Introduction
Atrial fibrillation (AF), the most prevalent
arrhythmia, is a risk factor for stroke (1,2).
Evidence indicates that subclinical hyperthyroidism is associated
with AF, heart failure and coronary disease events (3). In addition, left atrial fibrosis is
prominent in patients with AF. Extensive atrial tissue fibrosis
identified by delayed enhancement magnetic resonance imaging has
been demonstrated to be associated with poor outcomes of AF
catheter ablation (4). Atrial
fibrosis is hypothesized to be important in the reoccurrence of AF
following cardioversion. It is reported that the expression levels
of matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs) may predict AF recurrence following
cardioversion and may be considered as candidate novel biomarkers
of AF stratification and therapy (5). In addition, the generation of atrial
fibrosis is followed by alterations in the expression of
apoptosis-associated genes, such as decreased expression of BCL2,
apoptosis regulator (BCL-2), and increased expression of BCL-2
associated X, apoptosis regulator (BAX) (6). Therefore, it is considered to be of
great clinical significance to investigate the correlation of MMPs
and apoptosis-associated gene expression levels with AF.
MMPs are a family of metalloproteinases reported to
be associated with numerous biological processes, including
ontogenesis morphogenesis, angiogenesis and cell growth (7). MMP-7 and MMP-9, two
metalloproteinases in the MMPs family, were identified to be
involved in extracellular matrix (ECM) homeostasis and in joint
disc remodeling (8). TIMP
metallopeptidase inhibitor 2 (TIMP-2), a non-glycosylated protein
with a molecular weight of 21 kd, is a specific inhibitor of MMPs,
which significantly contributes to the inhibition of tumor invasion
and metastasis (9). Previous
studies investigated the association of MMPs and their inhibitors
(TIMPs) with the development and occurrence of AF, and the results
indicate that the circulating levels of MMPs and TIMPs are
associated with atrial remodeling and prediction of AF recurrence
(5,10). BCL-2 functions as a negative
regulator in cell apoptosis, which in turn results in the
disturbance of homeostatic cell growth. Furthermore, the
anti-apoptotic mechanism of BCL-2 involves the inhibition of BAX
homo-oligomerization and mitochondrial membrane poration (11).
The aim of the current study was to investigate the
role of MMP-7 and apoptosis-associated gene expression levels in
the pathogenesis of AF in a Beagle dog model, in order to provide a
meaningful experimental basis for clinical treatment of AF.
Materials and methods
Ethical approval
The present study was performed in accordance with
the approved animal protocols and guidelines established by the
Medicine Ethics Review Committee for animal experiments of
Zaozhuang Municipal Hospital (Zaozhuang, China).
Animal grouping and establishment of
Beagle dog models of AF
AF is usually treated with drugs, thus it is
relatively difficult to collect surgery tissue samples. Therefore,
Beagle dog models of AF were established in the present study. A
total of 20 adult male Beagle dogs (age, 1–3 years; weight, 8–10
Kg; purchased from Ya Dong Laboratorial Animal Research Center,
Nanjing, China) were selected and randomly divided into the AF
group (n=10) and the control group (n=10). Beagles were separately
housed at cages at a temperature of 13–16°C and humidity of 40–70%,
under 12-h light/dark cycles. Sufficient drinking water was
provided for each Beagle dog, and high-quality adult dog food (3–5%
of its weight) was provided twice a day. In the AF group, burst
stimulation was used to induce AF. Dogs in the AF group were
anaesthetized with ketamine (1 mg/kg; Jiangsu Hengrui medical Co.,
Ltd', Jiangsu, China; batch no. 20101105). Organon Vecuronium (0.1
mg/kg; Merck & Co., Inc., Whitehouse Station, NJ, USA; batch
no. 403138) was used to inhibit spontaneous breathing. Tracheal
intubation cannulae were used to assist with respiration and
Propofol (200 µg/kg/min; Xi'an Libang Pharmaceutical Co, Ltd.,
Xi'an, China; batch no. 0711192) was used to maintain anesthesia.
The dog hearts were exposed by opening the pericardium through the
right fourth intercostal space. Pacemaker electrodes were sutured
subcutaneously and placed in the right ventricle. The pacemaker
electrode was also sutured in the right atrial appendage as an
alternate to induce AF. After closing the chest, the YKE201 pulse
generator (Shanghai Yiwu Medical Equipment Co., Ltd, Shanghai,
China) was connected and ventricular pacing was set at a frequency
of 250 times/min. After surgery, the dogs were clothed with a
recovery vest to protect the incision, the pulse generator and the
electrode wire. The dogs in the control group underwent the same
surgical procedure with the pseudo electrodes stitched. No
ventricular pacing was performed in the two groups within 5 weeks
of surgery. Five weeks after surgery, Burst stimulation was used to
induce AF in the AF group (Pacing cycle length, 100 msec; pacing
threshold, four times, 1–10 sec). AF was induced in each Beagle dog
in the AF group five times and the mean duration of AF was
recorded. If the mean duration was >15 min, it was recorded as
15 min and the AF was defined as persistent AF (12). Following measurement of myocardial
physiological indices, dogs were sacrificed and the heart was
quickly removed. The left and right atrial free walls were
isolated. Following the removal of blood and fat tissue, the left
and right atrial free walls were quickly frozen with liquid
nitrogen and stored at −80°C.
