Introduction
Skeletal muscle is the most abundant tissue in the
body, the mass of which represents a determinant of strength,
endurance, and physical performance (1). Skeletal muscle is a plastic tissue to
be hypertrophy or atrophy with the dynamic balance between protein
synthesis and degradation (2–4).
Muscle atrophy has been characterized as decreased protein content,
decreased insulin sensitivity and increased fatigability (2,5). It
is noteworthy that the protein level in skeletal muscle is
dependent on mammalian target of rapamycin (mTOR) and forkhead box
O (FoxO) family. mTOR plays a critical role in promoting the
protein synthesis of skeletal muscle through its downstream S6,
P70S6K1, 4E-BP1, and eIF4E (6–8),
while FoxO1 and FoxO3a induce the degradation of protein by
regulating expression of MuRF1 and MAFbx (9).
Though skeletal muscle growth was regulated by
hormone signals (10,11) and nutrients availability (12,13),
endurance training has been proven to be the most effective method
to attenuate the skeletal muscle atrophy. Several evidences showed
that only one session of raise exercise could dramatically increase
the content of TCA metabolites, such as octanoylcarnitine,
glutamate, succinate and rotenone (14). In addition, both high-intensity
interval training and moderate-intensity continuous training
significantly increased citrate synthase activity and succinate
content (15). Succinate, a vital
intermediate in the tricarboxylic acid (TCA) cycle, is downstream
product of the α-ketoglutarate dehydrogenase complex, which has
been reported to play a crucial role in the process of cell
metabolism, substrate-level phosphorylation, ketone bodies
utilization and heme metabolism (16). However, the effects of succinate on
skeletal muscle hypertrophy and protein synthesis was rarely
excavated.
Recently, a series study delineated that succinate
could induce intracellular Ca2+ by G protein coupled
receptor and PLCβ (17,18). In skeletal muscle,
[Ca2+]i is a vital second messenger to active Erk1/2 and
promote the differentiation and protein synthesis of myotubes
(19,20). So, the present evidences led to the
hypothesis that succinate might be involved in skeletal muscle
protein synthesis and muscle hypertrophy. To test this hypothesis,
we first examined the effect of succinate on skeletal muscle
protein turnover signaling pathway. We found succinate could
dose-dependently promote skeletal muscle protein synthesis in
vitro and in vivo, which was mediated by Erk/Akt
signaling pathway. Therefore, our investigation initiates the
identification of succinate in promoting skeletal muscle protein
synthesis and highlights its nutrition value and biological
significance.
Materials and methods
Animals
C57BL6/J male mice were purchased from the Animal
Experiment Center of Guangdong Province [permission no. 8 SYXK
(Yue) 2016–0136). All experiments were conducted in accordance with
‘The Instructive Notions with Respect to Caring for Laboratory
Animals’ issued by the Ministry of science and Technology of the
Peoples Republic of China. All experimental protocols were approved
by the College of Animal Science, South China Agricultural
University. The mice were maintained under constant light for 12 h
and a 12 h dark cycle at a temperature of 23±3°C during the
experimental period. The mice were given access to standard pellets
(crude protein 18%, crude fat 4%, and crude ash 8%). In the acute
experiment, 30 8-week-old male mice were randomly divided into
three groups (n=10) and injected by intraperitoneal with saline,
150 and 300 mg/kg of succinate (Sigma, St. Louis, MO, USA) for 3 h.
The animals were sacrificed after 3 h injection to collect
gastrocnemius samples.
Cell culture and treatment
Murine skeletal muscle cell line C2C12 was cultured
in high glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100,000 U/l of penicillin
sodium, and 100 mg/l of streptomycin sulfate (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere that contained
5% CO2. The C2C12 myoblasts were induced differently to
myotubes by a medium that contained high glucose DMEM and 2% horse
serum (HS; Gibco; Thermo Fisher Scientific, Inc.) for 6 days. The
C2C12 cells were treated with the mTOR inhibitor rapamycin and Erk
inhibitor U0126 for 24 h, respectively.
Total protein content
C2C12 cell were treated with 0.5 and 2 mM succinate
for 48 h. Cells were washed twice with cold PBS and lysed using 200
µl radio immunoprecipitation assay (RIPA) lysis buffer that
contained protein phosphatase inhibitor complex (Biosino
Bio-Technology and Science Inc., Beijing, China) and 1 mM PMSF. The
total protein of the cell lysate was detected using a commercial
kit (Thermo Fisher Scientific, Inc.) and normalized through DNA
content.
Myotubes diameter
C2C12 cell were treated with 0.5 and 2 mM succinate
for 48 h. Cells were washed twice with cold PBS. Analyze using
Nikon Eclipse Ti-s microscopy with Nis-Elements BR software (Nikon
Instruments, Tokyo, Japan). Ten images were randomly captured on
each coverslip (replicate sample). The diameter of each myotube
(6–10) in each image was measured and used
to calculate the mean myotube diameter for each sample replicate
(n=5).
