Introduction
Cardiovascular diseases are a global cause of
mortality, and acute myocardial infarction (AMI) affects >1.5
million people in the United States annually (1). The effects of AMI are usually
attributable to the detrimental effects of acute myocardial
ischemia-reperfusion injury. In the process of myocardial
ischemia/reperfusion injury mitochondrial function is disturbed,
ROS overexpression and finally causing oxidative stress, energy
metabolism disorder, inflammation and ion channel dysfunction,
which may lead to DNA oxidation, promoting chain reactions of
membrane lipid peroxidation. Mitochondrial uncoupling proteins
(UCPs) are mitochondrial inner membrane proteins that maintain
calcium homeostasis and dissipate the proton electrochemical
gradient, resulting in the generation of heat, decreased ATP
production and reduced generation of mitochondrial reactive oxygen
species (ROS) (2). UCP-2 is a
member of the UCP family and has functions including regulation of
insulin metabolism, modulation of mitochondrial Ca2+
uptake and modulation of apoptosis (3).
Sirtuin 1 (SIRT1) is a nicotinamide adenine
dinucleotide-dependent histone deacetylase (4). It has multiple biological functions,
including transcription regulation, cell cycle regulation and
anti-apoptosis functions (5).
SIRT1 represses UCP-2 by binding directly to the UCP-2 promoter
(6), and its activation mediates
sildenafil-induced delayed cardioprotection against
ischemia-reperfusion injury in mice (7).
The aim of the present study was to explore the
functional significance of the SIRT1-UCP-2 axis in hypoxia-induced
myocardial infarction.
Materials and methods
Cell culture and in vitro ischemia
injury model
Q293T was obtained from the Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai, China)
and used for lentivirus amplification. Rat H9c2 cell lines were
obtained from the ATCC (American Type Culture Collection, Manassas,
VA, USA), cultured in high glucose Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and supplemented with 10% (v/v) fetal bovine serum (FBS;
Gibco/Invitrogen; Thermo Fisher Scientific, Inc.), 100 µg/ml
penicillin, 100 µg/ml streptomycin at 37°C in a humidified
incubator with 5% CO2.
When the H9c2 cells were cultured to 70–80%
confluence, the medium was changed to Krebs-Ringer Bicarbonate
buffer (115 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM
KH2PO4, 1.2 mM MgSO4, 24 mM
NaHCO3, 10 mM HEPES; pH 7.4) and cultured at 95%
N2 and 5% CO2, creating an anoxic environment
to stimulate hypoxia (8).
In vitro cell growth assays
When the cells were cultured to 70–80% confluence,
SIRT1 or UCP2 shRNA were transfected into the medium to overexpress
SIRT1 or decrease expression of UCP2, using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.). 24 h following transfection,
cells underwent 2% O2 hypoxia treatment for 2, 4, 6, 8,
12 or 24 h. Cell number was then counted under a light microscope
following trypan blue staining. All counts were performed on
triplicate wells and repeated in three independent experiments at
×100 magnification with Image J (version, 1.6.0.24; National
Institutes of Health, Bethesda, MD, USA).
In vitro cell survival was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were treated with 50 µM MTT for 4 h at 37°C, and the
formazan crystals were dissolved with 200 µl dimethyl sulfoxide for
10 min at room temperature. Absorbance was measured at a wavelength
of 570 nm.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from heart tissue treated with or without
I/R and H9c2 cells were extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). RNA concentration was determined
by UV spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA), reverse-transcribed using M-MMLV
reverse transcriptase with RNasin Ribonuclease Inhibitors (Promega
Corporation, Madison, WI, USA) at 42°C for 30 min, 95°C for 5 min.
qPCR amplifications were carried out using the StepOnePlus™
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) and Thunderbird SYBR Master Mix (Toyobo Co., Ltd, Osaka,
Japan). Primer sequences were: UCP-2, forward
5′-TGTGGTAAAGGTCCGCTTCC-3′ and reverse 5′-CCCGAAGGCAGAAGTGAAGT-3′;
Sirt-1, forward 5′-TGAGGTAGGGTCCCCGTTTA-3′ and reverse
5′-TTGAGGCCGGTTTGGCTTAT-3′; and GAPDH, forward
5′-GATCCCGCTAACATCAAATG-3′ and reverse 5′-GAGGGAGTTGTCATATTTCTC-3′.
qPCR was performed with the following cycles: 95°C for 10 min,
followed by 95°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec
for 40 cycles. GAPDH expression was used as an internal control.
