Introduction
The incidence of new onset peripheral nerve injury
is increasing worldwide. More than one million cases with new onset
peripheral nerve injury is presented annually worldwide (1). The peripheral nerve injuries can
result from trauma, cancer or congenital defects. Regarding the
clinical and basic research, the sciatic nerve injury and
regeneration process has been a focus of major investigation in
medicine. The nerve fiber growth velocity is very slow and is
estimated to 1–2 mm/day in humans (2,3). The
autologous nerve graft is considered the best treatment option,
although significant drawbacks are encountered, such as the limited
donor source, the donor site morbidity, the multiple surgical sites
and the possible size mismatch (4). Consequently, novel methods to replace
the autologous nerve graft for nerve gap reconstruction are
continuously evaluated.
In the previous study conducted by our group, the
RSC96 cell line was used to evaluate the biological applications of
the polyaniline/regenerated cellulose (PANI/RC) that enhanced RSC96
cell adhesion and proliferation (5). Scanning electron microscopy
demonstrated the adhesion of RSC96 cells via pseudopodium extension
on the PANI sub-micrometer dendritic particles (5). It is well established that Schwann
cells serve a critical role in the peripheral nerve regeneration
(6). In the present study, it was
hypothesized that PANI is involved in the latter process via the
regulation of Schwann cells. The specific function of PANI in nerve
regeneration was detected at the molecular level. During the
aforementioned process, the involvement of a complex interlinked
cell signaling pathway was identified. The recruitment of cytokines
and neurotrophins at the site of the lesion resulted in the
activation of a series of molecular and cellular signaling pathways
involved in neuronal and non-neuronal cell communication (7,8).
Such mechanisms may include the generation of new neurons, glia,
axons, myelin or synapses that in turn promote nerve regeneration
(8).
Neurotrophins are proteins that help stimulate and
control neurogenesis. Brain derived neurotrophic factor (BDNF) is
one of the most active neurotrophins (9) that supports the survival of existing
neurons and promotes the growth and differentiation of new neurons
and synapses (10,11). In addition, the application of
exogenous BDNF to the nerve topical lesion has been reported to
potentiate axon regrowth and maturation during peripheral nerve
regeneration (12).
Cytokine ciliary neurotrophic factor (CNTF) is an
additional neurotrophin that is required for nerve regeneration
(13). CNTF is secreted by Schwann
cells and astrocytes, and the application of exogenous CNTF to the
nerve lesion has also been reported to promote axon regrowth and
maturation during peripheral nerve regeneration (14–17).
However, it remains unclear whether the role of endogenous CNTF in
the activation of cell growth and differentiation is associated
with PANI, following peripheral nerve injury.
In the present study, the ability of PANI/RC to
promote rat sciatic nerve regeneration was demonstrated by
monitoring the morphological and molecular changes of the
regenerated nerve fibers using histological, immunohistochemical
and western blotting techniques.
Materials and methods
Preparation of PANI/RC conduit and
cellulose conduit
The PANI/RC hydrogels were prepared through the
interfacial polymerization via a U tube, of which a cellulose
hydrogel was sandwiched in the middle as previous described
(5). The PANI/RC and RC hydrogels
were rolled to fabricate PANI/RC and RC conduits. The final length
of conduits was 12 mm, with an inner diameter of 1.5 mm and a tube
wall thickness of 1 mm.
Animals
Adult male Sprague-Dawley rats, (age, 8–10 weeks,
weight, 210±10 g, n=45) were obtained from the Hubei Provincial
Center for Disease Control and Prevention in Wuhan, China. The
experimental procedures involving animals were carried out in
accordance with National Institutes of Health (Bethesda, MD, USA)
guide for the care and use of laboratory animals and The Code of
Ethics of the World Medical Association. The study received ethics
approval from the Ethics Committee of Zhongnan Hospital of Wuhan
University (Wuhan, China).
Surgical procedure
The animals were divided into three groups (15 rats
for each group): Sham (the sciatic nerve was exposed in the absence
of nerve tissue disposal), RC (the defected sciatic nerve was
connected with the RC conduit) and PANI/RC (the defected sciatic
nerve was connected with the PANI/RC conduit). The 5 mm defects in
the sciatic nerve were produced by removing the nerve tissue which
was repaired with the nerve conduits as reported previously
(18–20).
Triceps weight analysis
The triceps surae muscles from the
experimental and the contra lateral sides (non-operated) were
weighed in order to estimate the relative weight ratio 3 months
following surgery, according to the following formula:
triceps weight%=triceps weight of the
operated legtriceps weight of the unoperated leg×100%
Histological assessment
The harvested nerve grafts were fixed in a cold
buffered 4% paraformaldehyde solution or cold buffered 3%
glutaraldehyde solution. Following fixation, the former part of
tissues in each group were embedded in olefin, cut in 4 mm slices
and stained with hematoxylin (10 min at room temperature) and eosin
(2 min at room temperature; H&E) or toluidine blue (3 min at
room temperature). The latter part of the nerve sections were fixed
in a cold buffered 3% glutaraldehyde solution for transmission
electron microscopy TEM examination (JEM-1200 EX, JEOL Ltd., Tokyo,
Japan). A total of 200 nerve fibers in 10 random areas were
selected by two pathologists for the fiber size analysis. All nerve
sections were observed under a light microscope (TE2000-U, Nikon
Corporation, Tokyo, Japan). An image analysis system (Image-Pro
Plus Version6.0, Media Cybernetics Inc., Rockville, MD, USA) was
used to determine the number and areas of the individual myelinated
axons.