Puromycin assay
SUnSET method was used to measure protein synthesis
in vitro and in vivo. For the in vitro study,
10 µg/ml puromycin was added to the medium 1 h before C2C12 was
collected. For the in vivo study, 100 µg/ml puromycin was
injected 1 h before gastrocnemius muscle tissue collection. The
incorporation of puromycin in the total protein was analyzed by
western blotting.
Western blot assay
C2C12 Cells were lysed in RIPA lysis buffer. And
total protein concentration was tested using BCA protein assays.
After separation on 10% sodium dodecyl sulfate (SDS)-poliacrylamide
gel electrophoresis gel, the protein was transferred to
polyvinylidene fluoride (PVDF) membrane and blocked with 5%
(wt/vol) non-fat dry milk in Tris-buffered saline that contained
Tween-20 for about 2 h at room temperature. The PVDF membranes were
then incubated with the indicated antibodies, including rabbit
anti-β-actin (Bioss, Beijing, China) and mouse puromycin antibody
12D10 (EMD Millipore, Billerica, MA, USA); rabbit anti-phospho-mTOR
(Ser2481) and mTOR, rabbit anti-phospho-S6 (Ser235/236) and S6,
rabbit anti-phospho-4E-BP1 (Thr37/46) and 4E-BP1, rabbit anti-Akt,
rabbit anti-phospho-Akt (Ser473), rabbit anti-phospho-Akt (Thr308),
rabbit anti-eIF4E, rabbit anti-phospho-eIF4E (Ser209), rabbit
anti-eIF4E, rabbit anti-phospho-eIF4E (Ser251), rabbit
anti-phospho-FoxO3a, rabbit anti-FoxO3a, rabbit anti-phospho-Erk,
rabbit anti-Erk. The primary antibodies were incubated at 4°C
overnight and followed by the incubations of the appropriate
secondary antibody (Bioss) for 1 h at room temperature. Protein
expression was measured using a FluorChem M Fluorescent Imaging
System (ProteinSimple, Santa Clara, CA, USA) and normalized to
β-actin expression.
Immunohistochemistry
C57BL6/J mice gastrocnemius was sliced to 8–10 µm by
a frozen slicer (LEICA CM 1850, Germany). The sections were rinsed
3 times in PBS and then blocked for 1 h at room temperature.
Subsequently, the sections were incubated in rabbit anti-phospho-S6
(Ser235/236) overnight at room temperature. The sections were
rinsed 3 times by PBS. The next day, the sections were transferred
to FITC second antibody (Bioss). Sections were then observed using
Nikon Eclipse Ti-s microscopy with Nis-Elements BR software (Nikon
Instruments). Up to four fields of view were captured from every
group.
Assay of [Ca2+]i
[Ca2+]i was measured by calcium
fluorometry using fluo-8 AM. The C2C12 cells were seeded in a
24-well plate and cultured in high glucose DMEM with 2% horse serum
for 6 days. The cells were washed twice with Hanks Balanced Salt
Solution (HBSS, pH=7.2–7.4) containing 8 g/l NaCl, 0.4 g/l KCl, 0.1
g/l mgSO4.7H2O, 0.1 g/l
MgCl2.6H2O, 0.06 g/l
Na2HPO4.2H2O, 0.06 g/l
K2HPO4, 1 g/l Glucose, 0.14 g/l
CaCl2 and 0.35 g/l NaHCO3, and then incubated
with 10 µM fluo 8-AM at 37°C. After incubation for 1 h, the C2C12
cells were washed twice with HBSS and the calcium response assay
was initiated by manual addition reagent equipped with Nikon
Eclipse Ti-s microscopy. Intracellular calcium responses were
measured at 37°C by quantification of the fluorescence emission
intensity at 525 nm after excitation of the samples at 494 nm, with
data collection every 5 sec over a 180 sec period.
Co-immunoprecipitation
Lysates containing 500 µg total protein were
immunoprecipitated with antibodies specific to Erk overnight at
4°C. Immune complexes were collected by incubation with a mixture
of protein A- and G-Sepharose for 6 h at 4°C, the immune complexes
were then washed three times with wash buffer [50 mM HEPES-NaOH (pH
7.6), 150 mM NaCl, and 0.1% Triton X-100] before being eluted in 2X
sodium dodecyl sulfate sample buffer. The immune complexes were
subjected to SDS-PAGE and transferred to a polyvinylidene
difluoride membrane for further protein detection.
