Introduction
The majority of patients with cancer respond to
initial chemotherapy (1); however,
many patients subsequently relapse following this initial response.
These patients are commonly characterized by the emergence of
drug-resistant cells and consequent resistance to multiple
anti-cancer agents, which may have various chemical structures and
mechanisms of action (2). This
phenomenon, defined as multidrug resistance (MDR), is a major cause
of chemotherapy failure. There are several potential mechanisms of
resistance; one involves elevated expression of membrane
transporter proteins and, therefore, declined intracellular drug
concentrations and cytotoxicity. Among these transporter proteins,
MDR protein 1 (MDR1), encoded by the MDR1 gene, has been
associated with the resistance phenotype (3).
Various inhibitors of the drug efflux pump,
including calcium channel blockers, anti-arrhythmics,
antidepressants and antipsychotics, have been demonstrated to
overcome drug resistance in vitro (4,5).
However, a number of these were demonstrated to exhibit high
toxicity in animal studies (6).
Others that belong to the class of MDR modulators or
chemosensitizers are less cytotoxic and are able to reverse
MDR1-associated resistance (7).
Podophyllotoxin is an interesting lead in the
development of anticancer antiviral agents. Toxicity issues and
side effects cause its limited use. Etoposide (VP-16) and
teniposide (VM-26), derivatives of podophyllotoxin, have been
successfully used in combination chemotherapy. Cancers like small
cell lung cancer, testicular cancer, acute leukaemia and malignant
lymphoma responded to them well. However, these derivatives have
not overcome limitations, such as narrow anticancer spectrum, low
solvability and development of resistance. In addition, major side
effects including gastroenteric reaction and leukopenia have
restricted their usage. The present study designed and filtered a
series of water soluble derivatives of podophyllotoxin. To the best
of our knowledge, there has been no report on the role of
podophyllotoxin or its analogues in MDR reversal, particularly in
MDR leukemia K562/A02 cells. Therefore, a number of novel
podophyllotoxin derivatives were synthesized and their cytotoxicity
in K563/A02 cells was tested. The present study proposed that the
novel derivative GMZ-1 may be an alternative to VP-16, a clinical
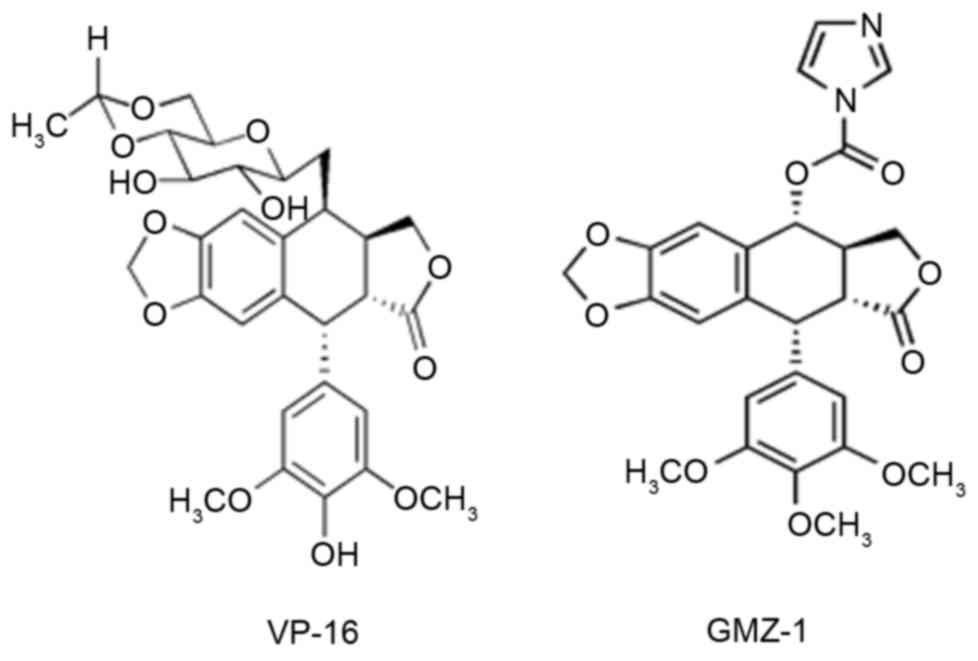
anti-cancer agent (Fig. 1). In
order to investigate this, the anti-proliferative capacity of GMZ-1
was assayed in a number of cancer cell lines; as GMZ-1 exhibited
high toxicity towards K562, an MDR cell line, these cells were
subsequently used to compare the effects of GMZ-1 and VP-16 in
vitro. It was observed that GMZ-1 inhibited proliferation and
induced apoptosis in K562/A02 cells in a time- and
concentration-dependent manner. The present study additionally
investigated the underlying mechanism of the anticancer activity of
GMZ.
