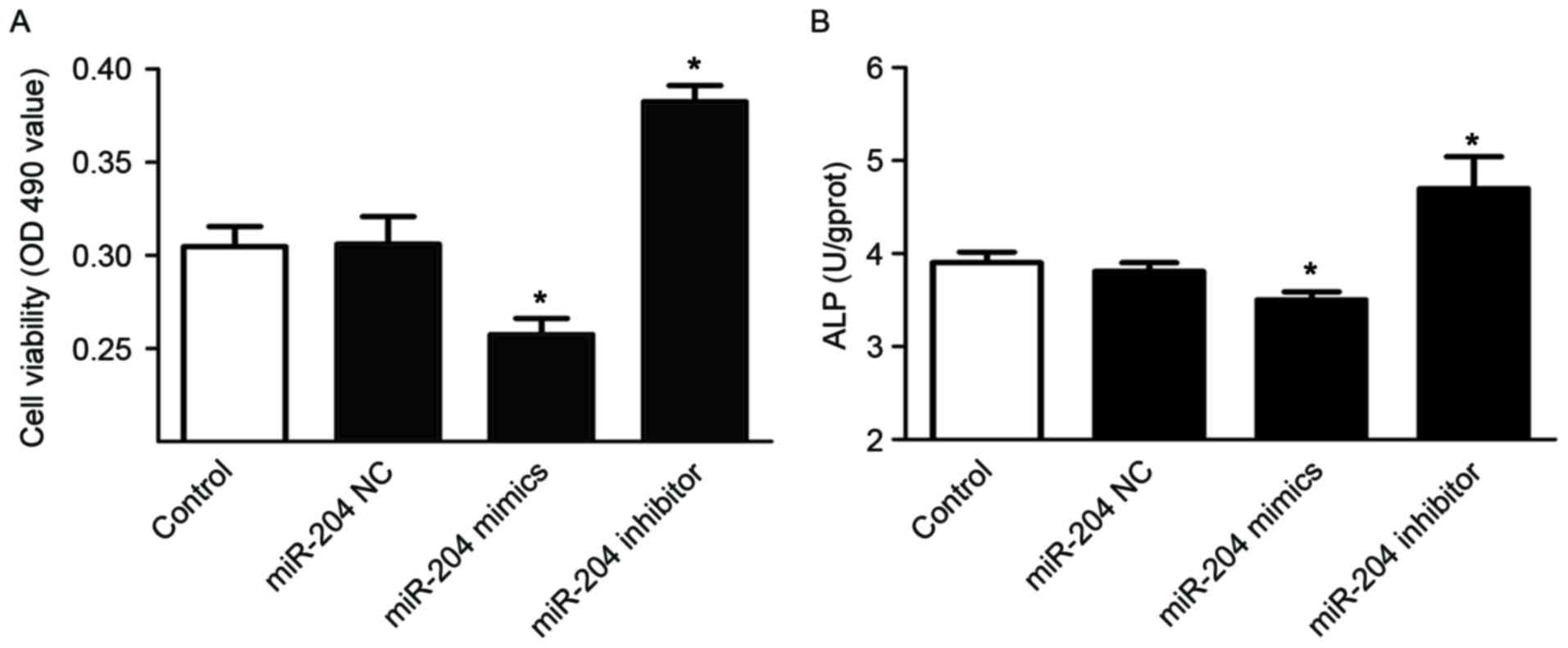
miR-204 inhibits the viability and
differentiation of osteoblasts
Finally, the authors investigated the effect of
miR-204 on osteoblasts. Following transfection with miR-204 mimics,
inhibitor or miR negative control for 48 h, the viability and ALP
activity of osteoblasts were tested. The cells that were
transfected with miR-204 mimics had significantly lower viability,
but cells transfected with miR-204 inhibitor had higher viability
compared with that of the control cells (Fig. 5A). Similarly, transfection with
miR-204 mimics significantly reduced ALP activity, whereas
transfection with miR-204 inhibitor had the opposite effect
(Fig. 5B). These results suggested
that miR-204 may inhibit osteoblast viability and
differentiation.
Discussion
Runx2 is an important bone regulatory factor, which
serves a crucial role in the regulation of transcription in
osteoblasts. Puerarin was reported to promote the proliferation and
osteoblastic differentiation in human bone marrow stromal cells by
increasing the expression of osteoblastic markers, including Runx2
(19). Runx2, BMP-2 and OPG gene
expression was found to be upregulated following treating mouse
osteoblasts 1×10−8 mol/l puerarin (20). However, other research reported
that puerarin significantly increased the expression of ALP, OPG
and RANKL, but not Runx2 in UMR106 cells (8). In addition, puerarin increased the
mRNA levels of osteoblast differentiation markers, such as ALP and
type I collagen, but not Runx2 or osterixin in primary baboon
osteoblasts (21). Obviously, the
effects of puerarin on Runx2 in different cells remains a
controversial issue. In the current study, puerarin significantly
increased the expression of Runx2 in osteoblast-like MC3T3-E1
cells, and the further study demonstrated that miR-204 was involved
in the regulatory mechanism.
