Introduction
Axonal regeneration in the central nervous system
(CNS) of adult mammals is very limited, leading to poor functional
recovery after CNS injury (1). In
addition, effective ways for promoting the regeneration of injured
neurons have not been developed yet (2). Therefore, novel strategies for
enhancing axonal outgrowth after injury are urgently required. CNS
axons have regeneration capacity, but fail to regenerate
predominantly due to environment obstacles that inhibit axonal
regeneration after injury (3). It
is reported that the inhibitory activity is closely associated with
CNS myelin components and other molecules at the injury site
(4). The three myelin-derived
inhibitors, reticulon 4 (Nogo), myelinassociated glycoprotein (MAG)
and oligodendrocyte myelin glycoprotein (OMgp), are potential
inhibitors of axonal outgrowth in vitro; they interact with
the lycosylphosphatidylinositol-anchored Nogo receptor (NgR) via
their C-terminal 66-residue loops (Nogo-66) (5). The inhibitory activities of MAG, OMgp
and the extracellular domain of Nogo-A (Nogo-66) on regeneration
may be regulated by common receptor complexes, which consist of NgR
and its signaling coreceptors (6).
Genetic deletion of NgR only exerted a modest disinhibitory effect,
and the axonal regeneration was partially blocked in neurons of
NgR-null mice (7). Similar to NgR,
leukocyte immunoglobulin like receptor B1 (PirB) functions as a
receptor of Nogo-66, MAG and OMgp (8). The combined inhibition of NgR and
PirB contributes to an almost complete regeneration of damaged
nerves (9). PirB, as a functional
receptor, is also capable of inhibiting neurite outgrowth (8,10).
However, the molecular mechnisms underlying the signaling that
inhibits axonal outgrowth through PirB needs to be identified.
Phosphoinositide 3-kinase (PI3K)/Akt
serine/threonine kinase (Akt)/mechanistic target of rapamycin
kinase (mTOR) signaling pathway has a critical role in regulating
axonal outgrowth of mammals (11).
Upregulation of mTOR signaling promotes axon regeneration (12). Considering that the importance of
PI3K/Akt/mTOR in regulation of axonal outgrowth, we hypothesized
that inhibition of PirB stimulates axonal outgrowth via
PI3K/Akt/mTOR signaling pathway.
To the best of our knowledge, the present study is
the first to investigated the effects of PirB on axonal outgrowth
via PI3K/Akt/mTOR signaling. The findings may provide a strategy to
develop novel therapeutics that enhance regeneration.
Materials and methods
Animals
BALB/c mice (1-day-old) obtained from the Shanghai
Experimental Animal Center (Shanghai, China) were used in the
experiments. Protocols were followed by the Animal Care and the
Guidelines of Animal Use and Protection of Fudan University. The
study was approved by the ethics committee of Minhang Hospital,
Fudan University (Shanghai, China). Every effort was made to
minimize the suffering of animals.
Isolation, culture and identification
of cortical neurons
Mice (1-day-old) were sacrificed by cervical
dislocation. The cortices were isolated and transferred in an
ice-cold calcium- and magnesium-free Hank's balanced salt solution.
The tissues were digested with 0.125% trypsin for 20 min, and were
washed three times with Dulbecco's minimal essential media (DMEM;
10 ml) with 20% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc, Waltham, MA, USA). The cells were suspended at
1.5×105 cells/cm2 in DMEM supplemented with
10% horse serum, 10% fetal bovine serum, 2 mM glutamine and 25 mM
glucose, and cultured in a humidified 5% CO2 incubator
at 37°C. On the second day, 10 µM cytarabine (Ara-C) was added to
the cultures to inhibit non-neuronal cell proliferation. Twice a
week, half of the medium was replaced with fresh fetal bovine
serum-free medium. The experiments on the cultured neurons were
performed after 10 days. Nogo inhibitory peptide (NEP1-40; Merck
KGaA, Darmstadt, Germany), a Nogo-66 receptor antagonist peptide
with sequence 1–40 amino acids, acts as the competitive antagonist
of NgR and blocks Nogo-66 inhibition of axonal outgrowth in
vitro (9). A total of 4 µmol/l
NEP1-40 was added at the time of cell plating, and cultures were
incubated for 2 h before anti-PirB antibody treatment. The neurons
were randomly divided into two groups: Negative control and
anti-PirB antibody treatment. For the anti-PirB antibody treatment
group, the cells (1.5×105 cells/cm2) were
incubated in Earle's culture medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 50 µg/ml 6C1, a specific
anti-PirB antibody (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) binding the PirB extracellular region, and incubated with a
PI3K inhibitor (LY294002, 50 µmol/l) and an mTOR inhibitor
(rapamycin, 100 nmol/l) after 4 h.
