Introduction
Age-related macular degeneration (AMD) is a leading
cause of visual impairment in the elderly in developed countries
(1). Epidemiological studies have
demonstrated that the prevalence of AMD in the aged population is
increasing steadily every year (2). The retinal pigment epithelium (RPE),
which forms a blood-retinal barrier between the neural retina and
choriocapillaris, serves a pathophysiological role in the process
of AMD (3). Oxidative stress is
crucial in the development of AMD (4,5).
Previous studies have reported that RPE cells are susceptible to
oxidative damage, and oxidative stress from hydrogen peroxide
(H2O2) leads to RPE cell death by causing
preferential damage to mitochondrial DNA (6,7).
Thus, strategies for protecting RPE cells against oxidative damage
may be helpful for preventing the progression of AMD.
Isorhamnetin is one of the major active components
isolated from herbal medicinal plants, such as Hippophae
rhamnoides and Ginkgo biloba (8). Previous studies have indicated that
isorhamnetin exhibits anti-inflammatory and antitumor effects, and
that it protects ventricular myocytes from ischemia and reperfusion
injury (9–11). In addition, isorhamnetin has been
reported to exhibit antioxidant properties (12–14).
Choi (15) reported that
isorhamnetin reverses H2O2-induced growth
inhibition and exhibits scavenging activity against intracellular
reactive oxygen species (ROS) in mouse-derived C2C12 myoblasts
(15). However, the effects of
isorhamnetin on RPE cells and the underlying molecular mechanism
remain unclear. Therefore, the aim of the present study was to
examine the effect of isorhamnetin against oxidative stress in
human RPE cells.
Materials and methods
Reagents and antibodies
Isorhamnetin (purity >98 %) was purchased from
Shanghai Tongtian Biotechnology Co., Ltd. (Shanghai, China).
Antibodies against phosphoinositide 3-kinase (PI3K; 1:2,000; cat
no. sc-365290), phosphorylated (p)-PI3K (1:2,000; cat no.
sc-293115), AKT serine/threonine kinase 1 (Akt; 1:2,000; cat no.
sc-5298), p-Akt (1:2,000; cat no. sc-52940), GAPDH (1:1,000; cat
no. sc-365062) and horseradish peroxidase-conjugated secondary
antibodies (1;3,000; cat no. sc-2370) were from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). All other chemicals and
reagents were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany).
Cell culture
The human RPE cell line ARPE-19 was purchased from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in DMEM/Nutrient Mixture F-12 (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (Sigma-Aldrich; Merck KGaA) and 1%
penicillin/streptomycin, in a 37°C incubator under a humidified
atmosphere containing 5% CO2.
Cell viability assay
Cell viability was evaluated using an MTT assay.
Briefly, ARPE-19 cells (4×104 cells/well) were
pretreated with various concentrations of isorhamnetin (25, 50 and
100 µM) for 24 h prior to exposure to 250 µM
H2O2 for 24 h. The medium was then replaced
with fresh medium containing 0.5 mg/ml MTT for 4 h at 37°C. The MTT
formazan crystals were dissolved in dimethyl sulfoxide, and the
absorbance was measured at 450 nm with a microplate reader (Omega
Bio-Tek, Inc., Norcross, GA, USA). The relative cell viability was
defined as the absorbance of treated cells divided by that of the
control untreated cells.
Measurement of intracellular ROS
generation
Intracellular ROS production was measured using the
2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA)
probe (Sigma-Aldrich; Merck KGaA). Briefly, following treatment,
ARPE-19 cells were washed twice with PBS, and then incubated with
10 mM H2DCFDA for 30 min at 37°C in the dark. Then, the
cells were harvested and fluorescence of the resulting
dichlorofluorescein (DCF) was measured using an F-2500
spectrophotometer (Hitachi, Ltd., Tokyo, Japan) at 488 nm
excitation and 525 nm emission. The results were expressed as the
fluorescence intensity of DCF of each sample relative to the
control.
