Introduction
Acute respiratory distress syndrome (ARDS) is a
common and life-threatening clinical syndrome, which accounts for a
large number of cases treated in Intensive Care Units; mild ARDS
was previously termed acute lung injury (ALI) (1,2).
Despite the use of lung-protective ventilation and comprehensive
treatments, the incidence and overall mortality of ARDS has not
changed substantially, with the mortality rate remaining at >40%
(3,4). Early detection and intervention is
important to prevent deterioration in patients with ARDS.
Therefore, the development of diagnostic tools to identify patients
at high risk of ARDS has been the subject of continuing research.
Various biomarkers, including receptors for advanced glycation end
products (RAGE), surfactant proteins, Clara cell secretory protein
(CC16) and certain cytokines, have been demonstrated to be useful
in ARDS diagnosis and prognosis (5–8). In
addition, proteomics approaches may be able to identify novel ARDS
protein biomarkers (9–11). One previous proteomic analysis of
lung tissues in animal models has provided novel insight into the
mechanisms underlying ALI (12).
Using the isobaric tag for relative and absolute quantitation
(iTRAQ) approach, a different study identified 132 plasma proteins,
among which 16 were differentially expressed in ARDS patients
compared with control subjects (10).
An ideal way to study specific diseases is by
histopathological examination of tissues from a disease-affected
area, which may directly reveal lesions; however, it is difficult
to obtain lung specimens from patients with ARDS. Therefore, owing
to the convenient accessibility and the ease of repeat sampling,
plasma samples have been a focus for biomarker identification. For
example, a previous study identified 30 plasma proteins that were
altered during the early phase of peritonitis-induced sepsis; the
majority of these proteins were revealed to serve important roles
in inflammatory responses, whereas other proteins were involved in
oxidative and nitrosative stress (13). It is well known that sepsis may
lead to multi-organ failure; however, the proteins identified in
plasma may not be specific indicators for single-organ damage.
Therefore, the present study aimed to determine whether any of the
altered plasma proteins were directly associated with the
occurrence of subsequent lung injury. It was hypothesized that
changes to certain immunogenic substances occurred in injured
lungs, and these substances may specifically bind with
corresponding proteins in the blood, such that lung
injury-associated changes may be detected in the blood.
Immunoproteomics is a technique that involves the
separation of proteins by two-dimensional electrophoresis (2-DE)
followed by western blotting, and has been used previously to
identify immunogenic proteins in various diseases (14,15).
The present study established a rat model of ARDS, which was
induced by cecal ligation and puncture (CLP) surgery, and used an
immunoproteomics approach to identify proteins that were altered
during lung injury. The ultimate goal was to clinically assess the
corresponding proteins identified in the blood plasma that may be
associated with lung injury.
Materials and methods
Establishment of an ARDS model
A total of 12 specific-pathogen-free, male
Sprague-Dawley rats (weight, 210–250 g) were used in the present
study. The rats were obtained from Medical Laboratory Animal Centre
of Anhui Medical University (Hefei, China) and housed in an
air-conditioned room at a constant temperature (23±2°C) under a 12
h light-dark cycle and with free access to food and water. Animals
were fasted for 12 h, but allowed free access to water prior to the
experiments. All experimental protocols were approved by The
Medical Ethics Committee of the First Affiliated Hospital of Anhui
Medical University (Hefei, China).
The CLP technique was conducted according to
described previously procedures (16,17).
Surgeries were performed following anesthetization of the rats via
intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g body
weight). The rats were randomly divided into two groups as follows
(n=6/group): i) CLP group and ii) Sham-operated control group. CLP
was conducted as follows: Under anesthesia, a longitudinal midline
incision was made in the skin and the cecum was isolated and
ligated below the ileocecal valve, so as not to ligate the
ileocecal valve itself, such that intestinal continuity was
maintained. Subsequently, the cecum was perforated by two
through-and-through punctures using a 20-gauge needle, and the
cecum was gently squeezed until a small amount of fecal matter
began to exude. The bowel was then repositioned and the abdominal
incision was closed. Sham-operated rats underwent laparotomy and
the cecum was manipulated without ligation and puncture. Following
surgery, sterile saline (2 ml/100 g body weight) was administered
subcutaneously to all rats in each group. Postoperatively, each rat
was placed in a clean cage and allowed free access to food and
water. Histopathological changes in the lungs caused by CLP begin
within 18–20 h, and high rates of lethality were reported at ~24 h
(16,17). Therefore, the endpoint of the
experiment was set at 24 h post-surgery. At the time of sacrifice,
animals were anesthetized and a laparotomy was performed to expose
the abdominal aorta, and blood samples (3–4 ml) were collected.
Lung tissues were obtained, washed twice with cold saline and
immediately stored at −80°C for proteomic analysis, or fixed in 10%
formalin for histopathological assessment. Blood samples were
centrifuged at 1,000 × g for 10 min at 4°C, and serum was aliquoted
and stored at −80°C.
Histopathological assessment of lung
injury
Histopathological alterations in the lungs were
assessed to determine whether the lung injury models had been
successfully established. Lung tissues were fixed in 10% buffered
formalin at room temperature for 24 h and embedded in paraffin.
Lung sections (5 µm) were stained with hematoxylin and eosin
(H&E) according to standard methods at room temperature, and
examined under a light microscope. Lung injury was assessed in a
blinded manner, and scored using the method described by Nishina
et al (18), which using
5-point scale according to combined assessment of alveolar
congestion, hemorrhage, infiltration or aggregation of neutrophils
in the airspace or vessel wall, and the thickness of the alveolar
wall/hyaline membrane formation: 0=minimum damage, 1=mild damage,
2=moderate damage, 3=severe damage, 4=maximum damage. The lung
injury scores between the two groups were analyzed using the
Wilcoxon rank sum test, performed using SPSS version 19.0 (SPSS
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Protein preparation
Lung tissue (200 mg) was homogenized in lysis buffer
containing 7 M urea, 2 M thiourea, 4% (w/v) 3-[(3-cholamidopropyl)
dimethylammonio)-1-propanesulfonate] (CHAPS), 40 mM dithiothreitol
(DTT), 1% Pierce Protease Inhibitor Cocktail (v/v) (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and 2% (v/v) Immobiline pH
Gradient (IPG) buffer (pH 3–10) on ice. Following vortexing at
maximum speed for 30 sec, the homogenate was centrifuged at 14,000
× g at 4°C for 20 min. The concentration of proteins in the
supernatant was quantified using the Bradford method.
