Introduction
Acute pharyngitis is a type of upper respiratory
tract infection, with the most common symptom of an acute sore
throat (1). Acute pharyngitis
refers to acute inflammation occurring in nasopharyngeal mucosa and
submucosal tissues, and usually involves pharyngeal lymphoid
tissues (2). It may occur alone or
secondary to acute rhinitis and acute tonsillitis (2). Acute pharyngitis is a common and
frequently-occurring disease, characterized by rapid incidence and
development (3). The main causes
of acute pharyngitis include bacterial and virus infection, as well
as non-infectious factors (such as breathing through the mouth,
allergic reaction, gastro-esophageal reflux, smoking, alcohol
intake, high temperature, dust, smoke and pungent gas) (4).
The activation and transcriptional regulation of the
nuclear factor (NF)-κB signaling pathway has been a primarily
research focus (5). In general,
NF-κB binds to inhibitor (I) κB in the cytoplasm under a resting
state to form a resting complex, which blocks the DNA binding sites
of the Rel dimer. Under certain stimulation, IκB will be
deactivated through phosphorylation by IκB kinase (6). NF-κB is released into the nucleus to
bind to specific sites in DNA, serving a transcription factor role.
Notably, the base sequences of κB sites are not exactly the same;
additionally, the Rel dimer cannot fully activate target genes
alone (7). NF-κB not only serves
an important role in the regulation of inflammatory response, but
also participates in many cellular activities, including
proliferation and apoptosis of cells. (8).
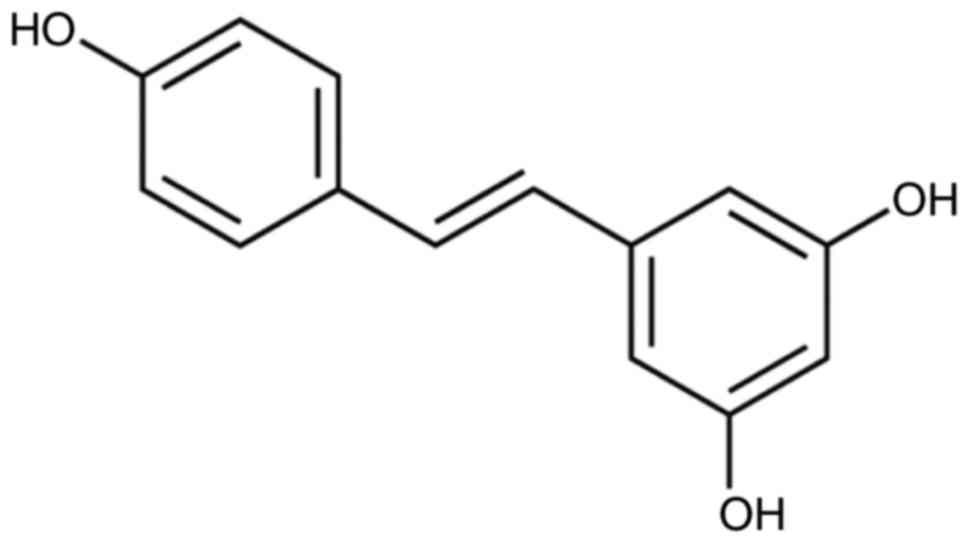
Resveratrol, a natural polyphenol compound (Fig. 1), exists in grapes, peanuts,
berries and other plants. Previous studies have demonstrated that
resveratrol regulates the activity of histone deacetylase, which
determines lifespan (9). As an
activator of deacetylase, resveratrol has been confirmed to prolong
the lifespan of yeast, nematode and mouse (9). Available studies have confirmed that
resveratrol has anti-inflammatory, anti-oxidant and anti-neoplastic
effects as well as protecting the heart (9). Due to their extensive effects, rich
and renewable sources and high compatibility with the environment,
botanical bactericide has become a hotspot in the field of the
research of pesticides (10). Many
plants with antibacterial and bactericidal activity have been
detected, and many active materials such as phenols, terpenoids and
flavonoids have been isolated (10). Stilbenes, one kind of active
components derived from many plants, has certain antibacterial
activity. Studies have demonstrated that resveratrol, a typical
component of stilbenes, has extensively biological activities and
pharmacological properties (10).
The present study observed that the anti-inflammatory activity of
resveratrol prevents inflammation in animal models of acute
pharyngitis.