Echocardiography
An EnVisor Ultrasound System (iE33, Philips Medical
Systems Nederland B.V. Measurements, Best, Netherlands) was used to
detect cardiac function parameters, including left atrial diameter
(LAD), LAD indexed (LADI) to body surface area, left ventricular
end-diastolic diameter (LVEDD), left ventricular septal and
posterior end-diastolic wall thickness (PWT of LV) and left
ventricular ejection fraction (LVEF) (12).
Atrial specimen collection
After the cardiac function parameters were measured
and recorded, the left and right atrial free walls were obtained,
blood and adipose tissue were removed, and frozen in liquid
nitrogen for preservation at −80°C. Preserved atrial tissue samples
were used for tissue staining, RNA extraction and protein
extraction, as previously described (13).
Mallory's trichrome staining
The preserved atrial tissues were washed with PBS
three times, fixed at room temperature for 15–30 min with 4%
paraformaldehyde, embedded in paraffin and sliced in 0.4-µm thick
sections. Mallory's trichrome staining was performed, as previously
described (13). The paraffin
sections were dewaxed with xylene and rehydrated. Lugol's iodine
salt crystals, alum, hematoxylin dye and acid fuchsin solution
(0.5%) were added successively for staining. Staining was performed
at room temperature for 1–5 min and full washing with water was
performed after each stain; when the fiber was colorless, washing
with distilled water was performed. Subsequent to Mallory's
trichrome staining, the sections were stained for 20 min with
Aniline Blue solution and alcohol (95%) was added for hydration.
Finally, anhydrous ethanol was used for dehydration. The tissue
block was cleared in xylene and mounted. After staining, the
collagen fiber was dark blue and the muscle tissues were bright
orange. Images were obtained using an optical microscope and the
fibrosis rate was calculated, as previously described (14).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The preserved atrial tissues were washed with PBS
three times. Total RNA was extracted using RNAiso Plus kit (Takara
Biotechnology Co., Ltd., Dalian, China) according to the
manufacturer's protocol, and RT was performed using the Prime
Script RT reagent kit (Takara Bio, Inc., Otsu, Japan), according to
the manufacturer's protocol. qPCR was performed on a StepOnePlus
PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), as previously described (13). The primer sequences that were used
in the present study are presented in Table I. The qPCR reaction included the
following: 1.6 µl cDNA solution, 5 µl 2X SYBR GREEN Taq PCR mix
(Takara Bio, Inc.), 0.2 µl of each forward and reverse primer (10
µM) and 3 µl ddH2O. The thermocycling conditions were as
follows: Pre-denaturation at 95°C for 5 min, followed by 60 cycles
at 95°C for 10 sec, at 58°C for 10 sec and at 72°C for 10 sec, with
a final extension step at 72°C for 10 min. MMP-7 (Gene ID 489432),
TIMP-2 (Gene ID 403633), BAX (Gene ID 403523) and BCL-2 (Gene ID
403416) were detected. GAPDH (Gene ID 403755) served as the
internal reference. Melting curve was applied to assess the
reliability of the PCR products. Relative gene expression was
calculated according to the comparative Cq method (15) and normalized to GAPDH.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence |
|---|
| MMP-7 | F:
5′-TGGTACCATAATGTCCTGAATG-3′ |
|
| R:
5′-TCGTTATTGGCAGGAAGCACACAATGAATT-3′ |
| TIMP-2 | F:
5′-GAAACGACATTTATGGCAAC-3′ |
|
| R:
5′-GATGTTCTTCTCTGTGACCC-3′ |
| BAX | F:
5′-TGGCTGGGGAGACACCTGAGC-3′ |
|
| R:
5′-TCAGCCCATCTTCTTCCAGATG-3′ |
| BCL-2 | F:
5′-GCGCTCAGCCCTGTGCCACC-3′ |
|
| R:
5′-TCATTCAACCAGACATGCAC-3′ |
| GAPDH | F:
5′-GGTCACTTGAAGGGTGG-3′ |
|
| R:
5′-CCATTCGTTGTCGTACCA-3′ |
Western blot analysis
The preserved atrial tissues were cleaned with
distilled water, and total proteins were extracted using
radioimmunoprecipitation assay lysis buffer (Gibco; Thermo Fisher
Scientific Inc.) with protease inhibitors (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) on ice for 5–15 min. Centrifugation was
performed at 4°C at a speed of 12,000 × g for 10 min. The
supernatants were collected and protein concentration was detected
using a bicinchoninic acid protein assay (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Equal amounts of extracted protein
samples (20 µg) were separated by 8% SDS-PAGE and transferred onto
nitrocellulose membranes. Membranes were probed with the following
primary antibodies at 4°C overnight: Anti-rabbit immunoglobulin
(Ig) G Fc antibody (RMG02; cat. no. ab190492; 1:500; Abcam,
Cambridge, MA, USA), anti-MMP-7 (cat no. ab189277; 1:500; Abcam),
anti-Col I (cat no. ab90395; 1:20; Abcam), anti-TIMP-2 (cat no.
