Introduction
Epithelial-mesenchymal transition (EMT) of renal
proximal tubular epithelial cells in adult kidneys is one of the
key events that occurs during the development of renal
tubulointerstitial fibrosis (1–5),
contributing to renal fibrogenesis. Previous research has
demonstrated that various injury factors (including high blood
glucose, proteinuria, hypertension, high blood cholesterol, high
protein intake and uremic toxins) can all lead to renal tubular
injury, which then aggravates the progression of renal fibrosis
(6–9). Recently, dysregulation of lipid
metabolism in the occurrence and development of renal interstitial
fibrosis, has received increasingly widespread awareness by
international kidney disease experts. Oxidized low-density
lipoprotein (Ox-LDL), the most widely studied of all lipoproteins,
has been reported to cause renal injuries through multiple
mechanisms. Ox-LDL, a LDL oxidation product with potent cell
cytotoxic properties, is considered an indicator of oxidative
stress levels in mesangial cells, which can trigger intracellular
lipid oxidation, release of reactive nitrogen and reactive oxygen
species (ROS), and deteriorative oxidative stress, thus further
promoting cell injury (10).
However, previous studies have focused on renal intraglomerular
mesangial cells, endothelial cells and monocyte/macrophage cells,
and explored the roles of Ox-LDL in promoting glomerular injuries
and sclerosis (10–12). Few research results have been
reported on the injury effects and mechanisms of Ox-LDL in human
renal proximal tubular epithelial cells and on whether antioxidant
therapy could alleviate the injury.
Lectin-like oxidized low-density lipoprotein
receptor-1 (LOX-1), a receptor of Ox-LDL that was first discovered
and identified in bovine aortic endothelial cells (13), is expressed in endothelial cells,
macrophages, vascular smooth muscle cells, platelets, and
adipocytes, but also in epithelial cells (14,15).
As LOX-1 can bind to multiple ligands, it has diverse physiological
functions and critical roles in signal transduction.
Oxidative stress, a major cause of cell damage,
aging and cell death, has recently received increasing attention
regarding its role in renal tubule injury. Kidney tissues require a
higher oxygen consumption to complete the active transport and
reabsorption of water and electrolytes. Therefore, renal tubules
are more susceptible to oxidative damage. As a second messenger in
a variety of signaling pathways, ROS participates in numerous
physiological and pathological processes, including proliferation,
differentiation and apoptosis (16–19).
It is generally accepted that damage of ROS to the kidney is
normally in the deposition of glomerular extracellular matrix (ECM)
and renal tubular EMT (20–22).
Probucol, as an antioxidant and anti-inflammatory drug, reduces ROS
production. It has been reported that probucol could ameliorate
renal tubular EMT in a diabetic nephropathy rat model (23).
Previous studies have suggested that the
dysregulation of lipid metabolism may also be involved in the renal
tubular transdifferentiation process, and ultimately may lead to
the occurrence of renal interstitial fibrosis (24). However, there is little evidence
supporting this hypothesis and the mechanism remains unclear. For
that purpose, the present study examined the EMT status and the
LOX-1 and ROS production in cultured renal proximal tubular cells
induced with Ox-LDL. In addition, the molecular mechanism and
downstream signaling pathways of LOX-1 and ROS were investigated,
as well as the potential protective effects of the antioxidant
reagent probucol on the above processes.
Materials and methods
Cell culture
Human HK-2 renal proximal tubule epithelial cells
(American Type Culture Collection, Manassas, VA, USA) were cultured
in Dulbecco's modified Eagles medium (DMEM)-F12 medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Biochrom, Ltd., Cambridge, UK), L-glutamine (2
mM), penicillin (100 U/ml), and streptomycin (100 µg/ml) (all from
Thermo Fisher Scientific, Inc.) in a humidified incubator
containing 5% CO2 at 37°C. Cells were pretreated with 20
µmol/l probucol or 250 µg/ml LOX-1 inhibitor polyinosinic acid
(both from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 h
in advance, and stimulated with Ox-LDL (0–100 µg/ml; Peking
Union-Biology Co., Ltd., Beijing, China) for 24 h.
MTT assay
Cells plated in 96-well plates
(2×104/well) in 200 µl medium were treated with 50 µg/ml
Ox-LDL for 0–72 h or 0–200 µg/ml Ox-LDL for 24 h. Cells were
incubated with 20 µl 5 g/l MTT solution (Sigma-Aldrich; Merck KGaA)
for 4 h. The supernatant was then removed, and 100 µl of DMSO was
added to each well. The absorbance at 570 nm was measured using a
spectrophotometric plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Results were quantified from four independent
experiments.
Nitric oxide (NO) measurement
NO was determined using the Total NO assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). HK-2 cells
were seeded in 96-well culture plates (8×104
cells/well), pretreated with 20 µmol/l probucol or 250 µg/ml LOX-1
inhibitor polyinosinic acid as mentioned above, and then stimulated
with 50 µg/m1 Ox-LDL for 24 h at 37°C. Then, the culture
supernatants were used to determine the concentration of NO. The
standard provided in the kit was diluted with DMEM-F12 medium to a
variety of concentrations, as indicated: 0, 1, 2, 5, 10, 20, 40, 60
and 100 µg/ml. Afterwards, the supernatant of each sample or 50 µl
of the above standard were added in a 96-well plate together with
50 µl Griess Reagent I and Griess Reagent II. The absorbance at 550
nm was measured using a spectrophotometric plate reader (Bio-Rad
Laboratories, Inc.). Results were quantified from four independent
experiments.