2−ΔΔCq was calculated for every sample. The mRNA
expression levels were indicated with 2−ΔΔCq and
normalized to GAPDH (9).
In-vitro UCP2 gene silencing and
retroviral infection of SIRT1
Lentivirus containing Rat UCP-2 shRNA (cat.
no. V3SR11242238929649; Dharmacon, Inc.; GE Healthcare Life
Sciences, Chalfont, UK) was used for UCP-2 gene silencing, and
Lentivirus containing a scrambled sequence (shScramble,
VSH11660, Thermo Scientific Open Biosystems) was used as the
negative control. Lentivirus was produced in transfected
293T packaging cells as described previously (10).
Q293A cells were transfected with retroviral vectors
expressing rat SIRT1 (cat. no. 4331182 Thermo Fisher Scientific,
Inc.) using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.),
and changed to fresh medium on the following day. Following 30 h
further incubation, retroviral particle-containing supernatant was
harvested and filtered through a 0.45 µm filter (EMD Millipore,
Billerica, MA, USA). H9c2 cells were transduced with virus
supernatant. Transduced cells were used for RT-qPCR or western blot
assays 48 h later.
Luciferase reporter assay
To determine whether the entire rat UCP-2
3′-untranslated region (UTR) segment was amplified by PCR, using
mouse genomic DNA as a template. The PCR products were inserted
into the p-MIR-reporter plasmid (Ambion; Thermo Fisher Scientific,
Inc.). 2×105 cells were seeded in 6-well plates and
transfected with 1 µg of firefly luciferase reporter plasmid and
0.5 µg of Renilla expression vector as a transfection control
(Ambion; Thermo Fisher Scientific, Inc.) for luciferase assays.
Cells were collected and measured 48 h following transfection
according to the manufacturer's instructions (Dual-Luciferase Assay
System; Promega Corporation, Madison, WI, USA).
ATP level assay and medium pH
measurement
ATP levels were measured with a Molecular Probes ATP
Determination kit (A22066; Invitrogen; Thermo Fisher Scientific,
Inc.). 100 µl lysis buffer was added into H9c2 cells to prepare the
total lysate at room temperature and was centrifuged at 2380 ×
g at 4°C for 15 min, and the supernatant was retrieved for
use in the ATP assay according to the manufacturer's instructions.
Protein concentration was measured via bicinchoninic acid (BCA)
assay. The pH of the extracellular medium was measured using pH
meter (pB-10, Sartorius AG, Göttingen, Germany).
Animals and in vivo myocardial
ischemia/reperfusion protocol
A total of 30 adult male C57BL/6 mice, 8 weeks old
and weighing 30.2±3.5 g were supplied by the Model Animal Research
Center of Nanjing University (Nanjing, China). All animal
experiments were conducted under the guidelines on humane use and
care of laboratory animals for biomedical research published by the
U.S. National Institutes of Health (NIH Publication No. 85–23,
revised 1996). All experimental preparations and protocols
involving animals were reviewed and approved by the Animal Care and
Use Committee of Nanjing University (Nanjing, China).
Resveratrol stock solution was dissolved in DMSO
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and then
diluted into 5 mg/ml with saline, and the DMSO was less than 5%.
Resveratrol (5 mg/ml) or saline was injected intraperitoneally
(i.p.) 24 h prior to subjection to 1 h of myocardial
ischemia and 24 h of reperfusion. Mice were anesthetized with
pentobarbital sodium (60 mg/kg). Ischemia was achieved through
ligation of the left anterior descending coronary artery (LAD)
using an 8-0 silk suture with a section of PE-10 tubing placed over
the LAD, 1 mm from the tip of the normally positioned left atrium.
Following ischemia for 1 h, reperfusion was initiated by releasing
the ligature. Sham-operated control mice underwent the same
surgical procedures except that the suture placed under the left
coronary artery was not tied. A total of 30 animals were included
and randomly distributed into the sham, I/R and RES+IR groups, with
10 animals in each group.
Hearts were harvested 24 h subsequent to this
directly for measurement of infarct size and stored in liquid
nitrogen immediately, and was processed using TRIzol (Thermo Fisher
Scientific, Inc.) for RT-qPCR assays or radioimmunoprecipitation
assay buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.) for
western blot assays.
Measurement of infarct size
Following reperfusion, myocardial infarct size was
evaluated by tetrazolium chloride (TTC) staining. The hearts were
immediately removed and sectioned into 5 mm thick short-axis
sections from the apex towards the base of the heart, then
incubated in 1% TTC for 20 min at 37°C. Following this, the
sections were photographed with a Nikon D810 digital camera (Nikon
Corporation, Tokyo, Japan). Areas of infarct size were measured
digitally using Image Pro Plus software 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA).