Materials and methods
Animals and acute pharyngitis
treatments
Male adult New Zealand white rabbits (n=18; age, 4–6
months; weight, 2.2–2.5 kg, 6 rabbits/group) were provided by the
Experimental Animal Center of Shandong University and all animal
experiments were performed according to protocols approved by the
Institutional Animal Care and Use Committee of Liaocheng People's
Hospital (Shandong, China). All rabbits were randomly assigned to
three groups: Control, acute pharyngitis model and resveratrol
treatment groups.
In the acute pharyngitis model and resveratrol
treatment groups, rabbits were anesthetized with 30 mg/kg of
pentobarbital sodium (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), then fixed at operating table at 37°C. The neck was cut,
the weasand was separated and endotracheal intubation was executed
at 3 µm from the cricoid cartilage. The weasand was cut and
exposed, and croton oil (0.1 ml; 2%) was used to wipe the weasand
and plated for 30 min. The wound was sterilized with iodophor and
rabbit placed back in the cage. In the resveratrol treatment group,
rabbits were treated with 4 mg/kg every two days
(intraperitoneally) resveratrol for 2 weeks.
Commercial ELISA kits
After treatment with resveratrol, peripheral blood
was acquired from the eye socket and was centrifuged at 2,000 × g
for 10 min at 4°C. ELISA kits were used to determine levels of
tumor necrosis factor (TNF)-α (cat. no. H052), interleukin (IL)-6
(cat. no. H007), macrophage inflammatory protein-2 (MIP-2, cat. no.
H112) and cyclooxygenase-2 (COX-2, cat. no. H200) activity, and ROS
production (cat. no. E004) and caspase-3/9 activity (cat. no.
G015/G018) in blood serum.
Western blot analysis
After treatment with resveratrol, rabbits were
sacrificed and weasand tissue samples were washed with PBS and
homogenized using ice-cold radioimmunoprecipitation assay buffer.
Protein content was measured using a Bicinchoninic Acid protein
assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Total protein (50 µg) was separated by 6–10% SDS-PAGE and
transferred to polyvinylidene fluoride membranes (BD Biosciences,
San Jose, CA, USA). The membranes were subsequently blocked with 5%
nonfat dry milk for 1 h at room temperature and overnight at 4°C
with primary antibodies against NACHT, LRR and PYD
domains-containing protein 3 (NLRP3; cat. no. ab214185; 1:1,000),
caspase-1 (cat. no. ab108362; 1:1,000), IL-1β (cat. no. ab200478;
1:1,000), IL-18 (cat. no. ab71495; 1:1,000), toll-like receptor 4
(TLR4; cat. no. ab13556; 1:1,000), myeloid differentiation primary
response protein 88 (MyD88; cat. no. ab2068; 1:1,000),
phosphorylated (p)-NF-κB (cat. no. ab86299; 1:1,000), p-IκB (cat.
no. ab75746; 1:1,000) and GAPDH (cat. no. ab8245; 1:2,000), all
purchased from Abcam (Cambridge, MA, USA). The membranes were
washed with TBST for 15 min and incubated with a horseradish
peroxidase-conjugated anti-rabbit secondary antibody (cat. no.
ab205718; 1:5,000, Abcam, Cambridge, MA, USA) for 1 h at room
temperature, and the results were detected using enhanced
chemiluminescence (Gibco; Thermo Fisher Scientific, Inc.). Band
intensity was calculated using Image J software version 1.42q
(National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
The results are expressed as the mean ± standard
deviation using SPSS version 19.0 software (SPSS, Inc., Chicago,
IL, USA). The data were analyzed using one-way analysis of variance
followed by Duncan's multiple range test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Vocal cord and absorption indexes in
animal models of acute pharyngitis
Firstly, the present study demonstrated that the
index of the vocal cords and absorption index in acute pharyngitis
model were higher compared with the control group (Table I). Resveratrol treatment
significantly inhibited the index of the vocal cords and absorption
index in acute pharyngitis rabbits, compared with the acute
pharyngitis model group (Table
I).
 | Table I.Vocal cord and absorption indexes. |
Table I.