Intracellular ROS measurement
The intracellular ROS levels were measured using the
specific probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA;
Beyotime Institute of Biotechnology) kit. HK-2 cells seeded
(8×105 cells/well) in 6-well cell culture plates were
pretreated by probucol or polyinosinic acid as mentioned above, and
then stimulated with 50 µg/m1 Ox-LDL for 24 h at 37°C. Afterwards,
serum-free medium containing 10 µM DCFH-DA was added to each well
for 20 min at 37°C in the dark. In order to fully remove the
remaining DCFH-DA, the cells were washed with serum-free medium
three times, then detached from the plates with 0.25% trypsin, and
collected by centrifugation at 300 × g for 5 min at 4°C. Positive
control cells were simultaneously treated with Rosup (a ROS
positive control provided by the kit). FACScan flow cytometric and
CellQuest Pro software v5.1 (both from BD Biosciences, San Jose,
CA, USA) analyses were conducted on the various cell treatment
groups to detect the average fluorescence intensity of
dichlorofluorescein (DCF), at an excitation wavelength of 488 nm
and at an emission wavelength of 525 nm. Results were quantified
from four independent experiments.
Western blotting
Proteins were extracted using a
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology) that contains the protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) and protein concentrations were
calculated by using the Pierce bicinchoninic protein assay kit
(Thermo Fisher Scientific, Inc.). An equal amount of protein (40
µg) was taken from each group. The samples were mixed with 5X SDS
sample buffer and heated at 100°C for 5 min, then loaded onto 10 or
12% Tris-glycine SDS-PAGE gel and separated at 100 V for 1.5 to 2
h. Primary antibodies against E-cadherin (cat no. 14472; 1:1,000),
extracellular signal-regulated kinase (ERK; cat no. 4696; 1:2,000),
phosphorylated (p-) ERK (cat no. 4094; 1:1,000), p38 (cat no. 9218;
1:1,000) and p-p38 (cat no. 9216; 1:2,000) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Primary antibodies
against LOX-1 (cat no. sc-20753; 1:300), NADPH oxidase 4 (NOX4; cat
no. sc-30141; 1:300), and p22phox (cat no. sc-271968; 1:200) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Primary antibodies against α-SMA (cat no. A2547; 1:500) and β-actin
(cat no. A5441; 1:1,000) were purchased from Sigma-Aldrich (Merck
KGaA). Following transferring of proteins to a polyvinylidene
fluoride membrane, nonspecific binding to the membrane was blocked
with 5% non-fat dry milk for 2 h at room temperature. The membranes
were then incubated overnight at 4°C with primary antibodies
against LOX-1, α-SMA, E-cadherin, NOX4, p22phox, ERK, p-ERK, ERK,
p-p38, and p38. Following washing, membranes were incubated with
goat anti-mouse (cat no. BA1051; 1:2,000) or anti-rabbit (cat no.
BA1055; 1:2,000) (both from Wuhan Boster Biological Technology,
Ltd., Wuhan, China) horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature. The signals were visualized
by enhanced chemiluminescence (Pierce; Thermo Fisher Scientific,
Inc.). ImageJ 1.37 (National Institute of health, Bethesda, MD,
USA) analysis system was used to analyze the signal absorbance.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 21.0; IBM SPSS, Armonk, NY, USA). All the data
represent at least three independent experiments and are expressed
as the means ± standard deviation unless otherwise indicated.
Differences between groups were determined using an unpaired
Student's t-test or a one-way analysis of variance (anova; S-N-K
post hoc test was used after the anova) when multiple comparisons
were required. P<0.05 was considered to indicate a statistically
significant difference.