Protein separation by two-dimensional
polyacrylamide gel electrophoresis (2-D PAGE)
Lung tissue proteins from each group (n=6/group)
were pooled and separated by 2-D PAGE as previously described
(14,19). A total of 100 µg of protein was
mixed with rehydration buffer [8 M urea, 2% CHAPS, 20 mM DTT, 0.5%
IPG buffer (pH 3–10) and 0.001% trace bromophenol blue] and applied
to IPG strips (pH 3–10; 13 cm; GE Healthcare Life Sciences,
Uppsala, Sweden). Isoelectric focusing was performed on an Ettan
IPGphor II system (GE Healthcare Life Sciences) at 20°C, according
to the following paradigm: 30 V for 6 h; 60 V for 6 h; 500 V for 1
h; 1,000 V for 1 h; and 8,000 V for 3 h. Immediately prior to the
second dimension sodium dodecyl sulfate (SDS)-PAGE, the IPG strips
were placed in 10 ml equilibration buffer [50 mM Tris-HCl (pH 8.8),
6 M urea, 30% (v/v) glycerol and 2% SDS] supplemented with 1% DTT
for 15 min at room temperature, and subsequently incubated in a
similar buffer, in which DTT was replaced with 2.5% iodoacetamide,
for 15 min at room temperature. The equilibrated strips were loaded
on top of the vertical slabs of 12.5% SDS-PAGE gels. 2-D gel
electrophoresis was conducted at 5 W per gel for 30 min and at 12 W
per gel until the dye front reached the bottom of the gels. 2-D
PAGE was repeated three times to minimize variation.
Following SDS-PAGE, some gels were electroblotted
onto a polyvinylidene fluoride (PVDF) membrane for western blotting
and the remaining gels were visualized by silver staining. The 2-DE
images were analyzed by ImageMaster 2D platinum software (Version
5.0, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Identification of immunogenic proteins
by western blotting
Proteins separated by SDS-PAGE were transferred to
PVDF membranes using Trans-Blot Turbo Transfer System RTA Transfer
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were
subsequently blocked with 5% non-fat dry milk in Tris-buffered
saline (TBS) for 2 h. The pooled serum (1:800) from the CLP group
or Sham-operated group was used as the primary antibody, and the
membranes were probed for 2 h at room temperature. Following three
washes in TBS containing 0.1% Tween-20, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rat
IgG H+L (1:1,500; OriGene Technologies, Inc., Beijing, China) for 1
h at room temperature. Immunogenic protein spots were visualized
using ECL Western Blot kit (Beijing CoWin Biotech Co., Ltd.,
Beijing, China) following three subsequent washing steps.
Differential analysis of the expression levels of protein spots was
performed using the ImageMaster 2D Platinum software (Version 5.0,
GE Healthcare Bio-Sciences).
In-gel enzyme digestion and
matrix-assisted laser desorption/ionization
time-of-flight/time-of-flight (MALDI-TOF/TOF) mass
spectrometry
Gel excision and derivatization steps were all
performed at room temperature, unless otherwise stated. The
differentially expressed immunogenic protein spots from silver
stained gels were excised and washed twice with double-distilled
H2O, placed in fresh solutions containing 30 mM
K3Fe (CN)6/100 mM
Na2S2O3 (1:1) for ~2 min until destained, and
washed again to halt the reaction. The gel pieces were dehydrated
with acetonitrile (ACN) for 5 min, the ACN was washed off and the
samples were air-dried. Subsequently, the gels were incubated in 10
mM DTT at 56°C for 1 h, followed by incubation in 55 mM
iodoacetamide (IAA) in the darkroom for 45 min. IAA was aspirated
off and the gel spots were dehydrated with 25 mM
NH4HCO3, followed by 50% ACN and finally 100%
ACN, and air-dried. Dried gel pieces were rehydrated with trypsin
(Promega Corporation, Madison, WI, USA) in 25 mM
NH4HCO3 at 4°C for 30 min. The excess liquid
was discarded and the samples were incubated at 37°C overnight
(10–14 h). A 0.1% concentration of trifluoroacetic acid (TFA) was
added to stop the reaction. Samples were spotted onto a 600 µm
AnchorChip MALDI probe (Bruker Daltonics GmbH, Bremen, Germany) for
mass spectrometry on a TOF Ultraflex II MALDI-TOF/TOF mass
spectrometer (Bruker Daltonics GmbH). The Bruker Peptide
Calibration Mixture was used for external calibration. The
resulting peptide mass lists were searched in the
NCBI-non-redundant sequence database (NCBI-nr 20150516: 66,926,000
sequences; 23,973,512,723 residues) using the MASCOT search engine
(http://202.195.183.2/mascot/) with the
following parameters: Trypsin as enzyme, cysteine
carbamidomethylation, methionine oxidation, minimum mass accuracy
100 parts/million and 1 missed cleavage site allowed. MASCOT
protein scores >62 were considered statistically significant
(P<0.05).
Bioinformatics analysis
To further explore the differentially expressed
immunogenic proteins, Ingenuity Pathway Analysis (IPA; www.ingenuity.com) was performed to characterize the
biological functions and pathways of these proteins. Associated
networks were built among the differentially expressed immunogenic
proteins and the IPA database proteins. The top canonical pathways
were presented with P-values calculated using a right-tailed
Fisher's exact test. IPA Biomarker Filter analysis was used to
optimize the candidate biomarkers from the differentially expressed
immunogenic proteins; ‘acute respiratory distress syndrome’ and
‘acute lung injury’ were used as filtered terms.
