Introduction
Previous clinical studies have revealed that
pulmonary complications, including acute respiratory distress
syndrome (1), severe pneumonia
(2) and respiratory failure, are
important causes of mortality in patients with ischemic stroke,
particularly in the elderly. Hilker et al (3) first reported the epidemiological and
prognostic impact of the incidence of pneumonia for the treatment
of acute stroke and demonstrated that the occurrence of
stroke-associated pulmonary complications deteriorate the clinical
outcomes in these patients. However, the mechanisms underlying the
fact that patients with acute ischemic stroke are vulnerable to
respiratory complications have not been clearly elucidated, despite
their clinical significance for the early prevention of
stroke-associated pulmonary syndrome and in improving clinical
outcomes (4,5).
Mucus hypersecretion has been recognized as one of
the important features of airway remodeling and is involved in the
pathogenesis of a number of pulmonary diseases, including chronic
pulmonary obstructive diseases (6)
and asthma (7). Although mucus
secretion by goblet cells in the airway epithelium is thought to
protect the respiratory system in physiological conditions, the
pathological overproduction of mucus often leads to increased
sputum production, airway narrowing due to sputum secretion and
increased airway wall thickness (8,9). The
majority of patients with acute stroke have increased sputum
retention in the airway, which is potentially induced by factors
involving the overproduction of the mucus, saliva secretion and
dysphagia secondary to the neurologic dysfunction (1). It is possible that the overproduction
of airway mucus may be an early sign of airway dysfunction that
occurs prior to onset and it may accelerate the onset of severe
pulmonary disorders. However, it remains unclear whether cerebral
ischemia induces airway mucus hypersecretion and the potential
mechanisms underlying this pathophysiologic process have not been
studied extensively.
A previous study indicated that the inflammatory
response has an important role in the pathogenesis of mucus
hypersecretion (10). T-helper 2
(Th2) cell-associated cytokines, particularly interleukin-13
(IL-13), are thought to be key regulators of mucus production in
the airway (11). In addition,
classical inflammatory signaling via the nuclear factor-κB (NF-κB)
signaling pathway, has also been indicated to be involved in the
regulation of mucus overproduction during pathological airway
remodeling (12,13). Increased levels of cytokines, such
as IL-13 and other factors, trigger the activation of the NF-κB
pathway in pulmonary tissues, which functions as a transcriptional
factor with the nuclear translocation of its key component p65.
This subsequently leads to cellular processes including the
overproduction of mucus and airway remodeling. Notably, cerebral
ischemia and reperfusion (I/R) injury, which frequently occurs in
patients following stroke, has been associated with systematic
activated inflammatory responses (14). Previous studies have indicated that
patients with acute ischemic stroke have a higher degree of
immunoinflammatory responses, which is reflected by increased serum
levels of inflammatory markers (15) and alterations in the peripheral
frequency of CD4+ cells (16). In addition, the degree of the
immunoinflammatory response, which is characterized by higher
peripheral white blood cell count at admission, has been associated
with increased in-hospital mortality and cognitive impairment at
discharge for patients with acute cerebrovascular syndromes
(17). Anti-inflammation
therapies, such as treatments targeting tumor necrotic factor-α
(TNF-α), have been proposed as a potential therapeutic strategy for
patients with brain injuries from strokes and trauma (18). These findings indicate that
activation of the inflammatory response serves a key role in the
pathogenesis and progression of cerebral I/R injury. Therefore, we
hypothesized that cerebral I/R injury may lead to airway mucus
hypersecretion via the activation of systematic inflammatory
responses, particularly those associated with the IL-13 and NF-κB
signaling pathways.
Therefore, in the present study, the aim was to
investigate the above hypothesis in a rat model of cerebral I/R by
evaluating the influence of cerebral I/R injury on mucus secretion
in the airway and the expression of its key component, mucin 5AC
(MUC5AC). To further elucidate the potential mechanisms involved,
the influence of cerebral I/R injury on systematic and pulmonary
IL-13 and activation of the NF-κB pathway were also examined.
Materials and methods
Ethics statement
All animal experiments were performed in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (19) and
the Helsinki Convention on the Use and Care of Animals. The Ethics
Committee of the Fourth Affiliated Hospital to China Medical
University approved the experimental protocols prior to the
commencement of the present study.
Animal models of cerebral I/R injury
and evaluation of neurological dysfunction
Healthy male Sprague Dawley rats (n=40; age, 3 to 4
months; weight, 280 to 300 g) were provided by the Animal
Experimental Research Center of China Medical University. Rats were
housed in the light for 14 h and the dark for 10 h, at a
temperature of 23±2°C and a humidity of 60–70%, with food and water
freely available. A total of 7 rats were randomly allocated to the
sham group (control), while the remaining 33 rats were allocated to
the cerebral I/R group. Following satisfactory anesthesia with 10%
chloral hydrate (350 mg/kg; intraperitoneal injection), the middle
cerebral artery occlusion (MCAO) method was used to establish the
model of cerebral I/R injury in rats. Rats in the sham group were
subjected to the same surgical procedures performed on the rats in
the I/R group, however, their blood vessels were not occluded. The
animals were not allowed to eat or drink 12 h prior to the
operation. A rat model of focal cerebral ischemia was induced
according to the modified Longa method, as previously described
[Longa et al (20)].
Briefly, a 0.23-mm diameter fish-thread was inserted
into the right common carotid artery through a mini-pore, which was
3-mm away from the bifurcation. Then, the thread was inserted
through the bifurcation and into the internal carotid artery until
it had advanced ~18 to 20 mm to induce focal ischemia in the brain.
Following MCAO for 2 h, the operator carefully removed the suture
to restore blood flow, sutured the skin and allowed the rat to wake
up. Sham-operated animals underwent the same surgical procedure,
except for arterial occlusion. The rectal temperatures of the rats
from each group were monitored during the surgical process. Rats in
the cerebral I/R group were further randomized into five subgroups
according to the different reperfusion time (6, 12, 24, 48 and 72
h). Neurological function was evaluated immediately following
reperfusion in the brains of rats in each group. Severity of
neurological dysfunction was assessed based on the standards of
Longa [Longa et al (20)]:
Score 0, the rat had no symptoms of neurological deficit; score 1,
the rat failed to fully to extend the left forepaw; score 2, the
rat circled to the left; score 3, the rat fell to the left; score
4, the rat did not walk spontaneously and had a depressed level of
consciousness.