Materials and methods
Cell lines and culture
The K562 cell line (courtesy of Professor Hong Chen,
Logistics University of Chinese People's Armed Police Forces,
Tianjin, China) was a clone from human chronic myelogenous
leukemia, previously established by alternate passages in nude mice
and in vitro culture. HeLa, A549, MCF-7, HepG2, SKOV3,
BGC-823, MGC-803 and the fibroblast cell line (3T3) were purchased
from Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). K562 was cultured in RPMI-1640 (catalog no.
31800-022) supplemented with 10% fetal bovine serum (catalog no.
10099141) (both from Gibco, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) at 37°C in a humidified 5% CO2
atmosphere. The MDR leukemia cell line K562/A02 (courtesy of
Professor Hong Chen also) was generated previously by incremental
adriamycin (ADM) treatments refer to Yangs et al paper
published in 1995 (1). K562/A02
was maintained in RPMI-1640 medium supplemented with 1 µg/ml ADM to
maintain its MDR phenotype.
Cell viability measurement
In order to evaluate the anti-proliferative activity
of GMZ-1 [molecular weight 508.15, white powder, insoluble in
water, purity >98%, supplied by Professor Hong Chen (Tianjin Key
Laboratory of Cardiovascular Remodeling and Target Organ Injury,
Tianjin, China). GMZ-1 was synthesized with imidazole-2-carboxy to
generate podophyllolox imidazole-2-carboxylate] in cancer cell
lines, cell viability was measured by determining mitochondrial
dehydrogenase activity using an MTT assay. Cells (5×103
cells/well) were plated in 96-well plates and cultured overnight.
Triplicate wells were treated with concentrations of GMZ-1 (10, 1,
0.1 and 0.01 µmol/l) and VP-16 (10, 1, 0.1 and 0.01 µmol/l) (cat
no. H32025583; prepared with normal saline; Jiangsu Hengrui
Medicine Co., Ltd., Lianyungang, China) or normal saline (control
vehicle) for 48 h. To perform the MTT assay, 20 µl MTT solution (5
mg/ml in PBS; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
added to each well, followed by incubation for 4 h at 37°C. A total
of 150 µl/well dimethyl sulfoxide was added at room temperature for
10 min to dissolve the formazan precipitate. Absorbance was
measured at a wavelength of 570 nm (Thermo Fisher Scientific,
Inc.).
Flow cytometry analysis
K562/A02 cells (5×103 cells/well) were
seeded in 6-well plates and cultured overnight. Triplicate wells
were treated with 0.05, 0.10 or 0.20 µM GMZ-1 and 10 µM VP-16 or
normal saline (vehicle control) for 12, 24 and 36 h. Cells were
collected and fixed in 70% ethyl alcohol at 4°C overnight, followed
by washing in PBS and incubation with 10 µg/ml RNA se at 37°C for
30 min. Cells were subsequently incubated with 10 µg/ml propidium
iodide (PI) for 30 min in the dark on ice. The stained samples were
analysed using a FACSCalibur (BD Biosciences, Franklin Lakes, NJ,
USA). Data was analysed using FlowJo version 7.6.3 software (FlowJo
LLC, Ashland, OR, USA).
Giemsa staining
K562/A02 cells were treated with varying
concentrations of GMZ-1 or normal saline (control vehicle) for 48
h, lifted from the plate and mounted on slides. Following rinsing
with water, the slides were stained with Giemsa solution (BDH;
Merck KGaA) for 5 min at room temperature. The slides were rinsed
with water three times and the cells were observed under an
inverted microscope (TMS; Nikon Corporation, Tokyo, Japan) at ×400
magnification.