Neurite outgrowth assay
The cultured cortical neurons (2×104)
were cultured on poly-L-lysine/laminin (PLL; a substrate for
neuronal outgrowth)-coated 6-well plates with or without purified
myelin, NEP1-40 and anti-PirB (8,13).
At 72-h post-plating, explants were incubated with Nogo-66 (4
µmol/l; cat no. sc-25659; Santa Cruz Biotechnology, Inc.). The
length of the longest neurite for each neuron was determined by
systematic scanning of the slide was analyzed by an inverted
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Neurite length quantitation was statistically analyzed as described
previously (8).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol according to
the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.), and the quality was assessed by spectrophotometric
absorbance at 260/280 nm. The extracted RNA was reverse transcribed
into cDNA using a RevertAid First Strand cDNA Synthesis kit at 37°C
for 15 min, followed by 85°C for 5 sec (Fermentas; Thermo Fisher
Scientific, Inc.). For each sample, 2 µg RNA was used to synthesize
the cDNA. RT-qPCR was performed with an ABI Prism 7700 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific.
Inc.). Reactions were performed in duplicate with SYBR Green PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific. Inc.).
PCR amplification were carried out accorrding to the following
program (40 cycles): Primary extension 94°C for 5 min; cycling at
94°C for 0.5 min, 55°C for 0.5 min and 72°C for 0.5 min; and final
extension at 72°C for 10 min. Amplification plots and cycle
threshold values from the exponential phase of the PCR were
assessed in ABI Prism SDS1.7 software using Genescan version 3.7
software (Applied Biosystems; Thermo Fisher Scientific. Inc.). Only
one product of the desired size was identified and one single peak
was observed in a melting curve for each primer. Each sample was
conducted three times. The relative mRNA expression of each gene
was calculated using the 2−ΔΔCq method and normalized to
β-actin (14). The primers
designed for RT-qPCR are presented in Table I.
 | Table I.Primers for qRT-PCR. |
Table I.
Primers for qRT-PCR.
| Gene | Sequence (5′-3′) | Tm (°C) | Size (bp) | Locus |
|---|
| PI3K |
| 60 | 241 | KF011500 |
| F |
ACCCAAGCGAGGATGAGG |
|
|
|
| R |
TGTTGCCCGTGTTGAATG |
|
|
|
| Akt1 |
| 60 | 215 | KF011501 |
| F |
TGCTGGATAAAGATGGAC |
|
|
|
| R |
CTGGTTGTAGAAAGGGAG |
|
|
|
| mTOR |
| 60 | 93 | KC424580 |
| F |
TCATTTGTTACTACCTCCCA |
|
|
|
| R |
TCTAGAGCAGCTTTGCGAGCCAC |
|
|
|
| Cofilin |
| 58.2 | 111 | KC424581 |
| F |
GTTCAGGCTCACCCCGTTCTT |
|
|
|
| R |
AGTAGAGTCATCTGGGCTATCAA |
|
|
|
| Myosin IIA |
| 53.9 | 129 | L21170 |
| F |
TTGGTGGAGCGATTTGTC |
|
|
|
| R |
ATCTCGGGTGGCTGAACG |
|
|
|
| β-actin |
| 59.6 | 92 | M26111 |
| F |
CAACGAGCGGTTCAGGTGT |
|
|
|
| R |
TGGAGTTGAAGGTGGTCTCGT |
|
|
|
Western blot analysis
The protein levels of PirB, PI3k, Akt, cofilin,
myosin IIA, mTOR and phosphorylated mTOR were assessed by western
blot. Briefly, cultured neurons were harvested in
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc.). The concentration of protein was determined
using bicinchoninic acid protein assay kit (cat no. 23225; Thermo
Fisher Scientific. Inc.). Equal amounts of protein (20 µg) were
denatured for 5 min at 95°C in sample buffer and separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Following electrophoresis, the proteins were transferred to a
polyvinylidene fluoride membrane, which was incubated in blocking
solution containing 5% non-fat milk in a Tris-buffered
saline/0.05%Tween 20 solution for 60 min at room temperature.