Results
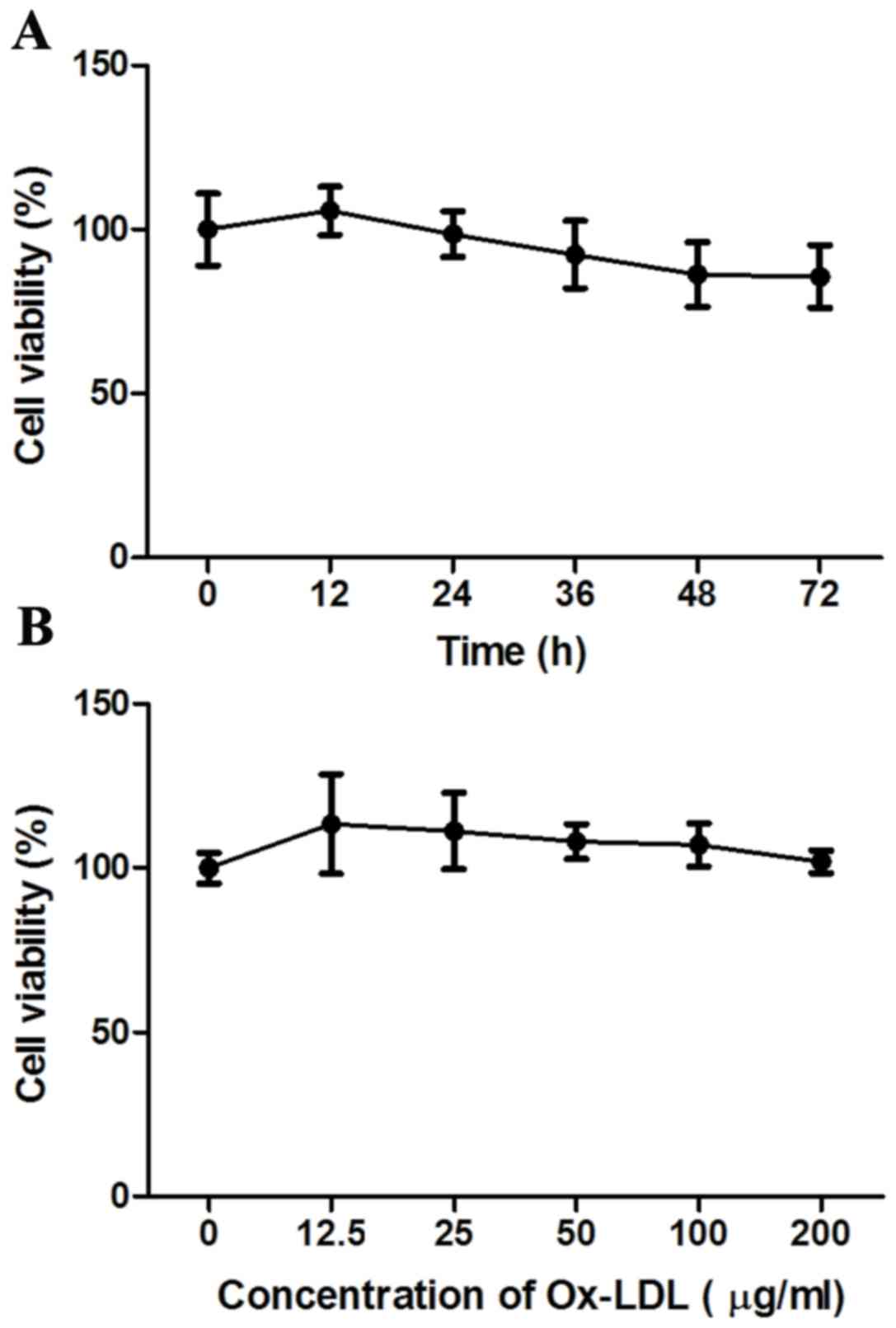
Effect of Ox-LDL on the viability of
HK-2 cells
The effect of Ox-LDL stimulation on the viability of
HK-2 cells was examined using the MTT assay. The results
demonstrated that there was a slight decrease in the total numbers
of viable cells in the Ox-LDL-stimulated groups in different times
(24–72 h) but over the range of different dosages (12.5–200 µg/ml)
after 24 h there was only a small change in cell viability,
compared with the control untreated group (Fig. 1). However, the differences were not
significant (P>0.05), suggesting that Ox-LDL had no effect on
the viability of HK-2 cells at the doses and times tested.
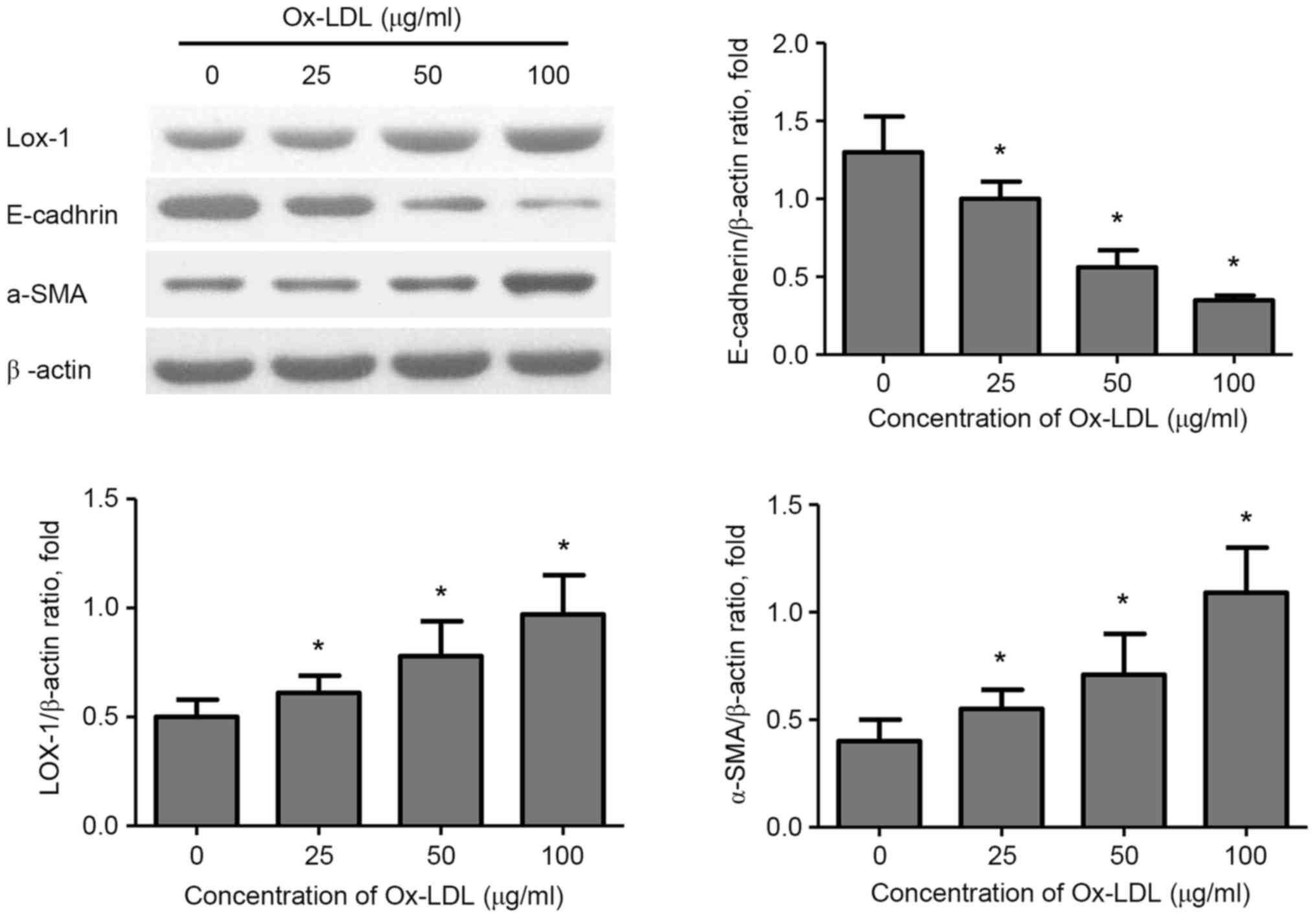
Effect of Ox-LDL on α-SMA, E-cadherin
and LOX-1 protein expression in HK-2 cells
To investigate the effects of Ox-LDL stimulation on
EMT in HK-2cells, the protein expression levels of the mesenchymal
marker α-SMA and of the epithelial marker E-cadherin, as well as
LOX-1 expression, were determined by western blotting. The results
demonstrated that stimulation with Ox-LDL (25–100 µg/ml) resulted
in a significant increase in the expression of LOX-1 and α-SMA in
HK-2 cells in a dose-dependent manner, while E-cadherin expression
was significantly decreased (Fig.
2).
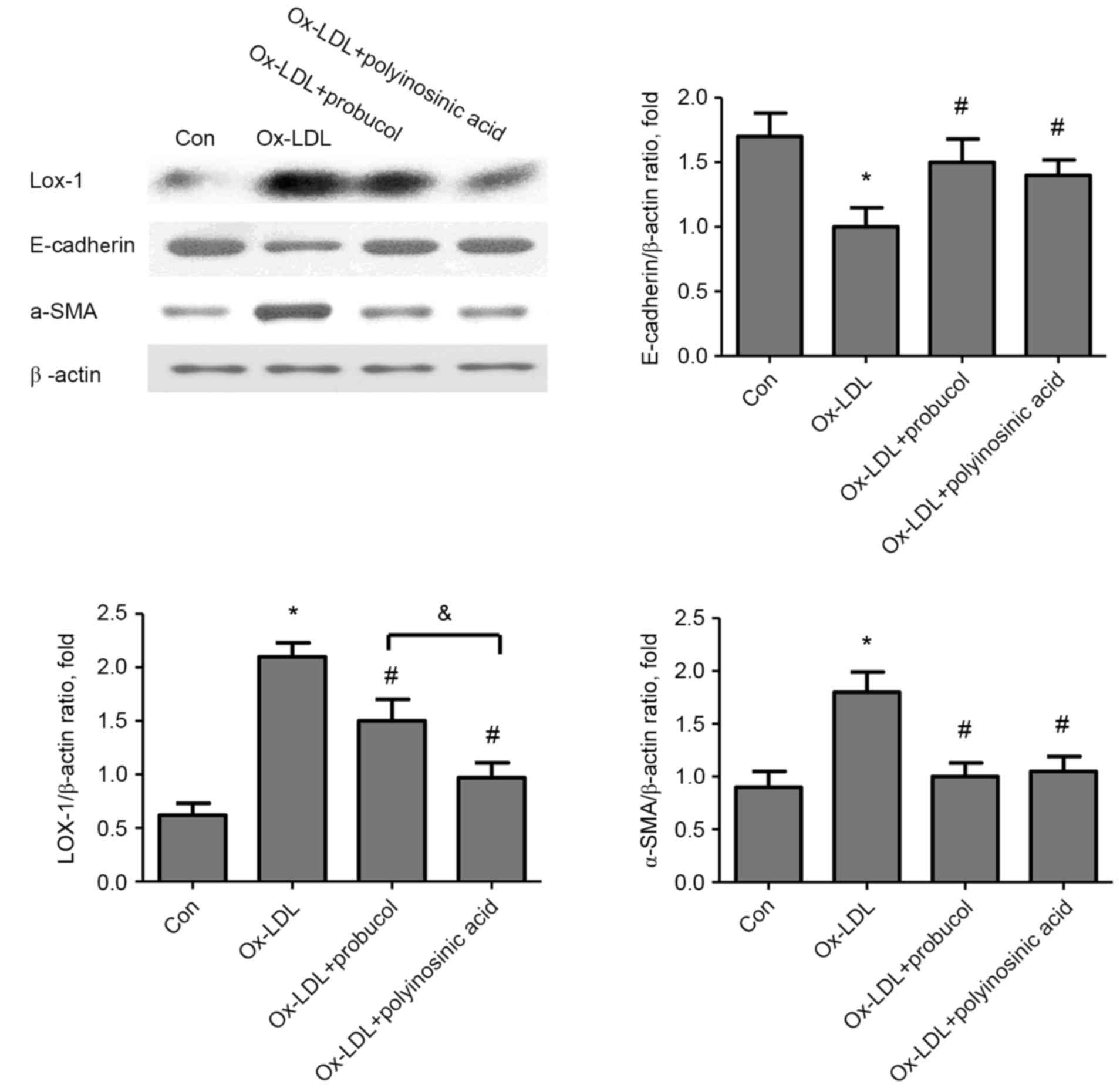
Effect of probucol on α-SMA,
E-cadherin, and LOX-1 protein expression in Ox-LDL-stimulated HK-2
cells
According to the above results, the dose of 50 µg/ml