Examination of MDR1 gene expression by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RNA was isolated from K562 or K562/A02 cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
RT-qPCR was performed using a SuperScript One-Step RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc.) in a 50 µl reaction
mix. The reaction mix contained 25 µl 2X RT-PCR buffer, 3 µl
template RNA, 0.6 µl forward and reverse primers each (β-actin 540
bp, sense, 5′-GTGGGGCGCCCCAGGCACCA-3′ and antisense,
5′-CTTCCTTAATGTCACGCACGATTTC-3′; MDR-1, 150 bp, sense,
5′-GTGGGGCGCCCCAGGCACCA-3′ and antisense,
5′-CTTCCTTAATGTCACGCACGATTTC-3′), 1 µl AMV/Taq mixture and 19.8 µl
deionised water. The thermocycling reaction protocol was as
follows: Reverse transcription for 35 min at 37°C; pre-denaturation
at 94°C for 3 min; 30 cycles of qPCR (1 min denaturation at 94°C,
30 sec annealing at 57°C and 1 min extension at 72°C); and 10 min
final extension at 72°C. PCR products were run on 1.5% agarose gels
with 0.01% Gel Red (cat no. G5560; Beijing Solarbio Science and
Technology Co., Ltd., Beijing, China), with β-actin (540 bp) as an
internal standard. Band intensity was quantified using Gel-Pro
Analyser 3.1 (Media Cybernetics, Inc., Rockville, MD, USA).
Examination of MDR1 protein expression
by western blot analysis
Cells were lysed using rdioimmunoprecipitation acid
lysis buffer (cat no. P0013B; Beyotime Institute of Biotechnology,
Haimen, China) and the extracted protein was quantified with a
bicinchoninic protein assay kit (cat no. P0010; Beyotime Institute
of Biotechnology). A total of 30 µg/well cell extracts were
separated by Bolt™ 12% Bis-Tris Plus 10-well gels (cat no.
NW00120BOX; Thermo Fisher Scientific, Inc.), and transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were blocked with 5% non-fat milk in PBS
containing 0.1% Tween-20 for 1 h at room temperature and incubated
overnight at 4°C with MDR1/ABCB1 (E1Y7B) rabbit monoclonal antibody
(cat no. 13342; Cell Signaling Technology, Inc., Danvers, MA, USA)
at 1:1,000 dilution or GAPDH (D16H11) XP® rabbit
monoclonal antibody (cat no. 5174; Cell Signaling Technology, Inc.)
at 1:1,000 dilution. Following washing, the membranes were
incubated with a horse radish peroxidase-conjugated secondary
antibody, anti-rabbit IgG, horse radish peroxidase-conjugated
antibody (cat no. 7074; Cell Signaling Technology, Inc.) at 1:3,000
dilution at room temperature for 1 h and visualized using
SuperSignal™ West Pico Plus Chemiluminescent substrate (cat no.
34580; Thermo Fisher Scientific, Inc.).
Statistics
All data are presented as the mean ± standard error.
Differences between groups were analysed using a one-way analysis
of variance followed by Student-Newman-Keuls and Least Significant
Difference post hoc tests using SPSS version 20 software (IBM
Corp., Armonk, NY, USA). P≤0.05 was considered to indicate a
statistically significant difference.
Results
GMZ-1 reduces cancer cell
viability
GMZ-1 demonstrated a marked effect on the viability
of several cancer cell lines, and the half-maximal inhibitory
concentration (IC50) values following treatment for 48 h
are presented in Table I. GMZ-1
displayed the highest efficacy in K562 and K562/A02 cells, with
IC50 values of 0.08±0.02 and 0.12±0.03 µM 48 h following
treatment, respectively (Table
II). Therefore, the K562/A02 cell line was selected as a model
to examine the impact of GMZ-1 on cell viability.
 | Table I.Cytotoxic activity of GMZ-1 on human
cancer cells and fibroblasts. |
Table I.
Cytotoxic activity of GMZ-1 on human
cancer cells and fibroblasts.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Cell line | GMZ-1 | VP-16 |
|---|
| HeLa |
0.07±0.01 |
1.33±0.86 |
| A549 |
0.18±0.07 |
1.06±0.73 |
| MCF-7 |
0.14±0.05 |
2.36±0.53 |
| HepG-2 |
0.093±0.012 |
2.03±0.55 |
| SKOV3 |
0.12±0.04 |
3.43±0.87 |
| BGC-823 |
0.083±0.009 |
2.06±0.59 |
| MGC-803 |
0.089±0.011 |
3.61±0.85 |
| 3T3 |
0.34±0.07 |
17.36±2.29 |
 | Table II.Reversion of drug resistance in
K562/A02 cells. |
Table II.