Western blot analysis was performed by overnight incubation at 4°C
using antibodies against PirB (cat no. 03-1647, 1:1,000; EMD
Millipore, Billerica, MA, USA), PI3K (cat no. 05-1563, 1:1,000; EMD
Millipore), Akt, (cat no. 05-591MG, 1:1,000; EMD Millipore),
cofilin (cat no. 07-326, 1:1,000; EMD Millipore), myosin IIA (cat
no. MABS755, 1:1,000; EMD Millipore), mTOR (cat no. 05-1592,
1:1,000; EMD Millipore), and phosphorylated mTOR (cat no. 09-345,
1:1,000; EMD Millipore) diluted in 10% horse serum in TBS-Tween
buffer (0.2 M NaCl, 25 mM Tris, pH 7.5, 0.5 ml/l Tween-20),
followed by incubation with a horseradish peroxidase-coupled mouse
secondary antibody (1:10,000, cat no. sc-2004; Santa Cruz
Biotechnology, Inc.). Blots were reprobed with β-actin antibody
(cat no. 612656; 1:4,000; BD Bioscience, San Jose, CA, USA),
α-tubulin III (ABT170; 1:1,000; Merck KGaA) GAPDH (cat no.
GB11002-50; 1:1,000; Merck KGaA) at room temperature for 2 h.
Signals were analyzed with the Beyotime ECL Plus detection kit
(Beyotime Institute of Biotechnology, Haimen, China). Protein were
normalized by comparison with the corresponding β-actin signal of
the samples; the data are presented as percentages of the
normalized control signal. Densitometric analysis was performed
using Image J software (version 2.1.4.7; National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard error.
An unpaired t-test and one way-analysis of variance (Bonferroni
post hoc test for equal variances assumed; Tamhane's T2 post hoc
test for equal variances not assumed) were used to compare the
groups using GraphPad Prism version 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA) and SPSS software v.22.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant different.
Results
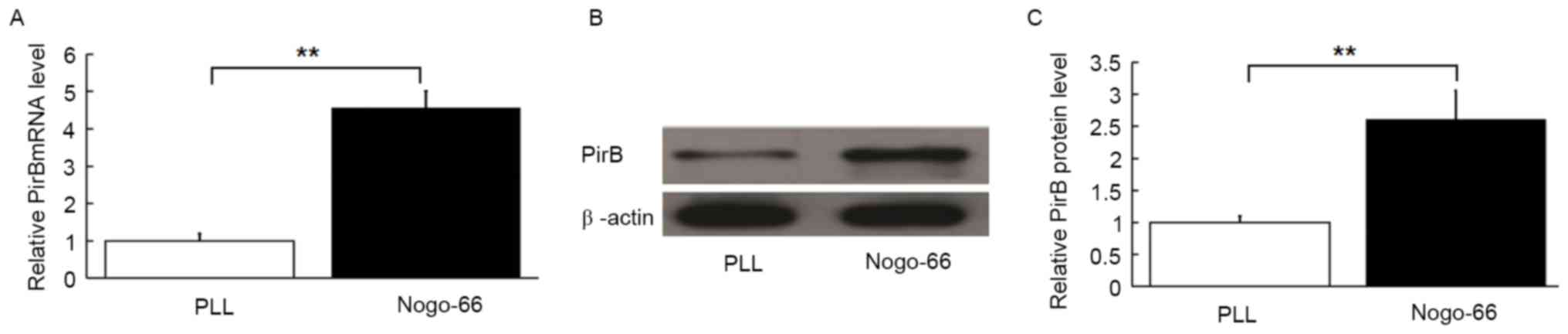
Nogo-66 induces endogenous PirB
expression in cultured neurons
Nogo-66 inhibits axonal outgrowth of cultured
neurons through NgR and PirB. NEP1-40 is a Nogo-66 receptor
antagonist peptide with the 1–40 amino acid sequence that acts as a
competitive antagonist of NgR and blocks Nogo-66 inhibition of
axonal outgrowth in vitro. In order to determine the role of
PirB, the cultured neurons were treated with NEP1-40 to block the
effect of Nogo-66 on axonal outgrowth. Regulation of PirB mRNA and
protein expression by exogenous Nogo-66 was investigated in
cultured neurons, treated with NEP1-40. The mRNA levels of PirB
were upregulated by treatment with exogenous Nogo-66 compared with
cells cultured on PLL (Fig. 1A;
P<0.01). PirB protein levels were also increased by Nogo-66
(P<0.01; Fig. 1B and C).
Therefore, Nogo-66 may enhance PirB expression in cultured
neurons.