ab180630; 1:500; Abcam), anti-BCL-2 (cat no. ab59348; 1:500; Abcam)
and anti-BAX (cat no. ab32503; 1:1,000; Abcam). Subsequently, they
were incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat. no. ab6721; 1:5,000; Abcam) at room
temperature for 30 min. Then Horseradish Peroxidase (HRP) (Bio-Rad
Laboratories, Inc.) was added for color rendering. Protein bands
were visualized using enhanced chemiluminescence. Blots were
semi-quantified by densitometry using Image Quant 350 and Image
Quant TL software version 1 (GE Healthcare Life Sciences, Little
Chalfont, UK). β-actin served as the internal control (13).
Statistical analysis
Statistical analyses were performed sing SPSS
software version 20.0 (IBM Corp., Armonk, NY, USA). Data are
expressed as the means ± standard deviation. The statistical
significance of the differences between groups was assessed
unpaired Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. In addition, the Pearson
correlation test was used for correlation analysis with
0.8<r<1.0 considered as a strong correlation.
Results
Comparison of cardiac function
parameters between the AF and control groups
LVEF and LAD in the control group were significantly
higher than those in the AF group (P<0.05); while LADI in the
control group was significantly lower than that in the AF group
(P<0.05). No significant difference was identified in LVEDD and
LVPWT between the two groups (P>0.05; Table II). The cardiac function of the AF
group was demonstrated to be significantly lower than that of the
control group, indicating the successful establishment of an AF
model.
 | Table II.Comparison of cardiac function
parameters between the AF and control groups. |
Table II.
Comparison of cardiac function
parameters between the AF and control groups.
| Parameter | Control group
(n=10) | AF group (n=10) | P-value |
|---|