Reversion of drug resistance in
K562/A02 cells.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Drug | K562 | K562/A02 | Fold change |
|---|
| Adriamycin |
0.26±0.10 |
28.62±4.27 | 110.08 |
| VP-16 |
2.02±0.83 |
22.81±4.23 | 11.29 |
| GMZ-1 |
0.08±0.02 |
0.12±0.03 | 1.52 |
GMZ-1 induces apoptosis in K562/A02
cells
A number of anti-cancer drugs impact upon
apoptosis-associated signaling pathways to induce apoptosis in
cancer cells. In order to examine whether the reduced viability of
K562/A02 cells was due to the induction of apoptosis, flow
cytometry analysis of PI-stained cells was performed. K562/A02
cells were treated with 0.05, 0.10 or 0.20 µM GMZ-1 for 12, 24 or
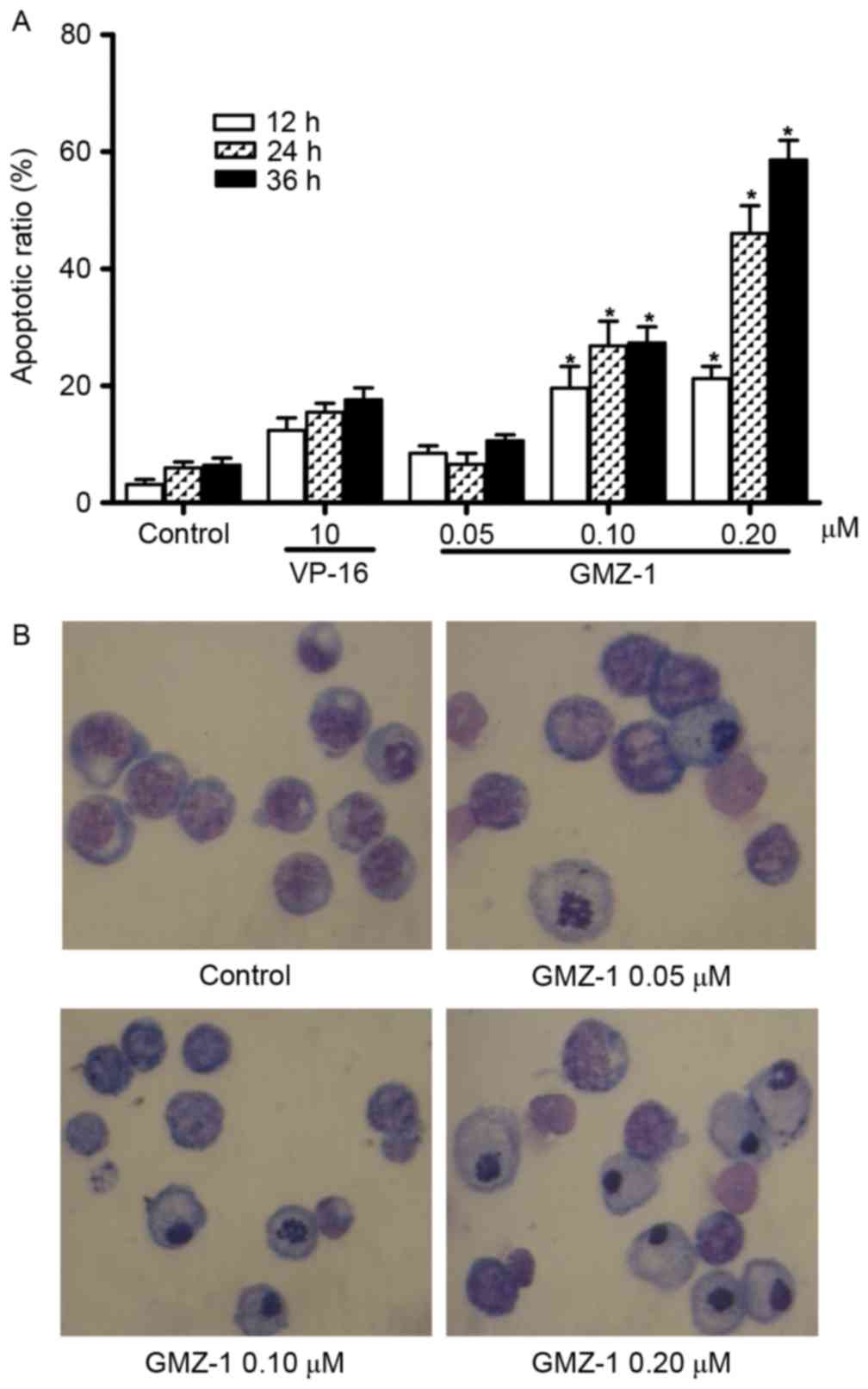
36 h. The flow cytometry results indicated that GMZ-1 may induce
apoptosis in K562/A02 cells in a time- and concentration-dependent
manner (Fig. 2A). Quantification
revealed a significant difference in the apoptotic rate between
control cells and cells treated with GMZ-1 (P<0.05; Fig. 2A).
