Introduction
Cerebral infarction, known as ischemic stroke, is
caused by various causes of local blood supply obstacles in the
brain tissue, which lead to cerebral ischemic necrosis, anoxic
lesions and even corresponding clinical nerve function loss
(1–3). Currently, the incidence rate of
cerebral infarction presents a rising trend with the growth in
living standards throughout the world (4,5).
Cerebral thrombosis has been identified as one of the most common
cardiovascular diseases and the most frequent disabling disease
that leads to mortality at the age of >60 years (6). Therefore, cerebral infarction
severely affects the lives of patients. The underlying cause is
rupture of atherosclerotic plaques following platelet adhesion and
thrombus formation or embolization in cerebral thrombosis (7,8).
Activation of platelets is essential for normal
hemostasis at sites of endothelial injury, however a congealing
clot in the blood can cause stoppage of flow leading to a heart
attack, aneurysm or stroke, depending on the location of the
blocked vessel (9–11). A clot in the blood is a major
pathomechanism underlying acute ischemic disease states including
stroke, atherosclerosis, myocardial infarction and cerebral
hemorrhage, which may be lead to severe disability. They cause the
majority of mortalities in clinical emergencies all over the world
(12,13). Glycoprotein VI (GPVI) has been
identified as the major signaling receptor for collagen and is
exclusively expressed on platelets and megakaryocytes, initiating
platelet recruitment at sites of vascular injury and demonstrating
numerous beneficial effects for patients with cerebral thrombosis
(14). Platelet GPVI is
upregulated in patients with acute stroke, coronary syndrome and is
associated with acute cerebral infarction (15). In addition, GPVI may be a potential
target and helpful to control infarct volume in patients with
myocardial necrosis and acute vascular syndromes (16,17).
The activation of platelets mediated by GPVI and
subsequent shedding of GPVI serves as a decisive factor in the
blood of patients with acute vascular syndromes (14). A previous study (18) reported that GPVI-Fc combined with
von Willebrand Factor (vWF) and inhibited platelet adhesion,
serving an essential role in vascular syndromes therapy and
eliminating thrombus formation. Therefore, the efficacy of GPVI-Fc
maybe a potential candidate target for the pharmacological
inhibition of pathological thrombus formation in patients with
vascular syndromes (19,20). The importance of GPVI-mediated
signals pathway has been investigated in a recent clinical study
(21).
Recently, polyethylene glycol (PEG) has been
reported as a small molecule, which can modify various protein
drugs to formed nanoparticles, leading to improved pharmacodynamics
in clinical outcomes (22,23). In addition, the effects of this
modification of pharmaceuticals by different PEG-containing
block-copolymers on the preparation of ovalbumin-loaded PLGA
nanoparticles has been studied and applied in clinical settings and
has demonstrated improved efficacy for patients (24). Therefore, protein modification by
PEG may be conducted to improve the therapeutic effects of protein
drugs.
In the present study, PEG-modified GPVI was tested
for the treatment of cerebral thrombosis and cerebral damage. The
preclinical outcomes demonstrated that experimental cerebral
thrombosis was relieved following treatment with PEG (2000)
modified GPVI-Fc (GPVI-Fc-PEG) in a cerebral thrombosis animal
model, suggesting that GPVI-Fc-PEG may be a potential candidate for
cerebral thrombosis therapy.
Materials and methods
Ethics statement
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(Bethesda, MD, USA). The protocol was approved by Chinese
Association for Laboratory Animal Sciences, Animal Health Products
and the committee on the Ethics of Animal Experiments Defense
Research. All surgery and euthanasia were performed under sodium
pentobarbital (30 mg/kg; Jiangsu Lianshui Pharmaceutical Co., Ltd.,
Lianshui, China) anesthesia followed by cervical dislocation, and
all efforts were made to minimize suffering.
Enzyme-linked immunosorbent assay
(ELISA)
In order to assess the capacity binding of
GPVI-Fc-PEG (cat. no. ab133065; Abcam, Cambridge, UK) or vWF (cat.
no. ab108918; Abcam) to collagen, commercially available ELISA kits
were used. The ELISA assays were performed according to the
manufacturer's instructions (25).
The result was measured at 450 nm in an ELISA reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and finally converted to the
affinity of GPVI-Fc-PEG for bovine and mouse collagen. Competitive
affinity analysis of GPVI-Fc-PEG to collagen with vWF was also
determined by competitive ELISA.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cerebroarterial cells
using an RNAeasy Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD,
USA) in experimental and control mice. Total RNA (1 µg) was reverse
transcribed into cDNA using a reverse transcription kit (Qiagen
Sciences, Inc.) at 37°C for 30 min and the quality was confirmed by
30% SDS-PAGE. The cDNA (10 ng) was subjected to qPCR with the SYBR
Green Master Mix system (Bio-Rad Laboratories, Inc.). Thermocycling
conditions were as follows: Initial denaturation at 95°C for 5 min,
followed by 35 cycles at 95°C for 20 sec, at 58°C for 20 sec and at
72°C for 20 sec, with a final extension at 72°C for 5 min. All the
forward and reverse primers were synthesized by Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA) and are presented in
Table I. Relative mRNA expression
changes were calculated by the 2−ΔΔCq method (26). The results are expressed as the
n-fold way vs. control.
 | Table I.Sequences of primers were used for
reverse transcription-quantitative polymerase chain reaction in the
present study. |
Table I.