| LAD (mm) |
31.61±4.19 |
23.91±3.36 | <0.001 |
| LADI
(cm/m2) |
2.47±0.14 |
3.72±0.38 | <0.001 |
| LVEDD (mm) |
1.05±0.10 |
0.98±0.11 | 0.156 |
| LVEF (%) |
57.77±4.39 |
51.57±5.31 | 0.011 |
| LVPWT (mm) |
7.35±0.85 |
7.31±1.33 | 0.937 |
Comparison of cardiac fibroses between
the AF and control groups
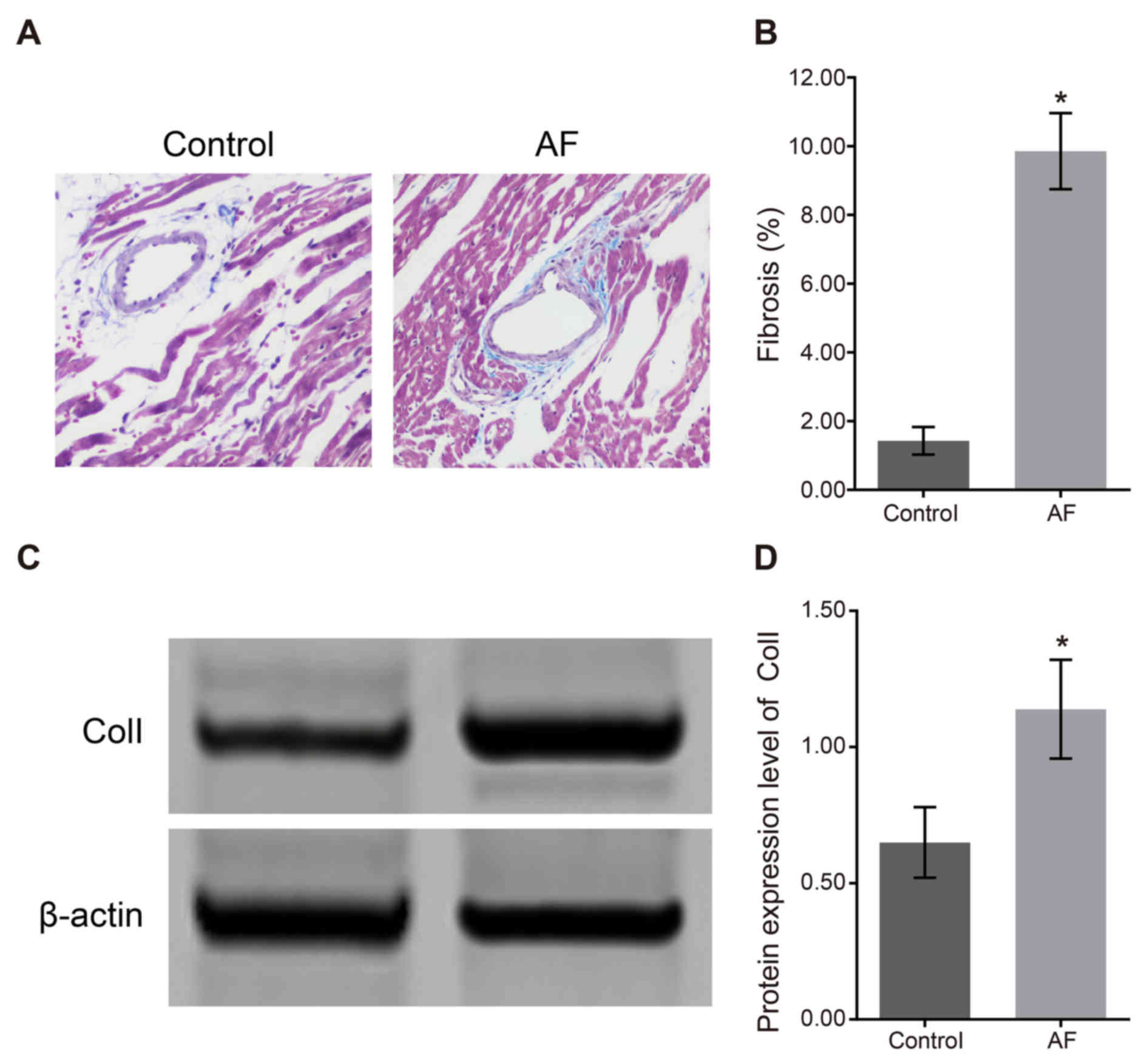
The Mallory's trichrome staining results of atrial
fibrosis (Fig. 1A) demonstrated
that the degree of atrial fibrosis in the control group was
significantly lower than that in the AF group. In the AF group, the
fibrous tissue samples in the left atrial myocytes in the
myocardial tissue were compact and intact, meanwhile muscle bundles
were separated by connective tissues, which was characterized by
over accumulation of collagen and deposition in connective tissues.
As shown in Fig. 1B, the
proportion of fibrotic tissue in the control group (1.43±0.40%) was
significantly lower than that in the AF group (9.86±1.11%;
P<0.05). The western blotting result of atrial fibrosis factor
Col I (Fig. 1C) demonstrated that
the relative expression of Col I protein in the control group was
0.65±0.13, which was significantly lower than that in the AF group
(1.14±0.18; P<0.05). The results of protein expression (Fig. 1D) indicated that the degree of
cardiac fibrosis was increased following AF.
Comparisons of the mRNA expression
levels of MMP-7 and other apoptosis-associated genes between the AF
and control groups
GAPDH mRNA expression in the control group was set
as 1. Compared with the control group, MMP-7 and BAX mRNA
expression levels in the AF group were elevated (P<0.05), while
BCL-2 and TIMP-2 mRNA expression levels were decreased (P<0.05;
Fig. 2). The results indicate that
the elevated mRNA expression levels of MMP-7 and BAX, and the
decreased mRNA expression levels of TIMP-2 and BCL-2 may lead to
AF.