Caspase-3 activity assay
Caspase-3 activity was detected using a caspase-3
cellular activity assay kit (Merck KGaA), as per the manufacturer's
instructions. Briefly, following treatment, ARPE-19 cells were
harvested and then suspended in the cell lysis buffer. Cell lysates
were incubated in the presence or absence of 5 µl
Asp-Glu-Val-Asp-p-nitroanilide at 37°C for 1 h. Following washing,
the fluorescence released by active caspase-3 was measured using a
microplate reader at 405 nm wavelength.
Western blot analysis
ARPE-19 cells were harvested, washed twice with PBS,
and lysed in radioimmunoprecipitation assay buffer containing 1 mM
phenylmethylsulfonyl fluoride (both from Shenneng Bocai
Biotechnology Co., Ltd., Shanghai, China) on ice for 10 min. Cell
lysates were centrifuged at 13,000 × g for 15 min at 4°C. The
supernatants were collected, and the protein content of each lysate
was measured by BCA protein assay (Pierce; Thermo Fisher
Scientific, Inc.). Protein (30 µg/lane) was separated by
electrophoresis on a 10% SDS-polyacrylamide gel and transferred
electrophoretically to a nitrocellulose membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membranes were blocked
in 5% non-fat dry milk and TBST buffer (5 mM Tris-HCl, pH 7.4, 136
mM NaCl and 0.1% Tween-20) for 1 h at room temperature, and
incubated with specific antibodies (p-PI3K, PI3K, p-Akt, Akt, and
GAPDH) overnight at 4°C. Then, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.) for another 1 h at room temperature. The blots
were visualized with an enhanced chemiluminescence system (GE