Sequences of primers were used for
reverse transcription-quantitative polymerase chain reaction in the
present study.
| Gene | Sequence |
|---|
| lgG | F:
5′-CTCCAGCAGTCTTCATGTTCCCCC-3′ |
|
| R:
5′-AAGCTTGATGGTCTTCTGCGTGTGGT-3′ |
| TGF-β | F:
5′-GCTTTGGATGCCGCCTATTGC-3′ |
|
| R:
5′-GCTGCATTTGCAAGACTTTAC-3′ |
| PDGF | F:
5′-AAGACCATGAGCCTGGGTACC-3′ |
|
| R:
5′-CTCGGTCACAGGCCGTGCTGC-3′ |
| β-actin | F:
5′-AGAAAATCTGGCACCACACC-3′ |
|
| R:
5′-TAGCACAGCCTGGATAGCAA-3′ |
Animal studies in vivo
A total of 6 eight-week-old female C57BL/6 mice
(weight, 30–35 g) were purchased (Bioray Laboratories, Inc.,
Shanghai, China) and housed in specific pathogen-free conditions.
All animals were housed in a temperature-controlled facility at
23±1°C with a relative humidity of 50±5%, under a 12-h light/dark
cycle with free access to food and water. A lesion of the
endothelium, induced by a transient ligature of the left common
carotid artery, was used to test the antithrombotic effect of
GPVI-Fc-PEG on an injured arterial wall. To visualize platelet
adhesion to the injured vessel wall under in vivo
conditions, platelets were fluorescently labeled and injected
intravenously and monitored in situ with an intravital
microscope over 45 min following the endothelial damage. The MTD of
GPVI-Fc-PEG was conducted as previously described (27). Administration of GPVI-Fc-PEG or
GPVI-Fc (0.18 mg) once daily was performed immediately prior to
inducing the endothelial lesion in the common carotid artery.
GPVI-Fc-PEG in vivo functional outcome
in mice with cerebral thrombosis
The influence of GPVI-Fc-PEG on arterial thrombosis
induced by deeper lesions of the arterial wall was investigated in
a mouse model of wire-induced different degrees of vascular injury.
Following preparation of the carotid artery, a coronary guiding
wire was introduced via the external carotid artery and rubbed over
the endothelium of the mouse common carotid artery. GPVI-Fc-PEG or
GPVI-Fc was injected intravenously prior to the intervention as in
a previous study (18). Thrombus
size was quantified following digital imaging and quantification
using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Determination of GPVI-Fc-PEG for
platelet function in vivo
An optical microscope was equipped with a video
camera recorder to analyze the role of GPVI-Fc-PEG in platelet
function in vivo. For determining the vessel diameter, a
video was captured at ×100 magnification. For the determination of
transiently adherent platelets, video sequences of 30 sec were
captured at ×200 magnification 5, 10, 15, 20 and 30 min following
endothelial damage. Transiently adherent platelets were counted in
slow motion during 30 sec video sequences within a 150×100
µm2 window, which was placed on the video screen