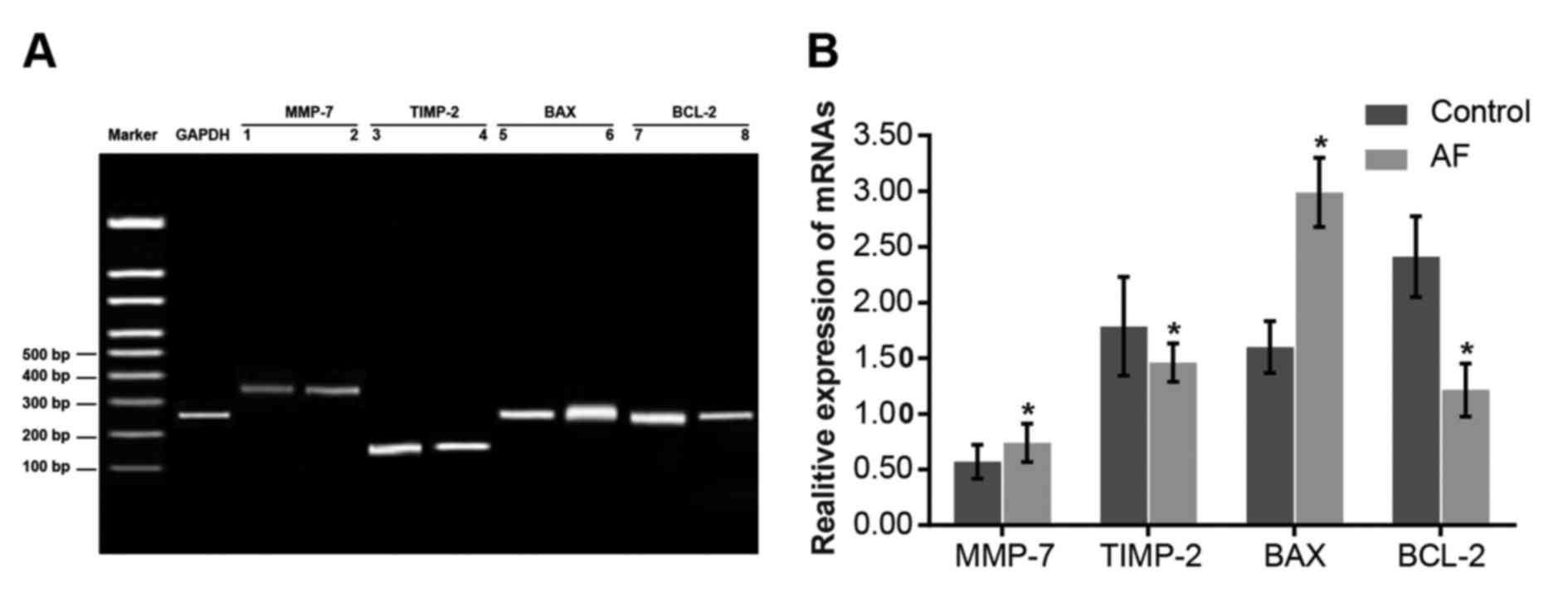 | Figure 2.Comparison of the mRNA expression
levels of MMP-7 and other apoptosis-associated genes between the AF
and control groups. (A) Reverse transcription-quantitative
polymerase chain reaction of MMP-7 and other apoptosis-associate
genes. The expression levels of the control group are presented in
bands 1, 3, 5 and 7, and the expression levels of the AF group are
presented in bands 2, 4, 6 and 8. (B) mRNA expression levels of
MMP-7 and other apoptosis-associated genes. Data are expressed as
the mean ± standard deviation. *P<0.05 vs. the control group.
AF, atrial fibrillation; MMP-7, matrix metalloproteinase-7; TIMP-2,
TIMP metallopeptidase inhibitor 2; BAX, BCL-2 associated X,
apoptosis regulator; BCL-2, BCL2, apoptosis regulator. |
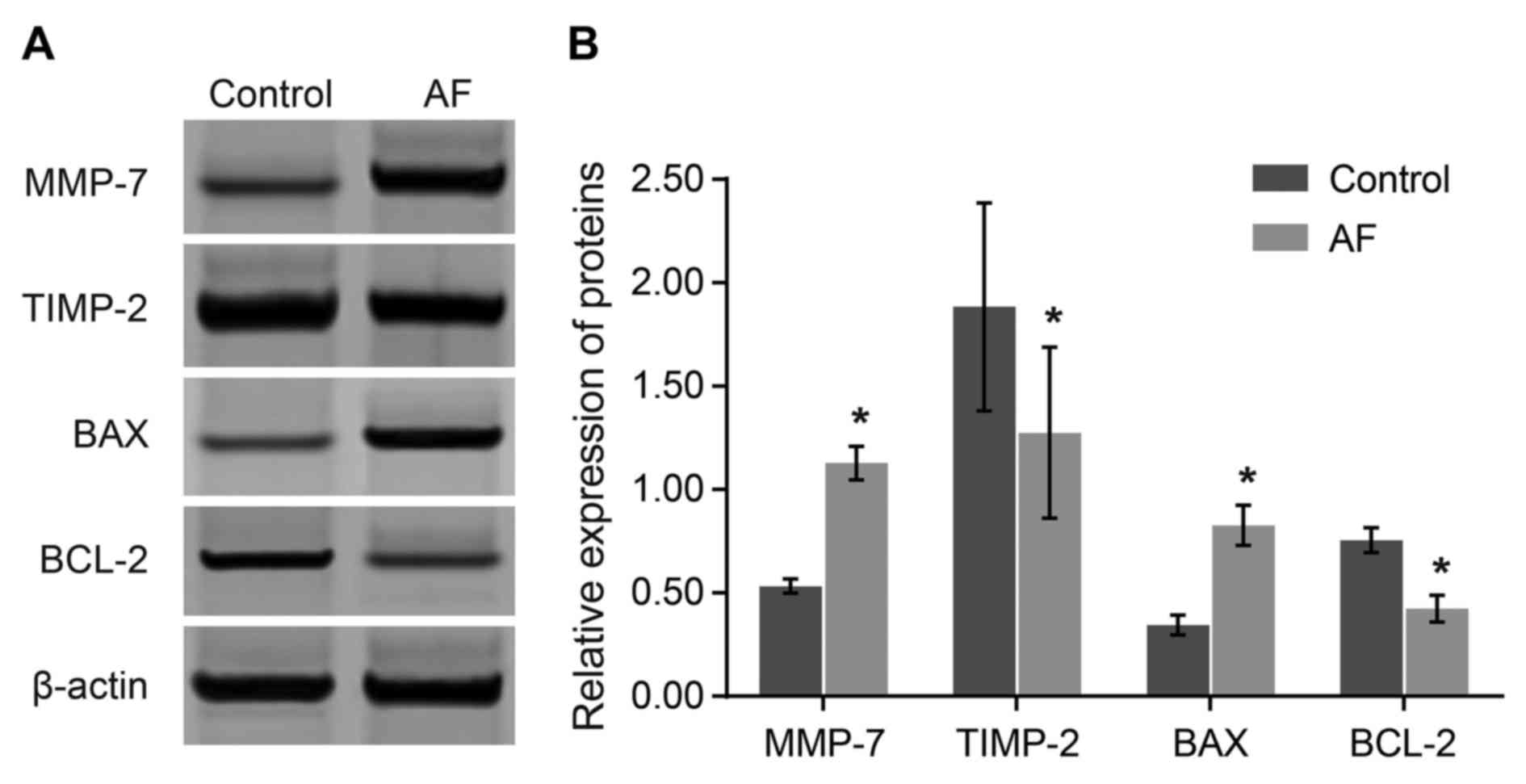
Comparisons of the protein expression
levels of MMP-7 and other apoptosis-associated genes between the AF
and control groups
The protein level of the relative reference, β-actin
in the control group was set as 1. Compared with the control group,
protein expression levels of MMP-7 and BAX were elevated
(P<0.05), while the protein expression levels of TIMP-2 and
BCL-2 were decreased in the AF group (P<0.05). The results
demonstrated that elevated expression levels of MMP-7 and BAX, and
decreased expression levels of BCL-2 and TIMP-2 may be associated
with AF occurrence (Fig. 3).
Correlation of the protein expression
levels of MMP-7 and other apoptosis-associated genes with the
cardiac function parameters of AF
Pearson correlation analysis was used to analyze the
