Introduction
Inflammation (Latin, inflammatio) represents
a complex biological response, which occurs in reaction to any type
of injury to the body, including damage to cells and from irritants
or pathogens (1). Inflammation is
a protective response, and immune cells, blood vessels and
molecular mediators are involved in this process. The acute stage
of inflammation is the initial response of the body to harmful
stimuli and persists for a short-term in which it mediates the host
defense against infections (2,3). The
inflammatory response requires active termination when it is no
longer required to prevent unnecessary ‘bystander’ damage to
tissues. Failure to do so results in chronic inflammation and
cellular destruction. Chronic inflammation is present in the long
term and can predispose the host to various chronic illnesses,
including cancer (4,5). For example, individuals who have
chronic inflammatory bowel diseases are at high risk of developing
colon cancer (4). Therefore,
inhibiting inflammatory activity may be a target in the treatment
of cancer and other diseases.
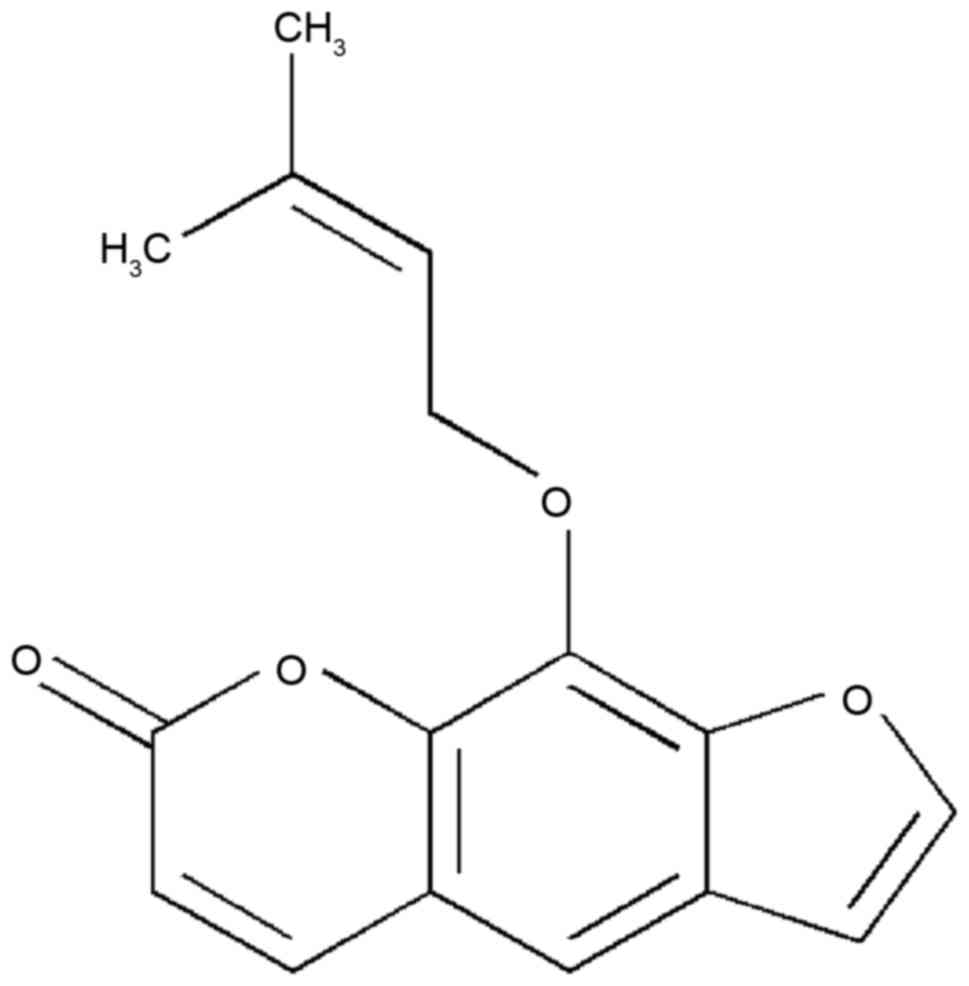
Imperatorin (IMT) is a bioactive furanocoumarin, the
structure of which is shown in Fig.
1. IMT is an effective component extracted from traditional
Chinese medicines and is widely distributed in the plant kingdom,
particularly in Angelica dahurica, A. archangelica,
Peucedani, Notopterygium and Radix Glehniae
(6,7). It has been reported that IMT has a
wide range of potent pharmacological activities, including
antibacterial, antitumor, anticonvulsant, acute neurotoxic effects
and abirritation (8–11). Of note, previous investigations
have demonstrated that IMT possesses notable anti-inflammatory
activity by inhibiting the production of nitric oxide (NO) and
prostaglandin E2 (PGE2), and decreasing the
expression of inducible nitric oxide synthase (iNOS),
cyclooxygenase (COX)-2 and microsomal prostaglandin E synthase
(12–14). However, few systemic investigations
of the molecular mechanisms underlying the anti-inflammatory
effects of IMT have been performed, which limits the clinical uses
of this compound for treating inflammatory diseases. Therefore, due
to the anti-inflammatory activity of IMT, the present study further
evaluated the inhibitory effects of IMT against inflammation in
vitro and in vivo, to understand the mechanism
underlying its effects.
The present study detected the anti-inflammatory
effects of IMT on dimethylbenzene-induced ear edema in mice, acetic
acid-induced vascular permeability in mice and cotton
pellet-induced granuloma in rats, and in LPS-induced RAW264.7
cells. In addition, the levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-6 and IL-1β in LPS-induced endotoxemic mice and
LPS-induced RAW264.7 cells were measured. The protein expression
levels of iNOS, COX-2, nuclear p65 [p65 (N)], cytosolic p65 [p65
(C)] and IκB (C) in LPS-induced RAW264.7 were also investigated to
elucidate the anti-inflammatory mechanism of IMT.
Materials and methods
Chemicals
IMT was purchased from Shanghai Bomaide Biotech.
Co., Ltd. (Shanghai, China); TNF-α, IL-6, and IL-1β enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Invitrogen;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA); dimethyl benzene
was purchased from the Sino Pharm. (Chengdu, China); Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum (FBS) and
trypsinase were from Gibco; Thermo Fisher Scientific, Inc.; the
Cell Counting Kit-8, bicinchoninic acid (BCA) protein assay reagent
and goat-anti-rabbit/rat horseradish-peroxidase (HRP)-conjugated
secondary antibodies (cat. nos. A0208 and A0192) were purchased
from Beyotime Institute of Biotechnology (Haimen, China);
lipopolysaccharide (LPS), dimethyl sulfoxide (DMSO) and Evans blue
were purchased from Sigma; Merck Millipore (Darmstadt, Germany);
COX-2 (cat. no. sc-19999), p65 (cat. no. sc-56735), IκB (cat. no.
sc-945) and Histone H1 (cat. no. sc-8030) antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA);
anti-β-actin antibody (cat. no. ab8226) and anti-iNOS antibody
(cat. no. ab15323) were purchased from Abcam (Cambridge, MA,
USA).