Western blot analysis
Protein concentrations of the complete lysate were
analyzed with the Bradford protein assay. Protein extracts were
incubated for 10 min at 100°C in sample buffer (10% (v/v) glycerol,
5% (w/v) sodium dodecyl sulfate (SDS), 0.25% (w/v) bromophenol
blue, 5% (v/v) 2-mercaptoethanol, and 0.0625 M Tris-HCl, pH 6.8)
prior to loading onto the gels. A total of 30 µg of homogenate
protein was loaded into each lane of a 12% SDS-PAGE mini-gel
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Electrophoresis
was conducted at 80 V in running buffer (0.025 M Tris-HCl, 0.2 M
glycine, 1 mM EDTA and 3.5 mM SDS). Proteins were subsequently
transferred to a polyvinylidene fluoride membrane (GE Healthcare
Life Sciences, Chalfont, UK) and blocked at room temperature with
5% milk in TBS with 5% Tween-20 (TBST) for 1 h. Western blotting
was performed with mouse monoclonal to GAPDH primary antibody
(1:2000; ab8245; Abcam, Cambridge, UK), polyclonal mouse anti-UCP2
primary antibody (1:1,000; ab67241; Abcam) or mouse anti-SIRT1
primary antibody (1:1,000; ab104833; Abcam), followed by goat
anti-mouse horseradish peroxidase-conjugated secondary antibody
(sc-3791; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
dilution of 1:5,000. Following washing, the bands were detected by
an enhanced chemiluminescence reagent (EMD Millipore). The band
intensities were quantified using the Quantity One 1-D software
(version, 4.6.6) in ChemiDoc™ MP System (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All results were expressed as the mean + standard
error from at least three independent experiments. Student's
t-test was used for normally distributed data, and the
Mann-Whitney U test was used for abnormally distributed data.
P<0.05 and P<0.01 were considered to indicate a statistically