Immunohistochemical evaluation
The expression of the extracellular signal-regulated
kinase (ERK) 1/2/mitogen-associated protein kinase (MAPK) was
evaluated in nerve paraffin sections of the regenerated nerve
tissues via immunohistochemical analysis. After being blocked (10
min at room temperature) using 5% sham goat serum (cat. no. AR1009;
Wuhan Boster Biological Technology Ltd., Wuhan, China), the
sections were incubated with a rabbit anti-rat ERK1/2 antibody
(cat. no. 16443-1-AP; 1:50; ProteinTech Group, Inc., Wuhan, China)
or MAPK (cat. no. 9212; 1:100; Cell Signaling Technology Ltd.,
Danvers, MA, USA) at 4°C overnight and then with biotinylated
secondary antibodies (cat. no. AB501-01A; 1:500; SinoBiological
Inc., Shanghai, China) for 20 min at 37°C. The sections were
reacted with a Strept Avidin Biotin Complex (100 µl; cat. no.
PV-9000; Zhongshan Gold Bridge Biological Technology Ltd., Beijing,
China) for 20 min at room temperature and finally stained with
3,3-Diaminobenzidine (cat. no. AR1022; Wuhan Boster Biological
Technology Ltd., Wuhan, China). The positive ratios (200 cells in
10 random areas counted) of ERK1/2(MAPK) were observed by
microscope (TE2000-U, Nikon Corporation, Tokyo, Japan) and analyzed
by an image analysis system (Image-Pro Plus version 6.0; Media
Cybernetics Inc., Rockville, MD, USA).
Western blot analysis
The total protein content was extracted from
regenerated or sham nerve in a lysis buffer (cat. no. P0013B;
Beyotime Biotechnology Ltd., Shanghai, China) containing 50 mM Tris
HCl, 1% NP-40, 150 mM NaCl, 0.1% SDS, 1 mM PMSF and 1 mM
Na3VO4. The protein concentration of the
samples was determined by the Bicinchoninic Acid assay kit. Bovine
serum albumin (5%; cat. no. 164210-100; Procell Life Science &
Technology Co., Ltd., Wuhan, China) was used as a standard. An
equal amount (30 µg) of protein was loaded on polyacrylamide gels
containing 10-12% SDS and transferred to polyvinylidene fluoride
membranes. The membranes were probed with specific antibodies
against nerve growth factor (cat. no. BA0611-2; 1:200; Wuhan Boster
Biotechnology Co., Ltd); tau (cat. no. ab32057; 1:5,000; Abcam,
Cambridge, MA, USA); S100 (cat. no. ab52642; 1:5,000; Abcam);
growth-associated protein-43 (GAP-43; cat. no. ab75810; 1:5,000;
Abcam); α-tubulin (cat. no. ab15246; 1:500; Abcam), BDNF (cat. no.
SC-546; 1:300; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
CNTF (cat. no. ab46172; 1:3,000; Abcam); ERK (cat. no. 16443-1-AP;
1:1,000; ProteinTech Group, Inc.); phosphorylated (p)-ERK1/2 (cat.
no. 4370; 1:2,000; Cell Signaling Technology Inc.); β-actin (cat.
no. BM0627; 1:200; Wuhan Boster Biotechnology Co., Ltd.) and GAPDH
(cat. no. AB-P-R 001; 1:1,000; Hangzhou Goodhere Biotechnology Co.,
Ltd., Hangzhou, China). GAPDH and β-actin were used as internal
controls. The protein detection was carried out using the enhanced
chemiluminescence detection system (cat. no. NCI5079; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and the membranes were exposed
on Kodak X-Omat films. The protein levels were semi quantified by
densitometric analysis of the integrated area (pixels) of the
protein bands using the BandScan software version 4.3 (http://download1.bio1000.com/soft/biology/BandScan5.0.rar).
The numerical values for the protein band intensities were produced
following normalization of the target protein expression with the
expression value corresponding to the GAPDH or β-actin band.