Animals and IMT treatment
A total of 150 Institute of Cancer Research (ICR)
mice (weight, 20±2 g; n=75 female; n=75 male) and 50 Sprague-Dawley
rats (weight, 220±20 g; n=25 female; n=25 male) were purchased from
the Shanghai Laboratory Animal Centre (Shanghai, China). Each
animal was housed under standard conditions (21±1°C, 50–10%
relative humidity, 12 h light/dark cycle) and had free access to
food and water. The experimental protocols were approved by the
Animal Care and Use Committee of The First Affiliated Hospital of
Xinjiang Medical University (Urumuqi, China).
The anti-inflammatory activity of IMT in vivo
was determined against dimethylbenzene-induced ear edema in mice,
acetic acid-induced vascular permeability in mice and cotton
pellet-induced granuloma in rats. A total of 50 mice or rats were
randomly divided into five groups of 10 animals, including a
control, positive control and three graded IMT treatments groups.
IMT was administered orally at doses of 15, 30 or 60 mg/kg. The
positive control group received indometacin (10 mg/kg/day) by
intraperitoneal (i.p.) injection and the control group received an
equal volume of vehicle (0.5% CMC-Na, 10 ml/kg/day), which had been
used to dilute IMT. For the assessment of dimethylbenzene-induced
ear edema and acetic acid-induced vascular permeability in mice,
mice received one dose of the treatment (1 dose of 15, 30 or 60
mg/kg for IMT, 10 mg/kg for indometacin). To assess the cotton
pellet-induced granuloma in rats, treatments were administered once
daily for 7 consecutive days (15, 30 or 60 mg/kg/day for IMT, 10
mg/kg/day for indometacin).
Assessment of dimethylbenzene-induced
ear edema in mice
The assessment of dimethylbenzene-induced ear edema
in mice was performed according to the method described in a
previous report (15). In brief,
dimethyl benzene was applied locally to the right ear 1 h following
final drug administration. The mice were sacrificed under
anesthesia via sodium pentobarbital injection (40 mg/kg i.p.) 1 h
following dimethylbenzene application. The right ear and left ear
were then amputated at the same location. Finally, the ear edema
was calculated by subtracting the weight of the left ear from that
of the right ear.
Assessment of acetic acid-induced
vascular permeability in mice
The acetic acid-induced vascular permeability
assessment in mice was performed according to a previously reported
method with modification (15). At
1 h following final drug administration, each mouse was injected
intravenously with Evans blue (2% in normal saline; 10 ml/kg) and
was injected abdominally with acetic acid (0.7% in saline; 10
ml/kg). Following injection of acetic acid for 20 min, all the mice
were sacrificed by cervical dislocation. The abdominal cavity was
then washed several times with 5 ml of saline solution per mouse.
The rinsed solution was collected and centrifuged for 15 min (780 ×
g at 4°C). Finally, the absorbance of Evans blue in the supernatant
was determined at 630 nm with a spectrophotometer (Model 721;
Shanghai Optical Instrument Factory Co., Ltd., Shanghai,
China).
Assessment of cotton pellet-induced
granuloma in rats
The assessment of cotton pellet-induced granuloma in
rats was performed to evaluate the chronic anti-inflammatory
activity of IMT and was performed as described previously (16). In brief, a small section of sterile
cotton pellet (50±1 mg) was applied subcutaneously to the
dorsolateral skin of the anesthetized rats (one on either side),
and drugs were administered once daily for 7 days consecutively. On
day 8, all rats were sacrificed and the pellets with granuloma were
excised. The increments of the wet and dry weights of the pellets
were used to evaluate granuloma formation.
Measurement of levels of TNF-α, IL-6
and IL-1β in LPS-induced endotoxemic mice
The mice were injected with IMT (15, 30 and 60
mg/kg) for 24 h in the presence of LPS (2 mg/kg). Subsequently, the
mice were sacrificed and serum was collected to measure blood
levels of TNF-α, IL-6 and IL-1β using ELISA kits, according to the
manufacturer's protocols.
Cell culture
The murine macrophage RAW264.7 cell line was
purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China), and the cells were cultured in DMEM
with 10% FBS, 1% penicillin and 1% streptomycin in a 5%
CO2 humidified atmosphere at 37°C.
Analysis of cell viability using a
Cell Counting kit-8 (CCK-8 assay)
The effects of IMT on cellular viability were
evaluated using a CCK-8 assay according to the manufacturer's
protocol. The RAW264.7 cells (5×103 cells/well) were
seeded into 96-well plates and incubated with either increasing
concentrations of IMT (18.5, 37, 74, 148, 296 and 592 µM) or
vehicle (DMSO) in the presence of LPS (100 ng/ml). Following
treatment for 72 h, CCK-8 solution was added to each well and
incubated for another 1 h in the incubator. The absorbance at 450
nm was then read using a 96-well plate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The results were determined
as a percentage of the DMSO control cells.
Quantitative analysis of the mRNA
expression levels of TNF-α, IL-6 and IL-1β in RAW 264.7 cells
The effects of IMT on the mRNA expression levels of
TNF-α, IL-6 and IL-1β were determined using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis. The RAW264.7 cells at a density of 3×105
cells/well were seeded into 6-well plates and incubated with either
increasing concentrations of IMT (55.5, 111 and 222 µM) or vehicle
(DMSO) in the presence of 100 ng/ml LPS for 72 h at 37°C.
Subsequently, the cells were collected and total RNA was extracted
using an RNAiso Plus kit (Takara Biotechnology Co., Ltd., Dalian,
Japan). The total RNA was then used to synthesize cDNA of TNF-α,
IL-6, IL-1β and β-actin using PrimeScript™ RT reagent kits (Takara
Biotechnology Co., Ltd.). The reverse transcription temperature
protocol was as follows: 37°C for 15 min and 85°C for 5 sec. The
synthesized cDNAs were amplified using SYBR Green mix on a CFX96
Touch Real-Time PCR detection system (Bio-Rad Laboratories, Inc.).