significant and a highly significant difference, respectively.
Results
Decreased expression of SIRT1 is
concomitant with increased expression of UCP-2 in hypoxic rat
cardiomyocytes (H9c2)
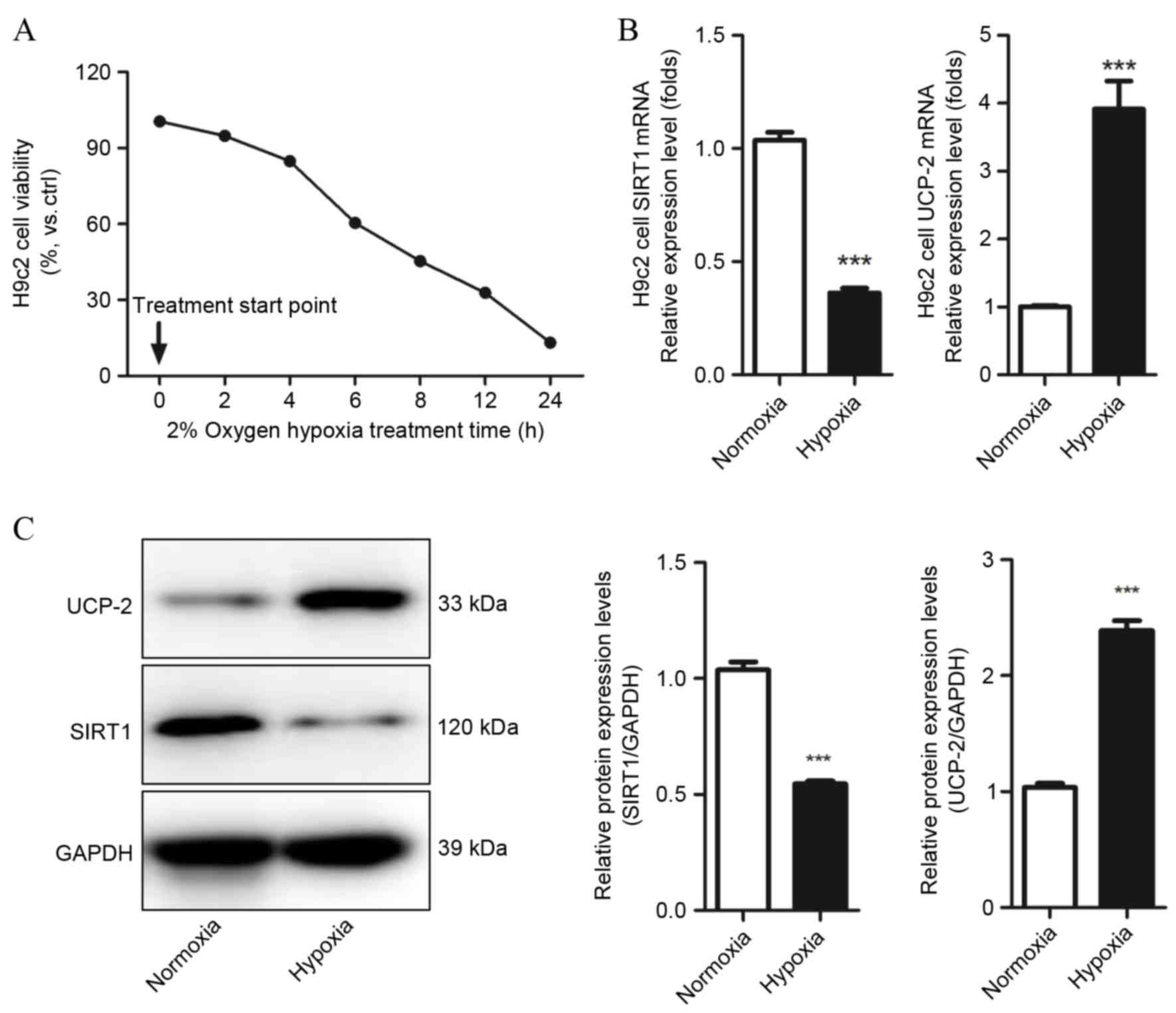
Cell viability was detected by MTT assays and cell
counting to evaluate whether H9c2 cells were suffering from hypoxic
injury, and determine which hypoxia condition should be used in
subsequent experiments. Hypoxia markedly decreased H9c2 cell
viability and number as time increased, and following 6 h hypoxia
treatment, cell viability decreased to 61.25±5.14% compared with
the control group (P=0.021 Fig.
1A). Therefore, this condition was used in subsequent
experiments. SIRT1 and UCP-2 mRNA and protein levels in H9c2 under
normoxic and hypoxic conditions were evaluated by qPCR and western
blot assays. Decreased expression of SIRT1 mRNA and protein was
observed in hypoxic H9c2 cells compared with normoxic H9c2 cells
(P=0.0001 and P=0.0004, respectively; Fig. 1B and C, respectively). UCP-2 mRNA
and protein levels were significantly increased in hypoxic H9c2
cells compared with normoxic H9c2 cells (P=0.0008 and P=0.0007,
respectively; Fig. 1B and C,
respectively). This result demonstrates that SIRT1 and UCP-2 may be
involved in the development of hypoxia-induced injury in
cardiomyocytes.
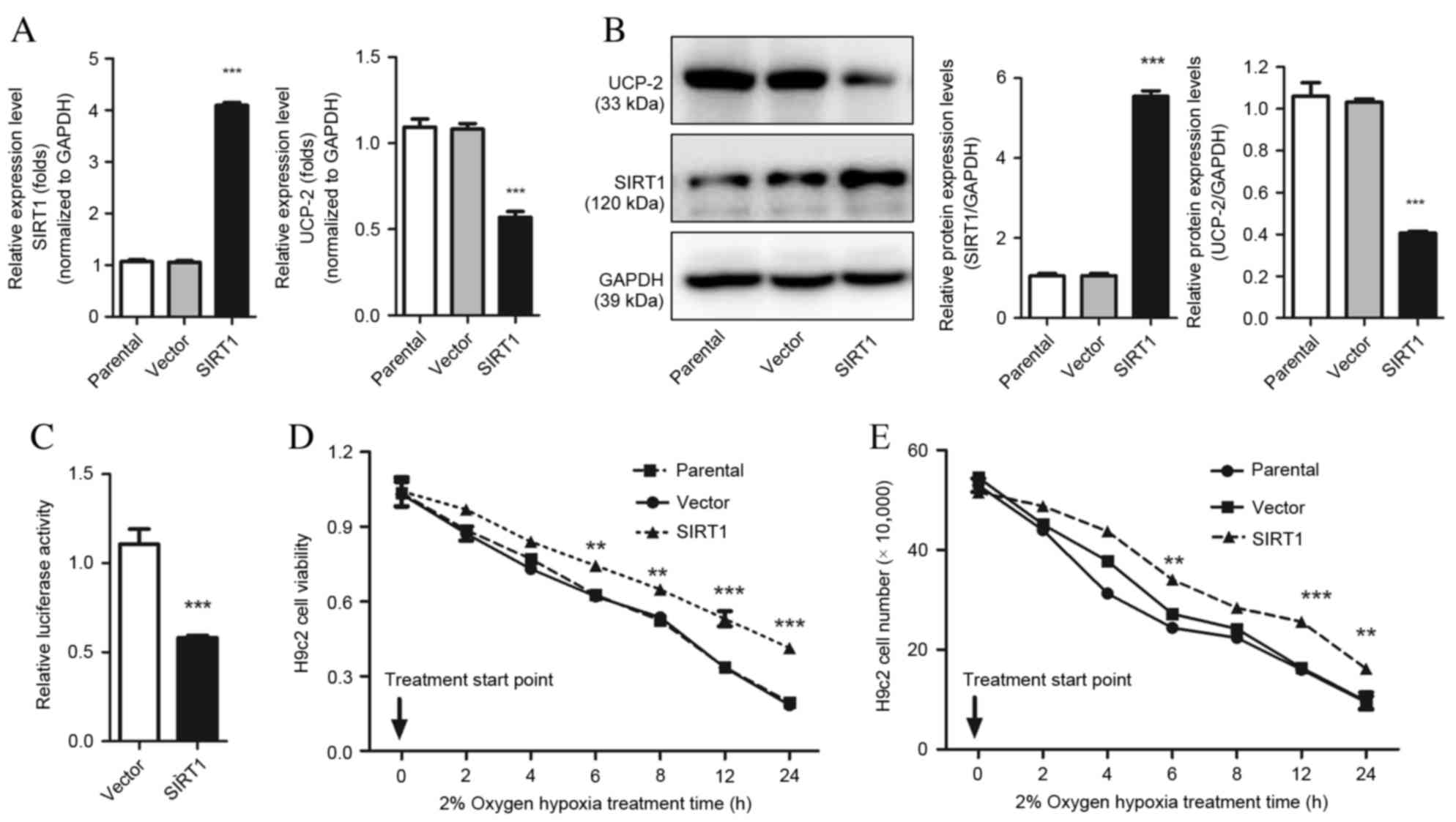
Overexpression of SIRT1 results in
increased cell survival following hypoxic injury by directly
regulating UCP-2 expression
The effect of SIRT1 in hypoxia-induced cardiomyocyte
injury was explored using gain-of-function methods in H9c2 cells.
UCP-2 mRNA and protein expression was significantly downregulated
in H9c2 cells overexpressing SIRT1, compared with scramble control