Statistical analysis
SPSS 21.0 statistical software was used for the
statistical analysis of the relevant data (IBM Corp., Armonk, NY,
USA). The data are expressed as the mean ± standard deviation. The
differences between the two groups were compared using the student
t-test. The differences among several groups were analyzed using
one-way analysis of variance followed by least significance
difference multiple-comparison tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
General post-operative
observations
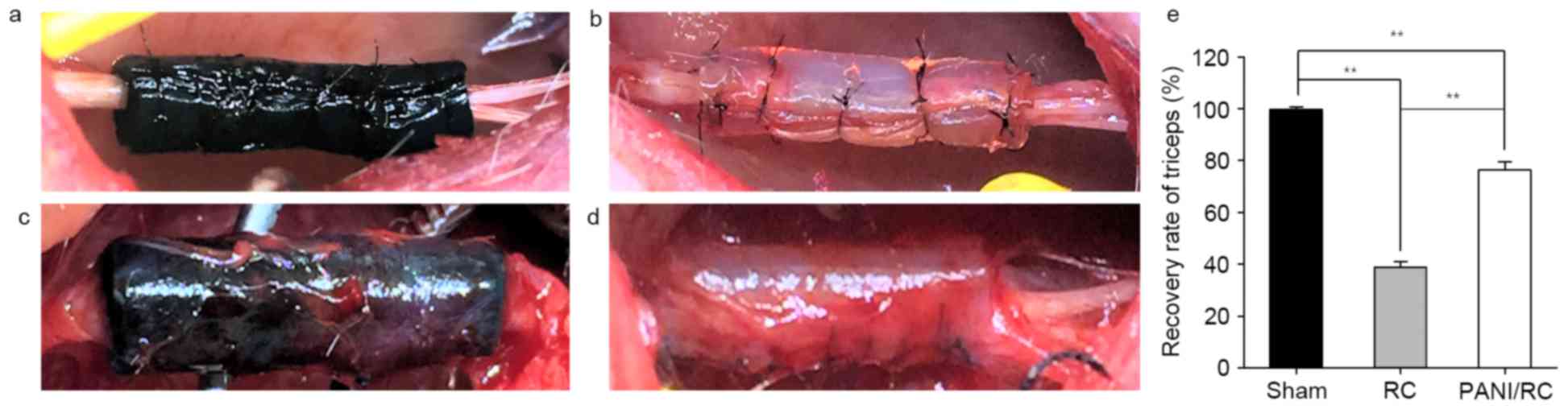
The conduits for nerve reconstruction are indicated
in the nerve condition intra-operatively (Fig. 1A and B) and 3 months post-operation
(Fig. 1C and D). Fig. 1 indicates the sciatic nerve
reconstruction that was conducted using the following materials:
PANI/RC (Fig. 1A and C) and RC
(Fig. 1B and D). The PANI/RC
conduit indicated favorable biocompatibility performance (Fig. 1C), while the RC conduit was wrapped
with thick fibrous connective tissue (Fig. 1D).
Triceps weight analysis
Nerve recovery is indirectly reflected by muscle
recovery. The triceps surae muscle weight ratio is estimated
by the formula: Operation side/contralateral side ×100%. Sham
group: 99.70±2.29; RC group: 38.88±4.76; PANI/RC group: 76.32±7.11,
P<0.01 (Fig. 1E). In terms of
triceps surae muscle weight ratio, the PANI/RC group
demonstrated better motor function recovery compared with the RC
group.
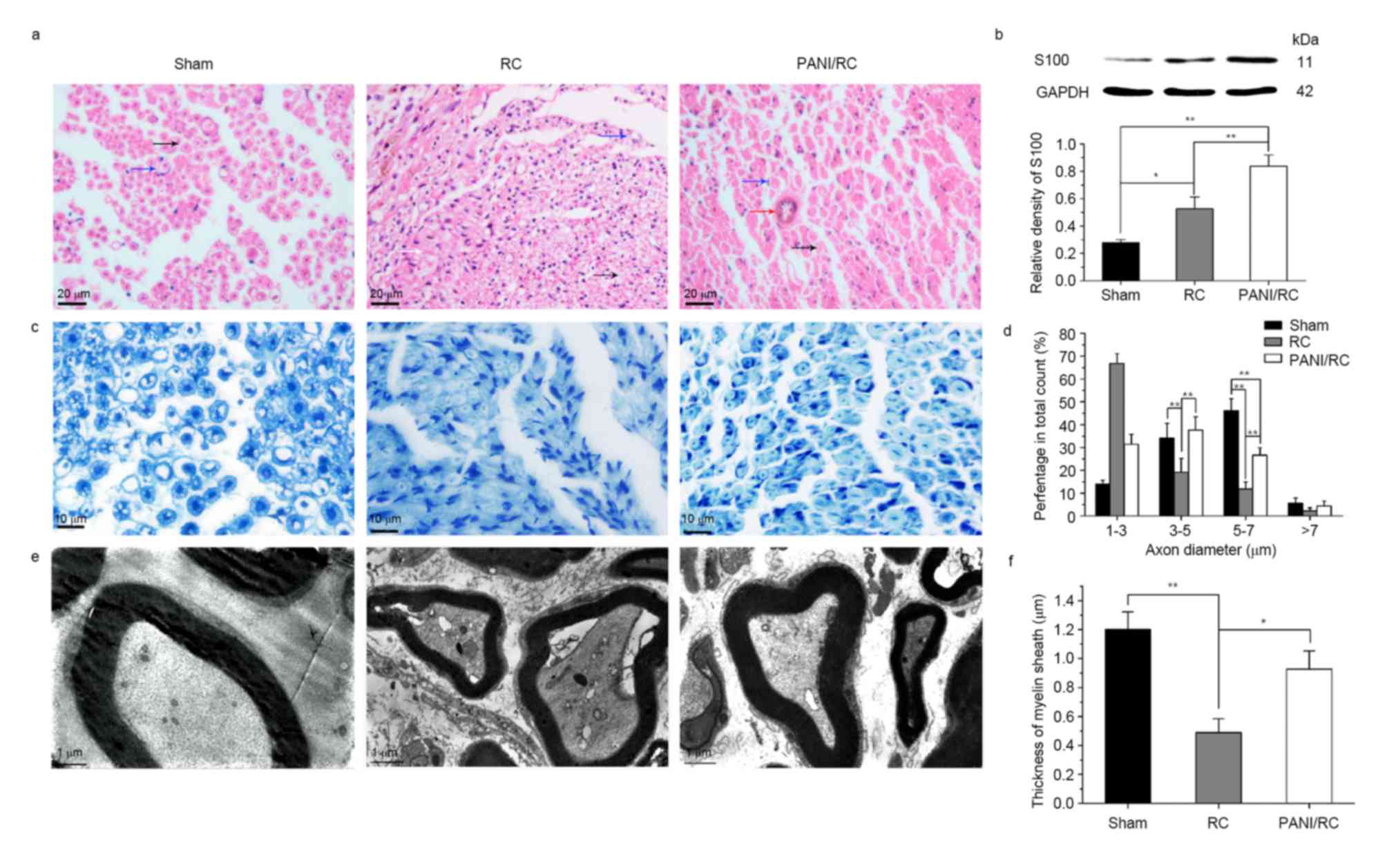
Axon regeneration, myelination and
activation of Schwann cells
The images of H&E sections indicated the
regenerated nerve fibers in the three groups (Fig. 2A). The number of Schwann cells
(blue arrow) detected in the PANI/RC group was greater than that in
the RC and sham groups. The axons (black arrow) regenerated more
efficiently in the PANI/RC compared with the RC group (Fig. 2A). The blood vessels that were