Results
Histopathological evaluation of lung
injury
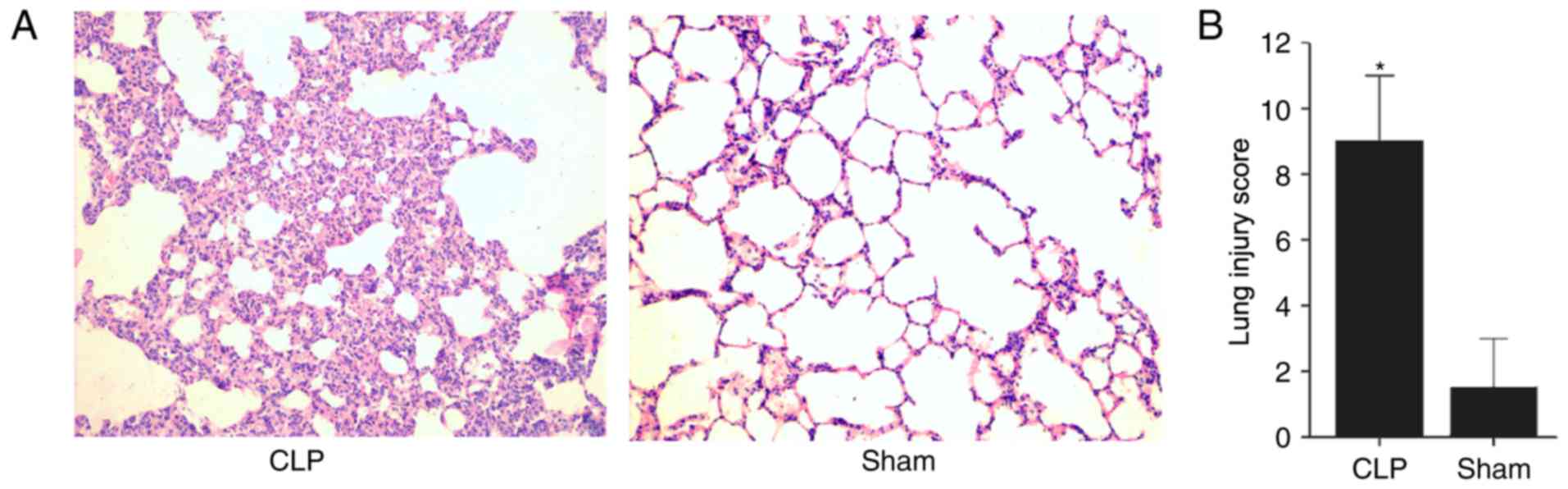
H&E staining was performed to observe
histopathological alterations in the lung tissues of each group at
24 h post-surgery. Examination of the tissues revealed vascular
congestion, interstitial edema, inflammatory cell infiltration and
pulmonary hemorrhage in the CLP group, whereas the lung tissues
from the Sham group exhibited minimal changes with scattered
interstitial infiltrates (Fig.
1A). The mean lung injury score of the CLP rats was 8.83
(range, 6–11), which was significantly higher compared with that of
the Sham group (mean, 1.33; range, 0–3; P<0.05; Fig. 1B). The histopathological results
indicated that the lung injury model was successfully established
and was suitable for the detection of protein expression
differences following CLP.
2-DE profiles and western blotting
analysis
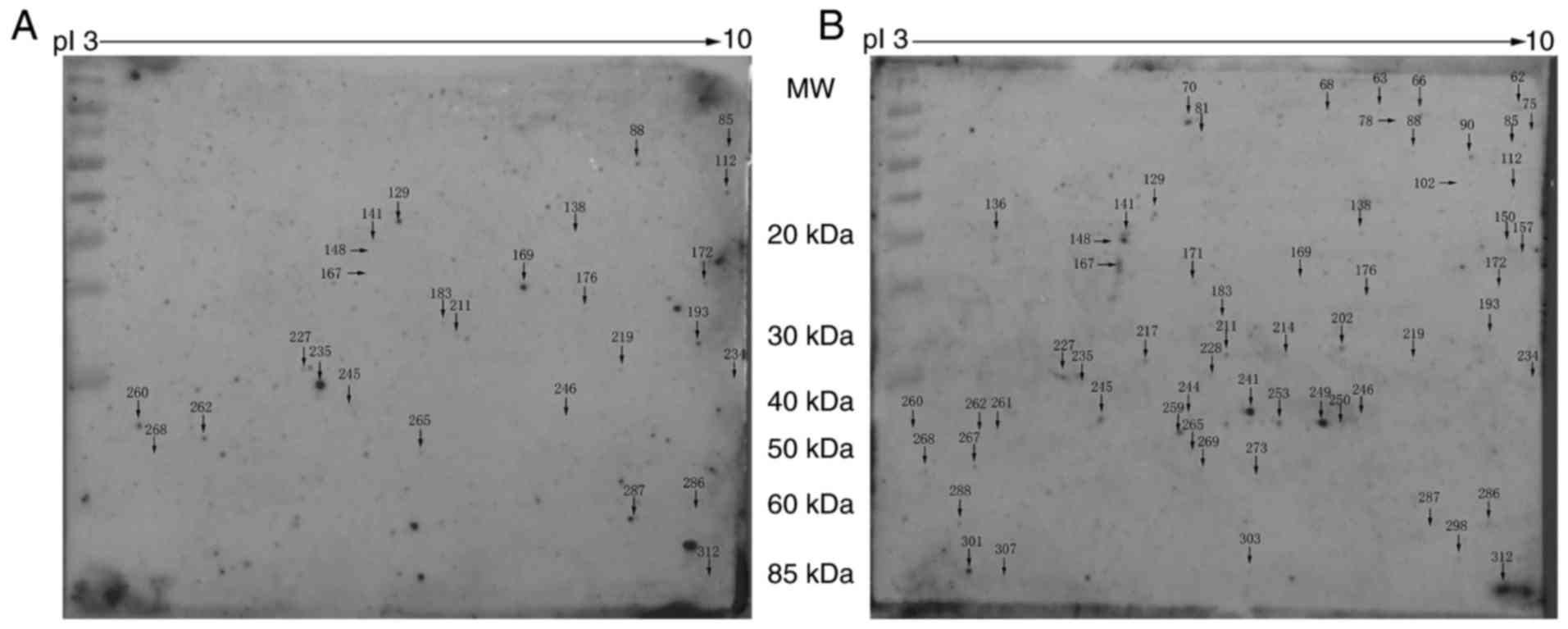
To identify immunogenic proteins in the lung
tissues, protein extracts were separated by 2-DE followed by
western blotting. Silver stained gels were scanned; image analysis
identified a total of 1,909 protein spots detected in both groups,
with molecular masses ranging from 10–100 kDa in the 3–10 pI range.
The proteins were transferred to a PVDF membrane and western
blotting was performed using pooled serum samples from the CLP or
Sham rats as the primary antibody. Positive spots recognized by
sera on the membranes were aligned and matched with the blots on
silver stained gels. A total of 27 immunogenic protein spots were
identified in the CLP group, and 60 immunogenic protein spots were
identified in the Sham group (Fig. 2A
and B, respectively).