correlation between MMP-7 together with apoptosis-associated genes
and the cardiac function parameters of AF. According to the
correlation analysis, MMP-7 and BAX expression levels were
negatively correlated with LVEF (r=−0.92; r=−0.96; P<0.001), and
TIMP-2 and BCL-2 expression levels were positively correlated with
LVEF (r=0.96; P<0.001; r=0.97; P=0.002). In addition, MMP-7 and
BAX expression levels were positively correlated with the degree of
cardiac fibrosis (r=0.88; P=0.001; r=0.92; P<0.001), while
TIMP-2 and BCL-2 expression levels were negatively correlated with
the degree of cardiac fibrosis (r=−0.92; r=−0.95; P<0.001;
Table III).
 | Table III.Correlation of the expression levels
of MMP-7 and apoptosis-associated genes, TIMP-2, BAX and BCL-2 with
LVEF and the degree of cardiac fibrosis between the AF and control
groups. |
Table III.
Correlation of the expression levels
of MMP-7 and apoptosis-associated genes, TIMP-2, BAX and BCL-2 with
LVEF and the degree of cardiac fibrosis between the AF and control
groups.
|
| LVEF | Degree of cardiac
fibrosis |
|---|
|
|
|
|
|---|
| Gene | r | P-value | r | P-value |
|---|
| MMP-7 | −0.92 | <0.001 | 0.88 | 0.001 |
| TIMP-2 | 0.96 | <0.001 | −0.92 | <0.001 |
| BAX | −0.96 | <0.001 | 0.92 | <0.001 |
| BCL-2 | 0.97 | 0.002 | −0.95 | <0.001 |
Discussion
By detecting the mRNA and protein expression levels
of MMP-7 and apoptosis-associated genes (TIMP-2, BAX and BCL-2),
the major finding of the current study is that MMP-7 and
apoptosis-associated genes (TIMP-2, BAX and BCL-2) may serve as
indicators of AF, and provide direction for the prognosis and
treatment of AF.