Statistical analysis
All data was expressed as means ± standard error of
the mean (SEM). Significant differences between the control and the
treated group were determined by Students t-test. One-way analysis
of variance was used to test the dosage effect of succinate on
protein synthesis (SPSS 18.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Succinate promoted protein synthesis
in C2C12 myotubes
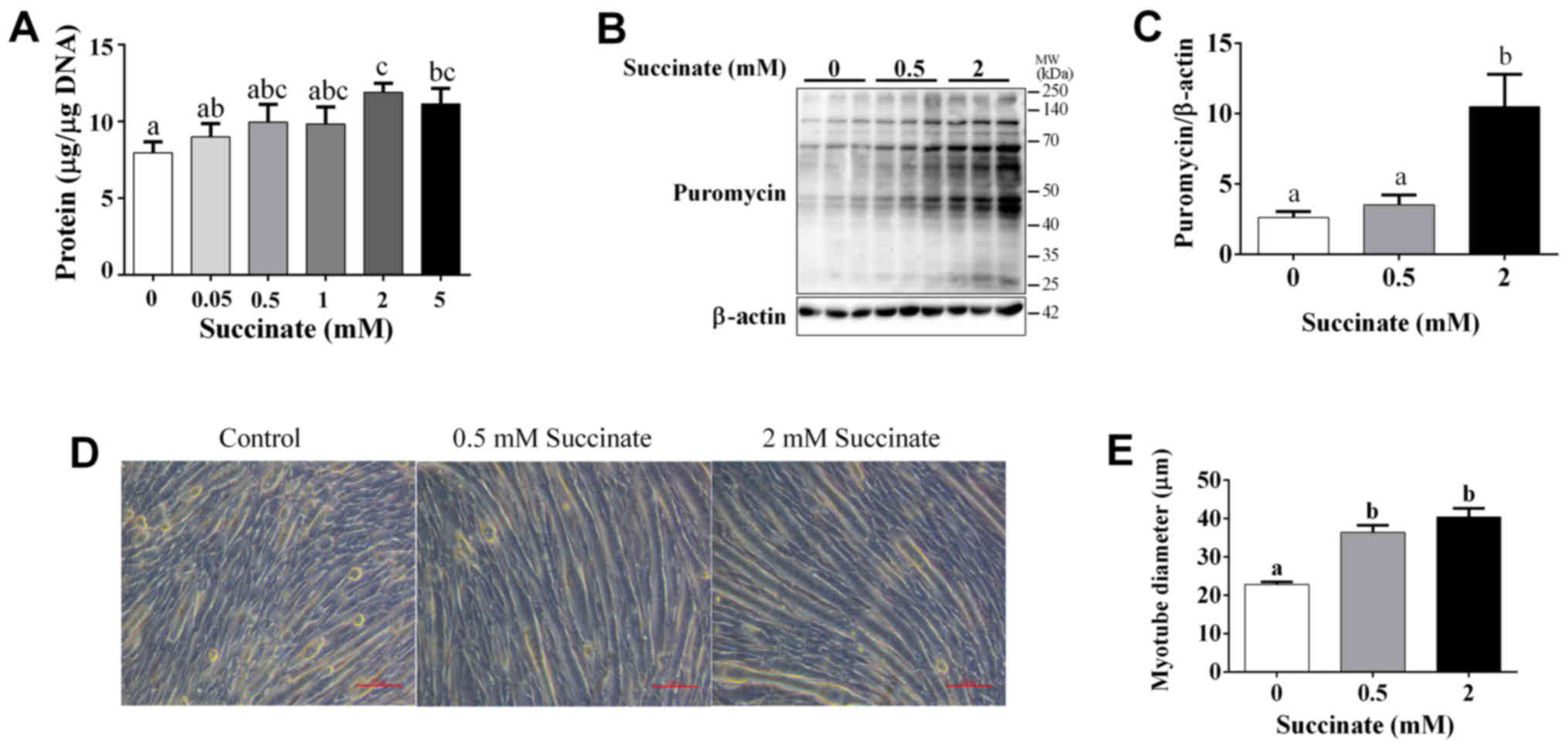
Our study demonstrated that succinate (0.5 and 2 mM)
could dose-dependently increase cellular protein levels of C2C12
cells (Fig. 1A). Further, the
puromycin test result showed that 2 mM succinate significantly
increased the puromycin incorporation in myotubes (Fig. 1B and C). In addition, succinate
(0.5 and 2 mM) significantly increased the myotube diameter
(Fig. 1D and E). These data
support the hypothesis that succinate promotes protein synthesis in
skeletal muscle.
Succinate actived protein translation
and suppressed protein degradation pathway in C2C12 myotubes
To further investigate the underlying mechanism, by
which succinate promotes protein synthesis in C2C12 myotubes, we
detected protein translation and degradation associated pathway.
Western blot data showed the phosphorylation level of Akt, mTOR,
S6, eIF4E, 4E-BP1 and FoxO3a were significantly increased in C2C12
myotubes when exposed to 2 mM succinate (Fig. 2A-G). Moreover, 0.5 mM succinate
significantly increased the phosphorylation level of Akt, 4E-BP1,
eIF4E (Fig. 2A, B, F and G).
Compared to 0.5 mM succinate group, 2 mM succinate significantly
increased the phosphorylation level of mTOR, S6, and FoxO3a
(Fig. 2A and C-E). As expected,
mTOR antagonist, rapamycin, effectively abolished succinate-induced
protein synthesis (Fig. 2H).