Ox-LDL was selected to stimulate HK-2 cells in the following
experiments. Cells were pretreated with probucol (20 µmol/l) or the
LOX-1 inhibitor polyinosinic acid (250 µg/ml), stimulated with
Ox-LDL and then analyzed by western blotting. The results
demonstrated a significant increase in α-SMA and LOX-1 protein
expression levels in HK-2 cells stimulated with 50 µg/ml Ox-LDL,
and this effect was significantly inhibited by probucol or
polyinosinic acid pretreatment (Fig.
3). Of note, the inhibition of LOX-1 expression by probucol was
weaker than by polyinosinic acid (Fig.
3). E-cadherin protein expression levels exhibited the opposite
effect, with decreasing levels following Ox-LDL stimulation and
increasing levels following probucol or polyinosinic acid
pretreatment (Fig. 3).
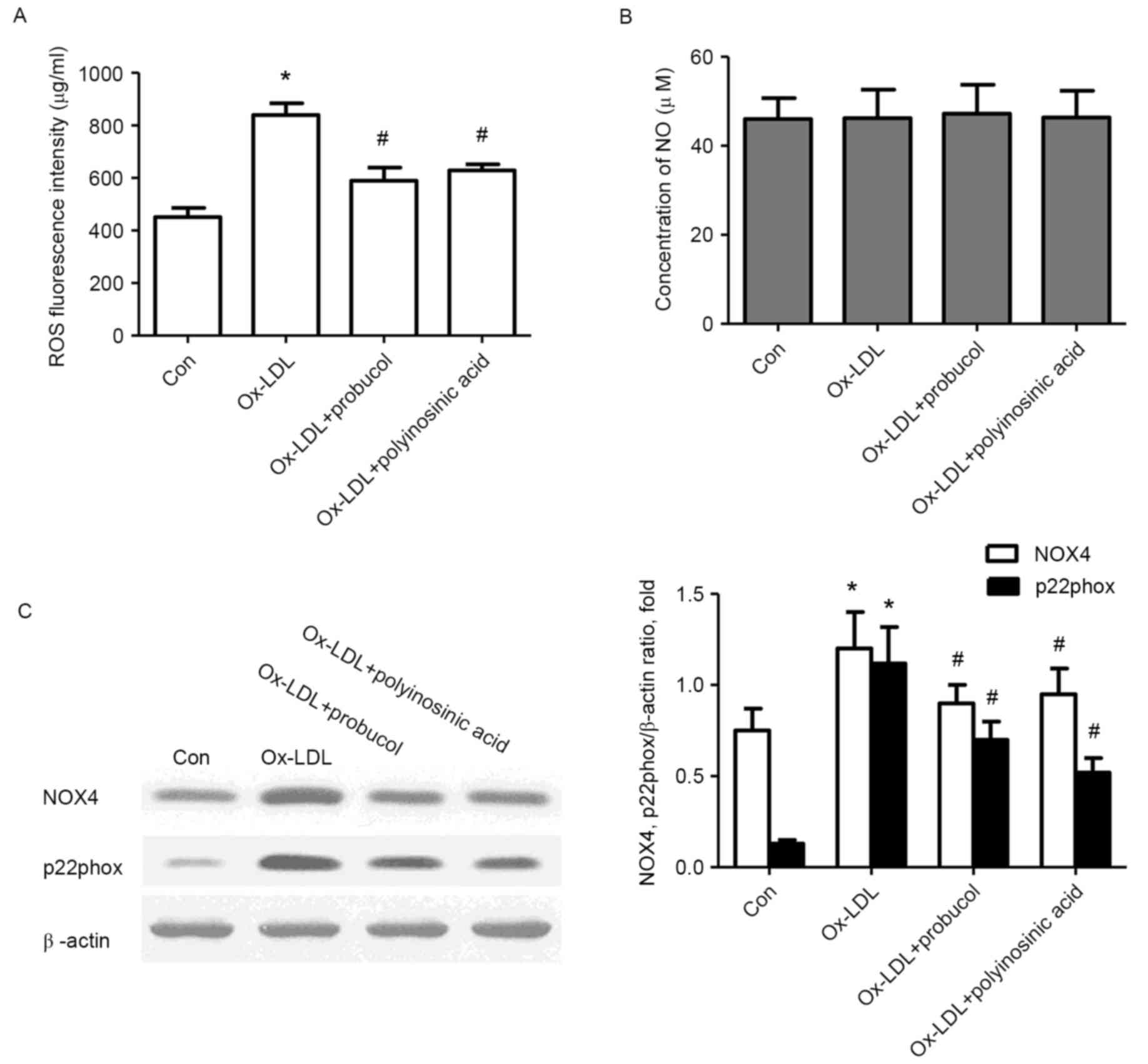
Effects of probucol on ROS and NO
production in Ox-LDL-stimulated HK-2 cells
To specify whether oxidative stress was involved in
the process of EMT in HK-2 cells induced by Ox-LDL, the production
of ROS was examined using the specific probe DCFH-DA and the
concentration of NO was detected using a biochemical colorimetric
method. The results revealed that the basic activity of ROS in HK-2
cells was 451.5 µg/ml. Following stimulation with 50 µg/ml Ox-LDL,
ROS activity increased to 839.8 µg/ml (P<0.05; Fig. 4A), however, this increase was
inhibited by probucol or polyinosinic acid pretreatment (P<0.05;
Fig. 4A). The concentration of NO
in the culture supernatant of the control unstimulated HK-2 cells