Collection of serum samples and
bronchoalveolar lavage fluid (BALF)
Following evaluation of neurological function, rats
from each group were sacrificed with an overdose of sodium
pentobarbital (150 mg/kg; Euthatal, Merial; Boehringer Ingelheim
Corporation, Ridgefield, CT, USA), which was injected
intraperitoneally. Serum was collected from the posterior vena cava
using a heparinized syringe and plasma was separated by
centrifugation (1,000 × g for 20 min at 4°C). The thoracic
cavity was carefully opened and the trachea was exposed. BALF was
collected by cannulating the upper part of the trachea and
performing lavage twice using 1 ml and 0.8 ml of phosphate-buffered
saline (PBS), respectively; 85 to 90% of the total input volume was
recovered. BALF samples were kept on ice during collection then
centrifuged at 400 × g for 5 min at 4°C.
Determination of dry-to-wet lung
weight ratio
The wet weight (ww) was recorded following
collection then 100 mg of lung tissue was dried in an oven at 60°C
for 48 h to reach a constant weight termed the dry weight (dw). The
dw/ww ratio was calculated, which was used as an indicator of
pulmonary mucus production.
Measurement of IL-13, TNF-α and MUC5AC
protein levels in serum and BALF
Serum and BALF supernatants were collected as
described above and stored at −70°C for use in cytokine assays.
IL-13 (cat. no. SEM03021A; Qiagen, Inc., Valencia, CA, USA), TNF-α
(cat. no. SEM03113A; Qiagen, Inc.) and MUC5AC (cat. no. fk2954Y;
R&D Systems, Inc., Minneapolis, MN, USA) levels were measured
using enzyme-linked immunosorbent assay (ELISA) kits, according to
the manufacturer's protocol. All assays were performed in
duplicate, and the mean values were used for statistical
analysis.
Immunohistochemical analysis
The right lower lung obtained from each rat was
fixed in 10% formalin for 10 min, embedded in paraffin, cut into
5-µm sections and stained with 0.2% hematoxylin at room temperature
for 10 min and 0.5% eosin at room temperature for 20 sec. MUC5AC,
aquaporin-5 (AQP-5), inhibitory proteins of NF-κB (IkBs) and NF-κB
p65 in lung tissues were detected by immunohistochemistry. Tissue
sections were deparaffinized, treated with
H2O2 and blocked with 5% normal rabbit serum
in PBS at room temperature for 1 h. Following washing with PBS,
tissue sections were incubated with rabbit anti-MUC5AC (cat. no.
sc-20118; 1:1,000), anti-AQP-5 (cat. no. sc-28628; 1:500),
anti-IkBs (cat. no. sc-847; 1:500) and anti-NF-κB p65 (cat. no.
sc-372; 1:500) antibodies (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) overnight at 4°C. Following rinsing with PBS, the sections
were incubated with biotinylated anti-rabbit IgG (cat. no. sc-2040;
Santa Cruz Biotechnology, Inc.) for at 4°C 2 h. Specific binding
was detected with an avidin-biotin-horseradish peroxidase complex
and diaminobenzidine kit (Vector Laboratories, Inc., Burlingame,
CA, USA). Slides were then counterstained with hematoxylin,
dehydrated using graded alcohol and xylenes, and mounted on
coverslips. The sections were examined using an LSM 5 PASCAL
confocal microscope (Carl Zeiss AG, Oberkochen, Germany). Negative
control staining was performed using normal bovine serum instead of
a primary antibody. Two blind investigators analyzed the sections
using the Image-Pro Plus software version 6.0 (Media Cybernetics,
Inc., Rockville, MD, USA). The positive areas were evaluated in ≥12
randomly selected tissue sections from each of the groups.
Western blot analysis
Total proteins in lung tissue samples were ground
and homogenized using a protein lysis solution (Nanjing KeyGen
BioTech Co., Ltd., Nanjing, China) for ≥12 randomly selected tissue
samples from each group studied. Briefly, the left upper lung
tissues were frozen in liquid nitrogen and homogenized in PBS with
a protease inhibitor cocktail (Roche Applied Science, Pleasanton,
CA, USA) using a tissue grinder. Homogenates were centrifuged at
21,920 × g for 15 min at 4°C. Nuclear and cytoplasmic proteins were
separated according to the protocol described in the Nuclear and
Cytoplasmic Extraction kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Supernatants were collected and assayed for
total protein using a bicinchoninic kit (Nanjing KeyGen BioTech
Co., Ltd.), according to the manufacturer's protocol. Equal amounts
of protein (125 µg) were resolved on 10% Tris-glycine-sodium
dodecyl sulfate (SDS) polyacrylamide gels, and protein bands were
blotted onto nitrocellulose membranes. With the aid of the
pre-stained markers, the gel for MUC5A, AQP-5, and β-actin was cut
at ~70 kDa. The larger molecular weight portion was used for
staining of MUC5A, and the smaller molecular weight portion was
used for staining of AQP-5. β-actin was subsequently stained after
stripping of the membrane. Similarly, the gel for cNF-κB, IkBs and
β-actin was cut at ~50 kDa. The larger molecular weight portion was
used for staining of cNF-κB, and the smaller molecular weight
portion was used for staining of IkBs. Again, β-actin was
subsequently stained after stripping of the membrane. The nuclear
gel was stained for nNF-κB and subsequently with Lamin A after
stripping of the membrane. Following blocking with 5% dried milk in
Tris-buffered saline containing 0.1% Tween-20 for 1 h at room
temperature, membranes were incubated for 24 h at 4°C with one of
the following antibodies to detect protein levels: Anti-MUC5AC
(cat. no. sc-20118; 1:500), anti-AQP-5 (cat. no. sc-28628;
1:1,000), anti-IkBs (cat. no. sc-847; 1:500), anti-NF-κB p65 (cat.
no. sc-372; 1:1,000), anti-β-actin (cat. no. sc-47778; 1:5,000) and
anti-Lamin A (cat. no. sc-293162; 1:500), purchased from Santa Cruz
Biotechnology, Inc. Membranes were incubated for 1 h at room
temperature with horseradish peroxidase-conjugated rabbit
anti-mouse (cat. no. sc358914; 1:10,000; Santa Cruz Biotechnology,
Inc.) or a donkey anti-rabbit secondary antibody (cat. no. sc2315;
1:10,000; Santa Cruz Biotechnology, Inc.). The internal controls
including β-actin and Lamin A were detected after stripping of the
membranes, which was performed using stripping buffer (cat. no.