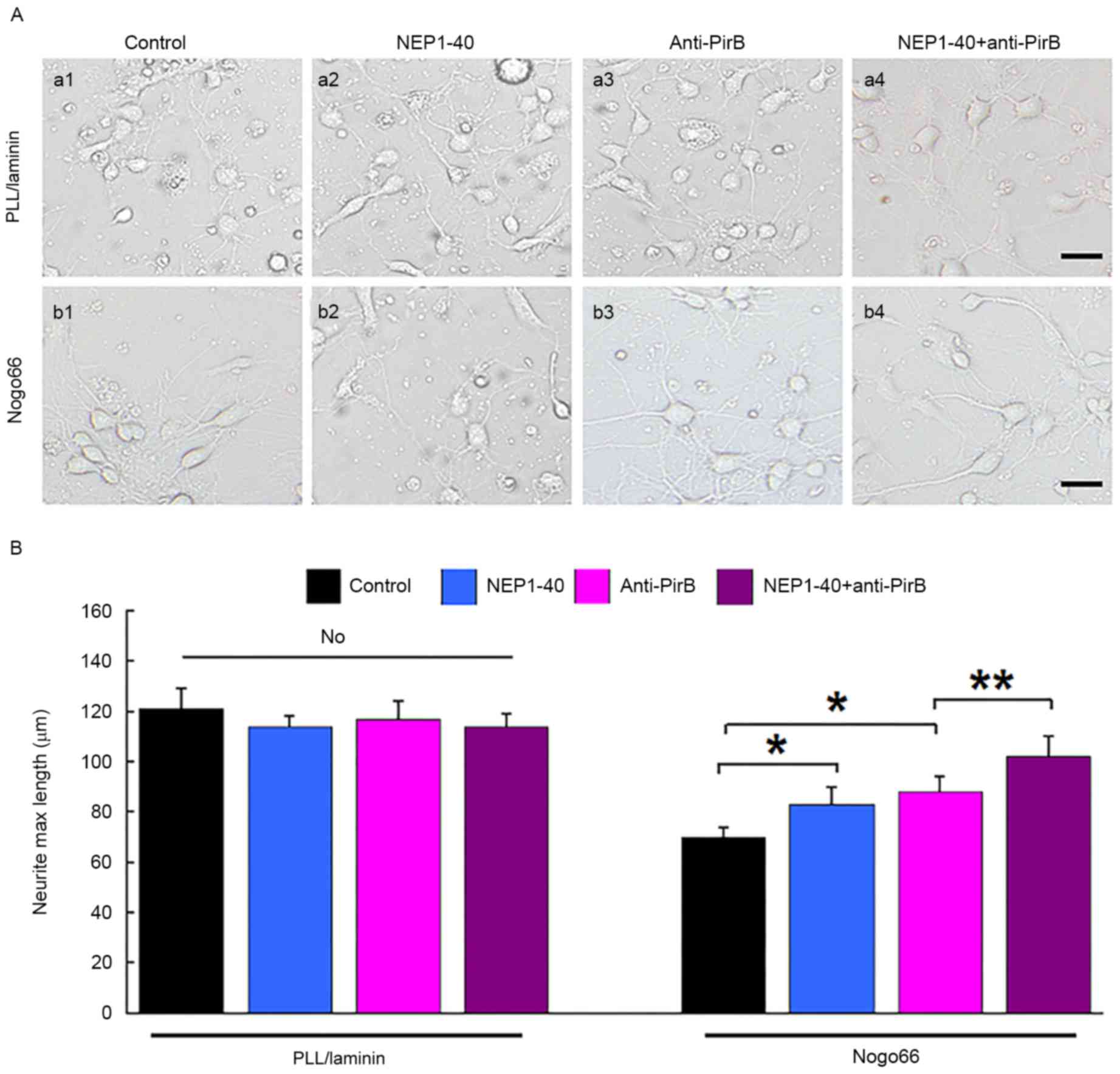
Inactivation of PirB induced axonal
outgrowth
To elucidate the role of PirB in axon growth, its
influence on axon outgrowth of cultured neurons was investigated.
No marked difference in the axonal length of neurons cultured on
PLL was observed when neurons were cultured in the presence of
NEP1-40, anti-PirB (50 mg/ml) or NEP1-40 + anti-PirB (Fig. 2). The axonal length of neurons was
decreased when they were placed on growth-non permissive (Nogo-66)
substrate. (70±4 µm on Nogo-66 compare with 121±10 on PLL;
P<0.01). NEP1-40 or anti-PirB treatment partially attenuated the
inhibitory effect of Nogo-66 and increased the axon length on
non-permissive substrate (83±7 and 88±6 µm, respectively, compared
with 70±4 µm with Nogo66 only; P<0.05; Fig. 2). NEP1-40 + anti-PirB treatment
almost fully attenuated the inhibitory effect of Nogo66 on axon
length, and increased the length compared with NEP1-40 or anti-PirB
treatment alone (115±11 µm; P<0.01) on non-pemissive substrate
(Fig. 2).