was 46.0 µmol/l (Fig. 4B).
Following stimulation with 50 µg/ml Ox-LDL, NO concentration was
46.2 µmol/l, which was not significantly different compared with
control (P>0.05; Fig. 4B).
Similarly, pretreatment with probucol or polyinosinic acid did not
have any effect on NO concentration (P>0.05; Fig. 4B).
Effects of probucol on NOX4 and
p22phox expression in Ox-LDL-stimulated HK-2 cells
Because NADPH oxidase activation is a major source
of ROS in cells, the effect of probucol on the oxidative
stress-related proteins NOX4 and p22phox was examined in HK-2 cells
induced by Ox-LDL. Western blot analysis demonstrated an obvious
increase in the protein expression levels of NOX4 and p22phox
following OX- LDL stimulation (Fig.
4C). However, pretreatment with probucol or polyinosinic acid
significantly suppressed these increases (P<0.05; Fig. 4C).
Effect of probucol on ERK1/2 and p38
MAPK signaling pathways in Ox-LDL-induced HK-2 cells
To further understand the mechanism responsible for
the Ox-LDL-induced EMT in renal tubular epithelial cells, the
activation status of the ERK1/2 and p38 MAPK signaling pathways was
investigated. Following Ox-LDL stimulation in HK-2 cells, an
increase in both ERK1/2 and p38 phosphorylation was observed at 5
min of stimulation, and peaking at 30 min (Fig. 5A). ERK1/2 and p38 phosphorylation
was significantly inhibited by pretreatment with probucol or
polyinosinic acid (Fig. 5B).
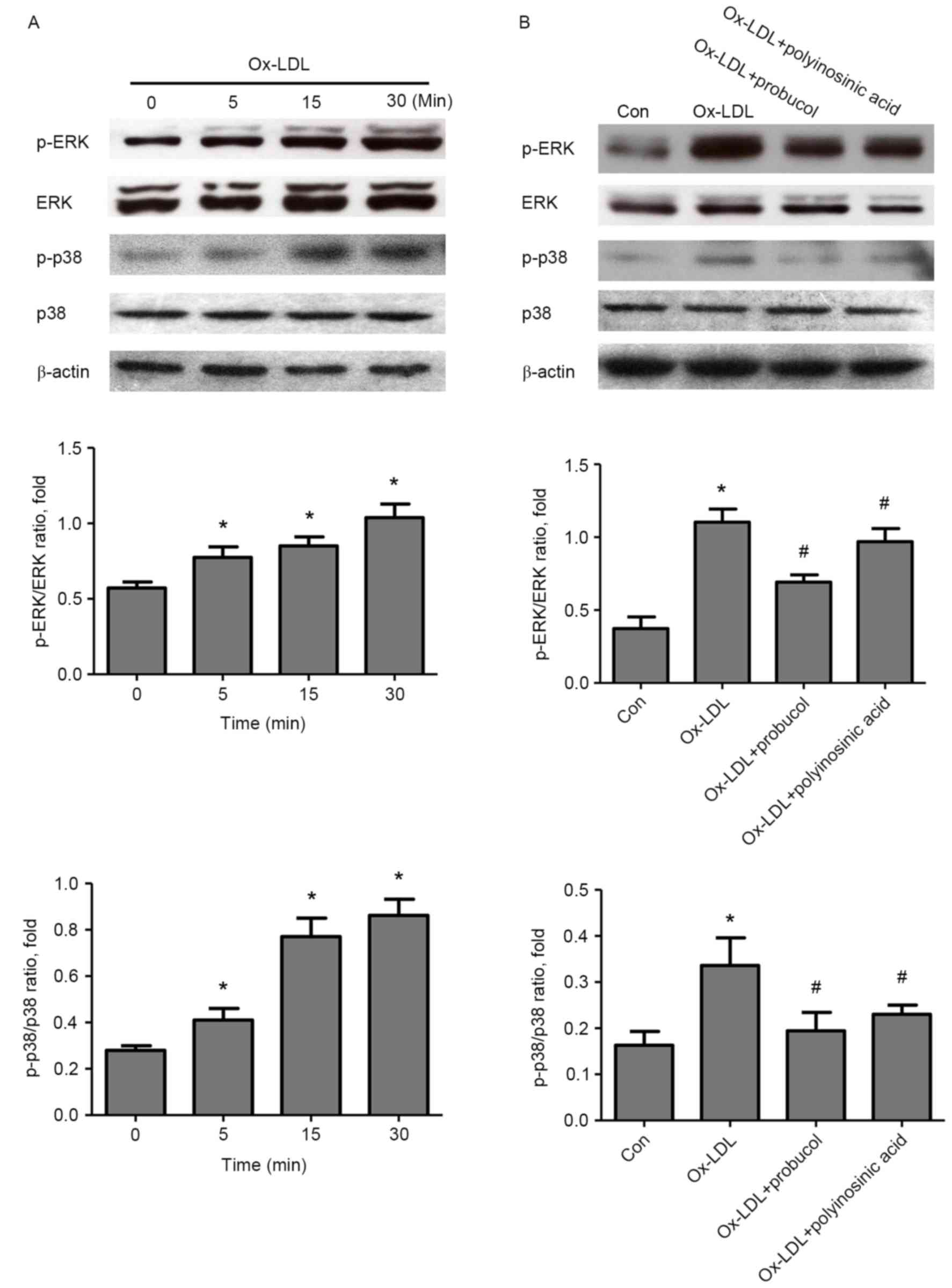 | Figure 5.Effect of probucol on ERK and p38