cells (P=0.0003 and P=0.0002; Fig. 2A
and B, respectively). Furthermore, the dual luciferase assay
revealed that overexpression of SIRT1 in H9c2 cells repressed UCP-2
(P=0.0004; Fig. 2C). This evidence
demonstrates that SIRT1 directly regulates the expression of UCP-2
by binding directly to the UCP-2 promoter. The viability of H9c2
cells overexpressing SIRT1 also increased significantly compared
with the parental strain and empty vector control, demonstrated by
MTT assay and cell counting (P<0.01; Fig. 2D and E).
Knockdown of UCP-2 in H9c2 cells
increases cell survival following hypoxia injury through
restoration of ATP biosynthesis
shRNA against UCP-2 was used to block the expression
of UCP-2 to study the effects of absence of UCP-2 on cell survival
under hypoxic conditions. UCP-2 specific shRNA significantly
reduced UCP-2 protein and mRNA expression levels compared with
scramble control cells (P=0.0008 and P=0.0009 respectively;
Fig. 3A and B, respectively).
Increased cell viability was observed in H9c2 cells transfected
with UCP-2 shRNA compared with scramble control cells from 4 h
(P<0.01; Fig. 3C) demonstrating
that UCP-2 knockdown improves cell resistance to hypoxia.
This enhanced cell survival in response to hypoxia
treatment, induced by the decreased expression of UCP2, may have
been mediated by the regulation of ATP biosynthesis. Under
normoxia, there was no significant difference between the UCP-2
shRNA and scramble shRNA groups (11). However, increased ATP accumulation
under hypoxic conditions was revealed in the UCP-2 knockdown group
compared with the scramble and parental controls (P=0.0006 and
P=0.0002 Fig. 3D). In addition,
the pH of the extracellular medium was increased compared to the
scramble and the parental controls (P=0.0006 and P=0.0003; Fig. 3D).