Each 10-µl reaction mixture contained 5 µl of SYBR Premix (Bio-Rad
Laboratories, Inc.), 1 µl of 2 µM forward and reverse primer mix, 1
µl of cDNA, and 3 µl of ddH2O. The PCR thermocycling
conditions were as follows: 95°C for 10 min, followed by 39 cycles
of 95°C for 15 sec and 60°C for 60 sec. All primers used for
RT-qPCR analysis are shown in Table
I. The relative mRNA expression levels of TNF-α, IL-6 and IL-1β
were assessed via 2−ΔΔCq relative quantitative analysis
(17) and all samples were
analyzed in triplicate.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequence
(5′-3′) |
|---|
| TNF-α | Forward:
CAGGTTCTGTCCCTTTCACTCACT |
|
| Reverse:
GTTCAGTAGACAGAAGAGCGTGGT |
| IL-6 | Forward:
TGGAGTACCATAGCTACCTGGAGT |
|
| Reverse:
TCCT-TAGCCACTCCTTCTGTGACT |
| IL-1β | Forward:
ATGGCAACTGTTCCTGAACTC |
|
| Reverse:
TTAGGAAGACACGGATTCCAT |
| β-actin | Forward:
GGGAAATCGTGCGTGACATCAAAG |
|
| Reverse:
CATACCCAAGAAGGAAGGCTGGAA |
Western blot analysis
The effects of IMT on the protein expression levels
of iNOS, COX-2, p65 (N), p65 (C) and IκB (C) were measured in the
LPS-induced RAW264.7 cells using western blot analysis. Following
treatment with either graded concentrations of IMT (55.5, 111 and
222 µM) or vehicle (DMSO) in the presence of 100 ng/ml LPS for 72
h, the cells were collected and total protein was extracted using
western blot and IP cell lysis buffer (Sangon Biotech Co., Ltd.).
Subsequently, the concentration of total protein was determined
using BCA protein assay reagent (Sangon Biotech Co., Ltd.). The
total protein (40 µg) in each sample was separated by 12% sodium
dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) and
blotted onto polyvinylidene difluoride (PVDF) filter membranes. The
proteins on the PVDF membrane were then probed with anti-iNOS
(1:1,000), anti-COX-2 (1:200), anti- p65 (1:200), anti-cytosolic
IκB (1:200), anti-β-actin (1:5,000) and anti-Histone H1 (1:500)
antibodies at 4°C for 12 h, followed by incubation with
corresponding HRP-conjugated secondary antibodies (1:7,000) for 2 h
at 37°C. Finally, the immunoreactive bands were visualized using
ECL-detecting reagents and optical density (OD) values were
analyzed using Image J 2X software. To normalize for protein
loading, the expression levels of iNOS, COX-2, p65 (C) and IκB (C)
were expressed as a relative value to that of β-actin, and the
expression level of p65 (N) was expressed as a relative value to
that of Histone H1.
Statistical analysis
All results are expressed as the mean ± standard
deviation of three independent experiments performed in triplicate.
Statistical analyses were performed using the SPSS 19.0 software
package (IBM SPSS, Armonk, NY, USA). One-way analysis of variance
with Dunnett's test was used to compare the means between two
groups. P<0.05 was considered to indicate a statistically
significance difference.
Results
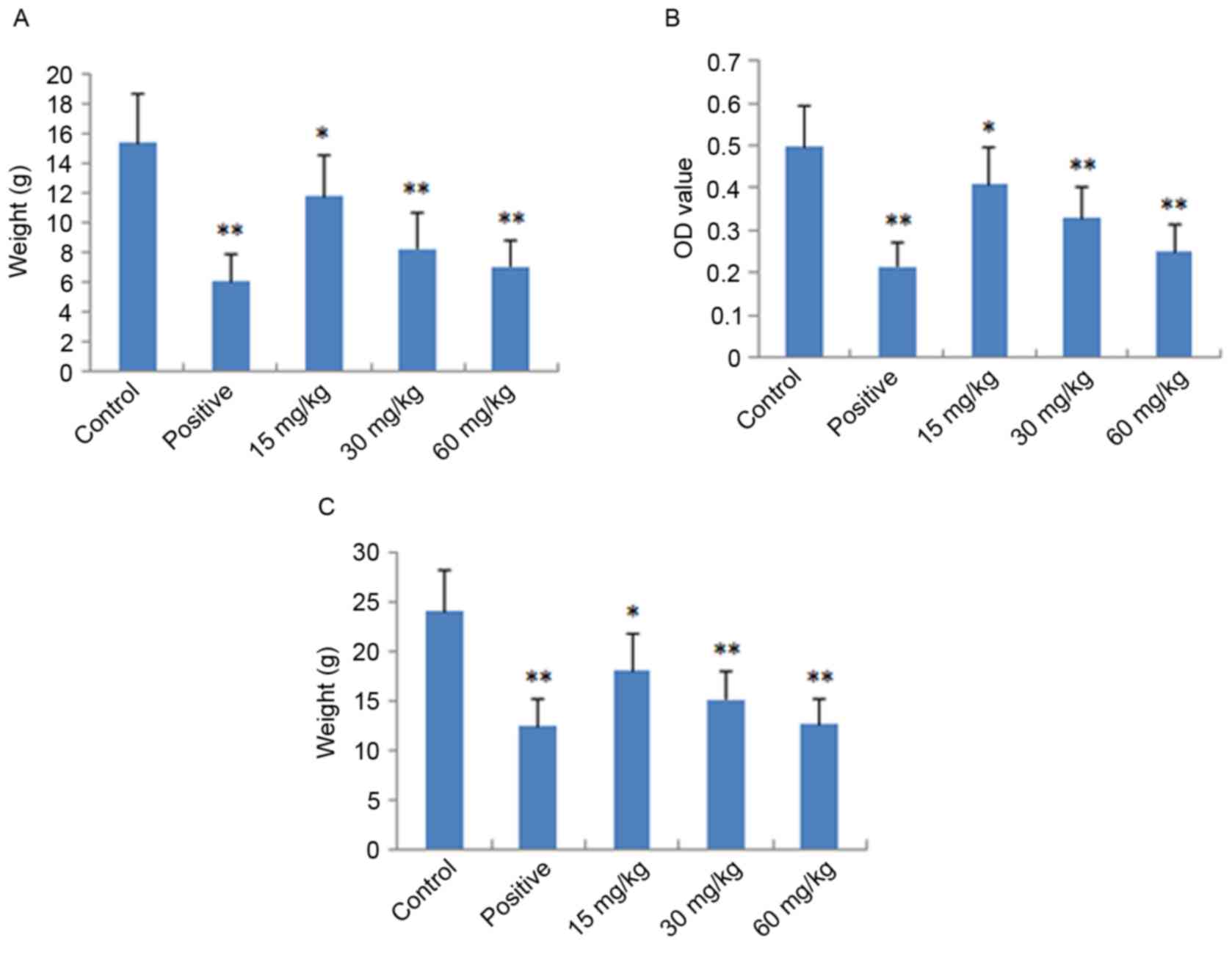
Assessment of dimethylbenzene-induced
ear edema
The local and acute anti-inflammatory activities of
IMT were evaluated by measuring its inhibition of
dimethylbenzene-induced ear edema in mice. As shown in Fig. 2A, IMT had significant and
dose-dependent inhibitory effects on ear edema at concentrations of
15, 30 and 60 mg/kg, compared with the control group (P<0.05,
P<0.01 and P<0.01, respectively). At a dose of 60 mg/kg, the
anti-inflammatory activity of IMT was comparable with that of
indometacin at a dose of 10 mg/kg.