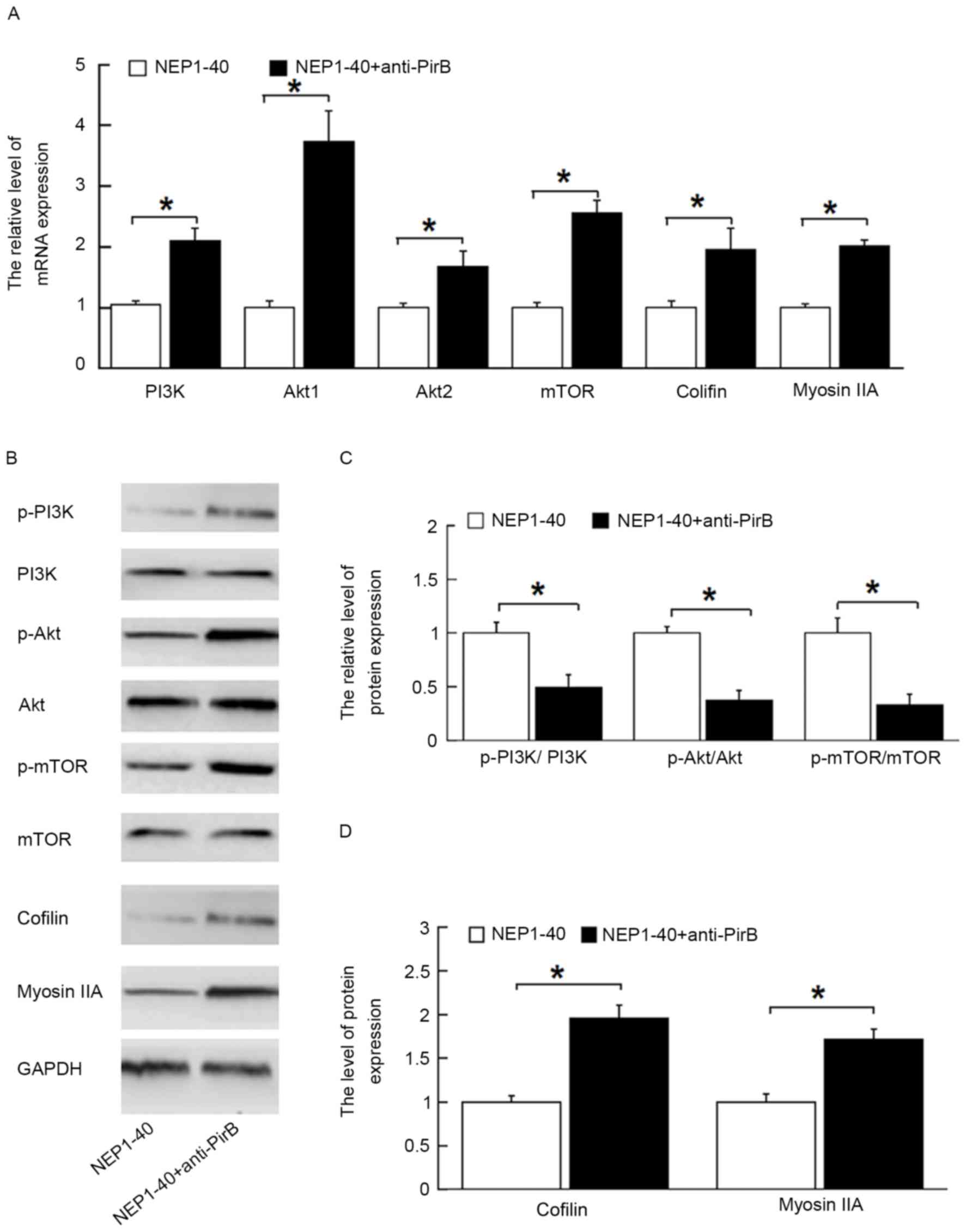
Inactivation of PirB activated
PI3K/Akt/mTOR signaling pathway
To study whether the PI3K/Akt/mTOR pathway is
involved in axonal outgrowth in the presence of anti-PirB, the mRNA
levels of the associated genes, including PI3K, Akt1, mTOR, cofilin
and myosin IIA, and phosphorylation of mTOR were detected by
RT-qPCR and western blot. As shown in Fig. 3A, anti-PirB treatment (50 µg/ml)
significantly increased the expression of PI3K, Akt1, Akt2, mTOR,
cofilin and myosin IIA mRNA in the NEP1-40 + anti-PirB group
compared with the NEP1-40 group (P<0.05). Anti-PirB treatment
promoted the phosphorylation of PI3K, Akt and mTOR, and enhanced
cofilin and myosin IIA protein expression (P<0.05; Fig. 3B-D). These results indicated that
the PI3K/Akt/mTOR pathway may mediate PirB-induced inhibition of
neurite outgrowth. No significant difference was found between the
AP-Nogo66 and AP-Nogo-66 + NEP1-40 groups (data not shown).