necessary for nutrient supply and neurite growth were detectable in
the PANI/RC group. A vast number of fibrous connective tissues
(white arrow) were crawled into the nerve that prevented nerve
regeneration (Fig. 2A). The
protein S100 (marker of Schwann cells) revealed a 3-fold increase
in the PANI/RC compared with the sham group (Fig. 2B). The aforementioned protein
demonstrated a 1.6-fold increase in the PANI/RC compared with the
RC group (P<0.01, Fig. 2B. The
images of toluidine blue sections (Fig. 2B) indicated that the regenerated
nerve fibers in the PANI/RC group were smaller and less uniform
compared with those in the sham group. However, the nerve fibers in
the RC group were the smallest in size and most irregular in shape
with numerous fibrous connective tissues. The fiber diameter
analysis (Fig. 2D) indicated that
the size range in the PANI/RC group was between 3 and 5 mm, whereas
the percentage was estimated to 37.6±5.8. The latter was similar to
the percentage of fiber diameter noted in the sham group (34.2±6.4)
and significantly greater compared with that in the RC group
(19.2±6; P<0.01).
TEM analysis of the regenerated nerve tissues
revealed that the formation of regenerated myelinated fibers
occurred at similar levels in both the PANI/RC and sham groups
(Fig. 2E). Statistical analysis
was carried out on the average axon diameter (Fig. 2D), the thickness of the regenerated
myelin sheath (Fig. 2F). A
significant difference between the PANI/RC and the RC groups was
noted for all of the parameters measured (P<0.05). A thicker
myelin sheath was observed in the PANI/RC group (0.93±0.28 µm)
compared with the RC group (0.49±0.21 µm, P<0.05), yet still
smaller than that in sham group (1.2±0.27 µm; P>0.05).
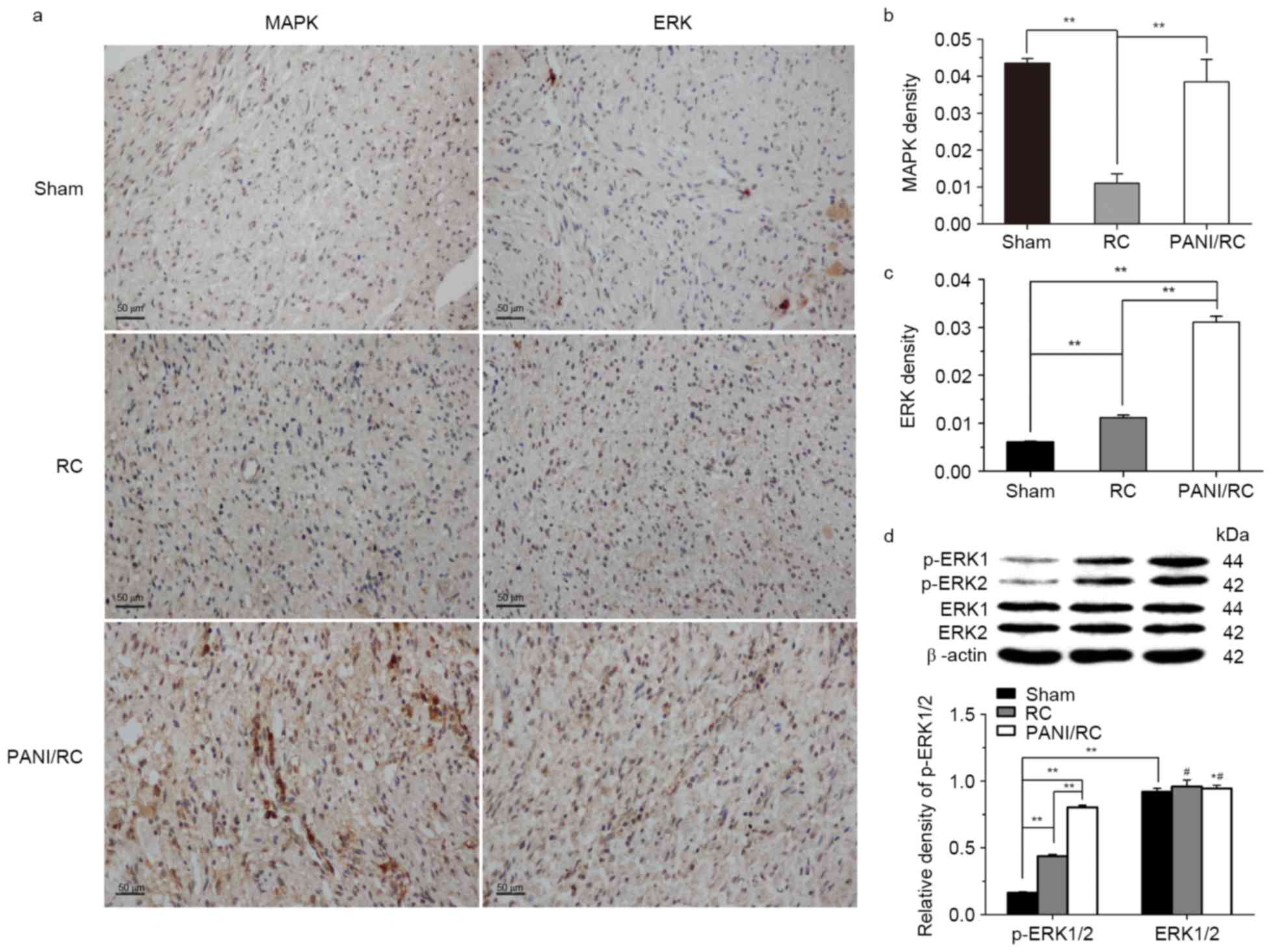
Activation of the ERK1/2/MAPK