P0025N; Beyotime Institute of Biotechnology, Haimen, China). In
brief, after rinsing the membrane with distilled water for 5 min,
they were incubated with stripping buffer for 10 mins at room
temperature and then washed with PBS 3 times. Peroxidase labeling
was detected using the enhanced chemiluminescence western blotting
detection system (Amersham Pharmacia Biotech; GE Healthcare Life
Sciences, Chalfont, UK) and analyzed by densitometry using
Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.).
Experiments were repeated three times. Optical density values were
normalized to that of β-actin or Lamin A.
Statistical analysis
Statistical analyses were performed using SPSS 11.0
(SPSS, Inc., Chicago, IL, USA). Results are presented as the mean ±
standard deviation. Differences were analyzed for significance by
one-way or two-way analysis of variance followed by the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
During the surgical procedure for the induction of
cerebral I/R injury, 1 rat in the sham group and 3 rats in the I/R
group did not survive. In total, 6 rats were included in the sham
group and 30 rats were included in the I/R group for the present
study. In the I/R group, rats were further divided into subgroups
with 6 rats in each subgroup according to the different reperfusion
times (6, 12, 24, 48 and 72 h). All of the rats that survived the
surgical procedures underwent subsequent neurological evaluation,
and pathological and molecular experiments.
Focal cerebral I/R injury-induced
neurological dysfunction
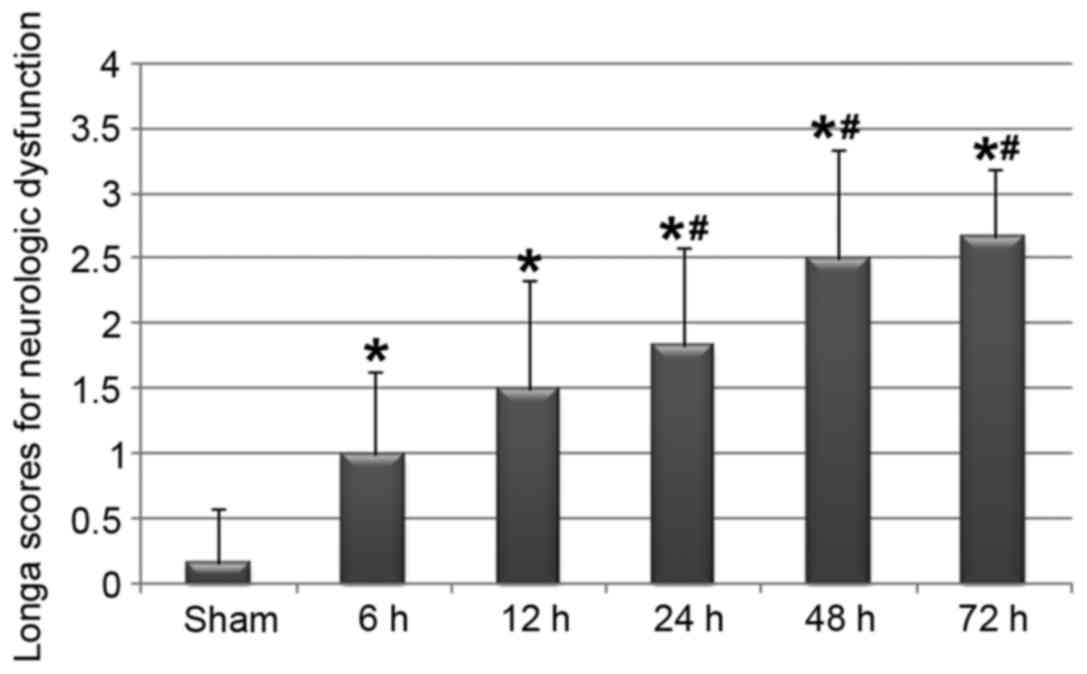
Using a conventional revisable MCAO model revealed
that focal ischemia, and the subsequent reperfusion to the brain,
was associated with various degrees of neurological dysfunction in
rats in the I/R group. Although rats in the sham operated group did
not exhibit any obvious symptoms of neurological disorder, rats
that underwent cerebral ischemia and subsequent reperfusion for ≥6
h presented with various degrees of neurological disorder, as
presented in Fig. 1. In addition,
the severity of neurological dysfunction may be dependent on the
reperfusion time of the brain.
Cerebral I/R injury was associated
with airway mucus hypersecretion
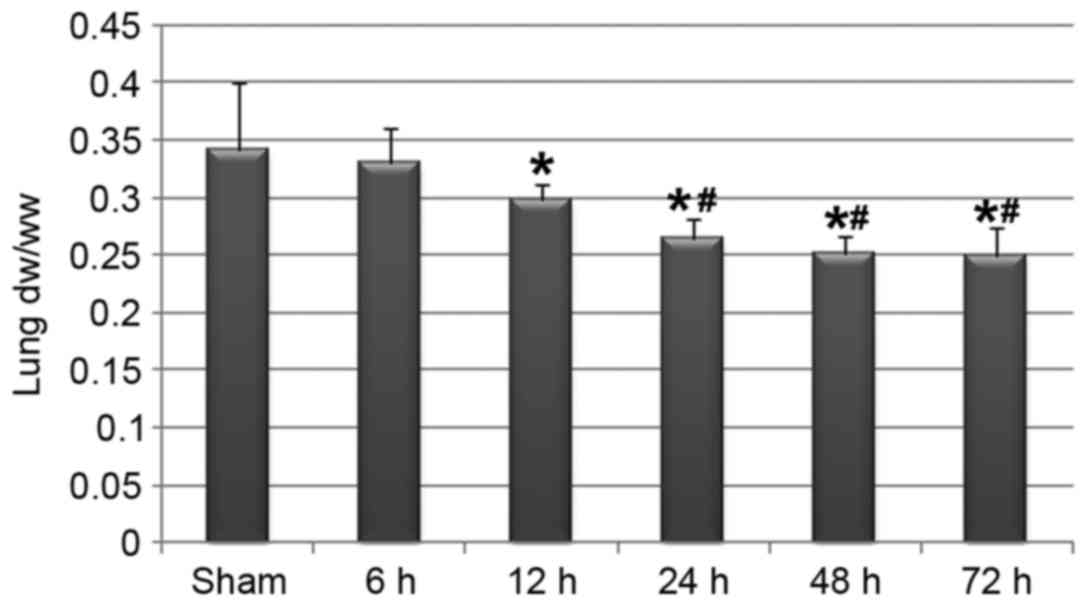
The dw/ww ratio of the lung is thought to be
reflective of the amount of mucus production in the airway. In the
present study, cerebral I/R injury and the neurological
dysfunctions were associated with a significant decrease in dw/ww
ratio in the lung (Fig. 2),
indicating that focal cerebral ischemia and the subsequent
reperfusion may induce airway mucus hypersecretion. In addition,
the reduction in the dw/ww ratio may be associated with the
reperfusion time of the brain.