Identification of the ARDS-associated
immunogenic proteins
The immunogenic protein spots that exhibited a
>2-fold difference in intensity between the CLP-operated group
and the Sham-operated group were manually excised and used for mass
spectrometry. In total, 38 proteins were successfully identified
from these spots. Among them, 14 proteins were highly expressed in
the CLP group, whereas 24 proteins were highly expressed in the
Sham group. Details of the experimental findings for these proteins
are shown in Table I. According to
the bioinformatics annotations, 10 proteins (26.32%) were enzymes,
6 proteins (15.78%) were kinases and transcription regulators
(7.89% respectively), and 4 proteins (10.52%) were phosphatases,
ion channels, transmembrane receptors, and transporters (2.63%
respectively). A total of 18 proteins (47.37%) were not classified
to any families, including sperm flagellar 2 (SPEF2; also known as
KPL2) and selenium-binding protein 1 (SELENBP1; also known as
SBP1).
 | Table I.Differentially expressed immunogenic
proteins in lung tissues from CLP-operated rats compared with
Sham-operated rats. |
Table I.
Differentially expressed immunogenic
proteins in lung tissues from CLP-operated rats compared with
Sham-operated rats.
| Spot ID | Accession
number | Score | Mr | Fold
changea | Protein symbol | Protein name |
|---|
| 66 | gi|6978511 | 168 | 12831 | −1,000 | SPEG | Striated
muscle-specific serine |
| 75 | gi|564388675 | 218 | 39366 | −1,000 | SLC25A42 | Mitochondrial
coenzyme A transporter SLC25A42 isoform X2 |
| 85 | gi|149062136 | 77 | 14970 | 3.419 | RGD1560544 | Similar to
chromosome 11 open reading frame 2 (predicted), isoform CRA_b |
| 88 | gi|149068094 | 205 | 26511 | 3.431 | LOC81691 | Similar to
exonuclease NEF-sp (predicted), isoform CRA_f |
| 112 | gi|149034857 | 306 | 16896 | 30.4 | RASL11A | RAS-like family 11
member A, isoform CRA_b |
| 129 | gi|238859563 | 129 | 19694 | 2.651 | DCTD | Deoxycytidylate
deaminase isoform 2 |
| 136 | gi|149026453 | 220 | 19686 | −1,000 | CDH18 | Cadherin 18, type 2
(predicted) |
| 138 | gi|149035234 | 263 | 21183 | −2.346 | FIP1L1 | FIP1-like 1 (S.
cerevisiae), isoform CRA_a |
| 141 | gi|149056821 | 95 | 20992 | −3.557 | PGLYRP1 | Peptidoglycan
recognition protein 1, isoform CRA_a |
| 148 | gi|149039413 | 216 | 22555 | −12.347 | C9ORF173 | Similar to
hypothetical gene supported by AK097565; BC033939, isoform
CRA_b |
| 157 | gi|157819619 | 199 | 22194 | −1,000 | BLVRB | Flavin reductase
(NADPH) |
| 167 | gi|157817749 | 72 | 22879 | −5.265 | EXOSC3 | Exosome complex
component RRP40 |
| 169 | gi|672082453 | 227 | 24158 | 11.429 | NUDT5 | Predicted:
ADP-sugar pyrophosphatase isoform X1 |
| 172 | gi|20750357 | 255 | 23927 | 5.31 | PLBD2 | Putative
phospholipase B-like 2 |
| 176 | gi|71043720 | 140 | 26432 | 2.074 | YIPF6 | Protein YIPF6 |
| 183 | gi|189011608 | 256 | 26522 | −2.742 | YEATS4 | YEATS
domain-containing protein 4 |
| 193 | gi|149057763 | 107 | 27597 | 4.37 | VDAC3 | Voltage-dependent
anion channel 3, isoform CRA_b |
| 211 | gi|7739682 | 233 | 28227 | −2.044 | A1BG | Liver
regeneration-related protein 1 |
| 217 | gi|157818187 | 174 | 31373 | −1,000 | TFAP2D | Transcription
factor AP-2-δ |
| 219 | gi|77404265 | 174 | 33493 | 3.31 | JAM2 | Junctional adhesion
molecule B precursor |
| 227 | gi|8393418 | 75 | 36090 | −2.492 | GAPDH |
Glyceraldehyde-3-phosphate
dehydrogenase |
| 228 | gi|42476181 | 140 | 36117 | −1,000 | MDH2 | Malate
dehydrogenase, mitochondrial precursor |
| 234 | gi|158138555 | 220 | 37513 | −2.33 | AKR1Cl | Aldo-keto reductase
family 1, member C-like |
| 235 | gi|672029914 | 66 | 38232 | 24.228 | LOC689092 | Predicted:
N-acetyllactosaminide β-1,6-N-acetylglucosaminyl-transferase,
isoform A-like |
| 241 | gi|274324174 | 220 | 40344 | −1,000 | WARS2 | Tryptophan-tRNA
ligase, mitochondrial |
| 245 | gi|19705521 | 213 | 14094 | −5.703 | Mk1 | Mk1 protein |
| 246 | gi|293342999 | 174 | 42109 | −6.374 | POTEF | Predicted: POTE
ankyrin domain family member F |
| 250 | gi|149035278 | 160 | 42158 | −1000 | TEC | TEC protein
tyrosine kinase, isoform CRA_a |
| 259 | gi|40254752 | 210 | 44909 | −1000 | PGK1 | Phosphoglycerate
kinase 1 |
| 260 | gi|60422786 | 134 | 47737 | 7.299 | NEMF | Nuclear export
mediator factor |
| 262 | gi|38649320 | 75 | 51736 | 9.404 | ENO1 | Eno1 protein |
| 265 | gi|18266692 | 112 | 53069 | 2.926 | SELENBP1 | Selenium-binding
protein 1 |
| 268 | gi|59808405 | 212 | 52825 | −2.224 | GATAD2A | GATA zinc-finger
domain-containing protein 2A |
| 286 | gi|58219535 | 125 | 55691 | −3.652 | PIGV | GPI
mannosyltransferase 2 |
| 287 | gi|66910999 | 175 | 61481 | 3.13 | SPEF2 | Sperm flagellar
protein 2 |
| 301 | gi|38454200 | 182 | 67088 | −1000 | CHDH | Choline
dehydrogenase, mitochondrial |
| 303 | gi|149034390 | 238 | 68243 | −1000 | Ankrd24 | Ankyrin repeat
domain 24 (predicted), isoform CRA_a |
| 312 | gi|293342784 | 161 | 85084 | −70.637 | EPC1 | Predicted: Enhancer
of polycomb homolog 1 isoform X2 |
Functional characteristics of the
differentially expressed immunogenic proteins
A total of 38 differentially expressed immunogenic
proteins were analyzed by IPA to determine their putative molecular
networks, molecular and cellular functions, and canonical pathways.