miRNAs are a large family of small non-coding RNAs
that regulate gene expression. miR-204, a negative regulator, is
involved in the regulation of many biological activities. It is
validated that the expression of miR-204 is downregulated in bone
tissue of ovariectomized mice (22) and it is also reported miR-204 has
no effect on bone formation in vivo (23). However, miR-204 has received little
attention in osteoblast viability and differentiation in
vitro. In the present study, puerarin promoted the viability
and differentiation of osteoblasts, and correspondingly increased
the expression of Runx2 and downregulated the expression level of
miR-204. The results indicated that miR-204 and Runx2 may be
involved in the regulation of the osteoblast viability and
differentiation, and then the authors sought to clarify the
relationship between miR-204 and Runx2. In the present study, the
bioinformatics analysis demonstrated that miR-204 could bind to the
3′-UTR of Runx2 through specific binding sites. miR-204
dramatically reduced the luciferase activity of wild type Runx2
3′-UTR transfected cells, but not that of the mutant ones.
Moreover, overexpression of miR-204 significantly decreased the
protein expression level of Runx2, while inhibition of miR-204
enhanced the level. The effect of alternation on protein levels,
rather than mRNA level, suggesting a role of miR-204 for
post-transcriptional regulation. Taken together, the current study
demonstrated that Runx2 is a direct target of miR-204.
Consistently, Huang et al (24) demonstrated that miR-204,
functioning as an endogenous negative regulator of Runx2, inhibited
osteogenesis and promotes adipogenesis of mesenchymal progenitor
cells and bone marrow mesenchymal stem cells (24). Wang et al (25) also confirmed that miR-204 inhibited
osteogenesis of human aortic valve interstitial cells by negatively
regulating the expression of Runx2.
In the current study, the authors observed and
explored the change of Runx2 targeted by miR-204; however, some
other osteoblast-related regulatory factors such as TGF-β1, Smad2
and Smad3 were upregulated following treatment of puerarin.
Although miR-204 could not directly target these factors based on
software prediction and bioinformatics analysis, it has been
revealed that miRNAs usually function in clusters (26). The mechanism whether the other
varied miRNAs among the miRNA expression profiles after puerarin
treatment has effects on TGF-β1, Smad2 and Smad3 remains unknown.
In fact, studies have indicated that Runx2, TGF-β1, Smad2 and Smad3
are capable of regulating the expression of miRNAs directly or
indirectly (27–29), suggesting that regulatory factors
and miRNAs are not independent and they regulate each other
bidirectionally. In view of the promoting effect of puerarin on
cellular viability and differentiation, its regulatory effects on
miR-204 and the downstream target Runx2, the detailed upstream
signaling pathway by which puerarin affects miR-204 downregulation
is still unknown, which remains to be elucidated. Besides, further
research on human osteoblasts is required to further confirm these
findings because of the differences between mouse and human
cells.
In conclusion, the authors demonstrated that
puerarin significantly promoted the viability and differentiation
of osteoblasts. Following treatment with puerarin in mouse
osteoblasts, the expression level of Runx2 was significantly
increased, which was negatively correlated with that of miR-204,
whose expression level was decreased. Moreover, the current study
demonstrated that miR-204 could directly target Runx2; Runx2 was
identified as a target of miR-204. The results suggested that
puerarin promoted the viability and differentiation of mouse
osteoblasts by increasing the expression of Runx2 via miR-204
downregulation. These findings provide a better understanding of
the biological effects of puerarin on bone formation in
vitro, and suggested the potential use of puerarin in
prevention and treatment of osteoporosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Jiangsu Province (grant no. BK20131417) and
by A Project Funded by the Priority Academic Program Development of
Jiangsu Higher Education Institutions (Integration of Chinese and
Western Medicine).
References
|
1
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haxaire C, Haÿ E and Geoffroy V: Runx2
controls bone resorption through the down-regulation of the Wnt
pathway in osteoblasts. Am J Pathol. 186:1598–1609. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wysokinski D, Pawlowska E and Blasiak J:
RUNX2: A master bone growth regulator that may be involved in the
DNA damage response. DNA Cell Biol. 34:305–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun C, Qiu Y, Yin G, Shu H, Liu Z, Wang
XH, Liu WJ and Li HB: Abnormal expression and significance of Runx2
in osteoblasts of adolescent idiopathic scoliosis patients.