Vocal cord and absorption indexes.
| Group | Index of the vocal
cords | Absorption index |
|---|
| Control | 0 | 0 |
| Model | 28.91 | 36.77 |
| Resveratrol | 16.67 | 20.09 |
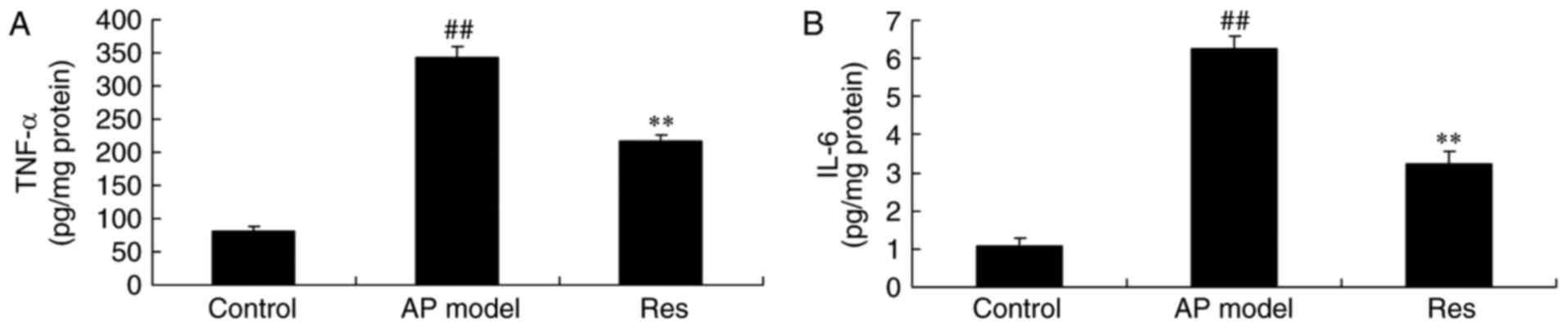
TNF-α and IL-6 serum levels in animal
models of acute pharyngitis
There was a significant increase of TNF-α and IL-6
serum levels in the acute pharyngitis model, compared with control
group (Fig. 2). Resveratrol
treatment significantly reduced TNF-α and IL-6 serum levels in
acute pharyngitis rabbits, compared with the acute pharyngitis
model group (Fig. 2).
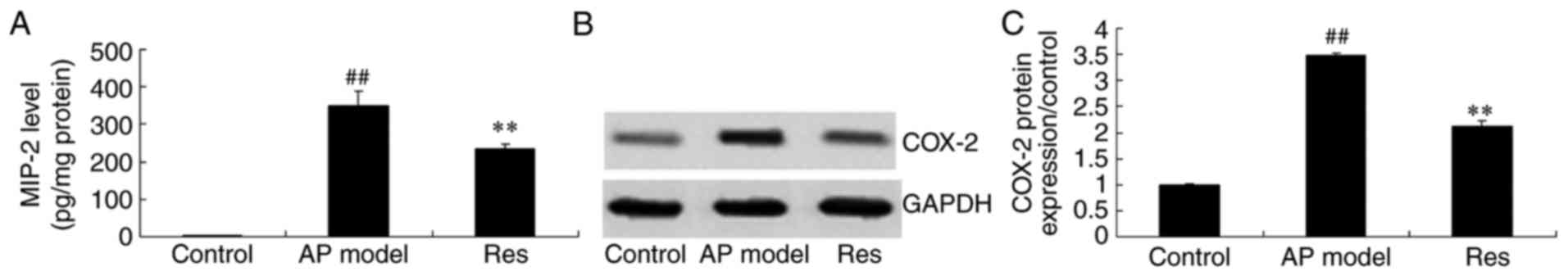
MIP-2 and COX-2 activity levels in
animal models of acute pharyngitis
It was observed that MIP-2 and COX-2 activity levels
of the acute pharyngitis model were significantly higher compared
with the control group (Fig. 3).
In acute pharyngitis rabbit model, treatment with resveratrol
significantly inhibited MIP-2 and COX-2 activity levels in acute
pharyngitis rabbits, compared with the acute pharyngitis model
group (Fig. 3).