With regard to the category of diseases and biofunctions, the top
five significant diseases and disorders associated with the
differentially expressed immunogenic proteins in the present study
were as follows: i) Hematological disease; ii) immunological
disease; iii) inflammatory disease; iv) inflammatory response and
v) and respiratory disease. Enolase 1 (ENO1),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and SELENBP1 were
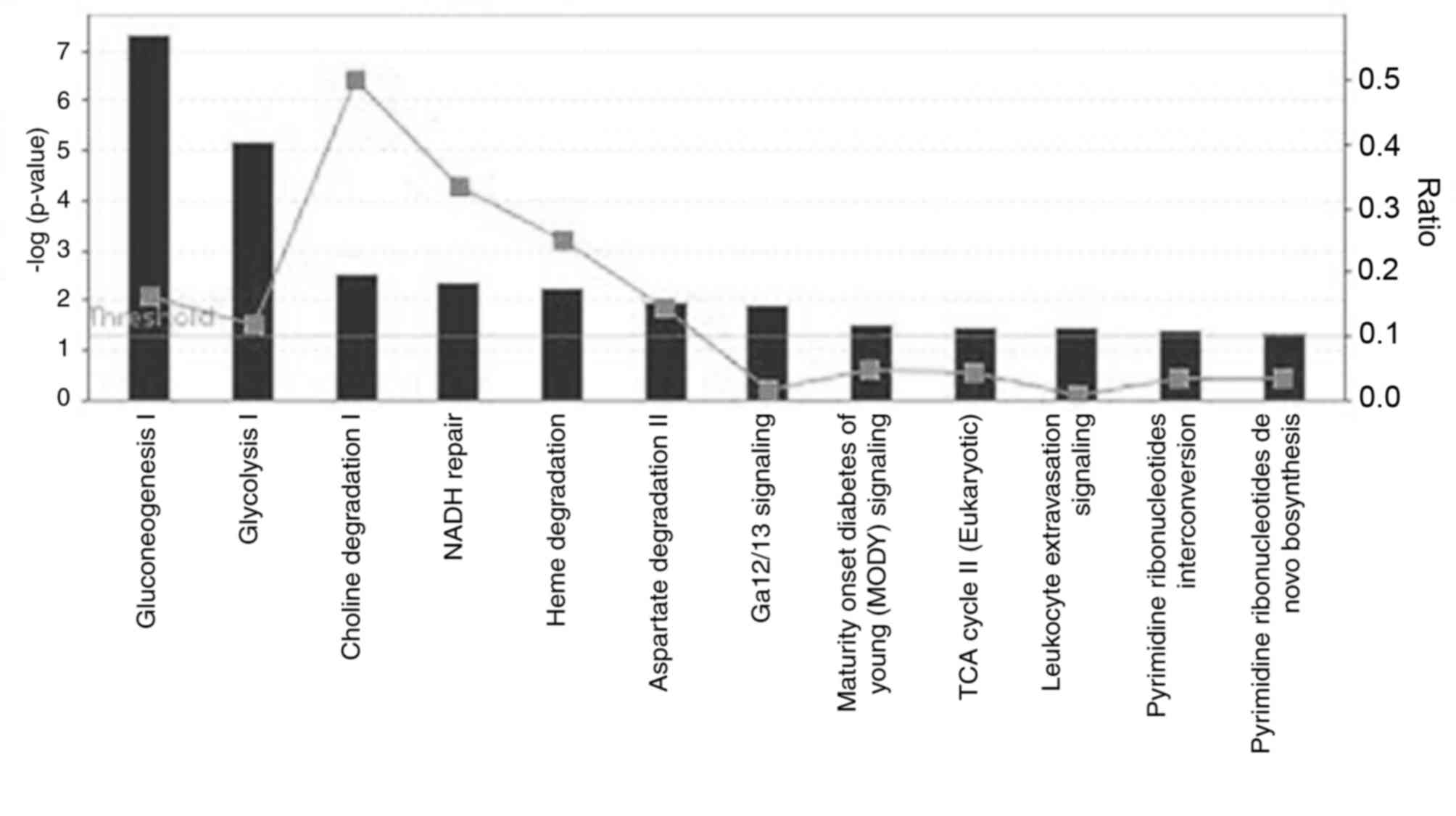
involved in these categories. The top five canonical pathways
included gluconeogenesis I, glycolysis I, choline degradation I,
NADH repair and heme degradation (Fig.