Cerebral I/R injury-activated
inflammatory factors in serum and BALF
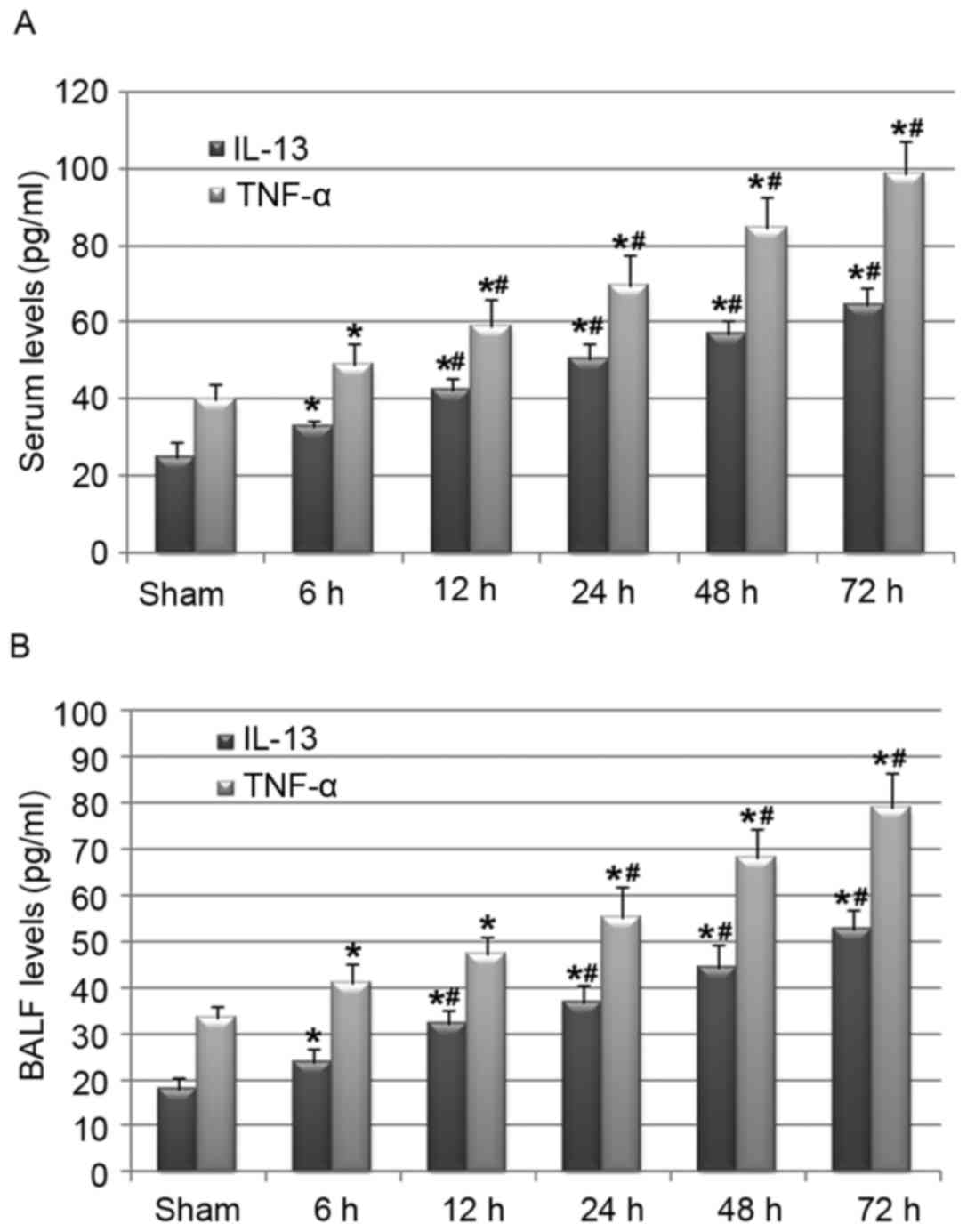
As the activation of the inflammatory response has
been recognized as an important mechanism underlying the
pathogenesis of cerebral I/R injury (14,21),
and the inflammatory factors IL-13 and TNF-α have been proposed to
be key regulators of pulmonary mucus production in a number of
pulmonary diseases (10), the
present study continued to evaluate whether cerebral I/R injury was
associated with the activation of these factors in the serum and
BALF of rats in the I/R group. Notably, cerebral I/R injury
upregulated IL-13 and TNF-α serum levels (Fig. 3A) and also increased BALF levels of
these cytokines (Fig. 3B) in a
reperfusion time-dependent manner. These results indicate that the
activation of the systematic inflammatory response, reflected by
the increase in IL-13 and TNF-α levels in serum and BALF, may
mediate the pathological process of cerebral I/R injury-induced
airway mucus hypersecretion.