Assessment of acetic acid-induced
vascular permeability
The acute anti-inflammatory activity of IMT was also
detected by measuring its inhibition of acetic acid-induced
vascular permeability in mice. As shown in Fig. 2B, IMT at doses of 15, 30 and 60
mg/kg significantly inhibited the increased vascular permeability
induced by acetic acid in mice, which also occurred in a
dose-dependent manner (P<0.05, P<0.01 and P<0.01,
respectively), compared with the control group. The inhibitory
effect of IMT on dye leakage at a dose of 60 mg/kg was comparable
with that of indometacin at the dose of 10 mg/kg.
Cotton granuloma assessment
The chronic anti-inflammatory activity of IMT was
performed by measuring its inhibition of cotton pellet-induced
granuloma in rats, the results of which are shown in Fig. 2C. The anti-inflammatory effect of
IMT was observed at the doses of 15, 30 and 60 mg/kg. Compared with
the control group, IMT significantly reduced ball fralunoma weight
at doses of 15, 30 and 60 mg/kg (P<0.05, P<0.01 and
P<0.01, respectively). The positive control drug, indometacin
(10 mg/kg), also manifested significant anti-inflammatory activity
(P<0.01).
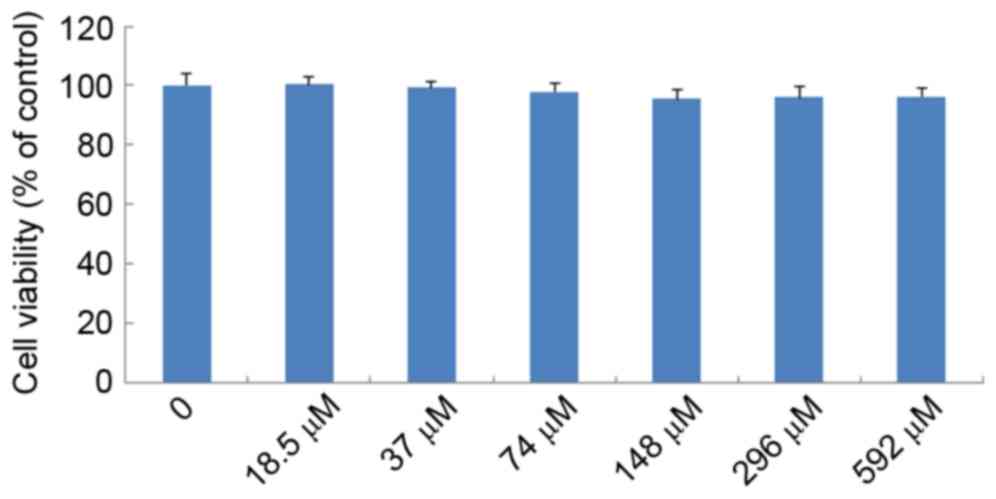
Effects of IMT on LPS-induced cell