According to the experimental results, the
expression level of MMP-7 in the AF group was increased when
compared with that of the control group, which indicated that the
mRNA expression levels of MMP-7 and other apoptosis-associated
genes are likely to be associated with AF. Increasing evidence
indicates that MMPs are important in normal embryonic development,
tissue remodeling, and pathological and physiological processes
occurring in the human body (16).
Regulating the expression and activity of MMPs and TIMPs may
represent an important clinical treatment strategy to prevent
atrial remodeling (17). A
previous study indicated that MMPs and TIMPs are essential for
cardiac ECM remodeling (18). ECM
is a dynamic structure, whose physiological turnover is mediated by
MMPs and their TIMPs. In addition to providing structural support,
the ECM also function as an extracellular reservoir for multiple
growth factors and cytokines (19). However, MMPs are crucial in the
collagen metabolism process of atrial tissue via gene
transcription, protein expression and activity elevation, causing
excessive degradation of normal collagen and abnormal myocardial
cell gaps in the ECM. The the gap is subsequently filled with other
fibrous tissues and fibrosis gradually increases (20). TIMPs bind to the active site of the
MMPs in a stoichiometric 1:1 molar ratio, thereby blocking access
to the ECM substrates, and also stimulate the proliferation and
differentiation of fibroblasts in cardiac tissue damage sites,
thereby promoting synthesis of the ECM (21,22).
In the infarcted myocardium, activation of the inflammatory cascade
clears the wound of dead cells, whereas stimulating matrix
degradation and chamber dilation contributes to the development of
heart failure (23).
The current study demonstrated that the low
expression level of BCL-2 and the high expression level of BAX were
associated with the occurrence and development of AF. Previous
studies indicate that there are two phylogenetically and
structurally distinct groups of proteins regulating the
stress-induced intrinsic apoptosis, i.e., the programmed
disassembly of cells, among which BCL-2 is an anti-apoptosis
regulator while BAX is a pro-apoptosis protein (24). As BAX and BCL-2 exert opposite
regulatory effects on cell apoptosis, it is considered that there
may be a balance between BAX and BCL-2, which co-regulates the
apoptosis of cells. A previous study found that in cases of atrial
aging or AF, the level of BCL-2 expression in the left atrial
myocardial tissue was downregulated, while the level of BAX
expression was elevated (13). In
addition, it was reported that apoptosis was significantly
elevated, as indicated by the increasing BAX/BCL-2 ratio, after
patients with gastric adenocarcinoma received crocin treatment
(25). Furthermore, a recent study
indicated that MMP may be involved in the occurrence and
development of AF via upregulating BAX expression levels and
downregulating BCL-2 expression levels, which is consistent with
the results of the current study (26). However, a limitation of the present
study was the relatively small number of subjects; thus, future
studies with a larger sample are required to verify the present
results.
In conclusion, the present study provides evidence
that MMP-7 and apoptosis-associated gene expression levels are
correlated with the cardiac function parameters of AF. However, the
underlying mechanisms of these genes in the occurrence and
development of AF remains unknown. Future investigations will focus
on the correlation analysis between the epidemiology of
cardiovascular disease and associated gene expression, with the aim
of improving knowledge of gene therapy for AF, as well as
myocardial infraction, dilated cardiomyopathy and heart
failure.
Acknowledgements
The authors would like to acknowledge the helpful
comments received from our reviewers for this study.
References
|
1
|
Gu J, Hu W and Liu X: The value of
magnetic resonance imaging in catheter ablation of atrial
fibrillation. Clin Cardiol. 38:190–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandolfo C, Balestrino M, Bruno C,
Finocchi C and Reale N: Validation of a simple method for atrial
fibrillation screening in patients with stroke. Neurol Sci.
36:1675–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carpi A, Cini G, Russo M, Antonelli A,
Gaudio C, Galetta F, Franzoni F and Rossi G: Subclinical
hyperthyroidism and cardiovascular manifestations: A reevaluation
of the association. Intern Emerg Med. 8 Suppl 1:S75–S77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marrouche NF, Wilber D, Hindricks G, Jais
P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, et
al: Association of atrial tissue fibrosis identified by delayed
enhancement MRI and atrial fibrillation catheter ablation: The
DECAAF study. JAMA. 311:498–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mukherjee R, Akar JG, Wharton JM, Adams
DK, McClure CD, Stroud RE, Rice AD, DeSantis SM, Spinale FG and
Gold MR: Plasma profiles of matrix metalloproteinases and tissue
inhibitors of the metalloproteinases predict recurrence of atrial
fibrillation following cardioversion. J Cardiovasc Transl Res.