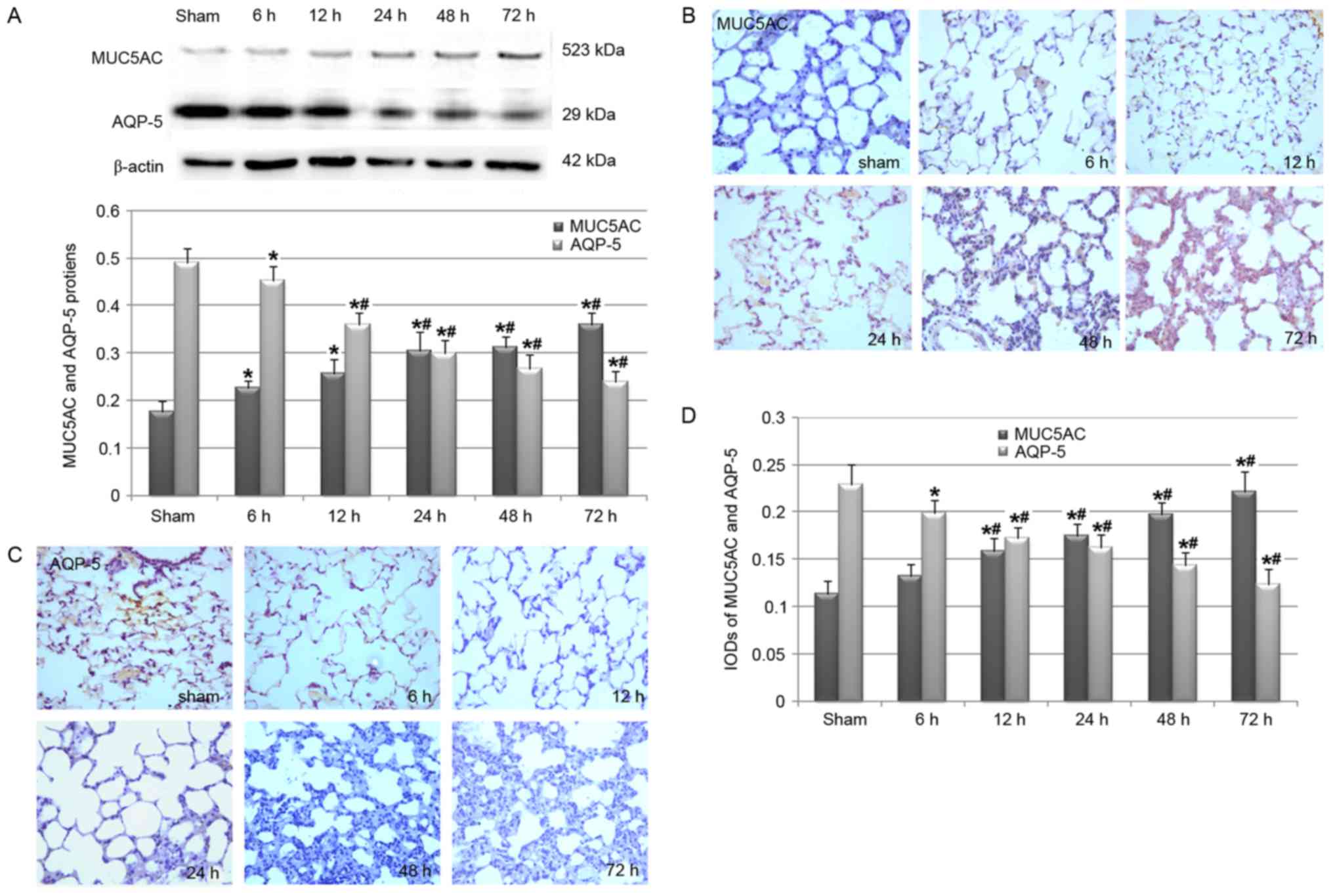
Cerebral I/R injury-induced pulmonary
overexpression of MUC5AC
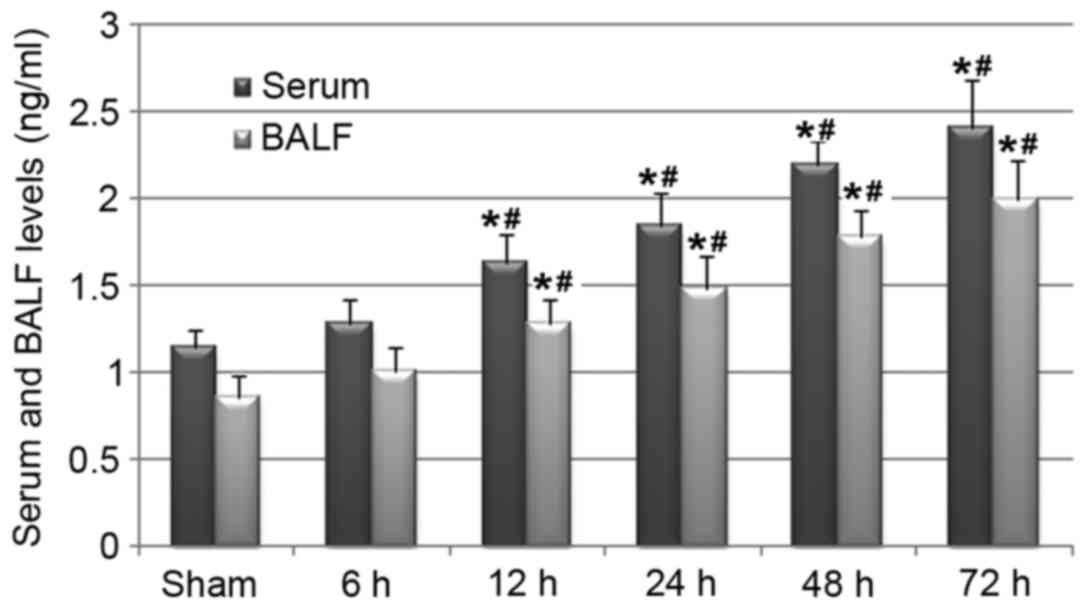
MUC5AC is the main component of mucus (7,22).
Therefore, the present study evaluated whether cerebral I/R injury
was associated with the systematic and pulmonary overexpression of
MUC5AC. ELISA analysis results revealed that rats in the 12–72 h
I/R groups had significantly increased levels of MUC5AC in serum
and BALF (Fig. 4). These results
were further confirmed by the western blot analysis of the MUC5AC
protein in lung tissues, which revealed that cerebral I/R injury
stimulated the pulmonary production of MUC5AC protein (Fig. 5A), potentially in a reperfusion
time-dependent manner. A subsequent immunohistochemical study also
confirmed that the pulmonary protein of MUC5AC was induced in rats
in the I/R group (Fig. 5B). In
addition, western blot analysis (Fig.
5A) and the immunohistochemical study (Fig. 5C and D) revealed that AQP-5, which
contributes to the volume of liquid secreted from the airways
(23), significantly decreased in
the I/R group rats when compared with the rats in the sham
group.
Cerebral I/R injury-activated NF-κB
inflammatory signaling pathway in lungs
As NF-κB inflammatory signaling has been implicated
in the regulation of airway mucus hypersecretion (12,13),
we hypothesized that cerebral I/R injury may lead to the
overproduction of mucus via activation of the pulmonary NF-κB
pathway. Western blot analysis revealed that cerebral I/R injury
was associated with decreased protein levels of IkBs and
cytoplasmic NF-κB p65, as well as increased levels of nuclear NF-κB
p65 (Fig. 6A). These results
indicate that there may be increased translocation of NF-κB p65 to
the nucleus and the subsequent activation of the NF-κB inflammatory
signaling pathway. In addition, changes in the expression of these
proteins may be dependent on reperfusion time. These results were
further confirmed by immunohistochemical analyses for IkBs and
cytoplasmic NF-κB p65 (Fig. 6B-D).