directly over the endothelial lesion. At 30 and 60 min following
endothelial damage, the platelet thrombus area was determined. For
the determination of the thrombus area, three screen shots were
captured and the area of mean total platelet thrombi were added up
for an overall thrombus area and evaluated using Image-Pro Plus
software version 6.0 (Media Cybernetics, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation of triplicate experiments. Unpaired data was assessed by
Student's t-test and comparisons of data between multiple groups
were analyzed by one-way analysis of variance followed by a post
hoc Dunnett's test for multiple comparisons. Kaplan-Meier was used
to estimate the risk of relapse and re-treatment during the 30 day
treatment. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
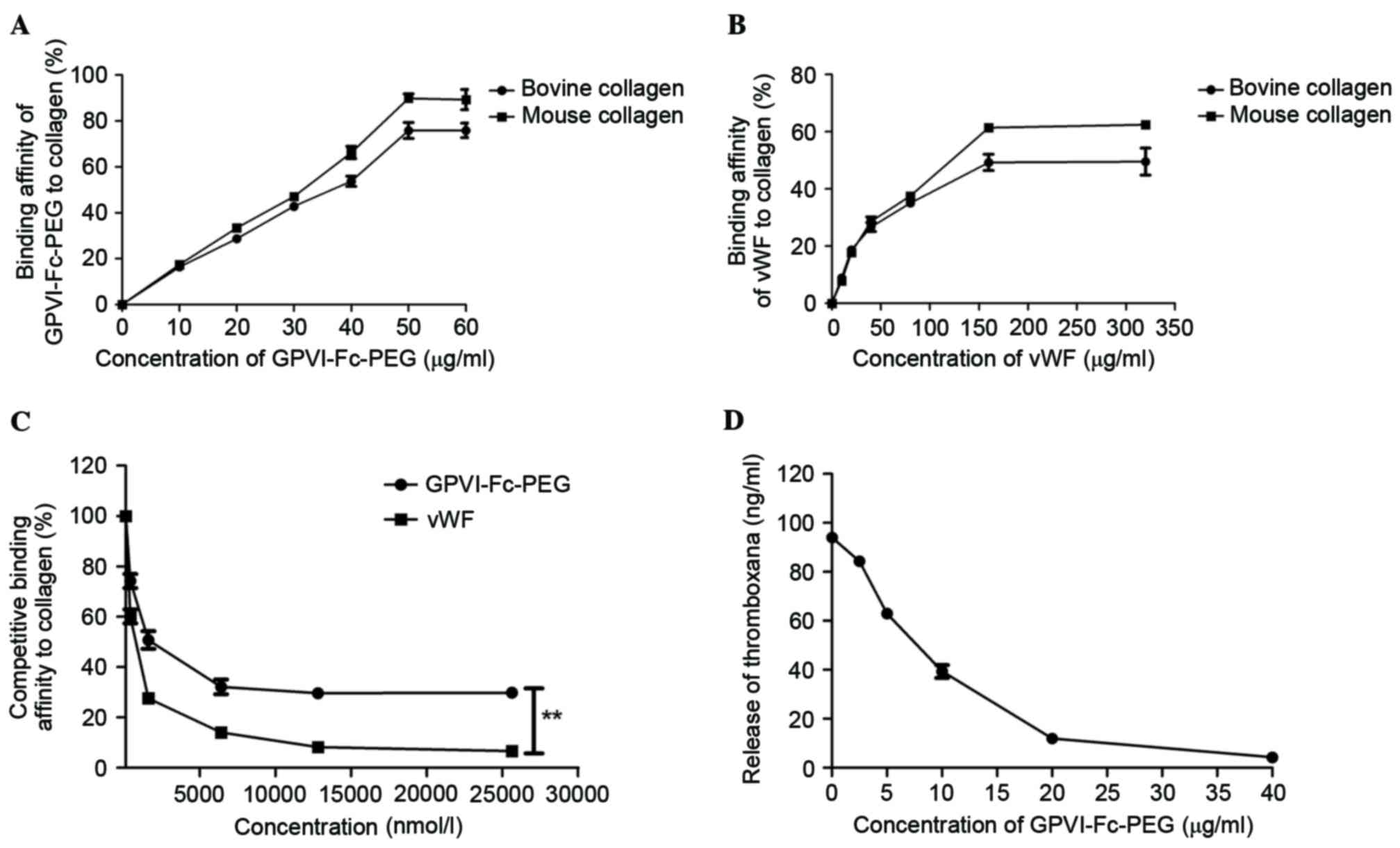
GPVI-Fc-PEG demonstrated completely
binding to collagen with vWF
GPVI demonstrated a high affinity with collagen in a
previous study (28) and in order
to test the affinity of GPVI-Fc-PEG with collagen, ELISA was
performed in the present study. The result, presented in Fig. 1A, revealed that GPVI-Fc-PEG
demonstrated a specific affinity to bovine and mouse collagen in a
linear dose-dependent manner. vWF specific affinity for bovine and
mouse collagen was demonstrated, with the maximum bindings of 241
and 76 ng/ml, respectively (Fig.
1B). A competitive ELISA experiment was conducted to
investigate the capacity of GPVI-Fc-PEG and vWF for completely
binding to collagen. As presented in Fig. 1C, GPVI-Fc-PEG presented competition
for the binding of vWF to collagen at increasing doses, while Fc
and PEG did not exhibit competitive effects. Additionally, the
results demonstrated that GPVI-Fc-PEG inhibited collagen-related
peptide (CRP)-stimulated thromboxane release from human platelets
in a dose-dependent manner (Fig.
1D).
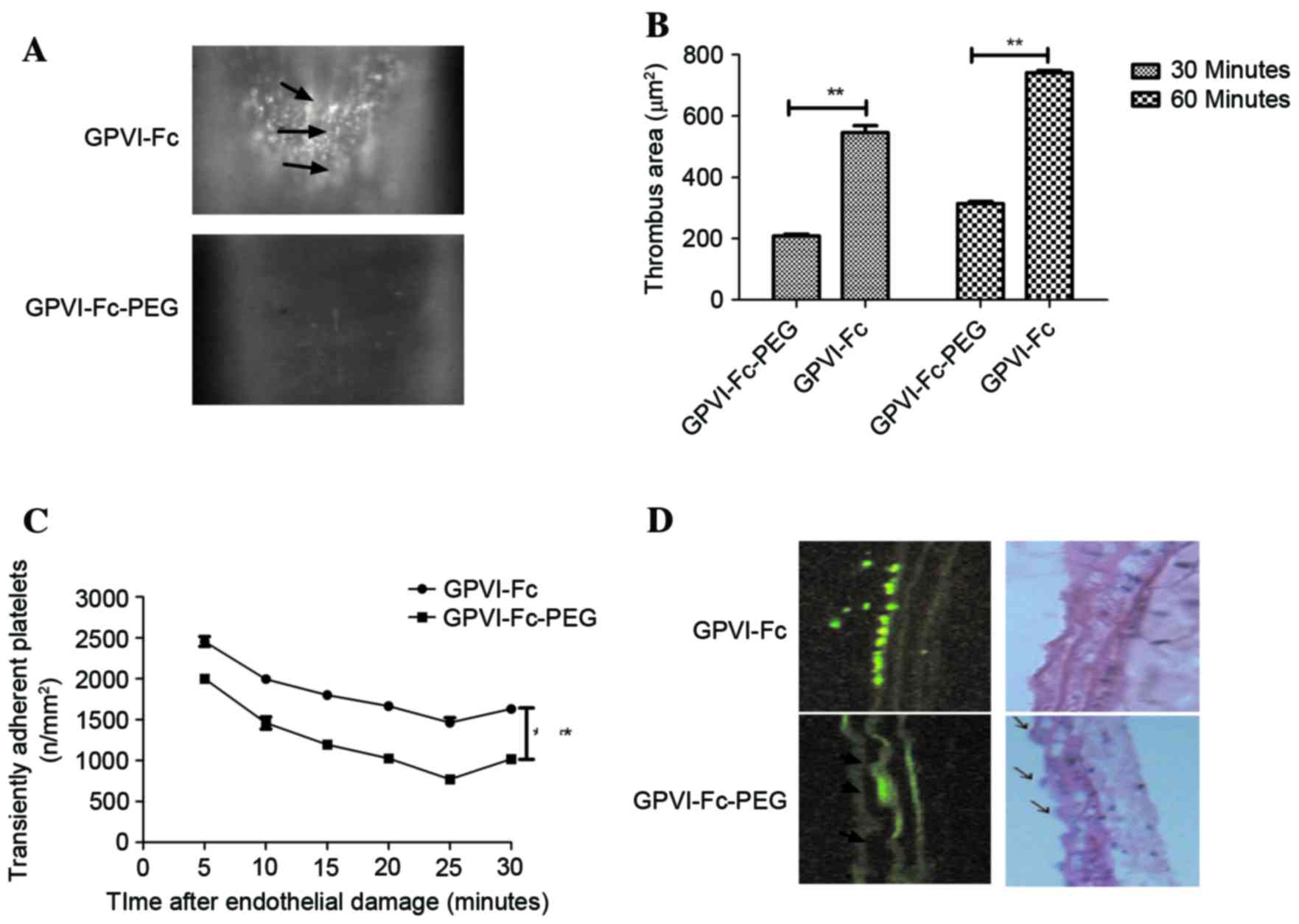
Effect of GPVI-Fc-PEG suppressed
thrombus formation on platelet-endothelial cell interactions
following endothelial lesion in mice in vivo
A previous study (18) demonstrated that GPVI-Fc inhibited
thrombus formation on platelet-endothelial cell interactions
following endothelial lesion in mice in vivo. In the present
study, GPVI-Fc-PEG was used to analyze its inhibition effects on
platelet-endothelial cell interactions and therapeutic effects in
mice model of cerebral thrombosis. The MTD of GPVI-Fc-PEG in
C57BL/6 mice was studied first and the median overall duration of
treatment was 7 days. The dosing cohort of GPVI-Fc-PEG was 0.08,
0.16, 0.32, 0.64 and 0.80 mg/animal. In the results, 0.18 mg of
GPVI-Fc-PEG once daily was identified as the MTD. The most common
treatment-related adverse events were hypertension, diarrhea,
vomiting, lethargy, constipation, proteinuria and vomiting
(Table II).