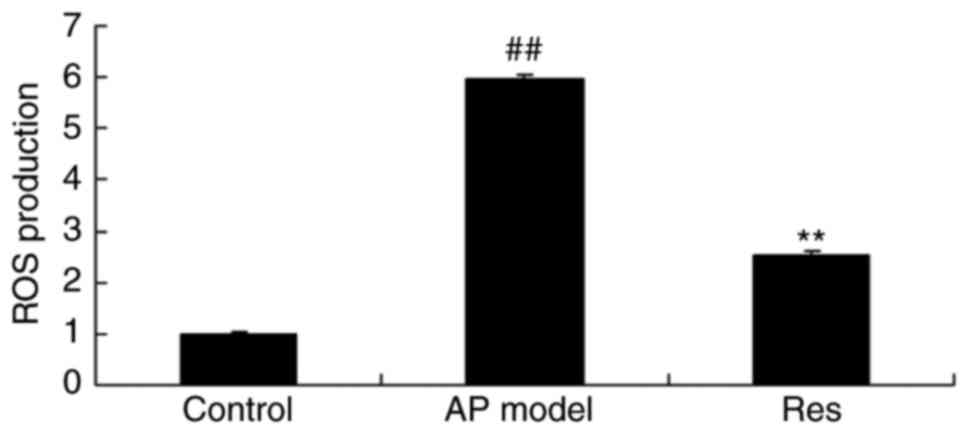
ROS production in animal models of
acute pharyngitis
After treatment with resveratrol, the present study
demonstrated that ROS production was significantly enhanced in the
acute pharyngitis model group, compared with the control group
(Fig. 4). Treatment with
resveratrol significantly inhibited the induction of ROS production
in acute pharyngitis rabbits, compared with the acute pharyngitis
model group (Fig. 4).
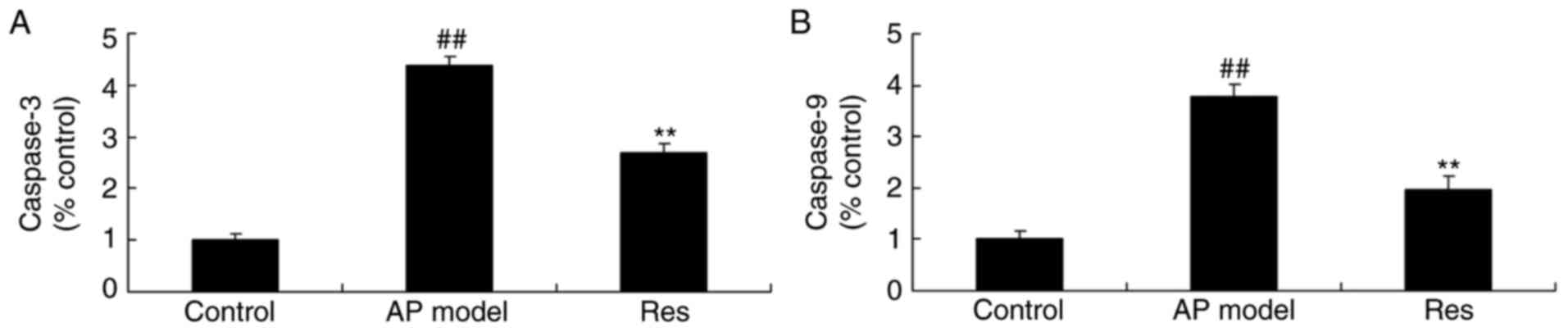
Caspase-3/9 activity in animal models
of acute pharyngitis
To investigate the apoptotic status of acute
pharyngitis by resveratrol, caspase-3/9 activity was measured using
ELISA kits. As presented in Fig.
5, caspase-3/9 activity was significantly promoted in the acute
pharyngitis model, compared with the control group. However,
resveratrol significantly reduced the increase of caspase-3/9
activity in acute pharyngitis rabbits, compared with the acute
pharyngitis model group (Fig.
5).
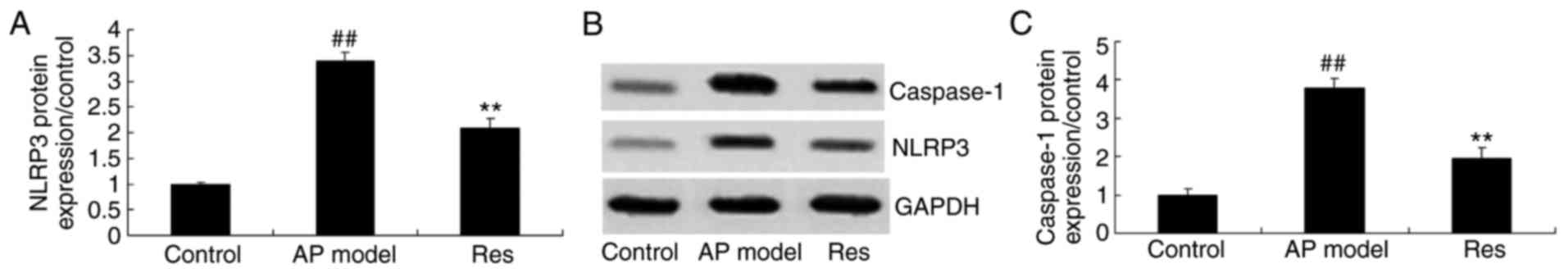
NLRP3 and caspase-1 protein expression
in animal models of acute pharyngitis
The present study investigated the effects of
resveratrol on the inflammasome in acute pharyngitis rabbits. NLRP3
and caspase-1 protein expression were measured using western
blotting. The results demonstrated that NLRP3 and caspase-1 protein
expression were significantly induced in the acute pharyngitis
model, compared with the control group (Fig. 6). Resveratrol treatment
significantly suppressed NLRP3 and caspase-1 protein expression in
acute pharyngitis rabbits, compared with the acute pharyngitis
model group (Fig. 6).