viability
The effects of IMT on LPS-induced cell viability
were evaluated using CCK-8. As shown in Fig. 3, no significant effect was observed
when the RAW 264.7 cells were treated with graded concentrations of
IMT (18.5–592 µM) in the presence of 100 ng/ml LPS for 72 h,
compared with the control group (P>0.05). This indicated that
IMT had no inhibitory effect on RAW 264.7 cell growth.
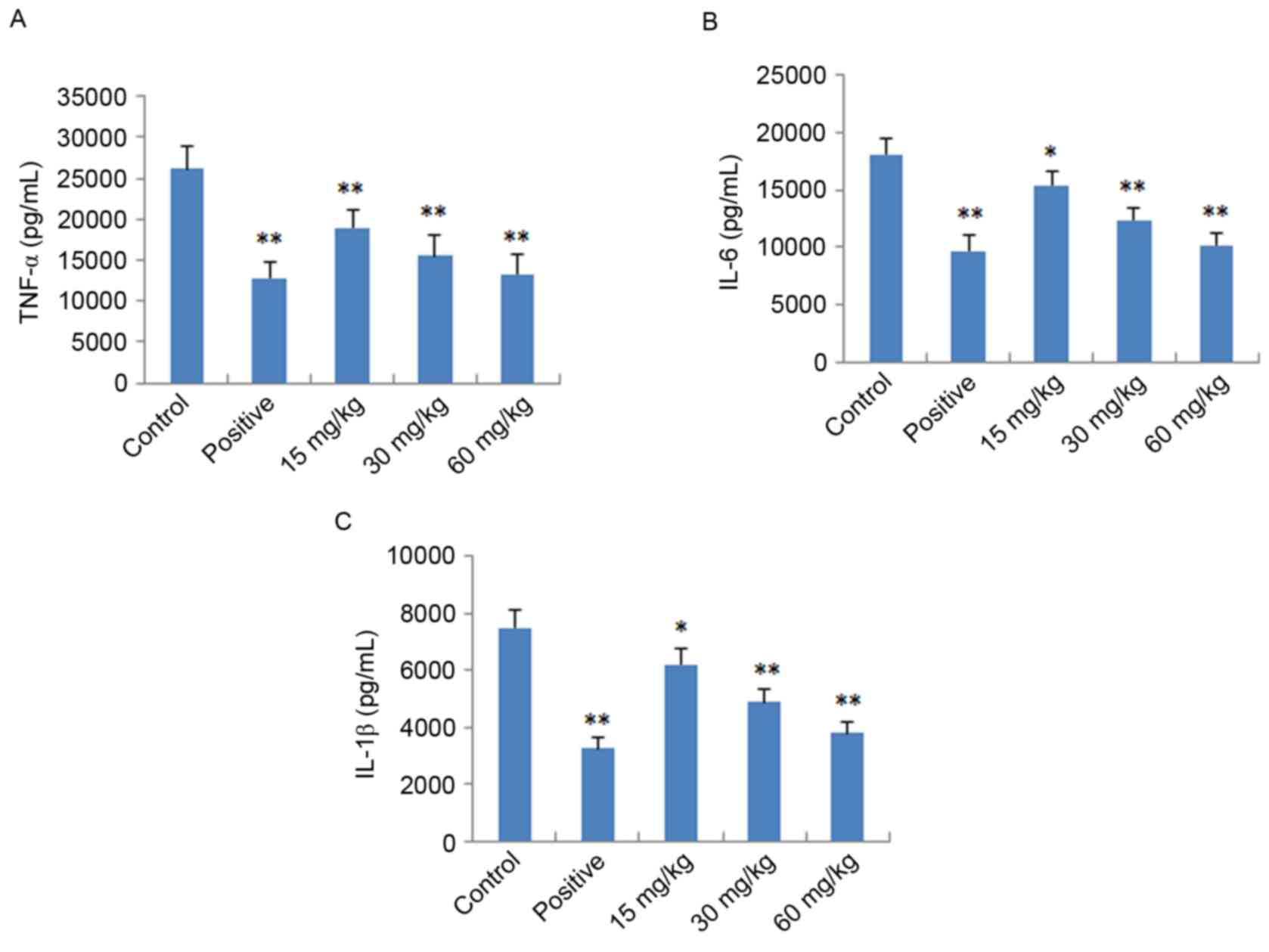
Effects of IMT on TNF-α, IL-6 and
IL-1β in vitro and in vivo
To further verify the anti-inflammatory effects of
IMT in vitro and in vivo, the expression levels of
pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β) were determined
in LPS-induced endotoxemic mice and RAW 264.7 cells using ELISA and
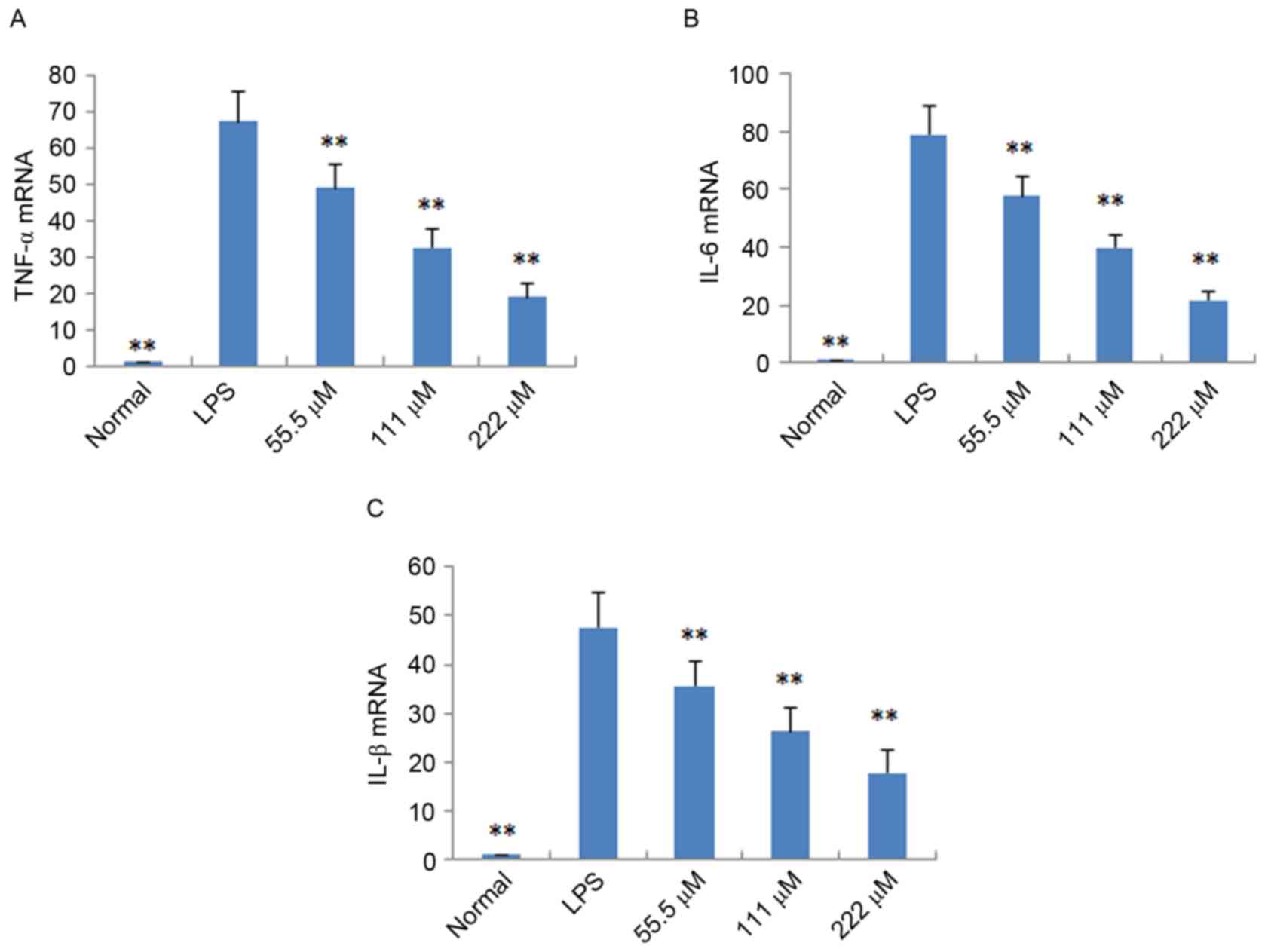
RT-qPCR analysis, respectively. As shown in Fig. 4, following treatment with IMT
(55.5, 111 and 222 µM), the levels of TNF-α, IL-6 and IL-1β induced
by LPS were significantly reduced, compared with those in the LPS
group (P<0.05) in the LPS-induced endotoxemic mice. In the
LPS-induced RAW 264.7 cells, the mRNA expression levels of these
pro-inflammatory cytokines were also significantly downregulated,
compared with those in the LPS group (P<0.01; Fig. 5).