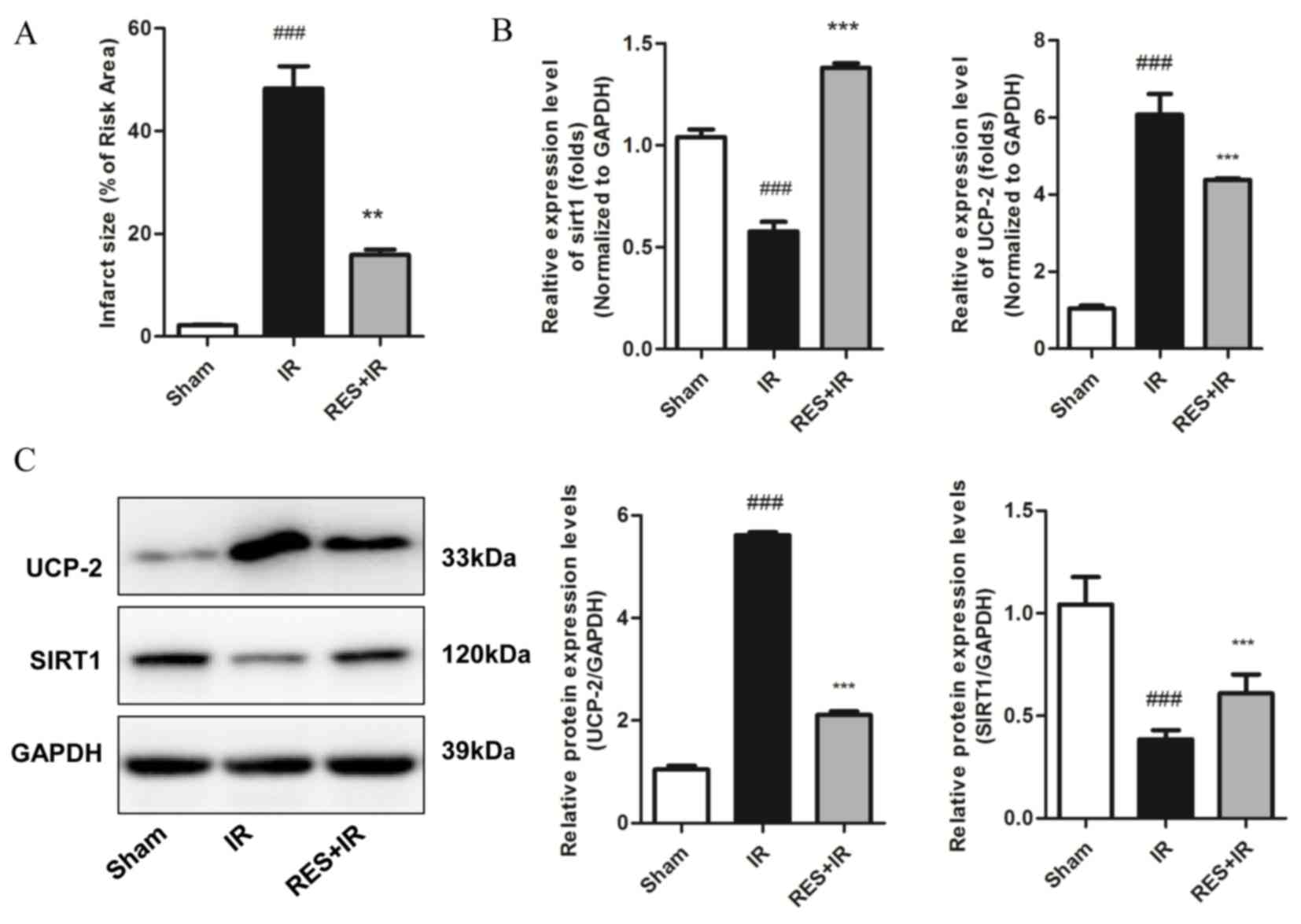
SIRT1 increases resistance to
ischemia/reperfusion injury in vivo by decreasing UCP-2
expression
Based on the finding that exogenous expression of
SIRT1 in H9c2 cells improved resistance to ischemia injury in
vitro, potentially through reducing the expression of UCP-2 and
therefore increasing ATP levels, the effect of a putative activator
of SIRT1, resveratrol, on ischemia/reperfusion injury was explored
in vivo. The results demonstrated that treatment with
resveratrol significantly reduced infarct size compared with the
saline control (P=0.003 Fig. 4A),
suggesting a protective effect against myocardial
ischemia/reperfusion injury. Higher mRNA and protein expression
levels of SIRT1 in resveratrol-treated myocardial tissue compared
with saline controls were validated by RT-qPCR and western blot,
respectively, and were concomitant with decreased expression of
UCP-2 mRNA and protein (P<0.05; Fig. 4B and C, respectively). These
findings demonstrate that the SIRT1/UCP2 axis may represent a novel
therapeutic target for myocardial ischemia/reperfusion injury
treatment.