Healthcare Life Sciences, Chalfont, UK) and grey intensity analysis
was performed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA).
Statistical analysis
The SPSS software package (version 17.0; SPSS Inc.,
Chicago, IL, USA) was used for statistical analysis. Data are
expressed as mean ± standard deviation of three independent
experiments performed in triplicate. Significant differences were
determined using a Student's t-test or one-way analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isorhamnetin is not cytotoxic in
ARPE-19 cells
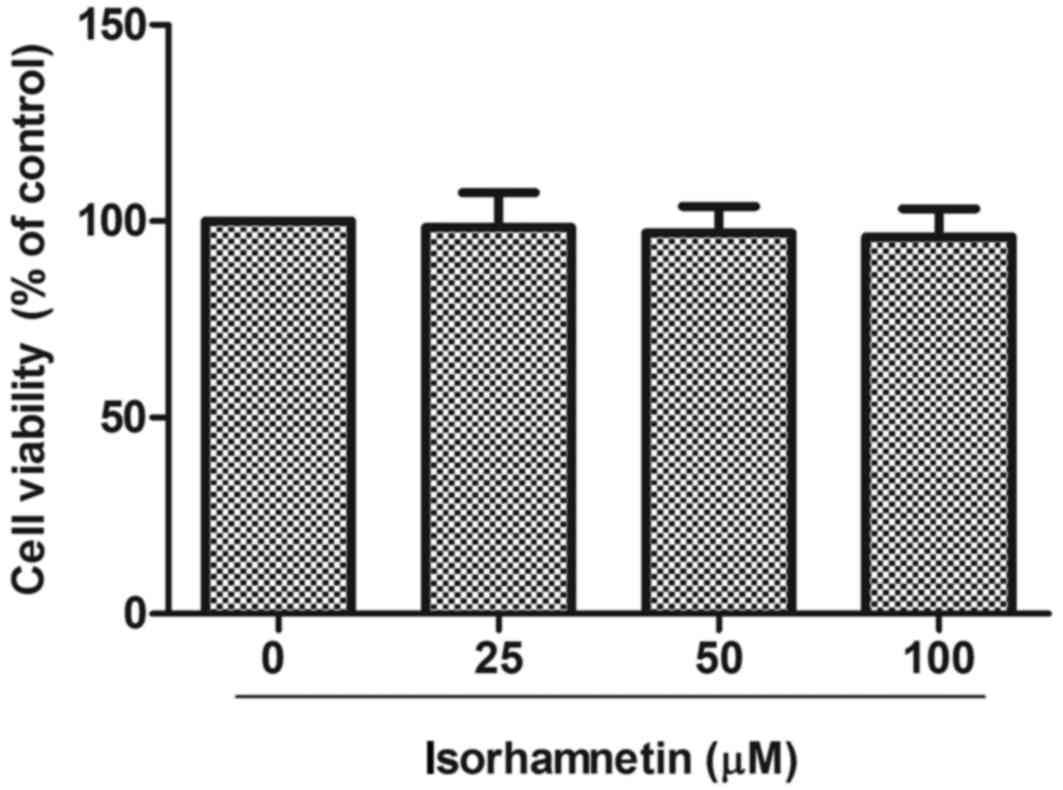
First, the potential cytotoxicity of isorhamnetin
was examined on human ARPE-19 cells using the MTT assay. As
demonstrated in Fig. 1,
isorhamnetin did not significantly affect the viability of ARPE-19
cells in doses up to 100 µM. Therefore, 25–100 µM of isorhamnetin
was used for treatments in the following experiments.
Isorhamnetin treatment protects human
RPE cells against oxidative stress
The effect of isorhamnetin on the cell viability of
H2O2-treated ARPE-19 cells was examined by
MTT assay. The results demonstrated that H2O2
treatment resulted in a significant decrease in cell viability
compared with untreated cells, whereas pretreatment with
isorhamnetin significantly reversed this decrease in a
dose-dependent manner (Fig.
2).
Isorhamnetin treatment inhibits
H2O2-induced ROS production in human RPE
cells
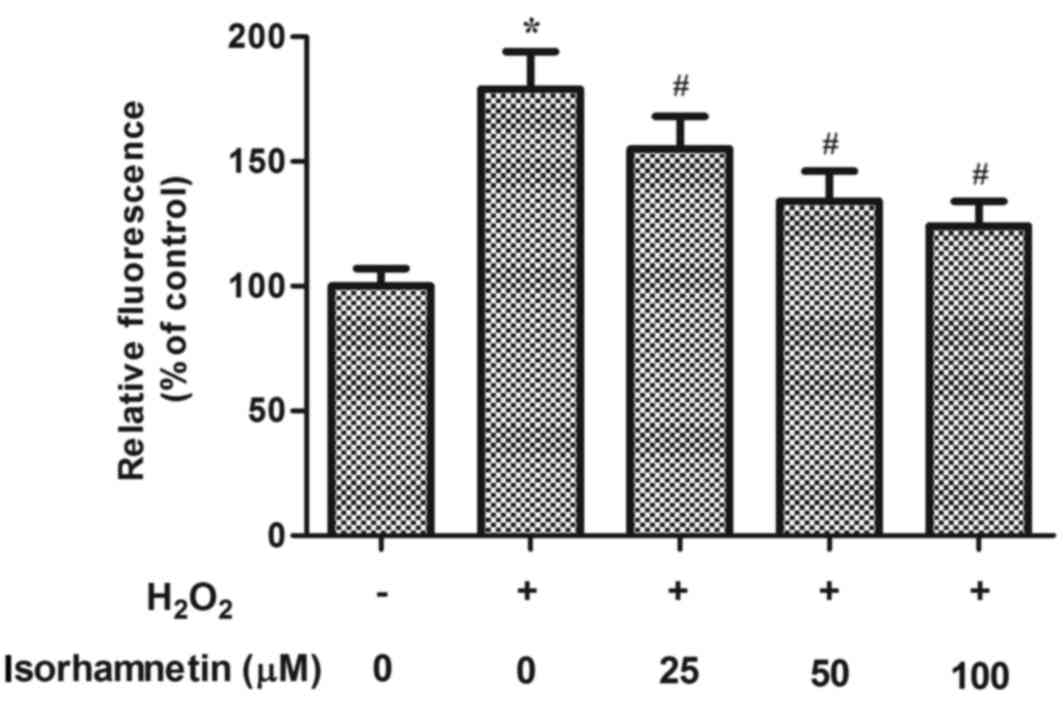
Increased ROS levels result in oxidative stress and
are hypothesized to be critical in the pathogenesis of AMD.