GMZ-1 treated K562/A02 cells were stained with
Giemsa solution to observe whether GMZ-1 induced the characteristic
morphology of apoptosis. The observed morphology in GMZ-1-treated
K562/A02 cells included nuclear condensation, cytoplasmic shrinkage
and the formation of apoptotic bodies (Fig. 2B), which were absent in the control
cells. Consistent with the MTT assay, these results indicated that
GMZ-1 may reduce the viability of K562/A02 cells via the induction
of apoptosis.
Expression analysis of the MDR1 gene
and MDR1 protein in K562 and K562/A02 cells
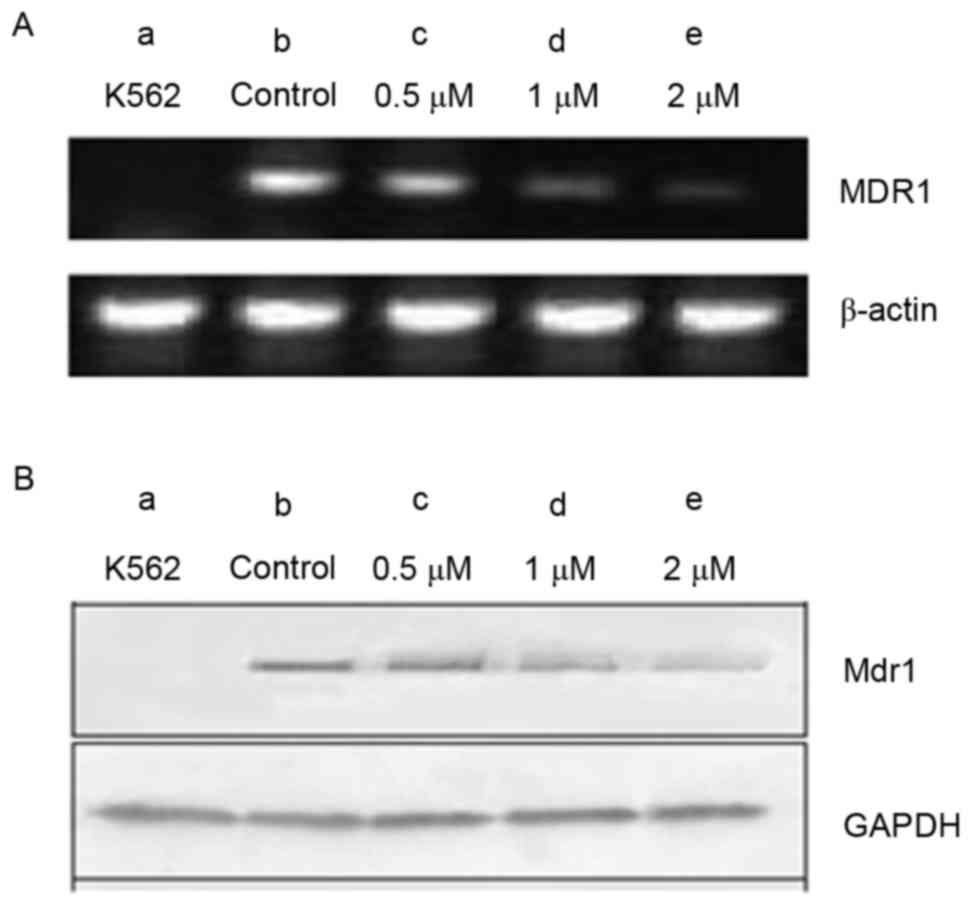
The MDR1 gene, encoding MDR1, is associated
with MDR. The present study examined MDR1 expression at the mRNA
and protein levels in K562 and K562/A02 cells, using RT-qPCR and
western blot analyses. Cells were treated with various
concentrations of GMZ-1 (0.05, 0.1 and 0.2 µM) for 24 h. A marked
increase in MDR1 gene and MDR1 protein expression was
observed in K562/A02 cells, compared with K562 cells (Fig. 3). Notably, MDR1 and MDR1
expression decreased with an increasing concentration of GMZ-1,
which suggested that K562 was an MDR1-negative cell line and
K562/A02 was an MDR1-overexpressing cell line.