Together, the result indicates that succinate promoted protein
synthesis was mediated by the activation of protein translation and
inactivation of protein degradation in C2C12 myotubes.
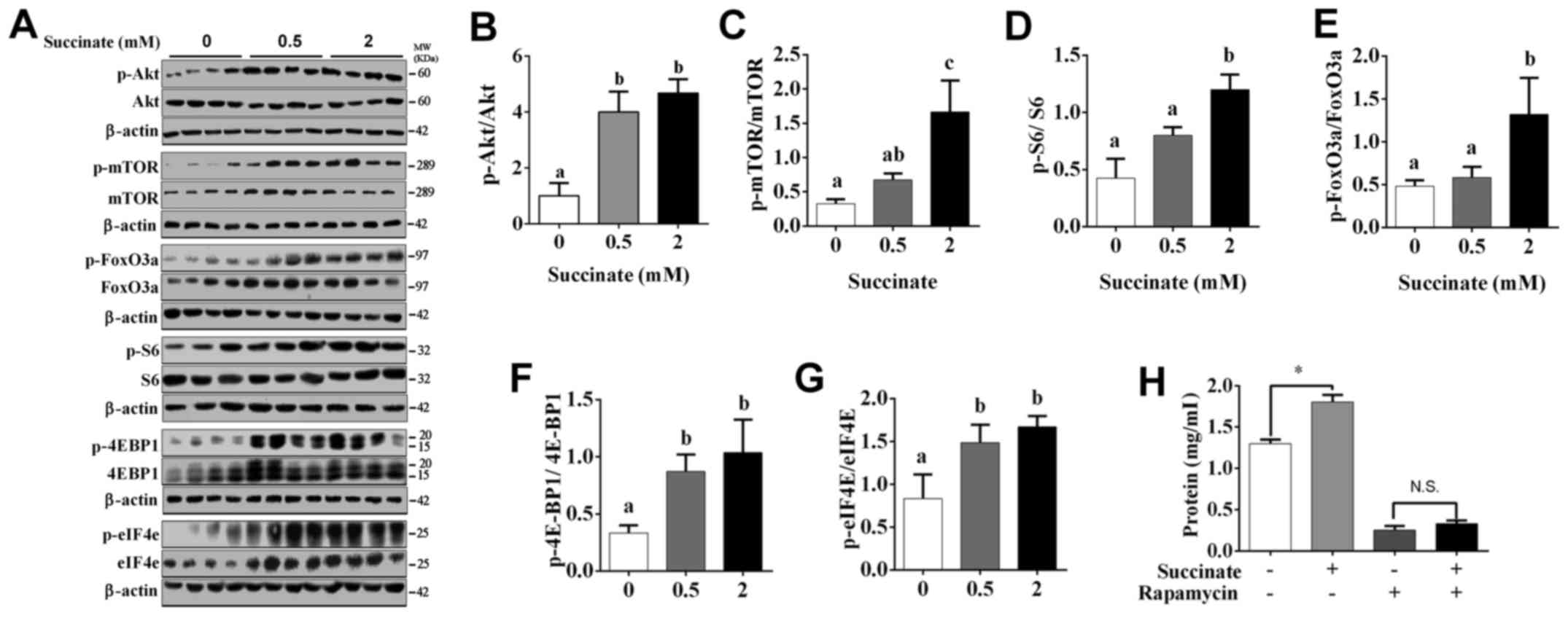 | Figure 2.Akt/mTOR/FoxO cascade was involved in
succinate-induced protein deposition in C2C12 myotubes. C2C12 cells
were cultured for 6 days in a differentiation medium. C2C12
myotubes were then exposed to succinate (0.5 and 2 mM) for 48 h.
(A) Western blot analysis of p-Akt, Akt, p-mTOR, mTOR, p-FoxO3a,
FoxO3a, p-S6, S6, p-4EBP1, 4EBP1, p-eIF4e and eIF4e. (B-G) The
statistical analyses results of the western blotting of the
phosphorylation level of Akt, mTOR, FoxO3a, S6, 4E-BP1 and eIF4e.
(H) The total protein level after C2C12 cells were co-treated with
succinate and rapamycin. a,bSignificant differences
between groups (P<0.05). *P<0.05 compared with the control.