Therefore, the effect of isorhamnetin on ROS generation was
examined in ARPE-19 cells exposed to H2O2. As
indicated in Fig. 3, treatment
with H2O2 for 24 h dramatically increased the
production of ROS compared with control untreated cells. However,
pretreatment with isorhamnetin significantly suppressed the
H2O2-induced ROS production in a
dose-dependent manner (Fig.
3).
Isorhamnetin treatment inhibits
H2O2-induced caspase-3 activity in human RPE
cells
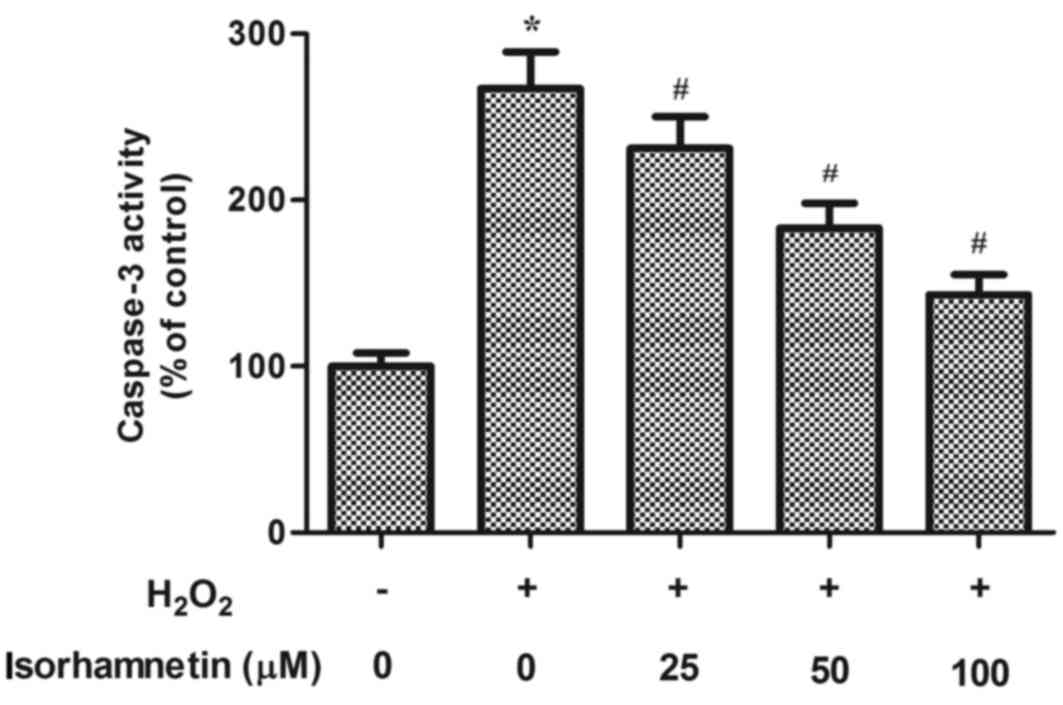
Caspase-3 is a main executor and an established
marker of cell apoptosis, so caspase-3 activity was measured as a
surrogate for cell death initiation (16). As demonstrated in Fig. 4, H2O2
treatment significantly increased caspase-3 activity, compared with
untreated control. This H2O2-induced increase
in caspase-3 activity, however, was dramatically attenuated by
isorhamnetin pretreatment in a dose-dependent manner (Fig. 4).