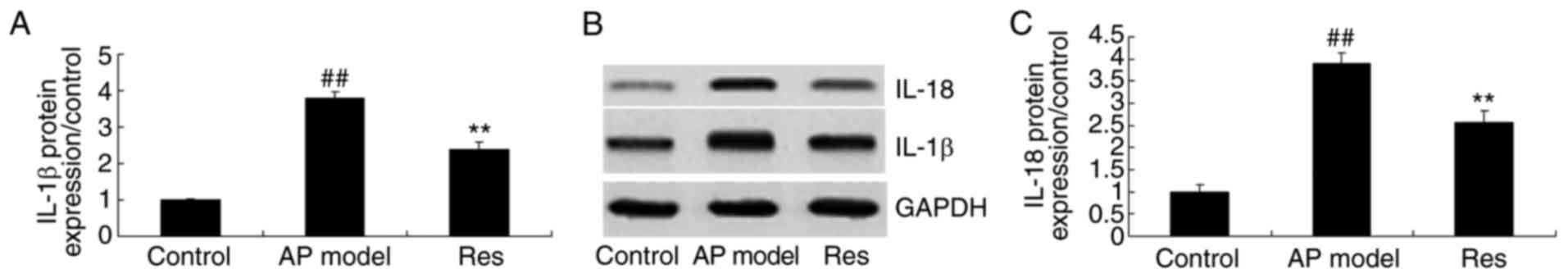
IL-1β and IL-18 protein expression in
animal models of acute pharyngitis
The present study investigated the effects of
resveratrol on IL-1β and IL-18 protein expression in animal models
of acute pharyngitis using western blotting. The results
demonstrated that the protein expression levels of IL-1β and IL-18
in the acute pharyngitis model group were significantly higher
compared with the control group (Fig.
7). Treatment with resveratrol significantly suppressed the
protein expression of IL-1β and IL-18 levels in acute pharyngitis
rabbits, compared with the acute pharyngitis model group (Fig. 7).
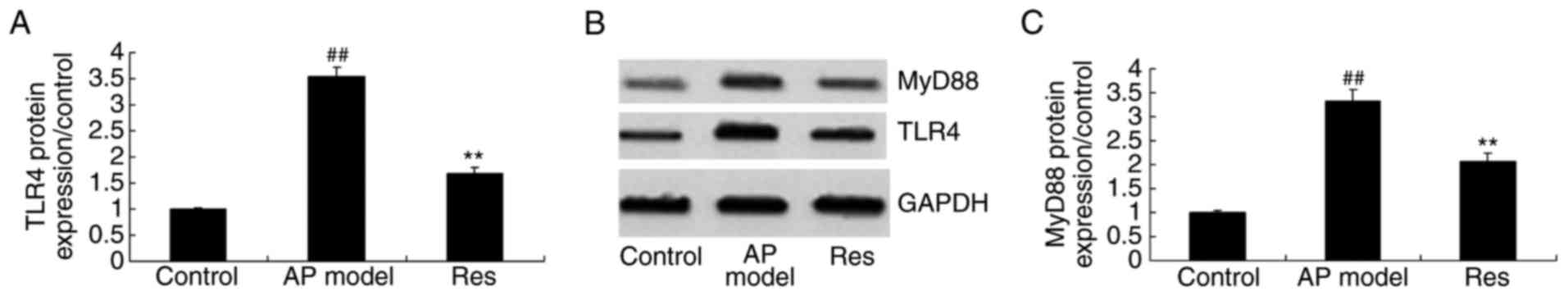
TLR4 and MyD88 protein expression in
animal models of acute pharyngitis
To further demonstrate the anti-inflammation effect
of resveratrol on the TLR4/MyD88 signaling pathway, acute
pharyngitis rabbits were treated with resveratrol. In the acute
pharyngitis model group, there was a significantly increase of TLR4
and MyD88 protein expression levels, compared with the control
group (Fig. 8). The induction of
TLR4 and MyD88 protein expression was significantly suppressed by
resveratrol in acute pharyngitis rabbits, compared with the acute
pharyngitis model group (Fig.