Discussion
The present study demonstrated a significant
increase in UCP-2 protein expression and reduced SIRT1 protein
expression in response to hypoxia treatment in the H9c2 cell line.
Overexpression of SIRT1 in H9c2 cells promoted cell proliferation
and confers resistance to hypoxia. Meanwhile, knockdown of UCP-2 by
short hairpin RNA (shRNA) in H9c2 cells increased cell
proliferation and significantly increased resistance to hypoxia,
potentially through increasing ATP levels. SIRT1 was demonstrated
to decrease the luciferase activity of the UCP-2 gene promoter and,
in vivo, resveratrol-induced increases in SIRT1 levels
reduced UCP-2 mRNA and protein levels in a murine
ischemia/reperfusion injury model. These findings suggest that
SIRT1 may promote hypoxia resistance in cardiomyocytes, mediated
via its regulation of UCP-2.
SIRT1 has been demonstrated to protect against
cardiovascular diseases, cancer, and neural dysfunction (12–14).
Despite its regulatory function in the cell cycle, apoptosis, DNA
repair, metabolism and oxidative stress, the involvement of SIRT1
in cardiac cell ischemia/reperfusion injury remains unclear. The
present study demonstrated that SIRT1 mRNA and protein levels
decreased in response to hypoxia. Furthermore, overexpression of
SIRT1 in cardiomyocytes was demonstrated to increase cell survival
under hypoxic conditions, indicating that SIRT1 is important in
protecting cardiomyocytes during hypoxia.
As a metabolic regulator, UCP-2 protects
cardiomyocytes from oxidative stress-induced cell death by reducing
the production of ROS in mitochondria (15). Overexpression of UCP-2 in A549
cells has also been demonstrated to promote resistance to hypoxia
by inhibiting ROS accumulation and apoptosis (16). However, UCP-2 deficiencies have
been demonstrated to increase hepatocyte survival and ATP levels
under conditions of ischemia/reperfusion, inhibiting steatosis
(2). Prior to the present study no
data were available on the function of UCP-2 in cardiac
ischemia/reperfusion injury. In the present study, increased
expression of UCP-2 was observed in cardiomyocytes under hypoxic
conditions in vitro. This was consistent with previous
findings stating that exposure to oxidative stress upregulates and
activate UCPs, potentially as a defensive mechanism against
oxidative injury (17,18).
However, the present study demonstrated that
exogenous expression of SIRT1 resulted in decreased expression of
UCP-2, and conferred protection to H9c2 cells under hypoxic
conditions in vitro. Furthermore, the function of UCP-2 was
further revealed by the finding that knockdown of UCP-2 increased
cell viability, augmented ATP concentration and ameliorated
acidosis in H9c2 cells under hypoxic conditions in vitro.
These findings were consistent with a previous study demonstrating
the function of the SIRT1/UCP-2 axis in protecting against cerebral
ischemia in rats (19). This is
direct evidence that the SIRT1/UCP-2 pathway may be a critical
component of the development of ischemia/reperfusion injury in
cardiomyocytes. To further investigate the effect of SIRT1 on the
expression of UCP-2 in H9c2 cells, dual luciferase assay results
revealed that overexpression of SIRT1 reduced the transactivation
of UCP-2 gene promoters, suggesting that the protective functions
of SIRT1 might be mediated by direct regulation of the UCP-2
expression.
Based on the in vitro evidence supporting the
involvement of the SIRT1/UCP-2 axis in resistance to hypoxia in
H9c2 cells, the in vivo function of SIRT1 in C57BL/6 mice
ischemia/reperfusion injury was investigated. Resveratrol treatment
was demonstrated to increase SIRT1 levels, decrease infarct size
and led to reduced expression of UCP-2 in cardiomyocytes, which
indicated that the SIRT1/UCP-2 axis may be a novel therapeutic
target for myocardial infarction treatment. However, UCPs protect
against oxidative stress-induced cell death (20), but downregulation of UCP-2 protects
against I/R injury, based on the observation of the present
results. The extent to which SIRT1/UCP-2 protects against
ischemia/reperfusion injury and the paradoxical role of UCP-2 in
the heart requires further investigation.