Isorhamnetin treatment activates the
PI3K/Akt pathway in human RPE cells
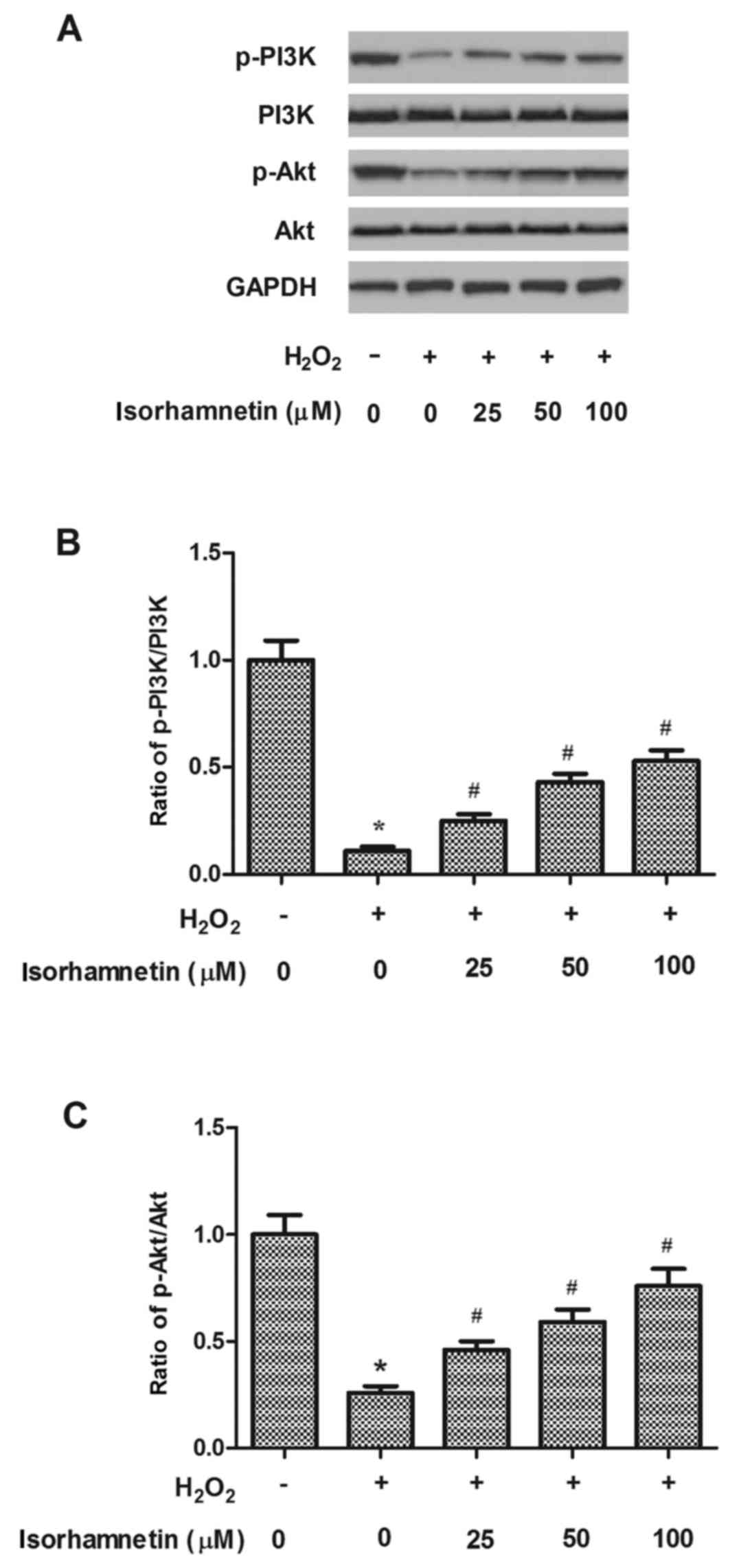
Activation of the PI3K/Akt signaling pathway is
important in protecting RPE cells from oxidative stress (17). Therefore, the hypothesis that the
PI3K/Akt pathway may be involved in the protective effects of
isorhamnetin on H2O2-induced cell death was
tested in ARPE-19 cells. As demonstrated in Fig. 5, phosphorylation of PI3K and Akt
were significantly decreased by H2O2
treatment, compared with the control group, as evident by the
decreased p-PI3K/PI3K and p-Akt/Akt ratios. However, pretreatment
with isorhamnetin for 24 h markedly increased phosphorylation of
both PI3K and Akt in ARPE-19 cells exposed to
H2O2, compared with cells treated with
H2O2 alone (Fig.