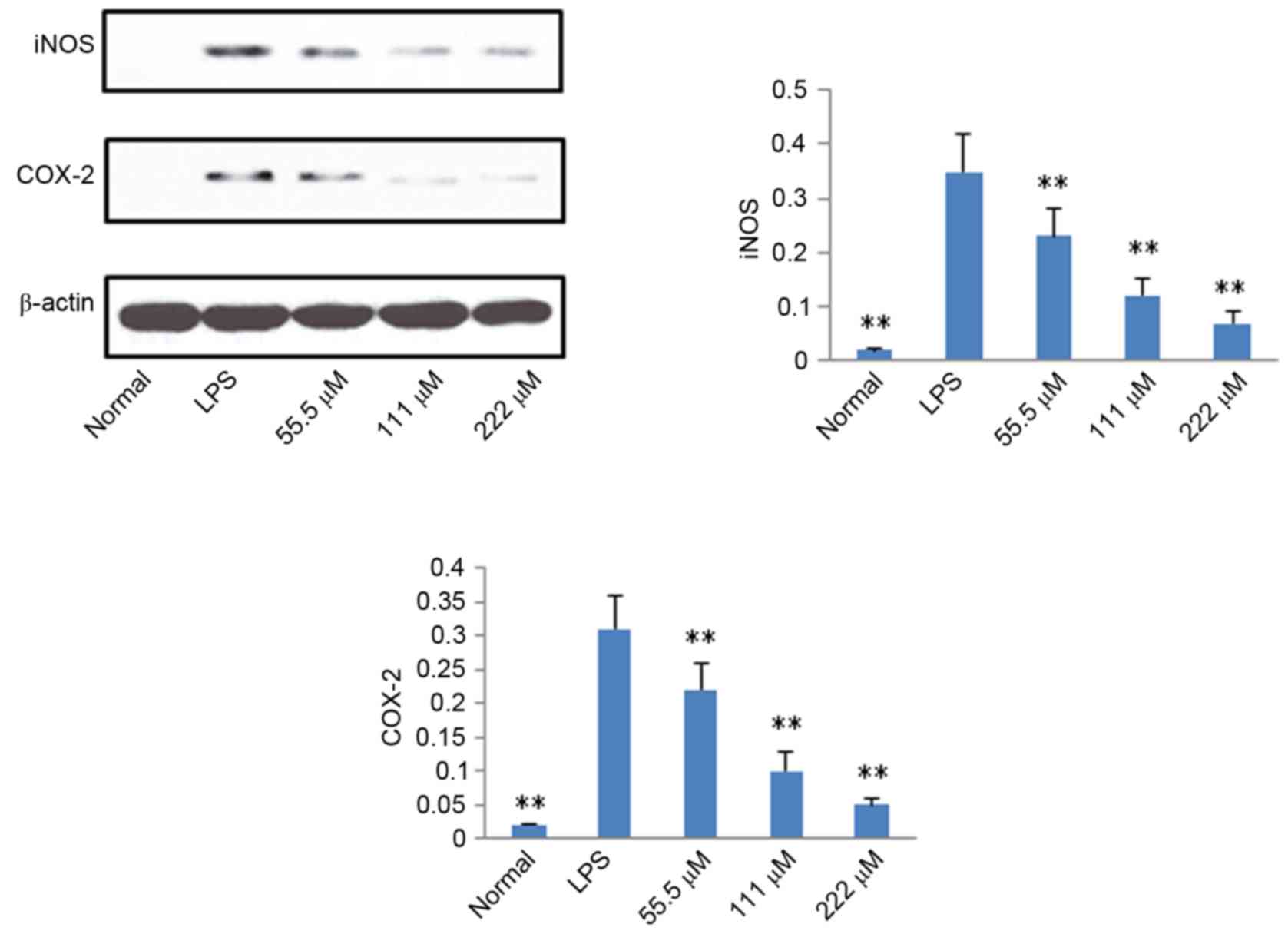
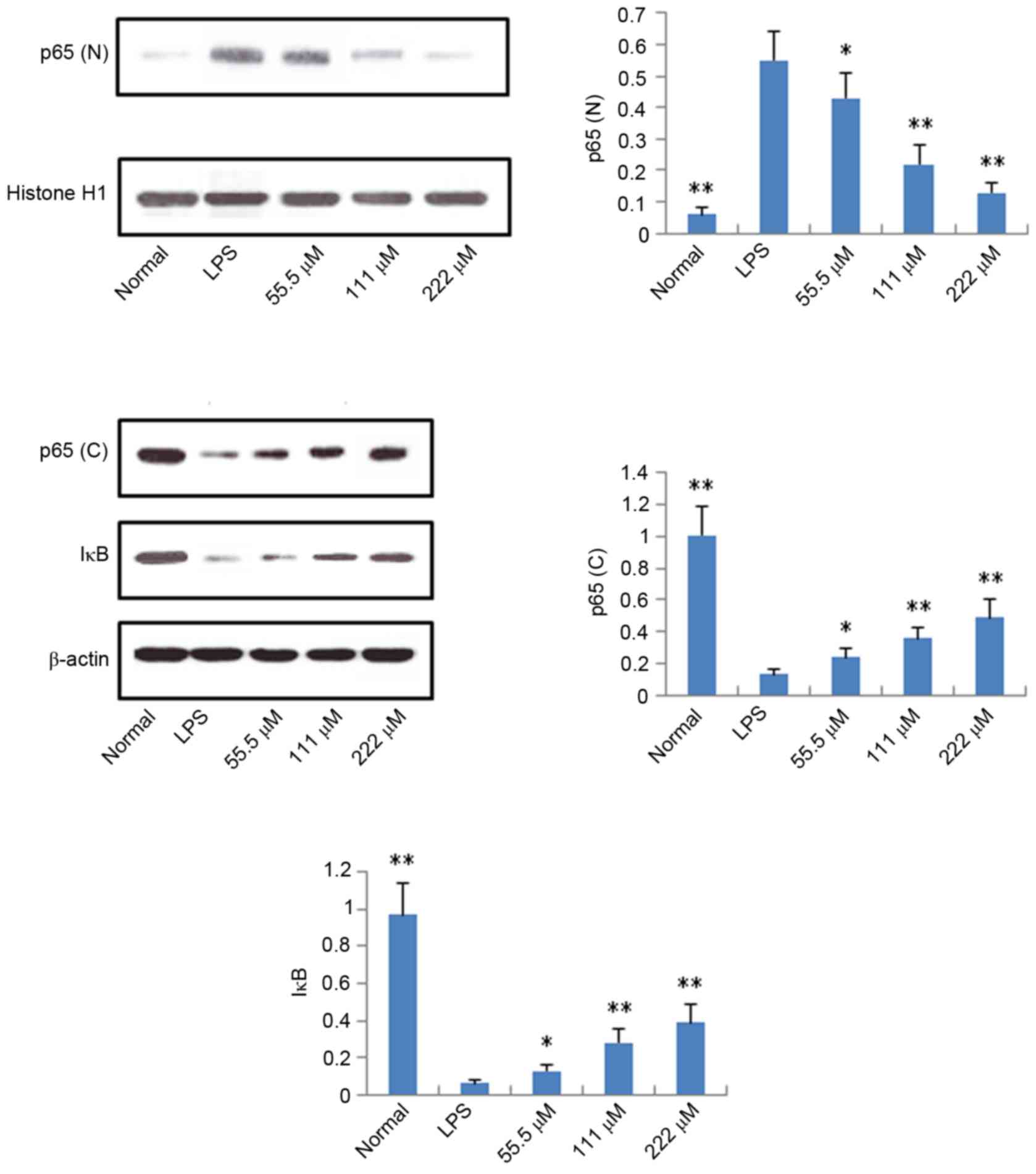
Protein expression levels of iNOS,
COX-2, p65 and IκB
According to the results described above, IMT not
only inhibited topical ear edema, vascular permeability and
granuloma in vivo, but also suppressed the expression levels
of pro-inflammatory cytokines TNF-α, IL-6 and IL-1β in vitro
and in vivo. To further investigate the possible mechanisms
underlying these changes, the protein expression levels of iNOS,
COX-2, p65 (N), p65 (C) and IκB (C) were measured using western
blot analysis. As shown in Figs. 6
and 7, compared with the LPS
group, the protein expression levels of iNOS, COX-2 and p65 (N)
were markedly downregulated in the IMT groups, whereas the protein
expression of p65 (C) and IκB (C) were significantly upregulated in
the LPS-induced RAW 264.7 cells.
Discussion
In the present study, comprehensive analyses of the
acute and chronic anti-inflammatory activities of IMT were
performed using mouse and rat models of inflammation. In addition,
the in vitro anti-inflammatory effect of IMT was
demonstrated using LPS-stimulated RAW264.7 macrophages. IMT
significantly inhibited the levels of pro-inflammatory cytokines
TNF-α, IL-6 and IL-1β in vitro and in vivo. The
expression levels of pro-inflammatory enzymes COX-2 and iNOS were
downregulated in the LPS-stimulated RAW264.7 macrophages treated
with IMT.
The anti-inflammatory effect of IMT was evaluated
using three popular in vivo models: Dimethylbenzene-induced
ear edema, acetic acid-induced vascular permeability, and cotton
pellet-induced granuloma (18,19).
The assessment of dimethylbenzene-induced ear edema in mice is a
useful model for preliminary experiments of acute anti-inflammatory
activity. In the inflammatory response, there is increased vascular
permeability; therefore, vascular permeability was assessed to
further demonstrate the anti-inflammatory effects of IMT. In
addition, cotton pellet-induced granuloma was assessed to evaluate
the chronic anti-inflammatory activity. The results of the present
study showed that IMT at doses of 15, 30 and 60 mg/kg led to topic
inhibition of dimethylbenzene-induced ear edema in mice in a
dose-dependent manner. At the same doses, IMT exhibited similar
inhibitory effects on the increased vascular permeability of mice.
IMT also significantly and dose-dependently reduced the weights of
the cotton pellet granuloma in the cotton pellet-induced model of
chronic inflammation in rats. These data suggested that IMT had
potential anti-inflammatory activity, therefore; the effect of IMT
on the release of inflammatory cytokines and mediators was also
examined to elucidate its anti-inflammatory mechanism.