8).
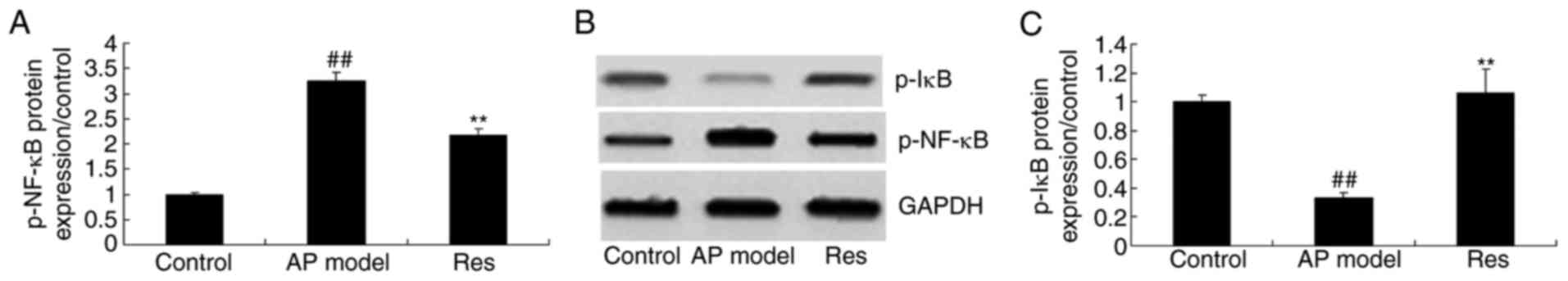
p-NF-κB and p-IκB protein expression
in animal models of acute pharyngitis
To further investigate the anti-inflammation effects
of resveratrol on the NF-κB signaling pathway, p-NF-κB and p-IκB
protein expression were analyzed using western blotting. Compared
with the control group, a significant increase of p-NF-κB protein
expression and inhibition of p-IκB in the acute pharyngitis model
group were observed (Fig. 9).
Resveratrol significantly suppressed p-NF-κB and induced p-IκB
protein expression in animal models of acute pharyngitis, compared
with the acute pharyngitis model group (Fig. 9).
Discussion
Acute pharyngitis, a common exogenous disease,
mainly occurs in the autumn and winter, as well as at the end of
spring and the beginning of summer (4). It is generally caused by the
decreased local or systemic immunity due to catching a cold,
fatigue, or excessive alcohol intake and smoking, which create a
favorable condition for pathogenic microorganisms (11). The preliminary symptoms mainly
include throat itching, dry cough, a sore throat, hoarseness,
fever, aversion to cold, malaise, joint pain, headache and loss of
appetite (12). At present,
patients with acute pharyngitis are mainly treated with symptomatic
therapies such as western anti-inflammatory and antiviral
medicines, as well as therapies of relieving cough and eliminating
phlegm, with slow alleviation of symptoms (13). The results of the present study
confirmed that resveratrol treatment significantly inhibited the
reduced MIP-2 and COX-2 activity levels and ROS production, and
suppressed the increase of caspase-3/9 activity in acute
pharyngitis rabbits.
Macrophages and epithelium produce large amounts of
cytokines and chemokines, as well as recruiting neutrophils,
monocytes, macrophages and lymphocytes under mechanical
stimulation, leading to lung inflammatory reaction, such as the
activation of IL-1β, IL-18, IL-6, IL-2, MIP-2, TNF-a and NF-kB,
which will be more severe if combined with inflammation (14,15).
At the beginning of the activation of the NLRP3 inflammasome, the
inflammasome complex is assembled, which requires the binding of
protein domains apoptosis-associated speck-like protein containing
a CARD (ASC) and NLRP3, as well as the structure domains ASC and
pro-caspase-1. Subsequently, caspase-1 is activated, and IL-1β and
IL-18 are released (16). The
present study demonstrated that resveratrol significantly inhibited
TNF-α and IL-6 serum levels and NLRP3/caspase-1/IL-1β/IL-18 protein
expression in acute pharyngitis rabbits. Similarly, Sui et
al (17) demonstrated that
resveratrol protects against sepsis and inhibits NLRP3/IL-1β in
microglia.