 | Table II.Treatment-related adverse events of
GPVI-Fc-PEG with an overall incidence ≥10%. |
Table II.
Treatment-related adverse events of
GPVI-Fc-PEG with an overall incidence ≥10%.
| Adverse event | Total (n=36) | GPVI-Fc-PEG
(0.04–0.12 mg) (n=12) | GPVI-Fc-PEG
(0.18–0.32 mg) (n=12) | GPVI-Fc-PEG (0.40
mg) (n=12) |
|---|
| Hypertension | 6 | 1 | 2 | 3 |
| Proteinuria | 7 | 2 | 2 | 3 |
| Diarrhea | 7 | 2 | 2 | 3 |
| Constipation | 4 | 1 | 1 | 2 |
| Lethargy | 10 | 2 | 3 | 5 |
| Diarrhea | 10 | 2 | 3 | 5 |
| Vomiting | 4 | 1 | 1 | 2 |
Subsequently, mice with cerebral thrombosis were
treated with GPVI-Fc or GPVI-Fc-PEG or with PBS as a control.
Endothelial erosion led to vascular injury with consecutive
thrombus formation and was verified by histological analysis
(Fig. 2A). As hypothesized,
GPVI-Fc-PEG resulted in a significant reduction of cerebral
thrombosis measured by platelet thrombus size following endothelial
damage in the right common carotid artery compared with other
drug-treated and control groups (Fig.
2B). In addition, the ability of platelets to adhere to the
endothelium was significantly decreased in the GPVI-Fc-PEG-treated
group from 10 min after treatment following endothelial injury
compared with the other groups (Fig.
2C). Histological analysis in Fig.
2D further confirmed the efficacy of GPVI-Fc-PEG in the
treatment of cerebral thrombosis in vivo.
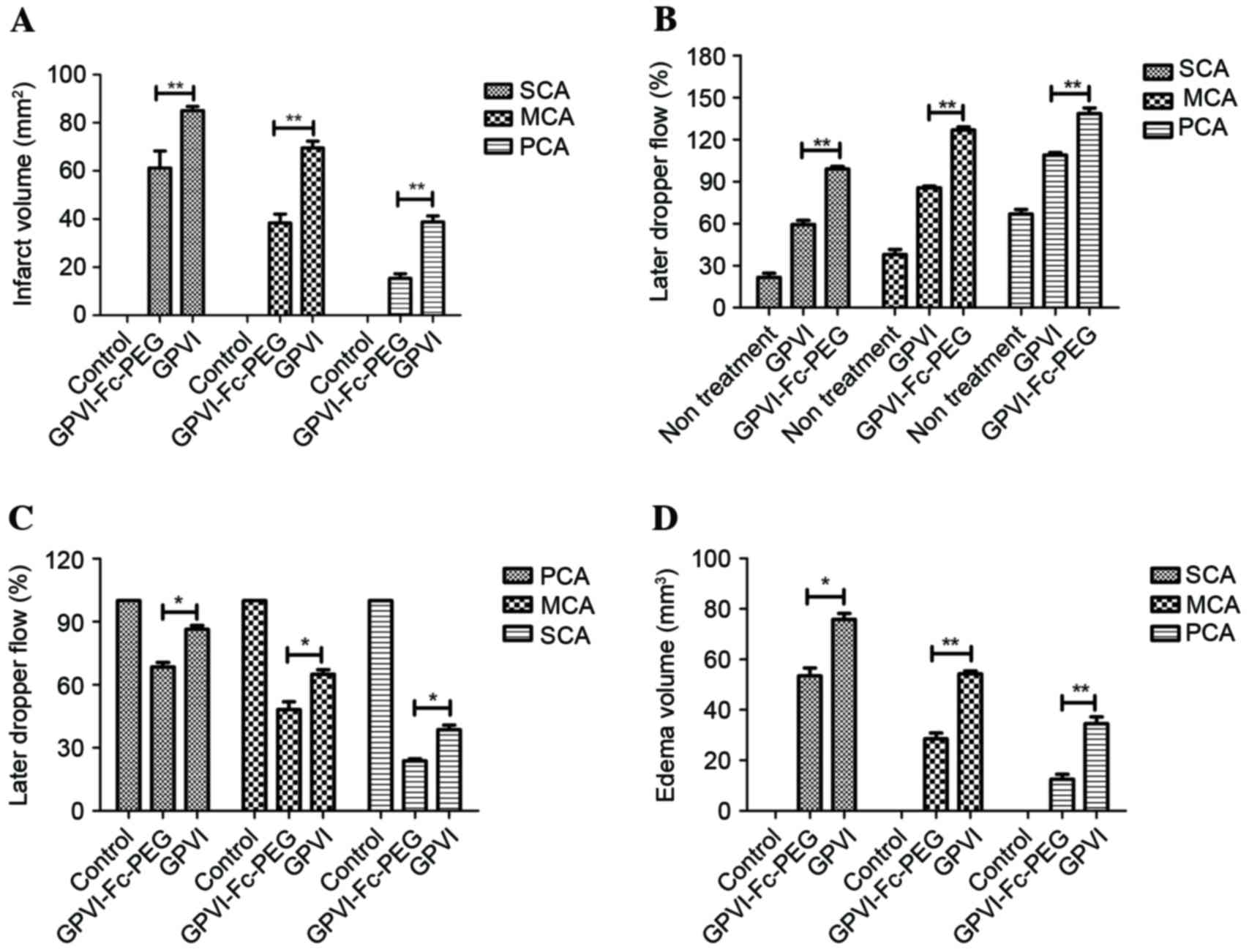
GPVI-Fc-PEG demonstrated efficacy for
thrombosis induced by vascular injury
The efficacy of GPVI-Fc-PEG on differing degrees of
vascular injury was investigated. Different degrees of vascular
injury could be induced by wire that led to exposure of severe,
moderate and primary layers of the vascular wall to the blood.