MAPK signaling in Ox-LDL-induced HK-2 cells. (A) Cells were
stimulated with Ox-LDL (50 µg/ml) for 5, 15 and 30 min and then
analyzed by western blotting, in order to determine the effect of
Ox-LDL on ERK and p38 MAPK pathway activation. (B) Cells were
pretreated with probucol (20 µmol/l) or with the LOX-1 inhibitor
polyinosinic acid (250 µg/ml) for 2 h, stimulated with Ox-LDL (50
µg/ml) for 30 min, and then analyzed by western blotting.
Representative images and quantification, with β-actin used as a
loading control. *P<0.05 vs. control; and #P<0.05
vs. Ox-LDL alone. ERK, extracellular signal-regulated kinase; MAPK,
mitogen-activated protein kinase; Ox-LDL, oxidized low density
lipoprotein; LOX-1, lectin-like oxidized low-density lipoprotein
receptor-1; p, phosphorylated; Con, control. |
Discussion
Renal fibrosis, characterized by glomerulosclerosis
and tubulointerstitial fibrosis, is the final common pathway of a
wide variety of chronic kidney disease (CKD) leading to the
complete destruction of kidney parenchyma and end-stage renal
disease, requiring dialysis or kidney transplant (25). Studies have demonstrated that in
renal fibrosis, the correlation of renal tubulointerstitial
fibrosis with renal function is much closer than of
glomerulosclerosis (26). The
renal proximal tubular epithelial cells, which are susceptible to
injuries by a wide variety of factors, are generally considered to
have a pivotal role in fibrogenesis. The proximal tubular