5).
Discussion
The present study focused on the effect of
isorhamnetin on oxidative stress in human RPE cells. The results
provide the first evidence that pretreatment of RPE cells with
isorhamnetin significantly protected cell viability against
oxidative stress. Furthermore, isorhamnetin inhibited
H2O2-induced ROS production and caspase-3
activation in ARPE-19 cells. This protective effect of isorhamnetin
may be mediated through PI3K/Akt signaling in ARPE-19 cells.
H2O2 is a well-established
model of oxidative stress in RPE cells because of its rapid
membrane permeability (18). A
vast array of studies have demonstrated that oxidative stress
induced by chemical oxidants, such as H2O2,
leads to RPE damage and cell death (5,19,20).
For these reasons, H2O2 was selected in the
present study as an inducer of oxidative stress in human RPE cells
to investigate the effect of isorhamnetin. The results demonstrated
that H2O2 treatment resulted in a significant
decrease in viability of ARPE-19 cells. However, pretreatment with
isorhamnetin significantly reversed this decrease in a
dose-dependent manner, implying that isorhamnetin exhibits a
protective effect against oxidative stress in RPE cells.
Oxidative damage is important in the pathogenesis of
AMD. ROS levels are increased in retinas of patients with AMD,
resulting in oxidization of key cellular components in local RPE
cells and severe RPE damage (21).
It has been reported that isorhamnetin pretreatment blocks
tertiary-butyl hydroperoxide-induced ROS production in hepatocytes
(12). Sun et al (22) reported that isorhamnetin
significantly decreases ROS generation in H9c2 cardiomyocytes
exposed to H2O2. In accordance with these
previous reports, the present results demonstrated that
H2O2 treatment significantly increased
intracellular ROS production in human RPE cells compared with
untreated cells, and that this effect was significantly inhibited
by isorhamnetin pretreatment. These results suggest that
isorhamnetin protected ARPE-19 cells from
H2O2-induced cell damage through its
antioxidant activity.
Caspase-3 activation is an initiative process of
cell apoptosis (23). It has been
reported that exposure of RPE cells to increased ROS results in
mitochondrial DNA damage and induces caspase-3 activation (24). In the present study, pretreatment
with isorhamnetin was demonstrated to significantly suppress
H2O2-induced caspase-3 activation, compared
with cells treated with H2O2 alone. These
data suggest that isorhamnetin protected ARPE-19 cells from
H2O2-induced cell damage via its
anti-apoptotic effect.
There is substantial evidence that the PI3K/Akt
signaling pathway is involved in regulating the antioxidant
function in RPE cells (25–27).
Akt becomes phosphorylated in a PI3K-dependent manner in response
to oxidants, such as H2O2, in human RPE
cells, and treatment with a PI3K inhibitor (LY294002) blocks
H2O2-mediated Akt phosphorylation and
significantly enhances caspase-associated RPE cell death (28); this result is different to that
observed in the present study which, may be due to the length of
H2O2 exposure. In the study by Yang et
al (28), when RPE cells were
exposed to H2O2 for 15 min, p-Akt levels
increased. However, in the present study, cells were exposed to
H2O2 for 24 h, and the phosphorylation of
PI3K and Akt significantly decreased. A recent study reported that
isorhamnetin attenuates atherosclerosis by suppressing apoptosis in
THP-1-derived macrophages via PI3K/Akt activation (29). In the present study, it was
demonstrated that pretreatment with isorhamnetin significantly
enhanced phosphorylation of PI3K and Akt in RPE cells exposed to
H2O2, compared to cells exposed to
H2O2 alone. These data suggest that
isorhamnetin may have protected human RPE cells from oxidative
stress-induced cell death, through activation of the PI3K/Akt
signaling pathway.