GPVI-Fc-PEG inhibited arterial thrombosis increase following 24 h
at MTD dose in wire-induced severe (S), moderate (M) and primary
(P) cerebral artery (CA) vascular lesion in mice compared with the
GPVI-Fc group (Fig. 3A). Blood
flow in the SCA, MCA and PCA was recorded following 30 min
occlusion and 30 min reperfusion. Fig.
3B demonstrates that the blood flow was increased by ~34, 40
and 30% in SCA, MCA and PCA, respectively compared with the GPVI-Fc
group during the occlusion time (45 min). In addition, the
reperfusion time was decreased ~15, 16 and 13% in SCA, MCA and PCA,
respectively compared with the GPVI-Fc group (Fig. 3C). Additionally, morphological
effects of GPVI-Fc-PEG on ischemic cerebral stroke by SCA, MCA and
PCA occlusion were observed (data not shown). The results (Fig. 3D) demonstrated that the edema
volume of mice was significantly reduced following treatment with
GPVI-Fc-PEG compared with the GPIV l group.
Effect of GPVI-Fc-PEG on cellular
inflammatory infiltration, reperfusion damage, functional outcome
and survival rate in mice following stroke induced by different
degree of occlusion
The therapeutic effects of GPVI-Fc-PEG on SCA, MCA
and PCA were evaluated at 6 and 18 h following the onset of
reperfusion. As presented in Fig.
4A grip strength was significantly increased in
GPVI-Fc-PEG-treated mice compared with the GPVI-Fc and control
groups following reperfusion. GPVI-Fc-PEG demonstrated beneficial
outcomes although with a trend to less positive motor activity
compared with GPVI-Fc-PEG. Changes in neurological function were
noted following GPVI-Fc-PEG-treatment. The results (Fig. 4B) demonstrated significant
differences between GPVI-Fc-PEG-treated and GPVI-Fc-treated mice.
Improvement of neurological function was observed following 24 h in
the GPVI-Fc-PEG-treated mice with GPVI-Fc as control. In addition,
the results (Fig. 4C) indicated
that the survival rate was prolonged following treatment with
GPVI-Fc-PEG in mice with different degrees of cerebral artery
lesion at 24, 48 and 72 h following reperfusion. Several factors
that indicate inflammatory response to injury were assessed by
RT-qPCR in brain sections of mice with SCM. A significant reduction
of immunoglobulin G, density of macrophages, transforming growth
factor (TGF)-β and platelet-derived growth factor was observed in
GPVI-Fc-PEG-treated mice with cerebral thrombosis (Fig. 4D).
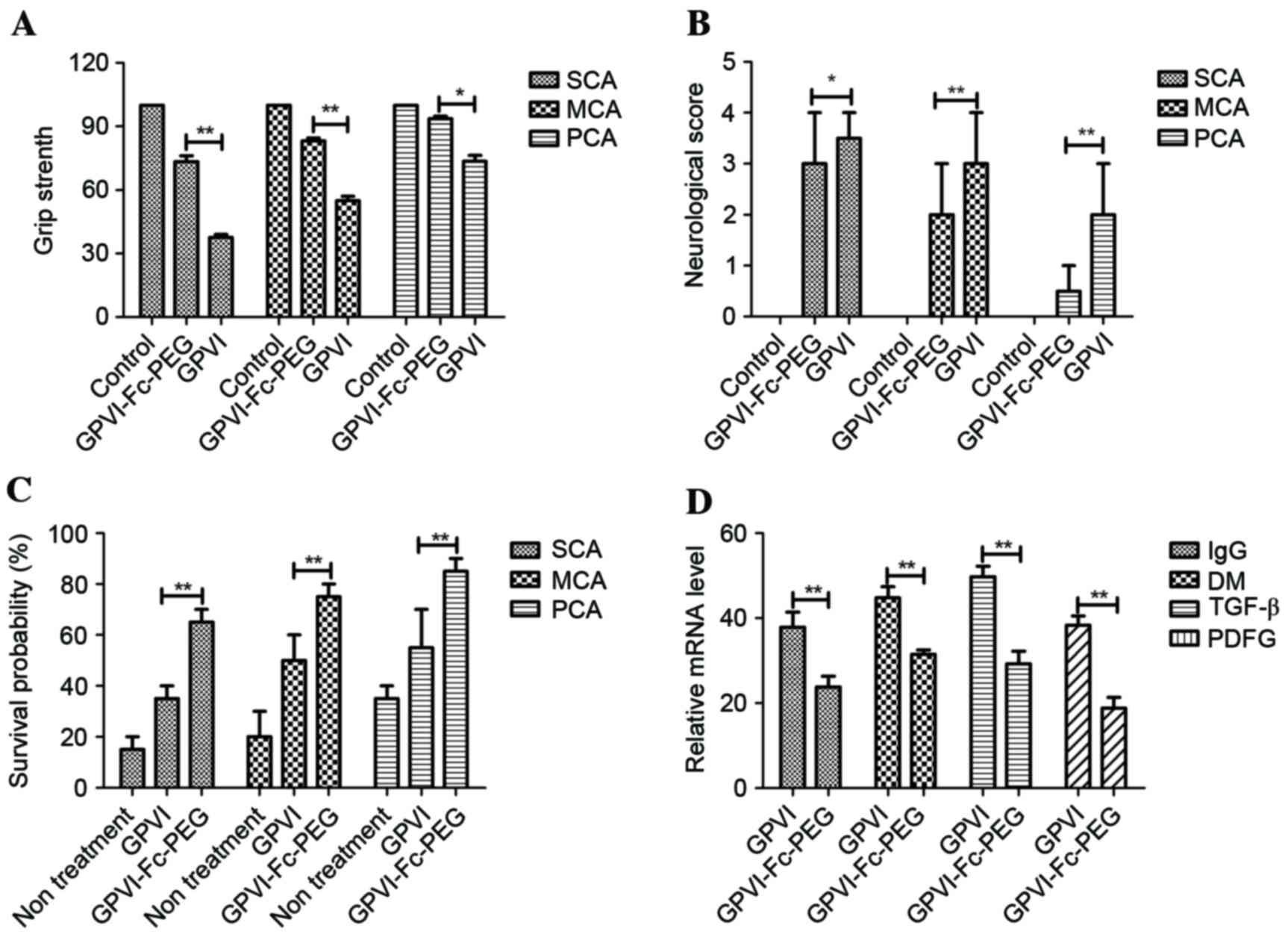 | Figure 4.Detection of parameters of
inflammatory factors in brain tissue by in situ
immunohistochemistry in mice. (A) Relaxing effects of GPVI-Fc-PEG
on the grip strength in mice with SCA, MCA and PCA. (B)
Neurological Bederson score (0, no deficit; 1, forelimb flexion; 2,
decreased resistance to lateral push without circling; 3, circling
and 4, no spontaneous movement) of mice treated with GPVI-Fc-PEG;
n=6 in each group and *P<0.05 was considered to indicate a
statistically significant difference. (C) Survival rate of mice
with SCA, MCA and PCA following treatment with GPVI-Fc-PEG in a
30-day observation. Kaplan-Meier was used to estimate the risk of
relapse and re-treatment during 30-day treatment. *P<0.05 and
**P<0.01 were considered statistically significant. (D) Decrease
of inflammatory factors IgG, TGF-β, DM and PDGF in brains from
GPVI-Fc-PEG- and GPVI-Fc-treated mice with SCA, MCA and PCA; n=6 in