Therefore, the results suggest that cerebral I/R injury may
contribute to increased mucus production in the airway by
activating the NF-κB inflammatory signaling pathway.
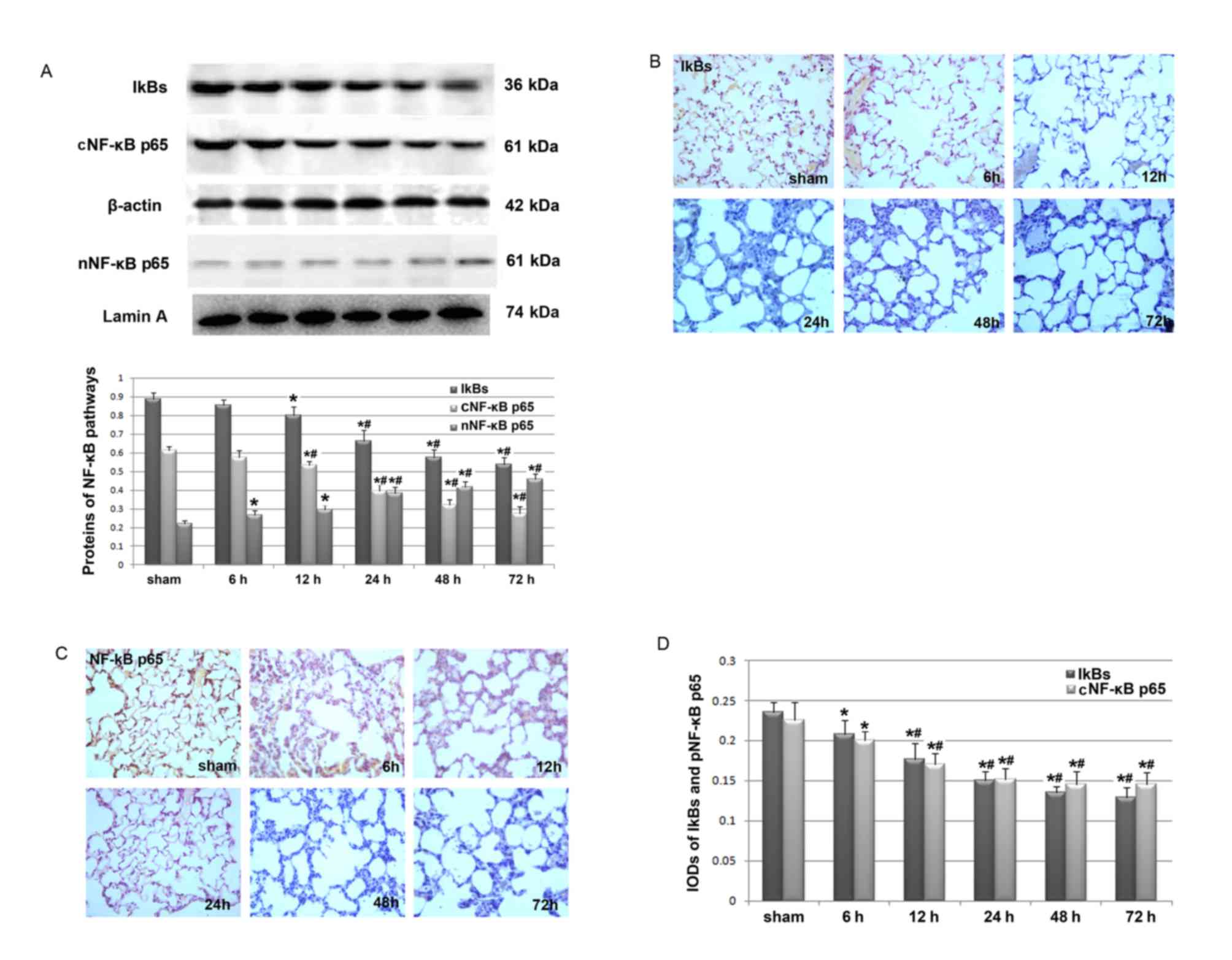 | Figure 6.Effects of cerebral I/R injury on the
pulmonary expression of IkBs and NF-κB p65. Western blot and
immunohistochemical analyses were performed in rats in the sham
group and the cerebral I/R group following 6, 12, 24, 48 and 72 h
of reperfusion (n=6/group). (A) Pulmonary expression of IkBs, and
cytoplasmic and nuclear NF-κB p65 detected by western blot
analysis. The upper panel shows the representative images of the
western blotting study, while the lower panel displays the
quantitative results of pulmonary IkBs, and cytoplasmic and nuclear
NF-κB p65 expression. Optical density values of IkBs, and
cytoplasmic NF-κB p65 were normalized to that of β-actin, while the
optical density of nuclear NF-κB p65 was normalized to that of
Lamin A. (B) Representative images (magnification, ×400) for the
immunohistochemical analysis of pulmonary IkBs in rat lung tissues
in each group. (C) Representative images (magnification, ×400) for
the immunohistochemical analysis of pulmonary cytoplasmic NF-κB p65
in rat lung tissues in each group. (D) Quantitative results of the
pulmonary expression of IkBs and NF-κB p65 presented as IOD values
detected by immunohistochemical analysis. Each experiment was
independently performed for 3 times. Data are presented as the mean
± standard deviation. *P<0.05 vs. sham group;
#P<0.05 vs. cerebral I/R group following 6 h of
reperfusion. I/R, ischemia and reperfusion; IkBs, inhibitory
proteins of NF-κB; NF-κB, nuclear factor-κB; IOD, integrated
optical density; cNF-κB, cytoplasmic NF-κB; nNF-κB, nuclear
NF-κB. |
Discussion
In the present study, the rat model of cerebral I/R
injury revealed that brain I/R injury-associated neurological
dysfunction may be associated with mucus overproduction in the
airway. In addition, the results of the subsequent mechanistic
evaluations indicate that the upregulation of the Th2-associated
inflammatory cytokine IL-13 and activation of the NF-κB signaling
pathway may be involved in the potential association between
cerebral I/R injury and airway mucus hypersecretion. To the best of
our knowledge, the present study is the first to elucidate the
influence and potential mechanisms of brain I/R injury on airway
mucus hypersecretion, which is of clinical significance. This
highlights the fact that inflammation has an important role in the
pathogenesis of brain I/R injury-induced mucus hypersecretion, and
thus targeting inflammation may be a potential strategy for the
early prevention of mucus hypersecretion and the associated
pulmonary disorders for patients with ischemic stroke.