References
|
1
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the Global
Burden of Disease Study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans ZP, Palanisamy AP, Sutter AG, Ellett
JD, Ramshesh VK, Attaway H, Schmidt MG, Schnellmann RG and Chavin
KD: Mitochondrial uncoupling protein-2 deficiency protects
steatotic mouse hepatocytes from hypoxia/reoxygenation. Am J
Physiol Gastrointest Liver Physiol. 302:G336–G342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Echtay KS: Mitochondrial uncoupling
proteins-what is their physiological role? Free Radical Biol Med.
43:1351–1371. 2007. View Article : Google Scholar
|
|
4
|
Haigis MC and Guarente LP: Mammalian
sirtuins-emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verdin E, Hirschey MD, Finley LW and
Haigis MC: Sirtuin regulation of mitochondria: Energy production,
apoptosis, and signaling. Trends Biochem Sci. 35:669–675. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bordone L, Motta MC, Picard F, Robinson A,
Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A,
et al: Sirt1 regulates insulin secretion by repressing UCP2 in
pancreatic beta cells. PLoS Biol. 4:e312006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shalwala M, Zhu S-G, Das A, Salloum FN, Xi
L and Kukreja RC: Sirtuin 1 (SIRT1) activation mediates sildenafil
induced delayed cardioprotection against ischemia-reperfusion
injury in mice. PLoS One. 9:e869772014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiu PY, Luk KF, Leung HY, Ng KM and Ko
KM: Schisandrin B stereoisomers protect against
hypoxia/reoxygenation-induced apoptosis and inhibit associated
changes in Ca2+-induced mitochondrial permeability transition and
mitochondrial membrane potential in H9c2 cardiomyocytes. Life Sci.
82:1092–1101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan Y, Yao YF, Hu SN, Gao J and Zhang LL:
MiR-133a is functionally involved in doxorubicin-resistance in
breast cancer Cells MCF-7 via its regulation of the expression of
uncoupling protein 2. PLoS One. 10:e01298432015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bodyak N, Rigor DL, Chen YS, Han Y,
Bisping E, Pu WT and Kang PM: Uncoupling protein 2 modulates cell
viability in adult rat cardiomyocytes. Am J Physiol Heart Circ
Physiol. 293:H829–H835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Onofrio N, Vitiello M, Casale R,
Servillo L, Giovane A and Balestrieri ML: Sirtuins in vascular
diseases: Emerging roles and therapeutic potential. Biochim Biophys
Acta. 1852:1311–1322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li C, Wang L, Zheng L, Zhan X, Xu B, Jiang
J and Wu C: SIRT1 expression is associated with poor prognosis of
lung adenocarcinoma. Onco Targets Ther. 8:977–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orozco-Solis R, Ramadori G, Coppari R and
Sassone-Corsi P: SIRT1 relays nutritional inputs to the circadian
clock through the Sf1 neurons of the ventromedial hypothalamus.
Endocrinology. 156:2174–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Teshima Y, Akao M, Jones SP and Marbán E:
Uncoupling protein-2 overexpression inhibits mitochondrial death
pathway in cardiomyocytes. Circ Res. 93:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng S, Yang Y, Han Y, Li X, Wang X, Li X,
Zhang Z and Wang Y: UCP2 inhibits ROS-mediated apoptosis in A549
under hypoxic conditions. PLoS One. 7:e307142012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McLeod CJ, Aziz A, Hoyt RF Jr, McCoy JP Jr
and Sack MN: Uncoupling proteins 2 and 3 function in concert to
augment tolerance to cardiac ischemia. J Biol Chem.
280:33470–33476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kukat A, Dogan SA, Edgar D, Mourier A,
Jacoby C, Maiti P, Mauer J, Becker C, Senft K, Wibom R, et al: Loss
of UCP2 attenuates mitochondrial dysfunction without altering ROS
production and uncoupling activity. PLoS Genet. 10:e10043852014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Della-Morte D, Dave KR, DeFazio RA, Bao
YC, Raval AP and Perez-Pinzon MA: Resveratrol pretreatment protects
rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling
protein 2 pathway. Neuroscience. 159:993–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teshima Y, Akao M, Jones SP and Marban E:
Uncoupling protein-2 overexpression inhibits mitochondrial death
pathway in cardiomyocytes. Circ Res. 93:192–200. 2003. View Article : Google Scholar : PubMed/NCBI
|