each group and *P<0.05 was considered to indicate a
statistically significant difference. GPVI-Fc-PEG, Fc and PEG
modified glycoprotein VI; SCA, severe cerebral artery lesion (CA);
MCA, moderate CA; PCA, primary CA; IgG, immunoglobulin G; DM,
density of macrophages; TGF-β, transforming growth factor-β; PDGF,
platelet derived growth factor. |
Discussion
Platelet activation is not only indispensable for
initiation, formation and stabilization of cerebral thrombus, but
also enhances the progression of vascular damage, increases
inflammatory factor expression and even occludes reperfusion of the
arteries (29). Platelet
activation is indispensable for initiation although vWF or GPVI
bind to platelet receptor glycoprotein Ib, leading to integrin
aIIbb3 activation and platelet aggregation in the platelet receptor
(30). Subsequently, pathological
thrombus formation is observed in the local blood supply causing
obstacles in brain tissue area and it has been suggested that
platelet activation is important in pathological thrombus
formation, however its exact in vivo function has long
remained undefined (31,32).
In the present study, the function of GPVI-Fc-PEG in
cerebral thrombosis was investigated in different degrees of
cerebral thrombosis (SCA, MCA and PCA). The findings demonstrated
that treatment with GPVI-Fc-PEG by intravenous injection led to an
evolutionary relegation of thrombus formation and inflammatory
response to injury following endothelial damage and a significant
improvement of neurological function and prognostic outcome in
addition to reduction of cerebral infarction area in mice with
cerebral thrombosis or ischemic stroke. In addition, the data
presented an improved anti-ischemic effect and greatly avoided the
risk of cerebral hemorrhage. Thus, GPVI-Fc-PEG markedly enhanced
the preclinical outcome of cerebral thrombosis without increasing
the risk of cerebral hemorrhage, achieved by nanoparticles modified
by PEG. According to the results of the present study, GPVI-Fc-PEG
competitively inhibited the binding capacity of vWF to collagen and
contributed to the improved therapeutic effects of GPVI-Fc-PEG for
cerebral thrombosis.
Previous studies (33–35)
have reported that the GPVI pathway is a potential treatment target
for cerebral thrombus by the administration of GPVI antibody, which
not only resulted in a decrease of GPVI protein level, but also
demonstrated suppressive effects on other platelet signal pathways,
including thrombin-dependent activation. In addition, a previous
review (36) considered the
complex signal pathway of GPVI and described the function,
structure, posttranslational, binding partners and modifications
presently known in cerebral thrombus. Furthermore, Walsh et
al (37) demonstrated that
Nox1 and Nox2 served an essential role in GPVI-dependent platelet
activation and thrombus formation, and their results demonstrated
that Nox1 is the key Nox homolog regulating GPVI-dependent reactive
oxygen species production, essential for CRP-dependent thromboxane
(Tx)A2 production, and was mediated in part through p38
mitogen-activated protein kinase signaling. Coincidentally, Goebel
et al (18) examined the
effect of GPVI-Fc on cerebral thrombus following vessel wall injury
in a mouse model of cerebral thrombus. However, the results for
GPVI-Fc did not present an ideal efficacy for the pharmacodynamics
of macromolecular particles.