signaling pathway
Immunohistochemical analysis was employed to analyze
the positive expression levels of ERK1/2, 3 months
post-implantation, in all groups in order to determine whether the
signaling pathways of MAPK/ERK1/2 were activated following the
increase in expression of CNTF and BDNF proteins. The positive
ratio of MAPK/ERK1/2 proteins in the PANI/RC group was
significantly greater than that of the RC group (P<0.01,
Fig. 3A-C). The results further
demonstrated that ERK1/2 is activated in the PANI/RC and RC group
compared with the sham group (Fig.
3C), as indicated by the increased phosphorylation levels of
ERK1/2 (Fig. 3D). The increase in
the p-ERK1/2 levels was highly significant between the PANI/RC and
the RC and the sham groups (P<0.01), while the levels of ERK1/2
were unchanged (Fig. 3D;
P>0.05).
Recruitment of growth factors,
cytokines and neurotrophins
The expression of the proteins CNTF, BDNF, Tau,
GAP-43, NGF and α-tubulin following implantation of the conduits
was examined using western blot analysis in order to determine the
activation of the Schwann cells following nerve injury. The
expression levels of the proteins corresponding to the PANI/RC
group were significantly higher compared with the expression levels
of the proteins corresponding to the sham and RC groups (Fig. 4). Specifically, the expression
levels of the proteins BDNF, CNTF, NGF, GAP-43, Tau and α-tubulin
were significantly increased in the RC and the PANI/RC groups
compared with the sham group (P<0.01, Fig. 4). The proteins BDNF, CNTF, NGF,
GAP-43, Tau and α-tubulin revealed a 3.07-, 2.17-, 3.05-, 3.01-,
3.48- and 2.44-fold increase in the PANI/RC group compared with the
sham group. The aforementioned proteins demonstrated a 1.27-,
1.18-, 1.43-, 1.59-, 1.64- and 1.24-fold increase in the PANI/RC
group compared with the RC group (Fig.
4).