Inflammation is a local response to irritants,
tissue injury and infection. During inflammation, circulating
macrophages, mast cells and neutrophils produce cytokines,
including TNF-α, IL-6 and IL-1β, which further exaggerate
inflammatory responses (20).
TNF-α, IL-6 and IL-1β are important in mediating acute inflammatory
reactions. IL-1β can induce fever through enhancing the synthesis
of PGE2. As with IL-1, TNF-α can trigger fever, either
directly by inducing PGE2 synthesis or indirectly by
stimulating the release of IL-1. Subsequently, TNF-α and IL-1
trigger secondary inflammatory effects by inducing the synthesis of
IL-6 (21). Therefore, inhibition
of these pro-inflammatory cytokines is an important treatment
strategy for inflammatory diseases. In the present study, IMT
suppressed the production of TNF-α, IL-6 and IL-1β in Raw264.7
cells and LPS-induced endotoxemic mice in a dose-dependent manner.
These results indicated that IMT in LPS-stimulated macrophages and
LPS-stimulated mice exerted anti-inflammatory effects by inhibiting
the expression and secretion of pro-inflammatory cytokines.
In addition to cytokines, the inflammatory response
induced by inflammatory mediators is generated through the
upregulation of several inducible genes, including iNOS and COX-2.
iNOS is expressed in macrophages under normal and pathological
conditions. In infectious and with pro-inflammatory stimuli, a high
protein expression level of iNOS is induced to produce NO, which is
an important messenger molecule with a critical function in the
host immune defence (22). COX is
also a molecular target for anti-inflammatory treatments, which
have been used for hundreds of years; for example, aspirin, a
selective COX-2 inhibitor and nonsteroidal anti-inflammatory drug,
has been used globally since 1899 (23). It is well known that the COX enzyme
consists of at least two isoforms, COX-1 and COX-2. In humans,
COX-1 protein is expressed at relatively stable levels in several
normal tissues, whereas the protein expression of COX-2 is low in
the majority of normal mammalian tissues in response to stimuli,
including LPS insult. In macrophages, the LPS-induced induction and
activation of signal transduction pathways results in activation of
the gene expression of the COX-2 (22). In the present study, LPS
significantly elevated the protein expression levels of iNOS and
COX-2 in macrophages, and these levels were markedly suppressed by
IMT in a dose-dependent manner. These results further supported the
ability of IMT to inhibit inflammation, and this inhibitory effect
may be associated with suppressing the production of inflammatory
mediators iNOS and COX-2.