Discussion
Although novel chemotherapeutic drugs are being
developed, chemotherapy remains a challenge in cancer treatment.
This is partially due to the development of MDR. Among the numerous
mechanisms underlying MDR, elevated expression of the
MDR1-encoded MDR1 protein in cancer cells has been
considered to be a frequent factor (8,9).
MDR1 serves to remove the drug from the cells, thereby assisting in
drug resistance. The weak potency and toxicity of developed MDR
modulators have limited their clinical use. The few non-toxic
compounds that downregulate the expression of MDR1 include curcumin
(10), tryptanthrin (11), estrogen (12) and perospirone (13).
VP-16, an aryltetralinelignan, is a clinical
antitumor drug used to treat testicular cancer and small cell lung
cancer (14,15). VP-16 elicits a few adverse effects,
including myelosuppression and the initiation of secondary
leukaemia (14,16–18).
In order to reduce damage to bone marrow cells, VP-16 has been
combined with other compounds in animal studies, such as quercetin,
dexrazoxane and wongonin (19–21).
The present study demonstrated that the novel
podophyllotoxin derivative GMZ-1 exhibited increased efficacy
compared with a traditional podophyllotoxin derivative (22). GMZ-1 has a similar IC50
value between MDR1-negative cell line K562 and overexpressing cell
line K562/A02; however, it exhibits decreased cytotoxicity in human
fibroblasts at therapeutic doses. Apoptosis is characterized by
specific morphological changes including plasma membrane blebbing,
chromatin condensation and fragmentation, and the emergence of
apoptotic bodies. The results of the present study suggested that
GMZ-1 may induce apoptosis in K562/A02 in vitro and
significantly decrease MDR1 expression from 24 h. To the best of
our knowledge, this is the first report demonstrating the
suppressive effect of GMZ-1 on MDR1 expression in K562/A02
cells.
In conclusion, GMZ-1, as a novel derivative of
podophyllotoxin, may have utility as an MDR modulator in
adriamycin-resistant K562/A02 cells. It may serve as an alternative
to the current treatment for treating patients with
MDR1-overexpressing tumors. Further work is required to validate
this drug, and to investigate whether GMZ-1 inhibits the functions
of other ATP binding cassette transporters.
Acknowledgements
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 30901985
and 81600051), the Tianjin Application Foundation and Advanced
Technology Research Project (grant no. 15JCQNJC13500), and the Open
Fund of Logistics University Central Laboratory of Chinese People's
Armed Police Forces (grant no. 2015ZXKF07).
References
|
1
|
Yang CZ, Luan FJ, Xiong DS, Liu BR, Xu YF
and Gu KS: Multidrug resistance in leukemic cell line K562/A02
induced by doxorubicin. Zhongguo Yao Li Xue Bao. 16:333–337.
1995.PubMed/NCBI
|
|
2
|
Hackl H, Astanina K and Wieser R:
Molecular and genetic alterations associated with therapy
resistance and relapse of acute myeloid leukemia. J Hematol Oncol.
10:512017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalton WS: Mechanisms of drug resistance
in hematologic malignancies. Semin Hematol. 34 4 Suppl 5:S3–S8.