6:528–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zhang H, Chai F, Liu X and Berk M:
The effects of escitalopram on myocardial apoptosis and the
expression of Bax and Bcl-2 during myocardial ischemia/reperfusion
in a model of rats with depression. BMC Psychiatry. 14:3492014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papazafiropoulou A and Tentolouris N:
Matrix metalloproteinases and cardiovascular diseases. Hippokratia.
13:76–82. 2009.PubMed/NCBI
|
|
8
|
Loreto C, Leonardi R, Musumeci G, Pannone
G and Castorina S: An ex vivo study on immunohistochemical
localization of MMP-7 and MMP-9 in temporomandibular joint discs
with internal derangement. Eur J Histochem. 57:e122013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Grady A, Dunne C, O'Kelly P, Murphy GM,
Leader M and Kay E: Differential expression of matrix
metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of
metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer:
Implications for tumour progression. Histopathology. 51:793–804.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CL, Huang SK, Lin JL, Lai LP, Lai SC,
Liu CW, Chen WC, Wen CH and Lin CS: Upregulation of matrix
metalloproteinase-9 and tissue inhibitors of metalloproteinases in
rapid atrial pacing-induced atrial fibrillation. J Mol Cell
Cardiol. 45:742–753. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barclay LA, Wales TE, Garner TP, Wachter
F, Lee S, Guerra RM, Stewart ML, Braun CR, Bird GH, Gavathiotis E,
et al: Inhibition of Pro-apoptotic BAX by a noncanonical
interaction mechanism. Mol Cell. 57:873–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lewkowicz J, Knapp M, Tankiewicz-Kwedlo A,
Sawicki R, Kamińska M, Waszkiewicz E and Musiał WJ: MMP-9 in atrial
remodeling in patients with atrial fibrillation. Ann Cardiol
Angeiol (Paris). 64:285–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu GJ, Gan TY, Tang BP, Chen ZH, Mahemuti
A, Jiang T, Song JG, Guo X, Li YD, Miao HJ, et al: Accelerated
fibrosis and apoptosis with ageing and in atrial fibrillation:
Adaptive responses with maladaptive consequences. Exp Ther Med.
5:723–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Myers RP, Patel K, Pianko S, Poynard T and
McHutchison JG: The rate of fibrosis progression is an independent
predictor of the response to antiviral therapy in chronic hepatitis
C. J Viral Hepat. 10:16–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tesfaigzi Y, Myers OB, Stidley CA, Schwalm
K, Picchi M, Crowell RE, Gilliland FD and Belinsky SA: Genotypes in
matrix metalloproteinase 9 are a risk factor for COPD. Int J Chron
Obstruct Pulmon Dis. 1:267–278. 2006.PubMed/NCBI
|
|
17
|
Wang W, Zhang HT and Yang XL: Effect of
matrix metalloproteinase and their inhibitors on atrial myocardial
structural remodeling. J Cardiovasc Med (Hagerstown). 14:265–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalogeropoulos AS, Tsiodras S, Rigopoulos
AG, Sakadakis EA, Triantafyllis A, Kremastinos DT and Rizos I:
Novel association patterns of cardiac remodeling markers in
patients with essential hypertension and atrial fibrillation. BMC
Cardiovasc Disord. 11:772011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takawale A, Sakamuri SS and Kassiri Z:
Extracellular matrix communication and turnover in cardiac
physiology and pathology. Compr Physiol. 5:687–719. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spinale FG: Myocardial matrix remodeling
and the matrix metalloproteinases: Influence on cardiac form and
function. Physiol Rev. 87:1285–1342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterisation of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vanhoutte D, Schellings M, Pinto Y and
Heymans S: Relevance of matrix metalloproteinases and their
inhibitors after myocardial infarction: A temporal and spatial
window. Cardiovasc Res. 69:604–613. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saxena A, Chen W, Su Y, Rai V, Uche OU, Li
N and Frangogiannis NG: IL-1 induces proinflammatory leukocyte
infiltration and regulates fibroblast phenotype in the infarcted
myocardium. J Immunol. 191:4838–4848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoshyar R, Bathaie SZ and Sadeghizadeh M:
Crocin triggers the apoptosis through increasing the Bax/Bcl-2
ratio and caspase activation in human gastric adenocarcinoma, AGS,
cells. DNA Cell Biol. 32:50–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diao SL, Xu HP, Zhang B, Ma BX and Liu XL:
Associations of MMP-2, BAX, and BCL-2 mRNA and protein expressions
with development of atrial fibrillation. Med Sci Monit.
22:1497–1507. 2016. View Article : Google Scholar : PubMed/NCBI
|