In summary, the present report demonstrated that
isorhamnetin protected human RPE cells from oxidative
stress-induced cell death, which may be associated with the
activation of PI3K/Akt signaling. Thus, isorhamnetin may be
considered as a potential antioxidant agent towards the prevention
of AMD.
References
|
1
|
Congdon NG, Friedman DS and Lietman T:
Important causes of visual impairment in the world today. JAMA.
290:2057–2060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fletcher EC and Scholl HP: Ophthalmic
disease in the ageing society. Ophthalmology and the Ageing
Society. 1–9. 2013. View Article : Google Scholar
|
|
3
|
Curcio CA, Zanzottera EC, Messinger JD,
Ach T, Smith T and Freund KB: Retinal pigment epithelium (RPE)
transdifferentiation and death in age-related macular degeneration
(AMD), seen in the Project MACULA grading system. Invest Ophth Vis
Sci. 56:893. 2015.
|
|
4
|
Beatty S, Koh HH, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarrett SG and Boulton ME: Consequences of
oxidative stress in age-related macular degeneration. Mol Aspects
Med. 33:399–417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang FQ and Godley BF: Oxidative
stress-induced mitochondrial DNA damage in human retinal pigment
epithelial cells: A possible mechanism for RPE aging and
age-related macular degeneration. Exp Eye Res. 76:397–403. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drobek-Słowik M, Karczewicz D and Safranow
K: The potential role of oxidative stress in the pathogenesis of
the age-related macular degeneration (AMD). Postepy Hig Med Dosw
(Online). 61:28–37. 2007.(In Polish). PubMed/NCBI
|
|
8
|
Park JC, Young HS, Yu YB and Lee JH:
Isorhamnetin sulphate from the leaves and stems of oenanthe
javanica in korea. Planta Med. 61:377–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen TL, Zhu GL, Wang JA, Zhang GD, Liu
HF, Chen JR, Wang Y and He XL: Protective effects of isorhamnetin
on apoptosis and inflammation in TNF-α-induced HUVECs injury. Int J
Clin Exp Pathol. 8:2311–2320. 2015.PubMed/NCBI
|
|
10
|
Saud SM, Young MR, Jones-Hall YL, Ileva L,
Evbuomwan MO, Wise J, Colburn NH, Kim YS and Bobe G:
Chemopreventive activity of plant flavonoid isorhamnetin in
colorectal cancer is mediated by oncogenic Src and β-catenin.
Cancer Res. 73:5473–5484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang N, Pei F, Wei H, Zhang T and Yang C,
Ma G and Yang C: Isorhamnetin protects rat ventricular myocytes
from ischemia and reperfusion injury. Exp Toxicol Pathol. 63:33–38.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JH, Shin BY, Han JY, Kim MG, Wi JE,
Kim YW, Cho IJ, Kim SC, Shin SM and Ki SH: Isorhamnetin protects
against oxidative stress by activating Nrf2 and inducing the
expression of its target genes. Toxicol Applied Pharmacol.