epithelial cells undergo EMT, resulting in matrix-producing
fibroblasts, and thereby contributing to the pathogenesis of renal
interstitial fibrosis and end-stage renal failure.
In the present study, the lipoprotein Ox-LDL was
demonstrated to induce EMT in tubular epithelial cells. A small
amount of LOX-1 expression was observed in control HK-2 cells,
which was then upregulated by Ox-LDL stimulation in a
dose-dependent manner, indicating that LOX-1 may have contributed
to the EMT of proximal tubular epithelial cells. In order to
determine the mechanism of LOX-1 in the injury of HK-2 cells, the
LOX-1 inhibitor polyinosinic acid and the antioxidant probucol were
used to pretreat the cells. The results indicated that pretreatment
suppressed both LOX-1 expression and EMT. In addition, Ox-LDL was
demonstrated to induce oxidative stress, with expression of p22phox
and NOX4 and production of ROS significantly increased in HK-2
cells following Ox-LDL stimulation. However, these increases were
inhibited by pretreatment with the LOX-1 inhibitor or probucol. No
significant differences were observed in NO concentration in any of
the treatment groups. Of note, the inhibition of LOX-1 by probucol
was weaker than by the LOX-1 inhibitor, suggesting that Ox-LDL
induced LOX-1 expression, which may have activated NADPH
oxidase-mediated ROS generation and LOX-1 upregulation. These
observations provide evidence for a positive feedback loop
involving Ox-LDL, NADPH oxidase-mediated ROS generation and
LOX-1.