β-actin was used as a loading control. |
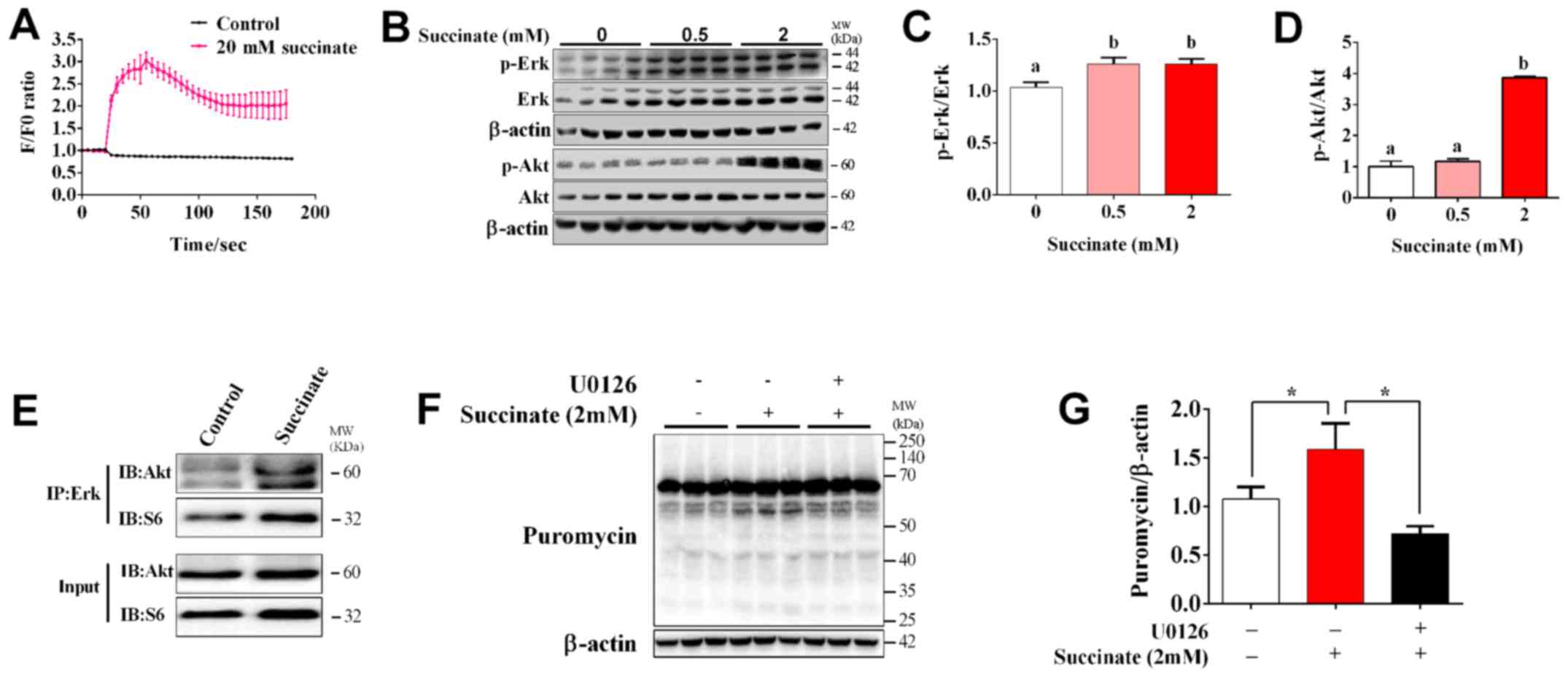
The role of Erk/Akt signaling pathway
in succinate-induced protein synthesis in C2C12 myotubes
To determine whether Ca2+ and Erk
signaling pathway are involved in succinate-induced protein
synthesis, we first detected the dynamic change of intracellular
Ca2+ concentration. The result showed that succinate
dramatically increased [Ca2+]i and reached the maximal
level at 30s post treatment (Fig.
3A). When compared to control, 2 mM succinate remarkably
increased the phosphorylation level of Erk and Akt, while 0.5 mM
succinate only remarkably increased the phosphorylation level of
Erk (Fig. 3B-D). In addition, 2 mM
succinate also significantly increased the phosphorylation level of
Akt compared to 0.5 mM succinate group (Fig. 3D). We further test the crosstalk
between Erk and Akt cascade by co-Immunoprecipitation. It was
interesting to find that succinate obviously promoted the binding
of Erk with Akt, but not that of Erk with S6 (Fig. 3E). Moreover, U0126, an Erk
inhibitor, could reverse succinate induced protein synthesis in
C2C12 myotubes (Fig. 3F and G).
These data indicated that Erk is crucial for succinate-induced
activation of Akt cascade and enhancement of protein synthesis.