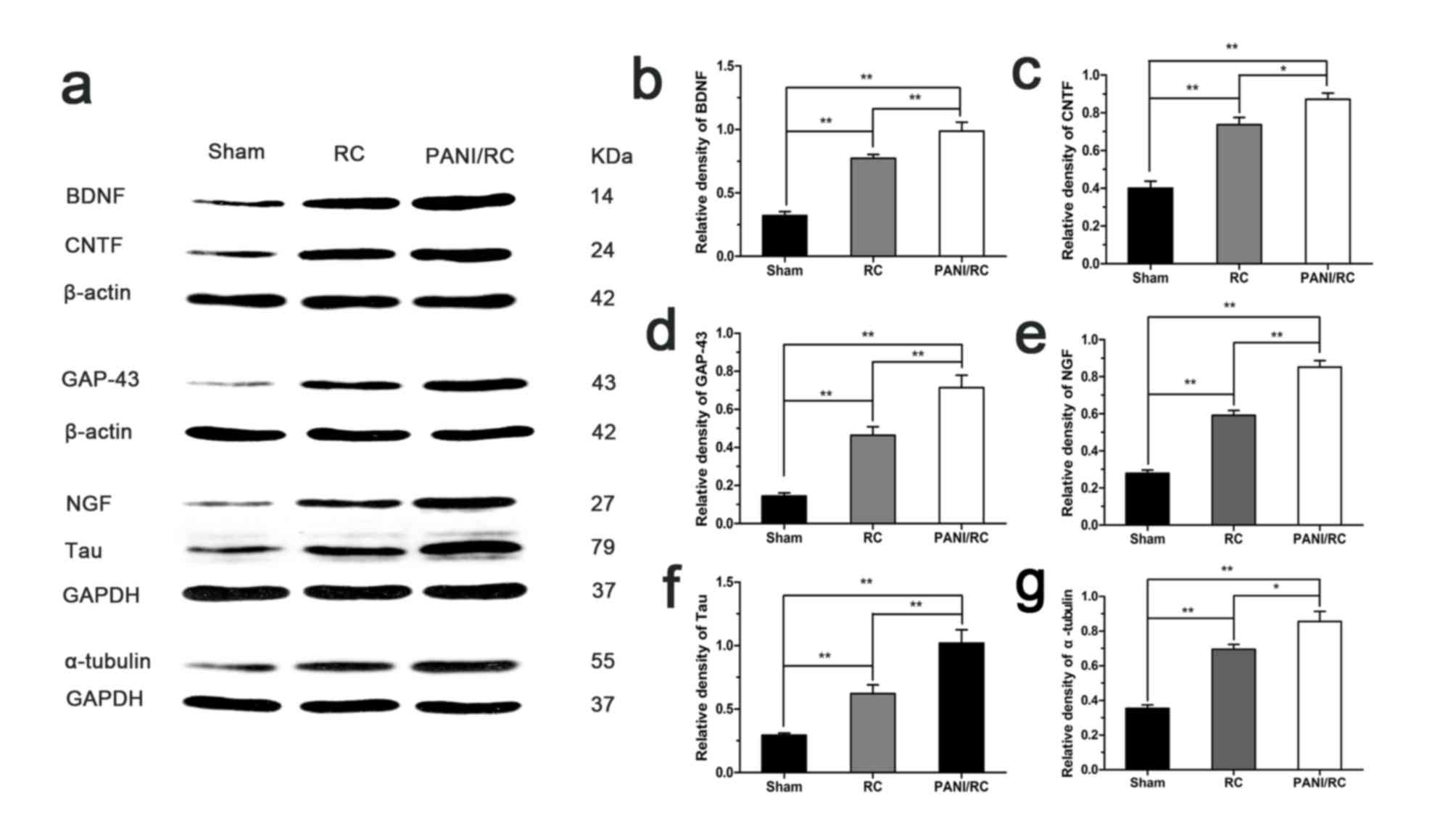 | Figure 4.Protein expression levels of BDNF,
CNTF, NGF, GAP-43, Tau and α-tubulin. (A) Representative western
blot images. Protein expression levels of (B) BDNF, (C) CNTF, (D)
NGF, (E) GAP-43, (F) Tau and (G) α-tubulin, in the three groups.
Data are expressed as the mean ± standard deviation. n=5,
*P<0.05, **P<0.01. PANI/RC, polyaniline/cellulose; BDNF,
brain-derived neurotrophic factor; CNTF, ciliary neurotrophic
factor; NGF, nerve growth factor; GAP, growth-associated protein;
RC, regenerated cellulose. |
Discussion
In the present study, the parameters of axons, axon
diameter and thickness of myelin sheath were greater in the PANI/RC
group compared with the corresponding parameters in the RC group
and lower than those noted in the sham group. The histological data
were similar with the findings reported in previous studies
(17–19).
In the present study, the PANI/RC conduit exhibited
two main characteristics: The hierarchical micro-nanostructure and
the conductivity (5). The
aforementioned factors may promote RSC96 cellular proliferation via
a synergistic mode of action (5).
Regarding conductivity, some authors have previously
reported that the conductive material promotes nerve regeneration
even without electrical stimulation (ES) (21–23).
The conductive scaffold in the absence of ES can in turn be used to
mediate a bioelectricity signal to the distal end for nerve
regeneration (21,22). Initial studies in the field of
nerve regeneration demonstrated the beneficial effects of a
conducting polymer with ES on neurite outgrowth (23). However, an implanted bioelectronic
conduit can establish a stable and long-term electrical
communication with living tissues, thereby allowing regeneration of
the tissues or an exchange of physiological signals in order to
recover damaged biological functions (24). The therapeutic actions of ES are
mediated by the enhanced BDNF-stimulated myelination that in turn
promotes the promyelination effect on Schwann cells at the onset of
myelination (25). The results
regarding the expression of NGF and BDNF are in agreement with the
aforementioned findings. Taken together, the data suggested that
the presence of PANI promotes the electrical conductivity of the
PANI/RC conduits on axon regeneration and myelination, compared
with the RC conduits.
In addition, BDNF activates the MAPK/ERK1/2 cascade,
which may lead to transcriptional regulation and protein synthesis
in the postsynaptic neuron (26).
In the present study, the expression level of BDNF in the PANI/RC
group was significantly increased compared with the RC and sham
groups. The ERK1/2 (MAPK) signaling pathway of the has demonstrated
to be involved in axon regeneration (27). Furthermore, Park et al
(28) revealed that CNTF promoted
retinal ganglion cell survival and axonal regeneration via the
ERK1/2/MAPK signaling pathway, which is similar with the present
results.