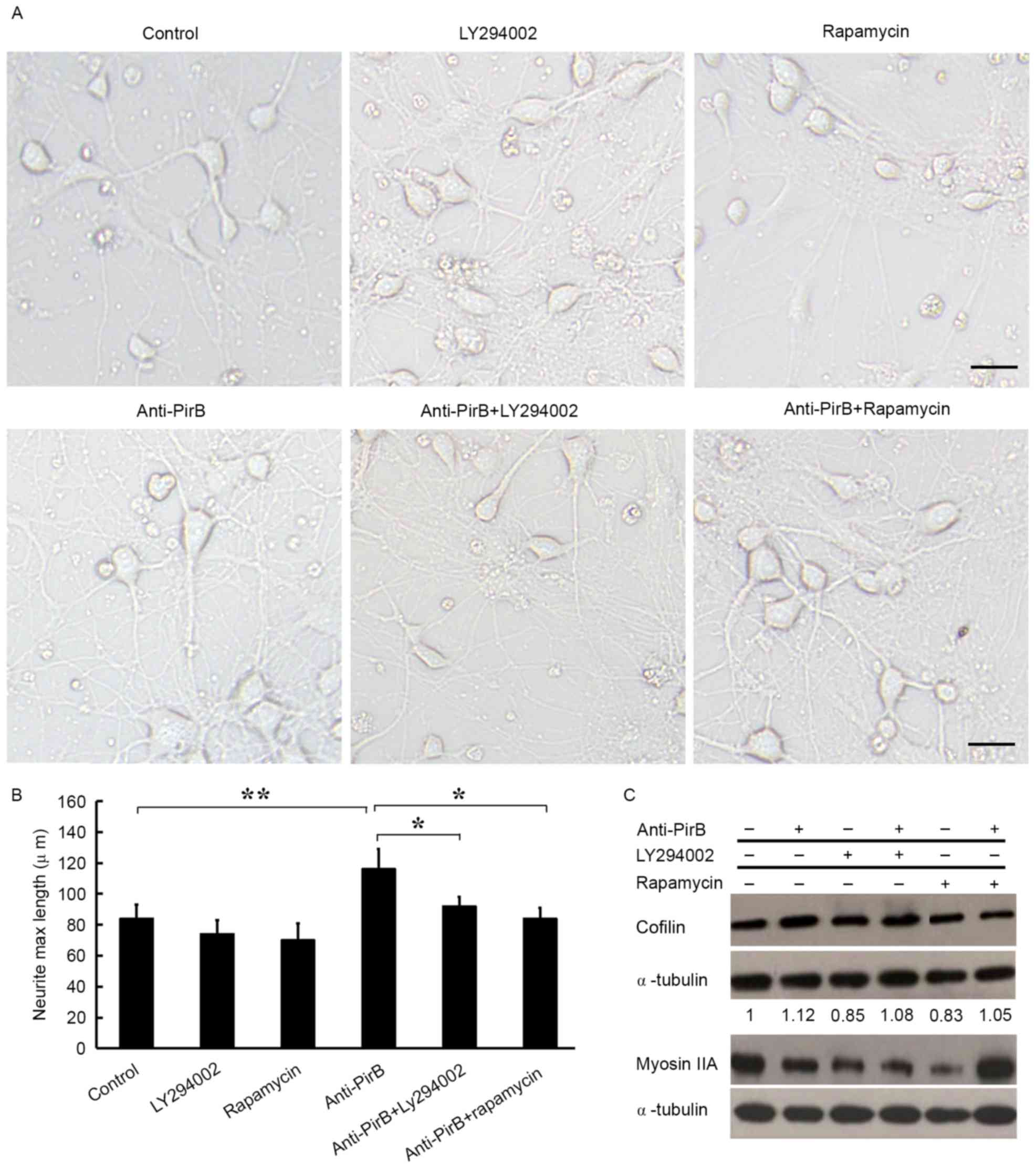
Blockade of PI3K/Akt/mTOR pathway
inhibits Anti-PirB-induced axonal outgrowth
To further explore the role of the PI3K/Akt/mTOR
pathway in anti-PirB-mediated promotion of neurite outgrowth, two
pharmacological inhibitors, a PI3K inhibitor (LY294002) and an mTOR
inhibitor (rapamycin), were used in the neurite outgrowth assay.
Both LY294002, and rapamycin significantly attenuated neurite
outgrowth induced by anti-PirB (P<0.05; Fig. 4A and B). In addition, western blot
analysis demonstrated that the protein expression of several axon
outgrowth-associated genes also decreased in the presence of either
LY294002 or rapamycin (Fig. 4C).