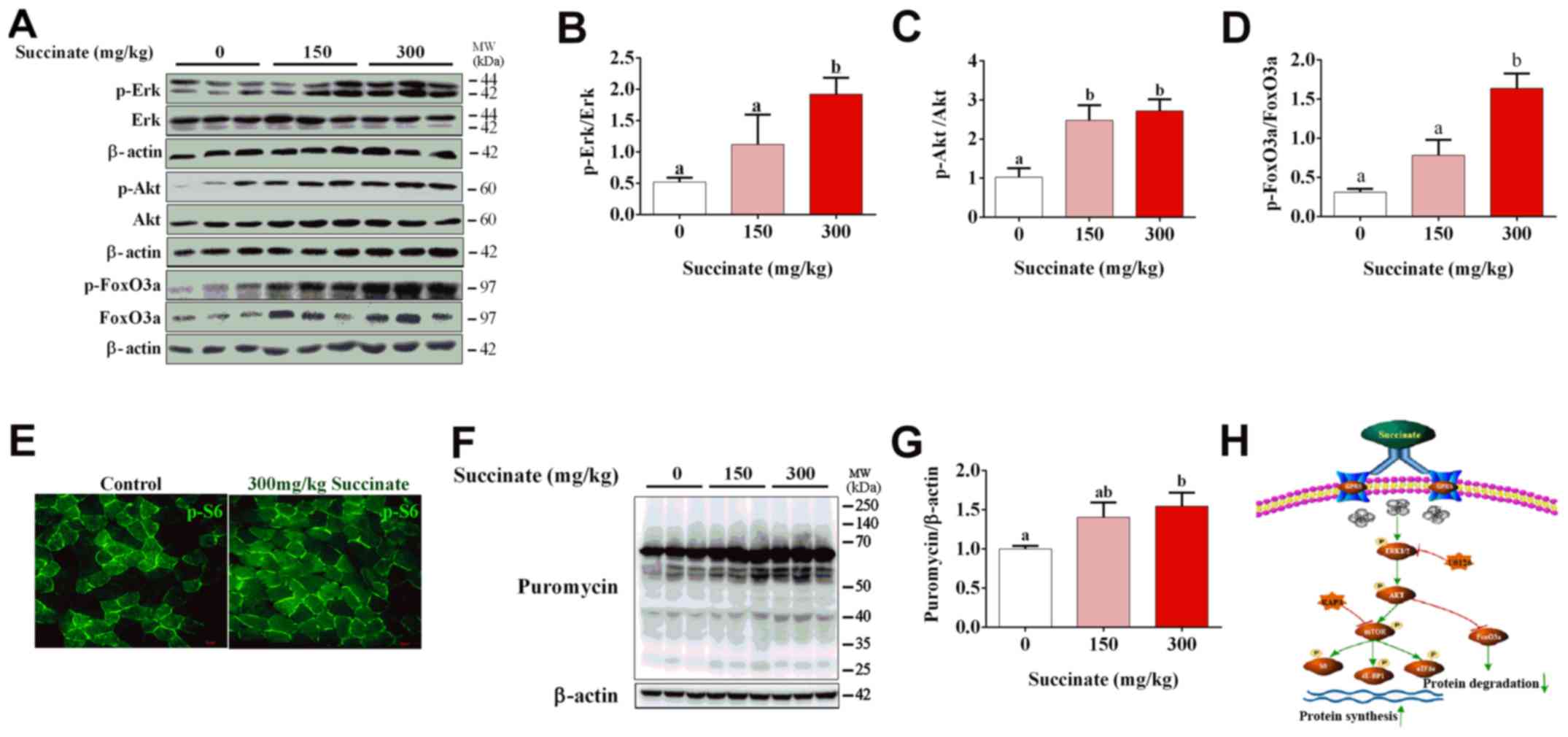
Succinate increased protein synthesis
in the skeletal muscle of mice
To further confirm the effect of succinate on
protein synthesis in vivo, C57BL6/J mice were injected with
150 and 300 mg/kg succinate, respectively. Consistent with previous
in vitro data, 300 mg/kg succinate could significantly
increase the phosphorylation level of Erk, Akt and FoxO3a in
gastrocnemius muscle compared to control, while 150 mg/kg succinate
could significantly increase the phosphorylation level of Akt
(Fig. 4A-D), as well as protein
synthesis (Fig. 4F and G).
Further, 300 mg/kg succinate significantly increased the
phosphorylation level of Erk and FoxO3a when compared to 150 mg/kg
succinate group (Fig. 4A, B and
D). Moreover, immunohistochemistry data illustrated that 300
mg/kg succinate increased phosphor-S6 levels in gastrocnemius
tissue (Fig. 4E). The mechanism
picture that succinate promotes the protein synthesis of skeletal
muscle myotubes (Fig. 4H). These
data verified that succinate increased protein synthesis and
deposition in the skeletal muscle of mice.
Discussion
Classically, hormones and nutrients, for example
insulin-like growth factors-1 (IGF-1) and amino acids, are crucial
regulator for skeletal muscle protein synthesis (21). It should be emphasized that several
metabolites of animo acids and TCA cycle, such as
α-ketoisocaproate, β-hydroxy-β-methylbutyrate and α-ketoglutarate,
have also been identified to enhance skeletal muscle growth by
increasing protein synthesis and inhibiting protein degradation
(3,22,23).
Succinic acid is another key TCA cycle metabolite, of which
exercise could dramatically increase the content (14). In this paper, we firstly identified
that succinate could significantly promote protein synthesis in
vitro and in vivo, accompanished with activation of
Akt/mTOR/S6 cascade and inhibition of FoxO3a.