Zhonghua Wai Ke Za Zhi. 47:1495–1498. 2009.(In Chinese). PubMed/NCBI
|
|
7
|
Li N, Luo D, Hu X, Luo W, Lei G, Wang Q,
Zhu T, Gu J, Lu Y and Zheng Q: RUNX2 and osteosarcoma. Anticancer
Agents Med Chem. 15:881–887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tiyasatkulkovit W, Charoenphandhu N,
Wongdee K, Thongbunchoo J, Krishnamra N and Malaivijitnond S:
Upregulation of osteoblastic differentiation marker mRNA expression
in osteoblast-like UMR106 cells by puerarin and phytoestrogens from
Pueraria mirifica. Phytomedicine. 19:1147–1155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang C, Meng MX, Tang XL, Chen KM, Zhang
L, Liu WN and Zhao YY: The proliferation, differentiation, and
mineralization effects of puerarin on osteoblasts in vitro. Chin J
Nat Med. 12:436–442. 2014.PubMed/NCBI
|
|
10
|
Zhan XQ, Qian KQ and Sun YM: Action of
puerarin on TGF-β1/Smad pathway in MC3T3-E1 cells. Chin Tradition
Patent Med. 35:1121–1124. 2013.(In Chinese).
|
|
11
|
Dong BS, Zhan XQ and Sun YM: Effects of
Puerarin on OPG/RANKL system with culture of osteoblast in vitro.
Jilin J Tratitional Chin Med. 32:388–390. 2012.(In Chinese).
|
|
12
|
Suthon S, Jaroenporn S, Charoenphandhu N,
Suntornsaratoon P and Malaivijitnond S: Anti-osteoporotic effects
of Pueraria candollei var. mirifica on bone mineral density and
histomorphometry in estrogen-deficient rats. J Nat Med. 70:225–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei J, Shi Y, Zheng L, Zhou B, Inose H,
Wang J, Guo XE, Grosschedl R and Karsenty G: miR-34s inhibit
osteoblast proliferation and differentiation in the mouse by
targeting SATB2. J Cell Biol. 197:509–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Huang Z, Chen D, Yang T and Liu G:
Role of microRNA-27a in myoblast Differentiation. Cell Biol Int.
38:266–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruderer M, Richards RG, Alini M and
Stoddart MJ: Role and regulation of RUNX2 in osteogenesis. Eur Cell
Mater. 28:269–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang YY, Zhou JB, Zeng XW, Zhao FM, Liu
GD and Zhan XQ: Effects of puerarin on proliferation of osteoblast
and Runx2-targeting miRNAs. Chin Pharmacol Bull. 32:1457–1462.
2016.(In Chinese).
|
|
19
|
Lv H, Che T, Tang X, Liu L and Cheng J:
Puerarin enhances proliferation and osteoblastic differentiation of
human bone marrow stromal cells via a nitric oxide/cyclic guanosine
monophosphate signaling pathway. Mol Med Rep. 12:2283–2290. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheu SY, Tsai CC, Sun JS, Chen MH, Liu MH
and Sun MG: Stimulatory effect of puerarin on bone formation
through co-activation of nitric oxide and bone morphogenetic
protein-2/mitogen-activated protein kinases pathways in mice. Chin
Med J (Engl). 125:3646–3653. 2012.PubMed/NCBI
|
|
21
|
Tiyasatkulkovit W, Malaivijitnond S,
Charoenphandhu N, Havill LM, Ford AL and VandeBerg JL: Pueraria
mirifica extract and puerarin enhance proliferation and expression
of alkaline phosphatase and type I collagen in primary baboon
osteoblasts. Phytomedicine. 21:1498–1503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
An JH, Ohn JH, Song JA, Yang JY, Park H,
Choi HJ, Kim SW, Kim SY, Park WY and Shin CS: Changes of microRNA
profile and microRNA-mRNA regulatory network in bones of
ovariectomized mice. J Bone Miner Res. 29:644–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui RR, Li SJ, Liu LJ, Yi L, Liang QH, Zhu
X, Liu GY, Liu Y, Wu SS, Liao XB, et al: MicroRNA-204 regulates
vascular smooth muscle cell calcification in vitro and in vivo.
Cardiovasc Res. 96:320–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364.
2010.PubMed/NCBI
|
|
25
|
Wang Y, Chen S, Deng C, Li F, Wang Y, Hu
X, Shi F and Dong N: MicroRNA-204 Targets Runx2 to Attenuate
BMP-2-induced osteoblast differentiation of human aortic valve
interstitial cells. J Cardiovasc Pharmacol. 66:63–71. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang FE, Zhang C, Maminishkis A, Dong L,
Zhi C, Li R, Zhao J, Majerciak V, Gaur AB, Chen S and Miller SS:
MicroRNA-204/211 alters epithelial physiology. FASEB J.
24:1552–1571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen P, Wei D, Xie B, Ni J, Xuan D and
Zhang J: Effect and possible mechanism of network between microRNAs
and RUNX2, gene on human dental follicle cells. J Cell Biochem.
115:340–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin X, Tian W, Wang L, Wang J, Zhang S,
Cao J and Yang H: Radiation quality-dependence of bystander effect
in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway
and miR-21 in irradiated keratinocytes. Sci Rep. 5:113732015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
García R, Nistal JF, Merino D, Price NL,
Fernández-Hernando C, Beaumont J, González A, Hurlé MA and Villar
AV: p-SMAD2/3 and DICER promote pre-miR-21 processing during
pressure overload-associated myocardial remodeling. Biochim Biophys
Acta. 1852:1520–1530. 2015. View Article : Google Scholar : PubMed/NCBI
|