Previous clinical observations have determined that
patients with neurological disorders are more vulnerable to
respiratory complications (1,2). In
addition, comorbidities of pulmonary diseases often deteriorate the
prognosis of patients with stroke (24). However, the potential
pathophysiological association between brain I/R injury and
pulmonary dysfunction has not been extensively investigated. In
light of the fact that airway mucus hypersecretion has been
suggested as an initial process in the pathogenesis of a number of
pulmonary disorders (7), the
present study investigated whether cerebral I/R-related
neurological dysfunction was associated with the overproduction of
mucus in the airway. Decreased dw/ww ratio of the lung and enhanced
expression of MUC5AC in pulmonary tissues were observed in rats
with I/R-associated neurological dysfunction in a reperfusion
time-dependent manner. Further studies are required to determine
the pathophysiological role of mucus hypersecretion in animal
models and patients with neurological disorders, and to determine
whether strategies targeting mucus hypersecretion could improve
prognosis in patients with early-stage cerebral I/R injury.
The activation of inflammation has been indicated to
be important in the pathogenesis of cerebral I/R injury (14,25)
and mucus hypersecretion-associated airway remodeling (26,27).
Therefore, the present study subsequently investigated whether the
activation of systematic and pulmonary inflammatory pathways was
responsible for the potential link between cerebral I/R
injury-associated dysfunction and airway mucus hypersecretion.
Th2-associated inflammatory factor IL-13 was induced in rat serum
and BALF. This is of particular interest as previous studies have
demonstrated that IL-13 induces a number of features of allergic
lung disease including airway hyperresponsiveness, goblet cell
metaplasia and mucus hypersecretion, which all contribute to airway
obstruction (28,29). An early in vivo study
demonstrated that CD4 Th cells stimulate mucus only through a
common IL-13-mediated pathway (11). Lin et al (30) demonstrated that the transmembrane
protein 16A may be involved in the process of IL-13-mediated mucus
hypersecretion. Notably, an animal study performed by Ma et
al (31) revealed that
suppression of IL-13 with a vaccine inhibits chronic airway
inflammation and the development of several key components of
airway remodeling including mucus hypersecretion. In addition, this
intervention was more effective during the earlier stages of
chronic inflammation (31). This
is of clinical significance as targeting the inhibition of IL-13
may be effective for the prophylaxis of dysfunction in patients
with cerebral I/R injury-associated neurological dysfunction.
Further studies are required to confirm this hypothesis.
The present study also revealed that the NF-κB
signaling pathway was activated in the pulmonary tissues of rats
with cerebral I/R injury-associated airway mucus overproduction.
This was to be expected as previous studies have indicated that the
activation of the NF-κB inflammatory signaling pathway serves an
important role in the pathogenesis of mucus hypersecretion
(32,33). The present study revealed that the
level of IkBs decreased and the translocation of NF-κB p65 from the
cytoplasm to the nucleus increased in the pulmonary tissues of rats
with cerebral I/R-associated mucus hypersecretion, indicating that
activation of the NF-κB signaling pathway may be involved. In fact,
a previous study has indicated that activation of the NF-κB
signaling pathway during the regulation of mucus overproduction may
be the downstream event following the induced systematic expression
of IL-13 (30). Further studies
are required to determine whether the upregulation of systematic
IL-13 and activation of the pulmonary NF-κB pathway was causative
in the pathogenesis of cerebral I/R injury-associated airway mucus
hypersecretion. In addition, it is important to further investigate
effective strategies that target the NF-κB signaling pathway for
the prevention of pulmonary disorders in patients with brain I/R
injury.
The current study has limitations that should be
noted when interpreting the results. Firstly, dw/ww ratios of the
lungs were used as an index of the extent of mucus hypersecretion.
However, this marker may not be directly associated with mucus
hypersecretion, and may be affected by other pulmonary processes
that occur following a stroke including neurogenic pulmonary edema
or inflammation. Other direct parameters or studies, such as
periodic acid-Schiff staining of the pulmonary tissues should be
performed in future studies. Secondly, the present study only
focused on the changes and the potential role of IL-13. The
potential involvement and function of the other Th2-associated
inflammatory factors should be evaluated in the future. Finally,
the sample size of the experimental animals in each group was
relatively small. Therefore, further investigations should be
performed with a larger sample size to confirm our results.
In conclusion, cerebral I/R injury may induce airway
mucus hypersecretion via the activation of the IL-13 and NF-κB
inflammatory signaling pathways. The present study revealed that
inflammatory-related mucus hypersecretion may be responsible for
the association between cerebral I/R-related neurological
dysfunction and the susceptibility of patients who have suffered a
stroke for respiratory disorders. Further studies are required to
determine whether targeting inflammation during the above
pathological process is effective for the early prevention of
cerebral I/R-associated pulmonary complications.
Acknowledgements
The present study was funded by the Scientific and
Technologic Program of Liaoning Province (grant no.
2013408001).
References
|
1
|
Zhao JN, Liu Y and Li HC:
Aspiration-related acute respiratory distress syndrome in acute
stroke patient. PLoS One. 10:e01186822015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maeshima S, Osawa A, Hayashi T and
Tanahashi N: Elderly age, bilateral lesions, and severe
neurological deficit are correlated with stroke-associated
pneumonia. J Stroke Cerebrovasc Dis. 23:484–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hilker R, Poetter C, Findeisen N, Sobesky
J, Jacobs A, Neveling M and Heiss WD: Nosocomial pneumonia after
acute stroke: Implications for neurological intensive care
medicine. Stroke. 34:975–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hannawi Y, Hannawi B, Rao CP, Suarez JI
and Bershad EM: Stroke-associated pneumonia: Major advances and
obstacles. Cerebrovasc Dis. 35:430–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith CJ, Kishore AK, Vail A, Chamorro A,
Garau J, Hopkins SJ, Di Napoli M, Kalra L, Langhorne P, Montaner J,
et al: Diagnosis of stroke-associated pneumonia: Recommendations
from the pneumonia in stroke consensus group. Stroke. 46:2335–2340.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin C, Frija-Masson J and Burgel PR:
Targeting mucus hypersecretion: New therapeutic opportunities for
COPD? Drugs. 74:1073–1089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergeron C, Tulic MK and Hamid Q: Airway
remodelling in asthma: From benchside to clinical practice. Can
Respir J. 17:e85–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aikawa T, Shimura S, Sasaki H, Ebina M and
Takishima T: Marked goblet cell hyperplasia with mucus accumulation
in the airways of patients who died of severe acute asthma attack.