These data strongly confirm that PirB exerts inhibitory effects on
axonal growth through the PI3K/Akt/mTOR pathway.
Discussion
Accumulating evidence suggests that the lack of
regeneration capacity in the adult mammalian CNS is due to the
presence of certain myelin-derived inhibitors, including the
repulsive trio (Nogo-66, MAG and OMgp) (4,5).
Inhibitory signaling is transduced via their receptors, NgR and
PirB. The trimeric receptor complexes stimulate the
RhoA-Rho-associated protein kinase (ROCK) pathway when
myelin-associated inhibitors bind with Nogo-A. It is reported that
PirB may have a role in inhibiting axonal regeneration in specific
PirB-expressing neurons in the CNS (15). However, the molecular mechanism
underlying PirB mediated inhibition of axonal outgrowth remains
unclear. In the present study, PirB expression was induced by
Nogo-66 in cortical neurons isolated from newborn mice.
Subsequently, PirB antibodies were used to block the effect of PirB
to determine how axonal regeneration was affected. The results of
the current study indicate that the PirB-mediated inhibitory
effects are mediated via PI3K/Akt/mTOR signaling pathway.
The inhibitory effects of PirB on axonal outgrowth
have been demonstrated. Attenuation of PirB activity through
antibody antagonism or genetic approaches can partially reduce the
inhibition of neurite outgrowth in vitro and in vivo
(9). PirB suppresses axonal
outgrowth via SH3 domain containing ring finger 1 (POSH)/ROCK or
and POSH/c-Jun N-terminal kinases pathway (16). Furthermore, the substrates of
tropomyosin receptor kinase B (TrkB) include tyrosine-kinase and
mitogen-activated protein kinase signaling complexes, such as PI3K
and Akt, ultimately leading to inhibition axonal regeneration
(9). PirB suppresses axon
regeneration through inhibition of Trk activity (17). The Trk receptor stimulates PI3K
heterodimers, which leads to the activation of pyruvate
dehydrogenase kinase 1 and Akt (18), subsequently activating mTOR
pathway. However, the effect of PirB on PI3K/Akt/mTOR on axonal
outgrowth remains poorly understood. The present study aimed to
examine the effects of PirB on axonal outgrowth in the presence NgR
inhibition and explored the mechanism of PirB-inhibited axonal
outgrowth via PI3K/Akt/mTOR pathway.
The results of the current study showed that
increased PI3K/Akt/mTOR activity in the cortical neurons of newborn
mice induced by Nogo-66. This finding suggests that PI3K/Akt/mTOR
is regulated by PirB and that PI3K/Akt/mTOR may be the signaling
pathway that transduces inhibitory signaling from PirB to the
intracellular part of neurons. PirB inhibited Trk activity in
process of suppressing axon regeneration (17). The inhibitory influence of
exogenous PirB on axonal outgrowth of cortical neurons induced by
Nogo-66 was investigated in the present study. Compared with the
control groups, anti-PirB treatment promoted axonal outgrowth.
Furthermore, myosin IIA and cofilin protein expression was
increased in following anti-PirB treatment in cortical neurons from
newborn mice induced by Nogo-66. Taken together, results from our
studies support the hypothesis that PirB has an important role in
inhibiting axonal outgrowth through PI3K/Akt/mTOR.
PI3K signaling cascades are important regulators
invovled in a host of cellular activities such as apoptosis,
proliferation, differentiation and axonal outgrowth (19). In mammals, PI3K/Akt/mTOR signaling
pathway has a crucial role in regulating axonal outgrowth (11). This study revealed that PirB
exerted the inhibitory effects through the PI3K/Akt/mTOR signaling
pathways. Nogo-66 treatment induced activation of the PI3K/Akt/mTOR
signaling in cortical neurons of newborn mice in the presence of
NgR blockade, as determined by quantitying the expression of PI3K,
Akt1, Akt2 and mTOR PI3K/Akt/mTOR pathway. Phosphorylated Akt
induced by PI3K can increase accumulation and activation of the
mTOR-raptor kinase complex, subsequently mediating phosphorylation
of S6 kinase to enhance protein synthesis (20). Furthermore, the pharmacological
inhibitors of PI3K and mTOR (LY294002 and rapamycin) were used to
confirm the involvement of PI3K/Akt/mTOR signaling pathway in
PirB-mediated inhibition of axonal outgrowth in cortical neurons of
newborn mice. The results indicated that activation of the
PI3K/Akt/mTOR pathway significantly reversed the inhibitory effects
of PirB on axonal outgrowth, since the blocked factors by PirB were
attenuated in the presence of either of two pharmacological
inhibitors, as determined by neurite outgrowth assay and measuring
the mRNA levels and protein associated with axonal outgrowth and
regulation of the PI3K/Akt/mTOR pathway. These findings confirm
that the PI3K/Akt/mTOR signaling pathway is involved in
PirB-mediated inhibition of axonal outgrowth.