3). In addition, phosphoglycerate kinase 1 (PGK1), ENO1, GAPDH
and malate dehydrogenase 2 (MDH2) participate in gluconeogenesis or
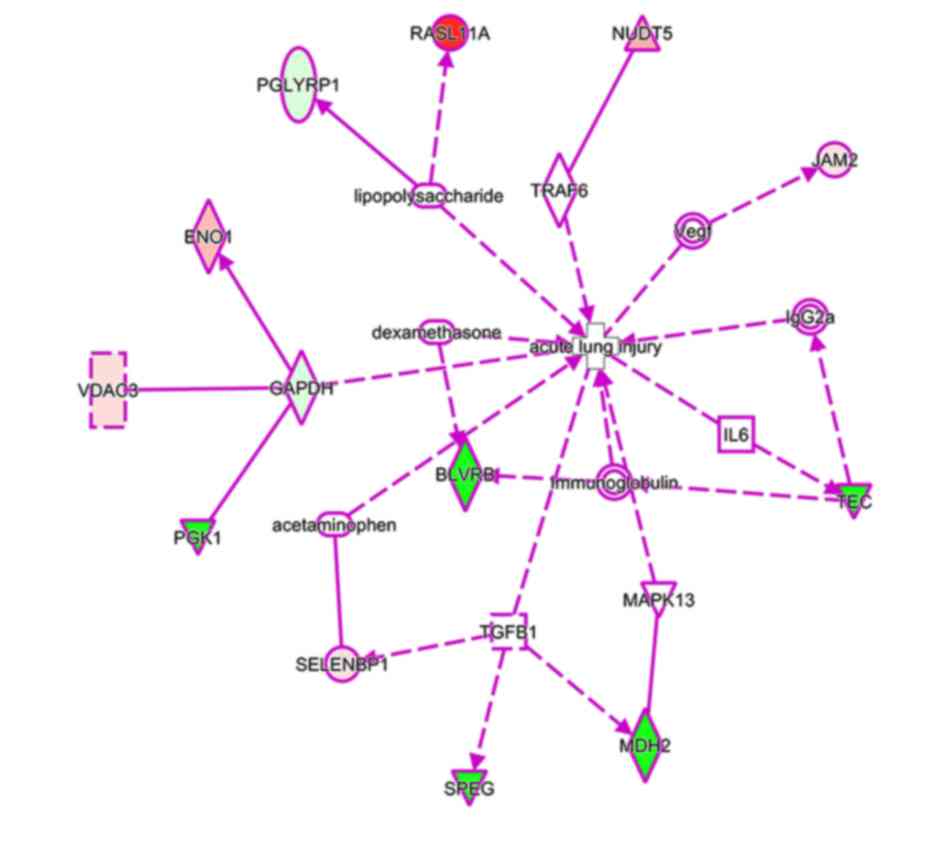
glycolysis pathways. Finally, using IPA Biomarker Filter analysis
with the terms ‘acute respiratory distress syndrome/acute lung
injury’, 13 proteins were identified as candidate biomarkers
(Fig. 4).
Discussion
To identify ARDS biomarkers, an immunoproteomics
approach was used to detect immunogenic proteins in an animal model
of ARDS. Through mass spectrometric analysis, 38 differentially
expressed immunogenic proteins were successfully identified.
Bioinformatics analysis demonstrated that the most significant
diseases and disorders in which the identified proteins were
associated with were immunological disease, inflammatory disease,
inflammatory responses and respiratory disease. Using IPA Biomarker
Filter analysis, 13 differentially expressed proteins in the
present study were identified as candidate biomarkers of ARDS.
Inflammatory cytokines, such as tumor necrosis
factor α (TNF-α), interleukin (IL)-1β and IL-6, and molecules
derived from injured lung tissues, such as surfactant proteins,
RAGE, CC-16, have been previously reported as candidate biomarkers
of ARDS (20,21). However, these factors were not
identified in the present study. The differentially expressed
proteins identified in this study were described as antigenic, and
among the evaluated proteins, ENO1 and enhancer of polycomb homolog
1 (EPC1) have been previously described as antigenic (22,23).
ENO1 is a multifunctional enzyme that has functions in various
processes, in addition to its role in glycolysis (24). Increased levels of ENO1
autoantibody have been observed in the serum of patients with
rheumatoid arthritis and cholangiocarcinoma (22,25).
A previous proteomics study identified sputum ENO1 as a potential
biomarker to aid in the diagnosis of early-stage lung cancer
(26). Increased ENO1 and protein
disulfide isomerase-associated 3 were also observed in alveolar
epithelial injury and remodeling, which is strongly associated with
chronic lung diseases (27). EPC1
is a chromatin protein that modulates skeletal muscle
differentiation and induces vascular smooth muscle cell (VSMC)
differentiation. EPC1 expression was demonstrated to be upregulated
during VSMC differentiation and decreased by platelet-derived
growth factor BB treatment (28,29).
Recently, EPC1 was characterized and isolated from Echinococcus
granulosus protoscoleces as a highly antigenic protein useful
in the diagnosis of cystic echinococcosis (23). Although the roles of ENO1 and EPC1
in ARDS require further investigation, the isolation and
identification of these antigenic proteins suggested that they may
be feasible for use in immunoproteomics.
The roles of the majority of the identified
immunogenic proteins in ARDS remain unclear. IPA analysis revealed
that PGK1, ENO1, GAPDH and MDH2 participated in canonical
glycolysis or gluconeogenesis pathways. However, these proteins
exhibited inconsistent regulatory changes in the present study,
which suggested that disordered carbohydrate metabolism may be
involved the pathogenesis of ARDS. A previous study reported that
during ALI, stretching of the pulmonary epithelial cells may result
in the inhibition of succinate dehydrogenase expression, in
association with the normoxic stabilization of hypoxia-inducible
factor (HIF)-1A. Alveolar epithelial HIF1A expression was reported
to enhance the glycolytic carbohydrate flux and optimize
mitochondrial respiration (30).
HIF-dependent prevention of mitochondrial dysfunction increases
alveolar epithelial ATP production and prevents the accumulation of
reactive oxygen species and lung inflammation (30).
A previous study reported that acrolein-induced ALI
is accompanied by a varied metabolomic pattern of energetic stress
(31). Four metabolites that
differed from the controls were identified in the plasma of
sepsis-induced ALI patients; computational data analysis to
identify the metabolic networks also provided information regarding
the enzymes involved in ALI and the genes that encoded them
(32). In addition to the changes
in carbohydrate metabolic enzymes aforementioned, the present study
identified other proteins involved in choline degradation,
aspartate degradation, pyrimidine ribonucleotide interconversion
and de novo biosynthesis that were also downregulated in
rats with ALI. Furthermore, molecular network analysis revealed
that the differentially expressed proteins were associated with one
another, directly or indirectly. Consistent with these previous
studies, data from the present study suggested that metabolic
dysregulation may be associated with the development of ARDS.
Diffuse alveolar damage is a morphological hallmark
of ARDS; however, bronchiolar cells may also be seriously damaged
in ARDS (33,34). Autopsy studies of the lung tissue
of subjects that succumbed to ARDS have revealed epithelial
denudation, inflammation and airway wall thickening with
extracellular matrix remodeling in distal airways (34). CC16 secreted by the Clara cells of
the distal respiratory epithelium is considered to be a marker of
lung injury (35). Therefore,
another protein identified in the present study, SPEF2, was of
interest. SPEF2 is predominantly expressed in ciliated tissues,
including lung, trachea, testis and brain tissue (36–38).
It is required for ciliary motility and spermatogenesis, and the
loss of SPEF2 function was reported to result in severe
spermatogenic defects (36). SPEF2
was also reported to have an important role in the differentiation
and function of ciliated cells in the airway (37). In the present study, the
differential expression of SPEF2 indicated that ciliated cells were
injured, and SPEF2 may also be a potential marker of lung
injury.