Succinate is the endogenous ligand for GPR91, which
mediated the effect of succinate on muscle hypertrophy (24), protein deposition (25) and metabolism regulation (26). Both the mRNA and protein level of
GPR91 are ubiquitously expressed in many tissues, including
skeletal muscle (27), which
indicates this receptor and its downstream signaling pathway might
be involved in succinate induced skeletal hypertrophy. Once
activated by succinate, GPR91 recruited Gαi protein to
inhibit cAMP production and increase intracellular Ca2+
(17). As expected, we detected a
dramatic elevation of [Ca2+]i of C2C12 myotubes in
response to succinate treatment, which might be the explaination to
elucidate effect of succinate on protein synthesis.
Skeletal muscle protein deposition is regulated by
multiple signaling pathways (28,29).
Among those, Erk1/2 pathway represented extracellular signal in
protein synthesis (30,31), which was sensitive to transient
elevation of [Ca2+]i (32). It has been reported activation of
GPR91 triggered [Ca2+]i elevation and subsequently, the
activation of Erk (33). In
consistent with previous evidence, we confirmed that succinate
dose-dependently increased phospho-Erk level in vitro and
in vivo. Moreover, our co-IP data also found that succinate
obviously promoted the binding of Erk with Akt. Notably, either Erk
antagonist (U0126) or mTOR inhibitor (rapamycin) could abolish the
effect of succinate on protein synthesis.
In conclusion, our study demonstrated that succinate
promoted skeletal muscle protein synthesis through Erk/Akt
signaling pathway.
Acknowledgements
This study was supported by National Basic Research
Program of China (no. 2013CB127306), National Key Point Research
and Invention Program (2016YFD0501205) and National Nature Science
foundation of China (no. 31572480).
References
|
1
|
Fanzani A, Conraads VM, Penna F and
Martinet W: Molecular and cellular mechanisms of skeletal muscle
atrophy: An update. J Cachexia Sarcopenia Muscle. 3:163–179. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandri M: Signaling in muscle atrophy and
hypertrophy. Physiology (Bethesda). 23:160–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai X, Zhu C, Xu Y, Jing Y, Yuan Y, Wang
L, Wang S, Zhu X, Gao P, Zhang Y, et al: Alpha-ketoglutarate
promotes skeletal muscle hypertrophy and protein synthesis through
Akt/mTOR signaling pathways. Sci Rep. 6:268022016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egerman MA and Glass DJ: Signaling
pathways controlling skeletal muscle mass. Crit Rev Biochem Mol
Biol. 49:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brooks NE and Myburgh KH: Skeletal muscle
wasting with disuse atrophy is multi-dimensional: The response and
interaction of myonuclei, satellite cells and signaling pathways.
Front Physiol. 5:992014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuentes EN, Einarsdottir IE, Paredes R,
Hidalgo C, Valdes JA, Björnsson BT and Molina A: The TORC1/P70S6K
and TORC1/4EBP1 signaling pathways have a stronger contribution on
skeletal muscle growth than MAPK/ERK in an early vertebrate:
Differential involvement of the IGF system and atrogenes. Gen Comp
Endocrinol. 210:96–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schiaffino S, Dyar KA, Ciciliot S, Blaauw
B and Sandri M: Mechanisms regulating skeletal muscle growth and
atrophy. FEBS J. 280:4294–4314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White JP, Gao S, Puppa MJ, Sato S, Welle
SL and Carson JA: Testosterone regulation of Akt/mTORC1/FoxO3a
signaling in skeletal muscle. Mol Cell Endocrinol. 365:174–186.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crossland H, Constantin-Teodosiu D,
Gardiner SM, Constantin D and Greenhaff PL: A potential role for
Akt/FOXO signalling in both protein loss and the impairment of
muscle carbohydrate oxidation during sepsis in rodent skeletal
muscle. J Physiol. 586:5589–5600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Long W, Fryburg DA and Barrett EJ:
The regulation of body and skeletal muscle protein metabolism by
hormones and amino acids. J Nutr. 136 1 Suppl:212S–217S.
2006.PubMed/NCBI
|
|
11
|
Suryawan A and Davis TA: Regulation of
protein degradation pathways by amino acids and insulin in skeletal
muscle of neonatal pigs. J Anim Sci Biotechnol. 5:82014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugden PH and Fuller SJ: Regulation of
protein turnover in skeletal and cardiac muscle. Biochem J.
273:21–37. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suryawan A and Davis TA: Regulation of
protein synthesis by amino acids in muscle of neonates. Front
Biosci (Landmark Ed). 16:1445–1460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Schaardenburgh M, Wohlwend M, Rognmo Ø
and Mattsson EJ: Mitochondrial respiration after one session of
calf raise exercise in patients with peripheral vascular disease
and healthy older adults. PLoS One. 11:e01650382016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai HH, Chang SC, Chou CH, Weng TP, Hsu
CC and Wang JS: Exercise training alleviates hypoxia-induced
mitochondrial dysfunction in the lymphocytes of sedentary males.