In conclusion, the findings of the current study
suggest that PirB exerts its inhibitory effects on axonal outgrowth
through PI3K/Akt/mTOR signaling pathway. These findings will
provide a novel insight into understanding the role of PirB in
axonal outgrowth and the molecular mechanisms of PirB signaling,
thus, potentially contributing to the development of novel
therapeutic strategies to encourage axonal regeneration following
CNS injury.
Acknowledgements
The present study was granted from the Youth Project
of Shanghai municipal Commission of Health and Family Planning
(grant no. 20144Y0229).
References
|
1
|
Pernet V and Schwab ME: Lost in the
jungle: New hurdles for optic nerve axon regeneration. Trends
Neurosci. 37:381–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shum JW, Liu K and So KF: The progress in
optic nerve regeneration, where are we? Neural Regen Res. 11:32–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li S, Yang C, Zhang L, Gao X, Wang X, Liu
W, Wang Y, Jiang S, Wong YH, Zhang Y and Liu K: Promoting axon
regeneration in the adult CNS by modulation of the melanopsin/GPCR
signaling. Proc Natl Acad Sci USA. 113:1937–1942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao SN and Pearse DD: Regulating axonal
responses to injury: The intersection between signaling pathways
involved in axon myelination and the inhibition of axon
regeneration. Front Mol Neurosci. 9:332016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang KC, Koprivica V, Kim JA, Sivasankaran
R, Guo Y, Neve RL and He Z: Oligodendrocyte-myelin glycoprotein is
a Nogo receptor ligand that inhibits neurite outgrowth. Nature.
417:941–944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Domeniconi M, Cao Z, Spencer T,
Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y,
et al: Myelin-associated glycoprotein interacts with the Nogo66
receptor to inhibit neurite outgrowth. Neuron. 35:283–290. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fournier AE, GrandPre T and Strittmatter
SM: Identification of a receptor mediating Nogo-66 inhibition of
axonal regeneration. Nature. 409:341–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Atwal JK, Pinkston-Gosse J, Syken J,
Stawicki S, Wu Y, Shatz C and Tessier-Lavigne M: PirB is a
functional receptor for myelin inhibitors of axonal regeneration.
Science. 322:967–970. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gou Z, Mi Y, Jiang F, Deng B, Yang J and
Gou X: PirB is a novel potential therapeutic target for enhancing
axonal regeneration and synaptic plasticity following CNS injury in
mammals. J Drug Target. 22:365–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Wang Y and Fu W: Axon regeneration
impediment: The role of paired immunoglobulin-like receptor B.
Neural Regen Res. 10:1338–1342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Xiong Y and Mu D: PirB restricts
neuronal regeneration in developing rat brain following
hypoxia-ischemia. Mol Med Rep. 6:339–344. 2012.PubMed/NCBI
|
|
12
|
Abe N, Borson SH, Gambello MJ, Wang F and
Cavalli V: Mammalian target of rapamycin (mTOR) activation
increases axonal growth capacity of injured peripheral nerves. J
Biol Chem. 285:28034–28043. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng Q, Cai W, Li S, Zhang Y and Su B:
Small Nogo-66-binding peptide promotes neurite outgrowth through
RhoA inhibition after spinal cord injury. Brain Res Bull.
99:140–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura Y, Fujita Y, Ueno M, Takai T and
Yamashita T: Paired immunoglobulin-like receptor B knockout does
not enhance axonal regeneration or locomotor recovery after spinal
cord injury. J Biol Chem. 286:1876–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rusanescu G, Yang W, Bai A, Neel BG and
Feig LA: Tyrosine phosphatase SHP-2 is a mediator of
activity-dependent neuronal excitotoxicity. EMBO J. 24:305–314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dickson HM, Zurawski J, Zhang H, Turner DL
and Vojtek AB: POSH is an intracellular signal transducer for the
axon outgrowth inhibitor Nogo66. J Neurosci. 30:13319–13325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita Y, Endo S, Takai T and Yamashita T:
Myelin suppresses axon regeneration by PIR-B/SHP-mediated
inhibition of Trk activity. EMBO J. 30:1389–1401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Segal RA: Selectivity in neurotrophin
signaling: Theme and variations. Annu Rev Neurosci. 26:299–330.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|