In the present study, the preclinical efficacy of
GPVI-Fc-PEG was synthesized and therapeutic outcomes of GPVI-Fc-PEG
was explored in cerebral thrombus mouse model. The results
demonstrated that the therapeutic outcomes of GPVI-Fc-PEG surpassed
GPVI-Fc in cellular inflammatory infiltration, reperfusion damage,
functional outcome and survival rate in mice following stroke
induced by different degree of occlusion. In addition, the findings
suggest that GPVI-Fc-PEG was a completive inhibitor with vWF in
platelet activation via binding to collagen exposed at vascular
injury.
In conclusion, the present study confirmed that
GPVI-Fc-PEG could efficiently block the GPVI-mediated and bind
competitively with vWF-mediated activation of platelets compared
with GPVI-Fc, and block thrombus formation by decreasing the level
of collagen following vascular injury. These improved efficacies
were also identified in the injured brain ischemic tissue during
cerebral thrombus and reperfusion, which presented less vascular
damage in SCA, MCA and PCA compared with a previous study (38). However, more studies are required
to further elucidate the mechanisms of the beneficial role of
GPVI-Fc-PEG during cerebral thrombus.
References
|
1
|
Kanamaru K, Suzuki H and Taki W: Cerebral
infarction after aneurysmal subarachnoid hemorrhage. Acta Neurochir
Suppl. 121:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee DH, Na DG, Ihn YK, Kim DJ, Kim EY, Kim
YS, Lim SM, Roh HG and Sohn CH: Stroke Study Group: Review of the
current status of intra-arterial thrombolysis for treating acute
cerebral infarction: A retrospective analysis of the data from
multiple centers in Korea. Korean J Radiol. 8:87–93. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakagomi T, Nakano-Doi A, Narita A and
Matsuyama T: Concise review: Are stimulated somatic cells truly
reprogrammed into an ES/iPS-like pluripotent state? Better
understanding by ischemia-induced multipotent stem cells in a mouse
model of cerebral infarction. Stem Cells Int. 2015:6306932015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Zhao D, Wu G, Liu J, Liu S, Qin L
and Wu Z: Trend analyses in the incidence of acute intracerebral
hemorrhage events and acute cerebral infarction events in urban
areas in Beijing. Zhonghua Liu Xing Bing Xue Za Zhi. 23:352–355.
2002.(In Chinese). PubMed/NCBI
|
|
5
|
Chang CC and Chen CJ: Secular trend of
mortality from cerebral infarction and cerebral hemorrhage in
Taiwan, 1974–1988. Stroke. 24:212–218. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
WHO publishes definitive atlas on global
heart disease and stroke epidemic. Indian J Med Sci. 58:405–406.
2004.PubMed/NCBI
|
|
7
|
Yamamoto K, Koh H, Shimada H, Takeuchi J,
Yamakawa Y, Kawamura M and Miki T: Cerebral infarction in the left
hemisphere compared with the right hemisphere increases the risk of
aspiration pneumonia. Osaka City Med J. 60:81–86. 2014.PubMed/NCBI
|
|
8
|
Quinn CT: Breakthrough: New guidance for
silent cerebral ischemia and infarction in sickle cell disease.
Hematology Am Soc Hematol Educ Program. 2014:438–443.
2014.PubMed/NCBI
|
|
9
|
Repossini A, Tononi L, Martinil G, Di
Bacco L, Girolettiz L, Rosati F and Muneretto C: Platelet
activation after sorin freedom solo valve implantation: A
comparative study with Carpentier-Edwards Perimount Magna. J Heart
Valve Dis. 23:777–782. 2014.PubMed/NCBI
|
|
10
|
Kinsella JA, Tobin WO, Hamilton G and
McCabe DJ: Platelet activation, function, and reactivity in
atherosclerotic carotid artery stenosis: A systematic review of the
literature. Int J Stroke. 8:451–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei HY and Han Y: Platelet activation
through signal transduction-review. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 12:704–707. 2004.(In Chinese). PubMed/NCBI
|
|
12
|
Chang MC, Lee AY, Chang WF and Chen TJ:
Embolic cerebral infarction and gastrointestinal hemorrhage
following thrombolytic therapy for acute myocardial infarction.
Echocardiography. 19:139–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McConnell ED, Wei HS, Reitz KM, Kang H,
Takano T, Vates GE and Nedergaard M: Cerebral microcirculatory
failure after subarachnoid hemorrhage is reversed by hyaluronidase.
J Cereb Blood Flow Metab. 36:1537–1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alshehri OM, Montague S, Watson S, Carter
P, Sarker N, Manne BK, Miller JL, Herr AB, Pollitt AY, O'Callaghan
CA, et al: Activation of glycoprotein VI (GPVI) and C-type
lectin-like receptor-2 (CLEC-2) underlies platelet activation by
diesel exhaust particles and other charged/hydrophobic ligands.