The aforementioned evidence demonstrated that the
release of CNTF and the activation of ERK1/2 were essentially
involved in axon growth modulation following nerve injury.
Furthermore, the ERK signaling pathway was reported to be involved
in the modulation of cytoskeletal protein expression, such as
GAP-43 and α-tubulin during axon regeneration (29,30).
GAP-43 is synthesized at high levels during axonal outgrowth and
transported to the growth cone (31). α-tubulin participates directly in
the regulation of the microtubule polymerization during neuronal
development and axonal regeneration (32). Tau is a highly soluble
microtubule-associated protein that modulates the stability of
axonal microtubules (33). The
proteins GAP-43, α-tubulin and Tau serve a crucial role in the
local cytoskeletal rearrangement at the axonal terminal, causing
the growth cone to reform and sprout (34). The level of protein synthesis of
GAP-43, Tau and α-tubulin during the axon regrowth suggested that
these proteins may directly participate in the processes of growth
cone formation or axon elongation (30,32,34).
In the present study, western blot analysis was carried out to
determine whether BDNF, CNTF, GAP-43, Tau and/or α-tubulin proteins
were activated during axon regeneration, so as to establish a link
between cytokine signaling and axon regeneration. The present study
indicated that the expression of the GAP-43, α-tubulin and Tau
proteins in the PANI/RC group was elevated compared with the
expression of the corresponding proteins in the sham and RC groups.
These findings are in agreement with previous studies that
investigated the expression levels of GAP-43 and α-tubulin
(~10-fold and ~3–5-fold increase, respectively) (35).
In the present study, the synthetic PANI/RC conduit
exhibited favorable efficacy for nerve tissue regeneration. The
aforementioned PANI conduit enhanced the sciatic nerve elongation
and provided a favorable environment for axon regeneration by
promoting the secretion of numerous cytokines, growth factors and
neurotrophins, as opposed to the RC conduit. Taken together, the
increasing levels of the CNTF and BDNF proteins induced by PANI
regulated the activation of the ERK1/2 (MAPK) signaling pathway
that in turn promoted the expression of the GAP-43, tau and
α-tubulin cytoskeletal proteins.
In conclusion, the findings of the present study
suggested that in the presence of PANI, the PANI/RC conduit serves
a pivotal role in the stimulation of Schwann cells, in the
activation of the ERK1/2 (MAPK) signaling pathway and in the
release of significant growth factors that are required for the
enhancement of axon regeneration and myelination following static
nerve injury. Despite the promising findings, additional research
is required to elucidate the exact mechanism(s) of the conduit
action and which characteristic is more important for nerve
regeneration: The hierarchical micro-nanostructure or the
conductivity.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China: United Foundation with
Xinjiang (grant no. U1403222).
References
|
1
|
Siemionow M and Brzezicki G: Chapter 8:
Current techniques and concepts in peripheral nerve repair. Int Rev
Neurobiol. 87:141–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buchthal F and Kühl V: Nerve conduction,
tactile sensibility, and the electromyogram after suture or
compression of peripheral nerve: A longitudinal study in man. J
Neurol Neurosurg Psychiatry. 42:436–451. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Evans GR: Peripheral nerve injury: A
review and approach to tissue engineered constructs. Anat Rec.
263:396–404. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burnett MG and Zager EL: Pathophysiology
of peripheral nerve injury: A brief review. Neurosurg Focus.
16:E12004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu D, Fan L, Gao L, Xiong Y, Wang Y, Ye Q,
Yu A, Dai H, Yin Y, Cai J and Zhang L: Micro-nanostructured
polyaniline assembled in cellulose matrix via interfacial
polymerization for applications in nerve regeneration. ACS Appl
Mater Interfaces. 8:17090–17097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhatheja K and Field J: Schwann cells:
Origins and role in axonal maintenance and regeneration. Int J
Biochem Cell Biol. 38:1995–1999. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mar FM, Bonni A and Sousa MM: Cell
intrinsic control of axon regeneration. EMBO Rep. 15:254–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makwana M and Raivich G: Molecular
mechanisms in successful peripheral regeneration. FEBS J.
272:2628–2638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Benraiss A, Chmielnicki E, Lerner K, Roh D
and Goldman SA: Adenoviral brain-derived neurotrophic factor
induces both neostriatal and olfactory neuronal recruitment from
endogenous progenitor cells in the adult forebrain. J Neurosci.
21:6718–6731. 2001.PubMed/NCBI
|
|
10
|
Acheson A, Conover JC, Fandl JP, DeChiara
TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD and Lindsay
RM: A BDNF autocrine loop in adult sensory neurons prevents cell
death. Nature. 374:450–453. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tom VJ, Sandrow-Feinberg HR, Miller K,
Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA and Houlé
JD: Exogenous BDNF enhances the integration of chronically injured
axons that regenerate through a peripheral nerve grafted into a
chondroitinase-treated spinal cord injury site. Exp Neurol.