Chest. 101:916–921. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jenkins HA, Cool C, Szefler SJ, Covar R,
Brugman S, Gelfand EW and Spahn JD: Histopathology of severe
childhood asthma: A case series. Chest. 124:32–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Turner J and Jones CE: Regulation of mucin
expression in respiratory diseases. Biochem Soc Trans. 37:877–881.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whittaker L, Niu N, Temann UA, Stoddard A,
Flavell RA, Ray A, Homer RJ and Cohn L: Interleukin-13 mediates a
fundamental pathway for airway epithelial mucus induced by CD4 T
cells and interleukin-9. Am J Respir Cell Mol Biol. 27:593–602.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen M, Lv Z, Zhang W, Huang L, Lin X, Shi
J, Zhang W, Liang R and Jiang S: Triptolide suppresses airway
goblet cell hyperplasia and Muc5ac expression via NF-κB in a murine
model of asthma. Mol Immunol. 64:99–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang JH, Hwang SM and Chung IY: S100A8,
S100A9 and S100A12 activate airway epithelial cells to produce
MUC5AC via extracellular signal-regulated kinase and nuclear
factor-κB pathways. Immunology. 144:79–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmad M, Dar NJ, Bhat ZS, Hussain A, Shah
A, Liu H and Graham SH: Inflammation in ischemic stroke:
Mechanisms, consequences and possible drug targets. CNS Neurol
Disord Drug Targets. 13:1378–1396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tuttolomondo A, Pecoraro R, Di Raimondo D,
Di Sciacca R, Canino B, Arnao V, Buttà C, Corte V Della, Maida C,
Licata G and Pinto A: Immune-inflammatory markers and arterial
stiffness indexes in subjects with acute ischemic stroke with and
without metabolic syndrome. Diabetol Metab Syndr. 6:282014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tuttolomondo A, Pecoraro R, Casuccio A, Di
Raimondo D, Buttà C, Clemente G, Corte V Della, Guggino G, Arnao V,
Maida C, et al: Peripheral frequency of CD4+ CD28-cells
in acute ischemic stroke: Relationship with stroke subtype and
severity markers. Medicine (Baltimore). 94:e8132015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuttolomondo A, Pedone C, Pinto A, Di
Raimondo D, Fernandez P, Di Sciacca R and Licata G: Gruppo Italiano
di Farmacoepidemiologia dell'Anziano (GIFA) researchers: Predictors
of outcome in acute ischemic cerebrovascular syndromes: The GIFA
study. Int J Cardiol Cardiol Cardiol. 125:391–396. 2008. View Article : Google Scholar
|
|
18
|
Tuttolomondo A, Pecoraro R and Pinto A:
Studies of selective TNF inhibitors in the treatment of brain
injury from stroke and trauma: A review of the evidence to date.
Drug Des Devel Ther. 8:2221–2238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals: Guide for the care and use
of laboratory animals. 8th. https://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf
|
|
20
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haqqani AS, Nesic M, Preston E, Baumann E,
Kelly J and Stanimirovic D: Characterization of vascular protein
expression patterns in cerebral ischemia/reperfusion using laser
capture microdissection and ICAT-nanoLC-MS/MS. FASEB J.
19:1809–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alagha K, Palot A, Sofalvi T, Pahus L,
Gouitaa M, Tummino C, Martinez S, Charpin D, Bourdin A and Chanez
P: Long-acting muscarinic receptor antagonists for the treatment of
chronic airway diseases. Ther Adv Chronic Dis. 5:85–98. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen Y, Wang Y, Chen Z, Wang D, Wang X,
Jin M and Bai C: Role of aquaporin 5 in antigen-induced airway
inflammation and mucous hyperproduction in mice. J Cell Mol Med.
15:1355–1363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Llinas RH: Ischemic stroke and ICU care.
Semin Neurol. 28:645–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tuttolomondo A, Di Sciacca R, Di Raimondo
D, Renda C, Pinto A and Licata G: Inflammation as a therapeutic
target in acute ischemic stroke treatment. Curr Top Med Chem.
9:1240–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Angelis N, Porpodis K, Zarogoulidis P,
Spyratos D, Kioumis I, Papaiwannou A, Pitsiou G, Tsakiridis K,
Mpakas A, Arikas S, et al: Airway inflammation in chronic
obstructive pulmonary disease. J Thorac Dis. 6 Suppl 1:S167–S172.
2014.PubMed/NCBI
|
|
27
|
McGovern AE and Mazzone SB: Neural
regulation of inflammation in the airways and lungs. Auton
Neurosci. 182:95–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitchell J, Dimov V and Townley RG: IL-13
and the IL-13 receptor as therapeutic targets for asthma and
allergic disease. Curr Opin Investig Drugs. 11:527–534.
2010.PubMed/NCBI
|
|
29
|
Rayees S, Malik F, Bukhari SI and Singh G:
Linking GATA-3 and interleukin-13: Implications in asthma. Inflamm
Res. 63:255–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Jiang Y, Li L, Liu Y, Tang H and
Jiang D: TMEM16A mediates the hypersecretion of mucus induced by
Interleukin-13. Exp Cell Res. 334:260–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Halayko AJ, Basu S, Guan Q, Weiss
CR, Ma AG, HayGlass KT, Becker AB, Warrington RJ and Peng Z:
Sustained suppression of IL-13 by a vaccine attenuates airway
inflammation and remodeling in mice. Am J Respir Cell Mol Biol.
48:540–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Janssen-Heininger YM, Poynter ME, Aesif
SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, Van Der
Velden J, Irvin CG and van der Vliet A: Nuclear factor kappaB,
airway epithelium, and asthma: Avenues for redox control. Proc Am
Thorac Soc. 6:249–255. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mortaz E, Masjedi MR, Allameh A and Adcock
IM: Inflammasome signaling in pathogenesis of lung diseases. Curr
Pharm Des. 18:2320–2328. 2012. View Article : Google Scholar : PubMed/NCBI
|