Biochem J. 468:459–473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bigalke B, Haap M, Stellos K, Geisler T,
Seizer P, Kremmer E, Overkamp D and Gawaz M: Platelet glycoprotein
VI (GPVI) for early identification of acute coronary syndrome in
patients with chest pain. Thromb Res. 125:e184–e189. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung SM, Tsuji K and Moroi M: Glycoprotein
(GP) VI dimer as a major collagen-binding site of native platelets:
Direct evidence obtained with dimeric GPVI-specific Fabs. J Thromb
Haemost. 7:1347–1355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bigalke B, Stellos K, Stakos D, Joos T,
Pötz O, Geisler T, Bischofs C, Kremmer E, Krämer BF, Seizer P, et
al: Influence of platelet count on the expression of platelet
collagen receptor glycoprotein VI (GPVI) in patients with acute
coronary syndrome. Thromb Haemost. 101:911–915. 2009.PubMed/NCBI
|
|
18
|
Goebel S, Li Z, Vogelmann J, Holthoff HP,
Degen H, Hermann DM, Gawaz M, Ungerer M and Münch G: The GPVI-Fc
fusion protein Revacept improves cerebral infarct volume and
functional outcome in stroke. PLoS One. 8:e669602013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furie B and Furie BC: Mechanisms of
thrombus formation. N Engl J Med. 359:938–949. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stoll G, Kleinschnitz C and Nieswandt B:
Molecular mechanisms of thrombus formation in ischemic stroke:
Novel insights and targets for treatment. Blood. 112:3555–3562.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rigg RA, Aslan JE, Healy LD, Wallisch M,
Thierheimer ML, Loren CP, Pang J, Hinds MT, Gruber A and McCarty
OJ: Oral administration of Bruton's tyrosine kinase inhibitors
impairs GPVI-mediated platelet function. Am J Physiol Cell Physiol.
310:C373–C380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rietscher R, Czaplewska JA, Majdanski TC,
Gottschaldt M, Schubert US, Schneider M and Lehr CM: Impact of PEG
and PEG-b-PAGE modified PLGA on nanoparticle formation, protein
loading and release. Int J Pharm. 500:187–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi J, Chen Z, Wang L, Wang B, Xu L, Hou L
and Zhang Z: A tumor-specific cleavable nanosystem of PEG-modified
C60@Au hybrid aggregates for radio frequency-controlled release,
hyperthermia, photodynamic therapy and X-ray imaging. Acta
Biomater. 29:282–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu L, Wu M, Zeng Y, Zhang D, Zheng A, Liu
X and Liu J: Multifunctional PEG modified DOX loaded mesoporous
silica nanoparticle@CuS nanohybrids as photo-thermal agent and
thermal-triggered drug release vehicle for hepatocellular carcinoma
treatment. Nanotechnology. 26:0251022015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashimoto K, Tagami T, Yamakage H,
Muranaka K, Tanaka M, Odori S, Kono S, Shimatsu A, Ogawa Y and
Satoh-Asahara N: Serum free thyroxine levels is associated with the
efficacy of weight reduction therapy in obese female patients.
Endocr J. 63:221–229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ledzewicz U, Schättler H, Gahrooi MR and
Dehkordi SM: On the MTD paradigm and optimal control for multi-drug
cancer chemotherapy. Math Biosci Eng. 10:803–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akbar H, Shang X, Perveen R, Berryman M,
Funk K, Johnson JF, Tandon NN and Zheng Y: Gene targeting
implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet
filopodia formation, secretion and aggregation. PLoS One.
6:e221172011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu H, Zhu L, Huang Z, Ji Q, Chatterjee M,
Zhang W and Li N: Platelets enhance lymphocyte adhesion and
infiltration into arterial thrombus. Thromb Haemost. 104:1184–1192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joglekar MV, Ware J, Xu J, Fitzgerald ME
and Gartner TK: Platelets, glycoprotein Ib-IX, and von Willebrand
factor are required for FeCl(3)-induced occlusive thrombus
formation in the inferior vena cava of mice. Platelets. 24:205–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae ON, Lim KM, Noh JY, Chung SM, Kim SH
and Chung JH: Trivalent methylated arsenical-induced
phosphatidylserine exposure and apoptosis in platelets may lead to
increased thrombus formation. Toxicol Appl Pharmacol. 239:144–153.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whyte CS, Swieringa F, Mastenbroek TG,
Lionikiene AS, Lancé MD, Van Der Meijden PE, Heemskerk JW and Mutch
NJ: Plasminogen associates with phosphatidylserine-exposing
platelets and contributes to thrombus lysis under flow. Blood.
125:2568–2578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fuly AL, Soares AM, Marcussi S, Giglio JR
and Guimarães JA: Signal transduction pathways involved in the
platelet aggregation induced by a D-49 phospholipase A2 isolated
from Bothrops jararacussu snake venom. Biochimie. 86:731–739. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newman PJ and Newman DK: Signal
transduction pathways mediated by PECAM-1: New roles for an old
molecule in platelet and vascular cell biology. Arterioscler Thromb
Vasc Biol. 23:953–964. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cardoso LE, Little PJ, Ballinger ML, Chan
CK, Braun KR, Potter-Perigo S, Bornfeldt KE, Kinsella MG and Wight
TN: Platelet-derived growth factor differentially regulates the
expression and post-translational modification of versican by
arterial smooth muscle cells through distinct protein kinase C and
extracellular signal-regulated kinase pathways. J Biol Chem.
285:6987–6995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mendoza E, Malong CL, Tanchee-Ngo MJ and
Mercado-Asis L: Acromegaly with cardiomyopathy, cardiac thrombus
and hemorrhagic cerebral infarct: A case report of therapeutic
dilemma with review of literature. Int J Endocrinol Metab.
13:e188412015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Walsh TG, Berndt MC, Carrim N, Cowman J,
Kenny D and Metharom P: The role of Nox1 and Nox2 in GPVI-dependent
platelet activation and thrombus formation. Redox Biol. 2:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ungerer M, Li Z, Baumgartner C, Goebel S,
Vogelmann J, Holthoff HP, Gawaz M and Münch G: The GPVI-Fc fusion
protein Revacept reduces thrombus formation and improves vascular
dysfunction in atherosclerosis without any impact on bleeding
times. PLoS One. 8:e711932013. View Article : Google Scholar : PubMed/NCBI
|