Another protein identified in the present study,
SELENBP1, was not classified into any of the functional families.
SELENBP1 is a member of the selenoprotein family and is expressed
in a variety of tissue types, including the lungs. It has been
previously suggested that this protein mediates the intracellular
transport of selenium (39,40).
Low SELENBP1 expression has been reported in several tumor types,
and was suggested to be a potential biomarker for cancer
progression and prognosis (41,42).
Recently, SELENBP1 was identified as a negative regulator of HIF1A
(42); inhibition of HIF1A has a
protective role in lung injury induced by trauma and hemorrhagic
shock, which may be associated with the regulation of the inducible
nitric oxide synthase/nitric oxide pathway by HIF1A in lung tissue
(43). In the present study,
SELENBP1 was revealed to be upregulated in the ARDS model compared
with the control. The relationships between SELENBP1, HIF1A and
ARDS require further investigation.
Certain limitations to the design of the present
study must be noted. By comparing the histopathological changes in
lung injury with the controls 24 h post-induction, a research time
point was selected for analysis and, thus, the immunoproteomic
study at that point did not reflect early ARDS, and no study was
conducted on sequential proteomic changes according to the course
of ARDS. Therefore, future studies must investigate the immunogenic
proteins identified in this experiment with regard to the early
detection of ARDS in sepsis patients.
In summary, 38 differentially expressed proteins
were identified in the rat model of ARDS using an immunoproteomic
method. These proteins were described as antigens, and paired
antibodies are predicted to be detected in the plasma of patients
at high risk of ARDS. Analysis of these identified proteins may
provide novel insights into the potential pathological mechanisms
of ARDS.
Acknowledgements
The authors would like to thank Mr. Fuqiang Wang
(Analysis Center of Nanjing Medical University, Nanjing, China) for
his assistance in the proteomics techniques and mass spectrometric
analysis.
References
|
1
|
Bernard GR, Artigas A, Brigham KL, Carlet
J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A and Spragg R:
Report of the American-European Consensus conference on ARDS:
Definitions, mechanisms, relevant outcomes and clinical trial
coordination. The Consensus Committee. Intensive Care Med.
20:225–232. 1994; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
ARDS Definition Task Force, . Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
3
|
Villar J, Blanco J, Añón JM, Santos-Bouza
A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa
S, et al: The ALIEN study: Incidence and outcome of acute
respiratory distress syndrome in the era of lung protective
ventilation. Intensive Care Med. 37:1932–1941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Villar J, Sulemanji D and Kacmarek RM: The
acute respiratory distress syndrome: Incidence and mortality, has
it changed? Curr Opin Crit Care. 20:3–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Determann RM, Millo JL, Waddy S, Lutter R,
Garrard CS and Schultz MJ: Plasma CC16 levels are associated with
development of ALI/ARDS in patients with ventilator-associated
pneumonia: A retrospective observational study. BMC Pulm Med.
9:492009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura T, Sato E, Fujiwara N, Kawagoe Y,
Maeda S and Yamagishi S: Increased levels of soluble receptor for
advanced glycation end products (sRAGE) and high mobility group box
1 (HMGB1) are associated with death in patients with acute
respiratory distress syndrome. Clin Biochem. 44:601–604. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McClintock D, Zhuo H, Wickersham N,
Matthay MA and Ware LB: Biomarkers of inflammation, coagulation and
fibrinolysis predict mortality in acute lung injury. Crit Care.
12:R412008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng IW, Ware LB, Greene KE, Nuckton TJ,
Eisner MD and Matthay MA: Prognostic value of surfactant proteins A
and D in patients with acute lung injury. Crit Care Med. 31:20–27.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bowler RP, Duda B, Chan ED, Enghild JJ,
Ware LB, Matthay MA and Duncan MW: Proteomic analysis of pulmonary
edema fluid and plasma in patients with acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 286:L1095–L1104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X, Shan Q, Jiang L, Zhu B and Xi X:
Quantitative proteomic analysis by iTRAQ for identification of
candidate biomarkers in plasma from acute respiratory distress
syndrome patients. Biochem Biophys Res Commun. 441:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhargava M, Becker TL, Viken KJ, Jagtap
PD, Dey S, Steinbach MS, Wu B, Kumar V, Bitterman PB, Ingbar DH and
Wendt CH: Proteomic profiles in acute respiratory distress syndrome
differentiates survivors from non-survivors. PLoS One.
9:e1097132014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu D, Mao P, Huang Y, Liu Y, Liu X, Pang
X and Li Y: Proteomic analysis of lung tissue in a rat acute lung
injury model: Identification of PRDX1 as a promoter of
inflammation. Mediators Inflamm. 2014:4693582014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thongboonkerd V, Chiangjong W, Mares J,
Moravec J, Tuma Z, Karvunidis T, Sinchaikul S, Chen ST, Opatrný K
and Matejovic M: Altered plasma proteome during an early phase of
peritonitis-induced sepsis. Clin Sci (Lond). 116:721–730. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Z, Liu H, Gu G, Wang G, Wu W, Zhang C
and Ren J: Immunoproteomic to identify antigens in the intestinal
mucosa of Crohn's disease patients. PLoS One. 8:e816622013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bunk S, Susnea I, Rupp J, Summersgill JT,
Maass M, Stegmann W, Schrattenholz A, Wendel A, Przybylski M and
Hermann C: Immunoproteomic identification and serological responses
to novel chlamydia pneumoniae antigens that are associated with
persistent C. pneumoniae infections. J Immunol. 180:5490–5498.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brooks HF, Osabutey CK, Moss RF, Andrews
PL and Davies DC: Caecal ligation and puncture in the rat mimics
the pathophysiological changes in human sepsis and causes
multi-organ dysfunction. Metab Brain Dis. 22:353–373. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishina K, Mikawa K, Takao Y, Maekawa N,
Shiga M and Obara H: ONO-5046, an elastase inhibitor, attenuates
endotoxin-induced acute lung injury in rabbits. Anesth Analg.