It is well known that NF-κB is a major regulator of
pathogen- and inflammatory cytokine-inducible gene regulation
(24). Several stimuli, including
pro-inflammatory cytokines, can activate NF-κB, and activated NF-κB
can induce the protein expression of pro-inflammatory cytokines,
COX-2 and iNOS (25). NF-κB is a
family of inducible dimeric transcription factors, which includes
five members: Rel (c-Rel), RelA (p65), RelB, NF-κB1 (p50/p105) and
NF-κB2 (p52/p100) (26). In
unstimulated cells, the NF-κB family members (homodimers or
heterodimers) are bound to ankyrin rich regions of IκB, an
inhibitor of NF-κB protein, which serves to retain NF-κB dimers in
the cytoplasm and thus inhibiting initiation of target gene
transcription (26). As IκB
dissociates from NF-κB, activated NF-κB is translocated to the
nucleus and exerts its function as a transcription factor. In the
present study, it was found that, following LPS stimulation of RAW
264.7 cells, the protein expression of p65 (N) was higher, compared
with that of the control group, whereas the protein expression
levels of p65 (C) and IκB (C) were significantly downregulated,
which indicated that LPS led to the activation of NF-κB. IMT
significantly increased the protein expression levels of p65 and
IκB in the cytoplasm of LPS-triggered RAW 264.7 cells, and
decreased the protein expression of p65 (N). These data indicated
that IMT inhibited the LPS-induced activity of NF-κB in
macrophages, and then suppressed the production of pro-inflammatory
cytokines and mediators.
In conclusion, IMT exhibited significant
anti-inflammatory effects in vitro and in vivo. IMT
reduced the release of proinflammatory cytokines (TNF-α, IL-6 and
IL-1β), inhibited the expression of inducible enzymes (iNOS and
COX-2), and suppressed the activity of NF-κB via upregulation of
the expression of p65 (C) and IκB (C), and downregulation of the
expression of p65 (N).
References
|
1
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
2
|
Ryan GB and Majno G: Acute inflammation. A
review. Am J Pathol. 86:183–276. 1977.PubMed/NCBI
|
|
3
|
Ward PA: Acute and Chronic
InflammationFundamentals of Inflammation. Cambridge University
Press; Cambridge: pp. 1–16. 2010, View Article : Google Scholar
|
|
4
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology (Williston Park). 16:217–226.
2002.PubMed/NCBI
|
|
5
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baek NI, Ahn EM, Kim HY and Park YD:
Furanocoumarins from the root of Angelica dahurica. Arch Pharm Res.
23:467–470. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang XH and Hu X: Advance in pharmacology
of imperatorin and isoimperatorin pharmacology. J Nanchang Uni Med
Sic. 52:95–97. 2012.
|
|
8
|
García-Argáez AN, Ramírez Apan TO, Delgado
H Parra, Velázquez G and Martínez-Vázquez M: Anti-inflammatory
activity of coumarins from Decatropis bicolor on TPA ear mice
model. Plant Med. 66:279–281. 2000. View Article : Google Scholar
|
|
9
|
Kawaii S, Tomono Y, Ogawa K, Sugiura M,
Yano M, Yoshizawa Y, Ito C and Furukawa H: Antiproliferative effect
of isopentenylated coumarins on several cancer cell lines.
Anticancer Res. 21:1905–1911. 2001.PubMed/NCBI
|
|
10
|
Luszczki JJ, Wojda E, Andres-Mach M,
Cisowski W, Glensk M, Glowniak K and Czuczwar SJ: Anticonvulsant
and acute neurotoxic effects of imperatorin, osthole and valproate
in the maximal electroshock seizure and chimney tests in mice: A
comparative study. Epilepsy Res. 85:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang MY, Ma YY and Li XB: Pharmcological
effect of four linear furocomarins in Radix Angelicae dahuricae.
Nat Prod Res Dev. 22:485–489. 2010.
|
|
12
|
Abad MJ, de las Heras B, Silván AM,
Pascual R, Bermejo P, Rodriguez B and Villar AM: Effects of
furocoumarins from Cachrys trifida on some macrophage functions. J
Pharm Pharmacol. 53:1163–1168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ban HS, Lim SS, Suzuki K, Jung SH, Lee S,
Lee YS, Shin KH and Ohuchi K: Inhibitory effects of furanocoumarins
isolated from the roots of Angelica dahurica on prostaglandin E2
production. Planta Med. 69:408–412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang GJ, Deng JS, Liao JC, Hou WC, Wang
SY, Sung PJ and Kuo YH: Inducible nitric oxide synthase and
cyclooxygenase-2 participate in anti-inflammatory activity of
imperatorin from Glehnia littoralis. J Agric Food Chem.
60:1673–1681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Q, Yang S, Yang S, Xin F and Wang M:
Anti-inflammatory activity of phlomisoside F isolated from Phlomis
younghusbandii Mukerjee. Int Immunopharmacol. 28:724–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Santos F and Rao VS: Antiinflammatory and
antinociceptive effects of 1,8-cineole a terpenoid oxide present in
many plant essential oils. Phytother Res. 14:240–244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winter CA and Porter CC: Effect of
alterations in side chain upon anti-inflammatory and liver glycogen
activities of hydrocortisone esters. J Am Pharm Assoc Am Pharm
Assoc. 46:515–519. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang QS, Yang L, Cui WY, Chen L and Jiang
YH: Anti-inflammatory and anti-nociceptive activities of methanol
extract from aerial part of Phlomis younghusbandii Mukerjee. PLoS
One. 9:e891492014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watkins LR, Maier SF and Goehler LE:
Immune activation: The role of pro-inflammatory cytokines in
inflammation, illness responses and pathological pain states. Pain.
63:289–302. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feghali CA and Wright TM: Cytokines in
acute and chronic inflammation. Front Biosci. 2:d12–d26. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murakami A and Ohigashi H: Targeting NOX,
INOS and COX-2 in inflammatory cells: Chemoprevention using food
phytochemicals. Int J Cancer. 121:2357–2363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vane JR and Botting RM: Mechanism of
Action of Nonsteroidal Anti-inflammatory Drugs. Am J Med.
104:2S–8S; 21S-22S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baeuerle PA and Baichwal VR: NF-kappaB as
a frequent target for immunosuppressive and anti-inflammatory
molecules. Adv Immunol. 65:111–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH,
Kim DS, Park JS and Cho HJ: Spinal NF-kB activation induces COX-2
upregulation and contributes to inflammatory pain hypersensitivity.
Eur J Neurosci. 19:3375–3381. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown KD, Claudio E and Siebenlist U: The
roles of the classical and alternative nuclear factor-kappaB
pathways: Potential implications for autoimmunity and rheumatoid
arthritis. Arthritis Res Ther. 10:2122008. View Article : Google Scholar : PubMed/NCBI
|