The TLR4/NF-κB signaling pathway also serves an
critical role in the inflammatory response, especially the
activation of macrophages in liver and adipose tissues (18). TLRs, a member of pattern
recognition receptor family, serve a key role in the innate immune
response (19). As the first TLR
protein identified, TLR4 is associated with a variety of
inflammatory reactions, and serves as the main receptor of the
lipopolysaccharide response (19).
TLR4 can activate multiple signaling pathways associated with
inflammation, and induce the expression and secretion of various
cytokines (20). Usually, TLR4
assists the binding of receptor cluster of differentiation 14 and
lymphocyte antigen 96 to form a polymer, and then MyD88 will be
recruited to the structure domain of toll/IL-1 receptor (TIR). The
interaction between TLR4 and MyD88 in the structure domain of TIR
triggers a cascade reaction of downstream signals, thus activating
the NF-KB signaling pathway to promote cells to secrete a variety
of inflammatory cytokines and chemokines (21). The present study demonstrated that
resveratrol significantly suppressed TLR4 and MyD88 protein
expression in acute pharyngitis rabbits. Zhang et al
(22) indicated that resveratrol
attenuates acute inflammatory injury through the TLR4 signaling
pathway in experimental subarachnoid hemorrhage.
NF-κB participates in the regulation of many
cellular functions, and serves an important role in the growth,
proliferation and apoptosis of cells, closely associated with the
innate and acquired immune response (23). NF-κB exists in the cytoplasm under
resting state and binds to IκB, of the inhibitory protein family,
to form a non-active complex (7).
Nearly 20 years of studies have demonstrated that the NF-κB
signaling pathway can be activated by various stimulating factors,
which results in the aggregation of NF-κB in the nucleus;
subsequently, NF-κB will be regulated by many signals in the
nucleus, serving the role of transcription factors (7). The present study revealed that
resveratrol significantly suppressed p-NF-κB and induced pn-IκB
protein expression in animal models of acute pharyngitis. Tian
et al (24) reported that
resveratrol inhibited the NF-κB inflammation pathway in mice with
fatty liver.
In conclusion, the findings of the present study
support that the anti-inflammatory activity of resveratrol prevents
acute pharyngitis and inflammation in a rabbit model of acute
pharyngitis through suppression of NLRP3/caspase-1/IL-1β and IL-18,
and of the TLR4/MyD88/NF-κB signaling pathway. These results
implicate resveratrol as a novel drug for the treatment of acute
pharyngitis.
References
|
1
|
Gu Y, Chen C, Yi S, Wang S, Gong L, Liu J,
Gu X, Zhao Q and Li S: miR-sc8 inhibits schwann cell proliferation
and migration by targeting Egfr. PLoS One. 10:e01451852015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao R, Wang L, Sun J, Nie K, Jian H, Gao
L, Liao X, Zhang H, Huang J and Gan S: MiR-204 promotes apoptosis
in oxidative stress-induced rat Schwann cells by suppressing
neuritin expression. FEBS Lett. 588:3225–3232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulaiman W and Nguyen DH: Transforming
growth factor beta 1, a cytokine with regenerative functions.
Neural Regen Res. 11:1549–1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lottrich M, Mawrin C, Chamaon K, Kirches
E, Dietzmann K and Freigang B: Expression of transforming growth
factor-beta receptor type 1 and type 2 in human sporadic vestibular
schwannoma. Pathol Res Pract. 203:245–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma J, Ma Y, Liu X, Chen S, Liu C, Qin A
and Fan S: Gambogic acid inhibits osteoclast formation and
ovariectomy-induced osteoporosis by suppressing the JNK, p38 and
Akt signalling pathways. Biochem J. 469:399–408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bott A, Erdem N, Lerrer S, Hotz-Wagenblatt
A, Breunig C, Abnaof K, Wörner A, Wilhelm H, Münstermann E,
Ben-Baruch A and Wiemann S: miRNA-1246 induces pro-inflammatory
responses in mesenchymal stem/stromal cells by regulating PKA and
PP2A. Oncotarget. 8:43897–43914. 2017.PubMed/NCBI
|
|
7
|
Chai S, Ng KY, Tong M, Lau EY, Lee TK,
Chan KW, Yuan YF, Cheung TT, Cheung ST, Wang XQ, et al: Octamer
4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in
liver cancer stem cells. Hepatology. 64:2062–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y, Gao F, Hao J and Liu Z:
microRNA-1246 mediates lipopolysaccharide-induced pulmonary
endothelial cell apoptosis and acute lung injury by targeting
angiotensin-converting enzyme 2. Am J Transl Res. 9:1287–1296.