84:1097–1103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu J, Wang F, Gong Y, Li D, Sha J, Huang X
and Han X: Proteomic analysis of changes induced by nonylphenol in
Sprague-Dawley rat Sertoli cells. Chem Res Toxicol. 22:668–675.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terpstra ML, Aman J, van Nieuw Amerongen
GP and Groeneveld AB: Plasma biomarkers for acute respiratory
distress syndrome: A systematic review and meta-analysis*. Crit
Care Med. 42:691–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujishima S: Pathophysiology and
biomarkers of acute respiratory distress syndrome. J Intensive
Care. 2:322014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rucksaken R, Pairojkul C, Pinlaor P,
Khuntikeo N, Roytrakul S, Selmi C and Pinlaor S: Plasma
autoantibodies against heat shock protein 70, enolase 1 and
ribonuclease/angiogenin inhibitor 1 as potential biomarkers for
cholangiocarcinoma. PLoS One. 9:e1032592014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Etebar F, Jalousian F, Hosseini SH,
Kordafshari S and Najafi A: Immunoproteomics approach for EPC1
antigenic epitope prediction of G1 and G6 strains of Echinococcus
granulosus. Parasitol Res. 112:3129–3135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Capello M, Ferri-Borgogno S, Cappello P
and Novelli F: α-Enolase: A promising therapeutic and diagnostic
tumor target. FEBS J. 278:1064–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JY, Choi IA, Kim JH, Kim KH, Lee EY,
Lee EB, Lee YM and Song YW: Association between anti-Porphyromonas
gingivalis or anti-α-enolase antibody and severity of periodontitis
or rheumatoid arthritis (RA) disease activity in RA. BMC
Musculoskelet Disord. 16:1902015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu L, Shen J, Mannoor K, Guarnera M and
Jiang F: Identification of ENO1 as a potential sputum biomarker for
early-stage lung cancer by shotgun proteomics. Clin Lung Cancer.
15:372–378, e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mutze K, Vierkotten S, Milosevic J,
Eickelberg O and Königshoff M: Enolase 1 (ENO1) and protein
disulfide-isomerase associated 3 (PDIA3) regulate
Wnt/β-catenin-driven trans-differentiation of murine alveolar
epithelial cells. Dis Model Mech. 8:877–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kee HJ, Kim JR, Nam KI, Park HY, Shin S,
Kim JC, Shimono Y, Takahashi M, Jeong MH, Kim N, et al: Enhancer of
polycomb1, a novel homeodomain only protein-binding partner,
induces skeletal muscle differentiation. J Biol Chem.
282:7700–7709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Joung H, Kwon JS, Kim JR, Shin S, Kang W,
Ahn Y, Kook H and Kee HJ: Enhancer of polycomb1 lessens neointima
formation by potentiation of myocardin-induced smooth muscle
differentiation. Atherosclerosis. 222:84–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eckle T, Brodsky K, Bonney M, Packard T,
Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M and
Eltzschig HK: HIF1A reduces acute lung injury by optimizing
carbohydrate metabolism in the alveolar epithelium. PLoS Biol.
11:e10016652013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fabisiak JP, Medvedovic M, Alexander DC,
McDunn JE, Concel VJ, Bein K, Jang AS, Berndt A, Vuga LJ, Brant KA,
et al: Integrative metabolome and transcriptome profiling reveals
discordant energetic stress between mouse strains with differential
sensitivity to acrolein-induced acute lung injury. Mol Nutr Food
Res. 55:1423–1434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stringer KA, Serkova NJ, Karnovsky A,
Guire K, Paine R III and Standiford TJ: Metabolic consequences of
sepsis-induced acute lung injury revealed by plasma ¹H-nuclear
magnetic resonance quantitative metabolomics and computational
analysis. Am J Physiol Lung Cell Mol Physiol. 300:L4–L11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarmiento X, Guardiola JJ, Almirall J,
Mesalles E, Mate JL, Soler M and Klamburg J: Discrepancy between
clinical criteria for diagnosing acute respiratory distress
syndrome secondary to community acquired pneumonia with autopsy
findings of diffuse alveolar damage. Respir Med. 105:1170–1175.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morales MM, Pires-Neto RC, Inforsato N,
Lanças T, da Silva LF, Saldiva PH, Mauad T, Carvalho CR, Amato MB
and Dolhnikoff M: Small airway remodeling in acute respiratory
distress syndrome: A study in autopsy lung tissue. Crit Care.
15:R42011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kropski JA, Fremont RD, Calfee CS and Ware
LB: Clara cell protein (CC16), a marker of lung epithelial injury,
is decreased in plasma and pulmonary edema fluid from patients with
acute lung injury. Chest. 135:1440–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sironen A, Kotaja N, Mulhern H, Wyatt TA,
Sisson JH, Pavlik JA, Miiluniemi M, Fleming MD and Lee L: Loss of
SPEF2 function in mice results in spermatogenesis defects and
primary ciliary dyskinesia. Biol Reprod. 85:690–701. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ostrowski LE, Andrews K, Potdar P,
Matsuura H, Jetten A and Nettesheim P: Cloning and characterization
of KPL2, a novel gene induced during ciliogenesis of tracheal
epithelial cells. Am J Respir Cell Mol Biol. 20:675–683. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Finn R, Evans CC and Lee L:
Strain-dependent brain defects in mouse models of primary ciliary
dyskinesia with mutations in Pcdp1 and Spef2. Neuroscience.
277:552–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang PW, Tsui SK, Liew C, Lee CC, Waye MM
and Fung KP: Isolation, characterization and chromosomal mapping of
a novel cDNA clone encoding human selenium binding protein. J Cell
Biochem. 64:217–224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Porat A, Sagiv Y and Elazar Z: A 56-kDa
selenium-binding protein participates in intra-Golgi protein
transport. J Biol Chem. 275:14457–14465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ansong E, Ying Q, Ekoue DN, Deaton R, Hall
AR, Kajdacsy-Balla A, Yang W, Gann PH and Diamond AM: Evidence that
selenium binding protein 1 is a tumor suppressor in prostate
cancer. PLoS One. 10:e01272952015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jeong JY, Zhou JR, Gao C, Feldman L and
Sytkowski AJ: Human selenium binding protein-1 (hSP56) is a
negative regulator of HIF-1α and suppresses the malignant
characteristics of prostate cancer cells. BMB Rep. 47:411–416.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang H, Huang Y, Xu H, Hu R and Li QF:
Inhibition of hypoxia inducible factor-1α ameliorates lung injury
induced by trauma and hemorrhagic shock in rats. Acta Pharmacol
Sin. 33:635–643. 2012. View Article : Google Scholar : PubMed/NCBI
|