Sci Rep. 6:351702016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tretter L, Patocs A and Chinopoulos C:
Succinate, an intermediate in metabolism, signal transduction, ROS,
hypoxia, and tumorigenesis. Biochim Biophys Acta. 1857:1086–1101.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sundström L, Greasley PJ, Engberg S,
Wallander M and Ryberg E: Succinate receptor GPR91, a Gα(i) coupled
receptor that increases intracellular calcium concentrations
through PLCβ. FEBS Lett. 587:2399–2404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aguiar CJ, Andrade VL, Gomes ER, Alves MN,
Ladeira MS, Pinheiro AC, Gomes DA, Almeida AP, Goes AM, Resende RR,
et al: Succinate modulates Ca(2+) transient and cardiomyocyte
viability through PKA-dependent pathway. Cell Calcium. 47:37–46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Espinosa A, Leiva A, Peña M, Müller M,
Debandi A, Hidalgo C, Carrasco MA and Jaimovich E: Myotube
depolarization generates reactive oxygen species through NAD(P)H
oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early
genes. J Cell Physiol. 209:379–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
May C, Weigl L, Karel A and Hohenegger M:
Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1
receptor in a Ca2+ independent manner in differentiated
human skeletal muscle cells. Biochem Pharmacol. 71:1497–1509. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishida H, Ikegami A, Kaneko C, Kakuma H,
Nishi H, Tanaka N, Aoyama M, Usami M and Okimura Y: Dexamethasone
and BCAA failed to modulate muscle mass and mTOR signaling in
GH-deficient rats. PLoS One. 10:e01288052015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Q, He L, Hou Y, Chen J, Duan Y, Deng
D, Wu G, Yin Y and Yao K: Alpha-ketoglutarate enhances milk protein
synthesis by porcine mammary epithelial cells. Amino Acids.
48:2179–2188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan Y, Li F, Li Y, Tang Y, Kong X, Feng
Z, Anthony TG, Watford M, Hou Y, Wu G and Yin Y: The role of
leucine and its metabolites in protein and energy metabolism. Amino
Acids. 48:41–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilissen J, Jouret F, Pirotte B and Hanson
J: Insight into SUCNR1 (GPR91) structure and function. Pharmacol
Ther. 159:56–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He W, Miao FJ, Lin DC, Schwandner RT, Wang
Z, Gao J, Chen JL, Tian H and Ling L: Citric acid cycle
intermediates as ligands for orphan G-protein-coupled receptors.
Nature. 429:188–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Littlewood-Evans A, Sarret S, Apfel V,
Loesle P, Dawson J, Zhang J, Muller A, Tigani B, Kneuer R, Patel S,
et al: Sarret GPR91 senses extracellular succinate released from
inflammatory macrophages and exacerbates rheumatoid arthritis. J
Exp Med. 213:1655–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Castro Fonseca M, Aguiar CJ, da Rocha
Franco JA, Gingold RN and Leite MF: GPR91: Expanding the frontiers
of Krebs cycle intermediates. Cell Commun Signal. 14:32016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ato S, Makanae Y, Kido K and Fujita S:
Contraction mode itself does not determine the level of mTORC1
activity in rat skeletal muscle. Physiol Rep. 4(pii): e129762016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agergaard J, Bülow J, Jensen JK,
Reitelseder S, Drummond MJ, Schjerling P, Scheike T, Serena A and
Holm L: Light-load resistance exercise increases muscle protein
synthesis and hypertrophy signaling in elderly men. Am J Physiol
Endocrinol Metab. 312:E326–E338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osterweil EK, Krueger DD, Reinhold K and
Bear MF: Hypersensitivity to mGluR5 and ERK1/2 leads to excessive
protein synthesis in the hippocampus of a mouse model of fragile X
syndrome. J Neurosci. 30:15616–15627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quan-Jun Y, Yan H, Yong-Long H, Li-Li W,
Jie L, Jin-Lu H, Jin L, Peng-Guo C, Run G and Cheng G: Selumetinib
attenuate skeletal muscle wasting in murine cachexia model through
ERK inhibition and AKT activation. Mol Cancer Ther. 16:334–343.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hegedũs L, Garay T, Molnár E, Varga K,
Bilecz Á, Török S, Padányi R, Pászty K, Wolf M, Grusch M, et al:
The plasma membrane Ca2+ pump PMCA4b inhibits the
migratory and metastatic activity of BRAF mutant melanoma cells.
Int J Cancer. 140:2758–2770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aguiar CJ, Rocha-Franco JA, Sousa PA,
Santos AK, Ladeira M, Rocha-Resende C, Ladeira LO, Resende RR,
Botoni FA, Melo M Barrouin, et al: Succinate causes pathological
cardiomyocyte hypertrophy through GPR91 activation. Cell Commun
Signal. 12:782014. View Article : Google Scholar : PubMed/NCBI
|