2017.PubMed/NCBI
|
|
9
|
Zhang H, Jiang Y, Nguyen HD, Poo DC and
Wang W: The effect of a smartphone-based coronary heart disease
prevention (SBCHDP) programme on awareness and knowledge of CHD,
stress and cardiac-related lifestyle behaviours among the working
population in Singapore: A pilot randomised controlled trial.
Health Qual Life Outcomes. 15:492017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishdorj G, Johnston JB and Gibson SB:
Cucurbitacin-I (JSI-124) activates the JNK/c-Jun signaling pathway
independent of apoptosis and cell cycle arrest in B leukemic cells.
BMC Cancer. 11:2682011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang H, Xu C, Pan Z, Zhang Y, Xu Z, Chen
Y, Li T, Li X, Liu Y, Huangfu L, et al: The antifibrotic effects
and mechanisms of microRNA-26a action in idiopathic pulmonary
fibrosis. Mol Ther. 22:1122–1133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Liu L, Shen Y, Wang T, Chen L, Xu D
and Wen F: MicroRNA-26a modulates transforming growth factor
beta-1-induced proliferation in human fetal lung fibroblasts.
Biochem Biophys Res Commun. 454:512–517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nie X, Deng M, Yang M, Liu L, Zhang Y and
Wen X: Axonal regeneration and remyelination evaluation of
chitosan/gelatin-based nerve guide combined with transforming
growth factor-β1 and schwann cells. Cell Biochem Biophys.
68:163–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song JL, Zheng W, Chen W, Qian Y, Ouyang
YM and Fan CY: Lentivirus-mediated microRNA-124 gene-modified bone
marrow mesenchymal stem cell transplantation promotes the repair of
spinal cord injury in rats. Exp Mol Med. 49:e3322017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao P, Wang S, Zhou Y, Zheng H and Zhao
G: MicroRNA-185 regulates spinal cord injuries induced by
thoracolumbar spine compression fractures by targeting transforming
growth factor-β1. Exp Ther Med. 13:1127–1132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koga K, Yokoi H, Mori K, Kasahara M,
Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, et al:
MicroRNA-26a inhibits TGF-beta-induced extracellular matrix protein
expression in podocytes by targeting CTGF and is downregulated in
diabetic nephropathy. Diabetologia. 58:2169–2180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sui DM, Xie Q, Yi WJ, et al: Resveratrol
Protects against Sepsis-Associated Encephalopathy and Inhibits the
NLRP3/IL-1beta Axis in Microglia. Mediators Inflamm.
2016:10456572016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reddy PH, Williams J, Smith F, Bhatti JS,
Kumar S, Vijayan M, Kandimalla R, Kuruva CS, Wang R, Manczak M, et
al: MicroRNAs, aging, cellular senescence and alzheimer's disease.
Prog Mol Biol Transl Sci. 146:127–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tigchelaar S, Streijger F, Sinha S,
Flibotte S, Manouchehri N, So K, Shortt K, Okon E, Rizzuto MA,
Malenica I, et al: Serum MicroRNAs reflect injury severity in a
large animal model of thoracic spinal cord injury. Sci Rep.
7:13762017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar S and Reddy PH: Are circulating
microRNAs peripheral biomarkers for Alzheimer's disease? Biochim
Biophys Acta. 1862:1617–1627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Z, Zhou H, Lu L, Li X, Fu Z, Liu J,
Kang Y, Wei Z, Pan B, Liu L, et al: The roles of microRNAs in
spinal cord injury. Int J Neurosci. 1–12. 2017.
|
|
22
|
Zhang XS, Li W, Wu Q, et al: Resveratrol
Attenuates Acute Inflammatory Injury in Experimental Subarachnoid
Hemorrhage in Rats via Inhibition of TLR4 Pathway. Int J Mol Sci.
17:2016.
|
|
23
|
Han K, Chen X, Bian N, Ma B, Yang T, Cai
C, Fan Q, Zhou Y and Zhao TB: MicroRNA profiling identifies MiR-195
suppresses osteosarcoma cell metastasis by targeting CCND1.
Oncotarget. 6:8875–8889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian Y, Ma J, Wang W, et al: Resveratrol
supplement inhibited the NF-kappaB inflammation pathway through
activating AMPKalpha-SIRT1 pathway in mice with fatty liver. Mol
Cell Biochem. 422:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|