1997.
|
|
4
|
Young AM, Allen CE and Audus KL: Efflux
transporters of the human placenta. Adv Drug Deliv Rev. 55:125–132.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SY, Rhee YH, Jeong SJ, Lee HJ, Lee HJ,
Jung MH and Kim SH, Lee EO, Ahn KS, Ahn KS and Kim SH:
Hydrocinchonine, cinchonine, and quinidine potentiate
paclitaxel-induced cytotoxicity and apoptosis via multidrug
resistance reversal in MES-SA/DX5 uterine sarcoma cells. Environ
Toxicol. 26:424–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dönmez Y, Akhmetova L, Iseri ÖD, Kars MD
and Gündüz U: Effect of MDR modulators verapamil and promethazine
on gene expression levels of MDR1 and MRP1 in doxorubicin-resistant
MCF-7 cells. Cancer Chemother Pharmacol. 67:823–828. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wibowo M, Levrier C, Sadowski MC, Nelson
CC, Wang Q, Holst J, Healy PC, Hofmann A and Davis RA: Bioactive
dihydro-β-agarofuran sesquiterpenoids from the Australian
rainforest plant Maytenus bilocularis. J Nat Prod.
79:1445–1453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pajeva I and Wiese M: Molecular modeling
of phenothiazines and related drugs as multidrug resistance
modifiers: A comparative molecular field analysis study. J Med
Chem. 41:1815–1826. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schondörf T, Kurbacher CM, Göhring UJ,
Benz C, Becker M, Sartorius J, Kolhagen H, Mallman P and Neumann R:
Induction of MDR1-gene expression by antineoplastic agents in
ovarian cancer cell lines. Anticancer Res. 22:2199–2203.
2002.PubMed/NCBI
|
|
10
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anuchapreeda S, Leechanachai P, Smith MM,
Ambudkar SV and Limtrakul PN: Modulation of P-glycoprotein
expression and function by curcumin in multidrug-resistant human KB
cells. Biochem Pharmacol. 64:573–582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu ST, Chen TM, Tseng SY and Chen YH:
Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in
breast cancer cells. Biochem Biophys Res Commun. 358:79–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mutoh K, Tsukahara S, Mitsuhashi J,
Katayama K and Sugimoto Y: Estrogen-mediated post transcriptional
down-regulation of P-glycoprotein in MDR1-transduced human breast
cancer cells. Cancer Sci. 97:1198–1204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou YG, Li KY and Li HD: Effect of the
novel antipsychotic drug perospirone on P-glycoprotein function and
expression in Caco-2 cells. Eur J Clin Pharmacol. 64:697–703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baldwin EL and Osheroff N: Etoposide,
topoisomerase II and cancer. Curr Med Chem Anticancer Agents.
5:363–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hande KR: Etoposide: Four decades of
development of a topoisomerase II inhibitor. Eur J Cancer.
34:1514–1521. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi K and Ratain MJ:
Pharmacodynamics and long-term toxicity of etoposide. Cancer
Chemother Pharmacol. 34 Suppl:S64–S68. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith MA, Rubinstein L, Anderson JR,
Arthur D, Catalano PJ, Freidlin B, Heyn R, Khayat A, Krailo M, Land
VJ, et al: Secondary leukemia or myelodysplastic syndrome after
treatment with epipodophyllotoxins. J Clin Oncol. 17:569–577. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choudhury RC, Palo AK and Sahu P:
Cytogenetic risk assessment of etoposide from mouse bone marrow. J
Appl Toxicol. 24:115–122. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Attia SM, Al-Anteet AA, Al-Rasheed NM,
Alhaider AA and Al-Harbi MM: Protection of mouse bone marrow from
etoposide-induced genomic damage by dexrazoxane. Cancer Chemother
Pharmacol. 64:837–845. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapiszewska M, Cierniak A, Papiez MA,
Pietrzycka A, Stepniewski M and Lomnicki A: Prolonged quercetin
administration diminishes the etoposide-induced DNA damage in bone
marrow cells of rats. Drug Chem Toxicol. 30:67–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enomoto R, Koshiba C, Suzuki C and Lee E:
Wogonin potentiates the antitumor action of etoposide and
ameliorates its adverse effects. Cancer Chemother Pharmacol.
67:1063–1072. 2011. View Article : Google Scholar : PubMed/NCBI
|