274:293–301. 2014. View Article : Google Scholar
|
|
13
|
Kong CS, Kim JA, Qian ZJ, Kim YA, Lee JI,
Kim SK, Nam TJ and Seo Y: Protective effect of isorhamnetin
3-О-β-d-glucopyranoside from Salicornia herbacea against
oxidation-induced cell damage. Food Chem Toxicol. 47:1914–1920.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bouhlel I, Skandrani I, Nefatti A, Valenti
K, Ghedira K, Mariotte AM, Hininger-Favier I, Laporte F,
Dijoux-Franca MG and Chekir-Ghedira L: Antigenotoxic and
antioxidant activities of isorhamnetin 3-O neohesperidoside from
Acacia salicina. Drug Chem Toxicol. 32:258–267. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi YH: The cytoprotective effect of
isorhamnetin against oxidative stress is mediated by the
upregulation of the Nrf2-dependent HO-1 expression in C2C12
myoblasts through scavenging reactive oxygen species and ERK
inactivation. Gen Physiol Biophs. 35:145–154. 2016. View Article : Google Scholar
|
|
16
|
Jänicke RU, Sprengart ML, Wati MR and
Porter AG: Caspase-3 is required for DNA fragmentation and
morphological changes associated with apoptosis. J Biol Chem.
273:9357–9360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faghiri Z and Bazan NG: PI3K/Akt and
mTOR/p70S6K pathways mediate neuroprotection D1-induced retianl
pigment epithelial cell survival during oxidarive stress-induced
apoptosis. Exp Eye Res. 90:718–725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MH, Chung J, Yang JW, Chung SM, Kwag
NH and Yoo JS: Hydrogen peroxide-induced cell death in a human
retinal pigment epithelial cell line, ARPE-19. Korean J Ophthalmol.
17:19–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanneken A, Lin FF, Johnson J and Maher P:
Flavonoids protect human retinal pigment epithelial cells from
oxidative-stress-induced death. Invest Ophthalmol Vis Sci.
47:3164–3177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sreekumar PG, Kannan R, Yaung J, Spee CK,
Ryan SJ and Hinton DR: Protection from oxidative stress by
methionine sulfoxide reductases in RPE cells. Biochem Bioph Res Co.
334:245–253. 2005. View Article : Google Scholar
|
|
21
|
Cai J, Nelson KC, Wu M, Sternberg P Jr and
Jones DP: Oxidative damage and protection of the RPE. Prog Retin
Eye Res. 19:205–221. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu
Y, Cao L, Yang ZH and Sun XB: Isorhamnetin inhibits
H2O2-induced activation of the intrinsic
apoptotic pathway in H9c2 cardiomyocytes through scavenging
reactive oxygen species and ERK inactivation. J Cell Biochem.
113:473–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kook D, Wolf AH, Alice LY, Neubauer AS,
Priglinger SG, Kampik A and Welge-Luüssen UC: The protective effect
of quercetin against oxidative stress in the human RPE in vitro.
Invest Ophth Vis Sci. 49:1712–1720. 2008. View Article : Google Scholar
|
|
25
|
Wang R, Peng L, Zhao J, Zhang L, Guo C,
Zheng W and Chen H: Gardenamide A protects RGC-5 cells from
H2O2-induced oxidative stress insults by
activating PI3K/Akt/eNOS signaling pathway. Int J Mol Sci.
16:22350–22367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zha X, Wu G, Zhao X, Zhou L, Zhang H, Li
J, Ma L and Zhang Y: PRDX6 protects ARPE-19 cells from oxidative
damage via PI3K/AKT signaling. Cell Physiol Biochem. 36:2217–2228.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y,
Hou D and Zhang X: Astaxanthin protects ARPE-19 cells from
oxidative stress via upregulation of Nrf2-regulated phase II
enzymes through activation of PI3K/Akt. Mol Vis. 19:1656–1666.
2013.PubMed/NCBI
|
|
28
|
Yang P, Peairs JJ, Tano R and Jaffe GJ:
Oxidant-mediated Akt activation in human RPE cells. Invest
Ophthalmol Vis Sci. 47:4598–4606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo Y, Sun G, Dong X, Wang M, Qin M, Yu Y
and Sun X: Isorhamnetin attenuates atherosclerosis by inhibiting
macrophage apoptosis via PI3K/AKT activation and HO-1 induction.
PLoS One. 10:e01202592015. View Article : Google Scholar : PubMed/NCBI
|