239:91–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Friedman B, Scherer SS, Rudge JS, Helgren
M, Morrisey D, McClain J, Wang DY, Wiegand SJ, Furth ME, Lindsay
RM, et al: Regulation of ciliary neurotrophic factor expression in
myelin-related Schwann cells in vivo. Neuron. 9:295–305. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Lineaweaver WC, Oswald T, Chen Z,
Chen Z and Zhang F: Ciliary neurotrophic factor for acceleration of
peripheral nerve regeneration: An experimental study. J Reconstr
Microsurg. 20:323–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cen LP, Luo JM, Zhang CW, Fan YM, Song Y,
So KF, van Rooijen N, Pang CP, Lam DS and Cui Q: Chemotactic effect
of ciliary neurotrophic factor on macrophages in retinal ganglion
cell survival and axonal regeneration. Invest Ophthalmol Vis Sci.
48:4257–4266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang CW, Lu Q, You SW, Zhi Y, Yip HK, Wu
W, So KF and Cui Q: CNTF and BDNF have similar effects on retinal
ganglion cell survival but differential effects on nitric oxide
synthase expression soon after optic nerve injury. Invest
Ophthalmol Vis Sci. 46:1497–1503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Y, Leaver SG, Plant GW, Hendriks WT,
Niclou SP, Verhaagen J, Harvey AR and Cui Q: Lentiviral-mediated
transfer of CNTF to schwann cells within reconstructed peripheral
nerve grafts enhances adult retinal ganglion cell survival and
axonal regeneration. Mol Ther. 11:906–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin KL, Yang DY, Chu IM, Cheng FC, Chen
CJ, Ho SP and Pan HC: DuraSeal as a ligature in the anastomosis of
rat sciatic nerve gap injury. J Surg Res. 161:101–110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oliveira EF, Mazzer N, Barbieri CH and
DelBel EA: The use of a muscle graft to repair a segmentary nerve
defect. An experimental study using the sciatic nerve of rats as
model. J Neurosci Methods. 133:19–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weber RA, Warner MR, Verheyden CN and
Proctor WH: Functional evaluation of gap vs. abutment repair of
peripheral nerves in the rat. J Reconstr Microsurg. 12:159–163.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JY, Bashur CA, Goldstein AS and
Schmidt CE: Polypyrrole-coated electrospun PLGA nanofibers for
neural tissue applications. Biomaterials. 30:4325–4335. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang J, Lu L, Zhang J, Hu X, Zhang Y,
Liang W, Wu S and Luo Z: Electrical stimulation to conductive
scaffold promotes axonal regeneration and remyelination in a rat
model of large nerve defect. PLoS One. 7:e395262012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmidt CE, Shastri VR, Vacanti JP and
Langer R: Stimulation of neurite outgrowth using an electrically
conducting polymer. Proc Natl Acad Sci USA. 94:8948–8953. 1997;
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu B, Luo SC, Zhao H, Lin HA, Sekine J,
Nakao A, Chen C, Yamashita Y and Yu HH: Large enhancement in
neurite outgrowth on a cell membrane-mimicking conducting polymer.
Nat Commun. 5:45232014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang J, Hu X, Lu L, Ye Z, Zhang Q and Luo
Z: Electrical regulation of Schwann cells using conductive
polypyrrole/chitosan polymers. J Biomed Mater Res A. 93:164–174.
2010.PubMed/NCBI
|
|
26
|
Mohajerani MH, Sivakumaran S, Zacchi P,
Aguilera P and Cherubini E: Correlated network activity enhances
synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1
connections in the hippocampus. Proc Natl Acad Sci USA.
104:13176–13181. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abe N and Cavalli V: Nerve injury
signaling. Curr Opin Neurobiol. 18:276–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park K, Luo JM, Hisheh S, Harvey AR and
Cui Q: Cellular mechanisms associated with spontaneous and ciliary
neurotrophic factor cAMP-induced survival and axonal regeneration
of adult retinal ganglion cells. J Neurosci. 24:10806–10815. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen ZL, Yu WM and Strickland S:
Peripheral regeneration. Annu Rev Neurosci. 30:209–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaneda M, Nagashima M, Mawatari K, Nunome
T, Muramoto K, Sugitani K and Kato S: Growth-associated protein43
(GAP43) is a biochemical marker for the whole period of fish optic
nerve regeneration. Adv Exp Med Biol. 664:97–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janke C and Bulinski JC:
Post-translational regulation of the microtubule cytoskeleton:
Mechanisms and functions. Nat Rev Mol Cell Biol. 12:773–786. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu MF, Zhou H, Hu CY, Liang YQ, Hu L and
Chen D: The mechanisms of EGFR in the regulation of axon
regeneration. Cell Biochem Funct. 32:101–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu K, Tedeschi A, Park KK and He Z:
Neuronal intrinsic mechanisms of axon regeneration. Annu Rev
Neurosci. 34:131–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Skene JH, Jacobson RD, Snipes GJ, McGuire
CB, Norden JJ and Freeman JA: A protein induced during nerve growth
(GAP-43) is a major component of growth-cone membranes. Science.
233:783–786. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fernandes KJL, Fan DP, Tsui BJ, Cassar SL
and Tetzlaff W: Influence of the axotomy to cell body distance in
rat rubrospinal and spinal motoneurons: Differential regulation of
GAP-43, tubulins, and neurofilament-M. J Comp Neurol. 414:495–510.
1999. View Article : Google Scholar : PubMed/NCBI
|