Recently, there has been increasing interest in the
role of LOX-1 in the progression of renal diseases. A previous
study has reported that high glucose levels can induce the
expression of LOX-1 at the gene promoter/transcription level via a
p38 MAPK-dependent mechanism, leading to Ox-LDL-induced apoptosis
in renal tubular epithelial cells (27). It has also been reported that LOX-1
and NADPH oxidase are upregulated in human renal proximal tubular
epithelial cells induced with angiotensin II (Ang II), and that the
activation of the MAPK pathway occurs downstream of the Ang
II/ROS/LOX-1 cascade (28). A
study in LDLr-null mice has demonstrated that high fat diet induced
renal fibrosis through activation of the LOX-1/p38, p44/42/nuclear
factor-κB pathway by Ox-LDL (29).
The above studies suggest that an increase in LOX-1 expression is
observed in renal tubular epithelial cells, and this is associated
with the pathogenesis and progression of chronic renal tubular and
interstitial diseases. The present results demonstrated that LOX-1
expression was increased following Ox-LDL stimulation and EMT
induction in HK-2 cells, which is consistent with previous
studies.
Renal tubules are known to be susceptible to injury
from oxidative stress, in which ROS are crucial cell-damaging
molecules. Studies reveal that ROS induces apoptosis and EMT in
renal tubular epithelial cells through the MAPK pathway (20,30,31).
High glucose induces EMT by decreasing intracellular ROS levels via
the downregulation of NADPH oxidase subunits NOX1 and NOX4, and
activation of ERK1/2 is associated with high glucose-induced EMT in
HK-2 cells (21). The present
results demonstrated that Ox-LDL stimulation triggered oxidative
stress and NOX4 and p22phox upregulation, but NO was not involved
in this process.
The MAPK pathway, which includes ERK, c-Jun
N-terminal kinase (JNK) and p38 MAPKs (32–34),
can be activated by ROS. Previous studies have demonstrated that
ERK and p38 MAPK signaling pathways have a vital role in EMT,
inflammation of renal proximal tubule epithelial cells and renal
injury (33,35–39).
Zhou et al (38) reported
that the knockdown of thioredoxin-interacting protein antagonized
the high glucose-induced EMT by inhibiting ROS production and by
activation of p38 MAPK and ERK1/2 (40). ROS is also important in
transforming growth factor-β1-induced EMT, primarily through
activation of p38, ERK MAPKs, and the subsequent ERK-directed
activation of the Smad pathway in proximal tubular epithelial cells
(20). Consistent with previous
studies, probucol and polyinosinic acid pretreatments were
demonstrated to prevent the Ox-LDL-mediated activation of ERK and
p38 MAPKs in HK-2 cells, indicating that probucol may protect HK-2
cells against Ox-LDL-induced EMT by modulating the ERK and p38 MAPK
signaling pathways.
Probucol is a phenolic lipid-lowering prototype
agent with anti-inflammatory and antioxidant properties, and has a
long history of clinical application for the treatment and
prevention of cardiovascular diseases. Its ability to consume
oxidation substances, such as oxygen free radicals, serves an
important role in its antioxidant, anti-inflammatory and
antiapoptotic functions. In a rat model of diabetic nephropathy,
probucol inhibited EMT in renal tubular epithelial cells by
downregulating specificity protein 1 expression in kidney (23). It has also been reported to
attenuate podocytes injury in type 2 diabetic nephropathy of db/db
mice through the suppression of NADPH oxidase NOX2 (41). The present data revealed that
probucol may have a protective role on the injury of HK-2 cells by
modulating LOX-1 and oxidative stress.
There is controversy regarding the use of probucol
in cardiovascular diseases and some western countries have stopped
its use because of the reduction in serum HDL-cholesterol (HDL-C).
However the HDL-C reduction may not be a ‘side effect’ but may
reflect a mechanism of action of probucol as it causes a decrease
in HDL-C by enhancing plasma cholesteryl ester transfer protein
activity and hepatic scavenger receptor class B type I. Probucol
also accelerates the antioxidant function of HDL via increasing
paraoxonase 1 activity (42). In
particular, a significant coronary artery disease risk reduction
has been demonstrated in the long-term treatment of patients with
heterozygous familial hypercholesterolemia (FH) in Japan with
probucol (43). And there is also
evidence for the pleiotropic and beneficial therapeutic effects of
probucol on the cardiovascular system and in chronic kidney disease
(42,44). As a result of these studies it is
clear that probucol may have beneficial therapeutic effects in
certain suitable CKD patients. In conclusion, probucol may postpone
renal interstitial fibrosis through the suppression of
Ox-LDL-induced EMT in HK-2 cells via the downregulation of LOX-1,
NADPH oxidase, and ROS production and the inhibition of MAPK
signaling activation. The present findings suggest that probucol
may serve as a useful antioxidant agent for postponing the
progression of renal interstitial diseases. However, further in
vivo and in vitro studies are needed to elucidate the
mechanism involved.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (grant no. 81473480),
Key Medical Discipline Project Shanghai Municipal Health Bureau
(grant no. ZK2015A18), Independent Innovation Research Fund of
Putuo District Science and Technology Committee (grant no.
2012PTKW006), Potential Health Professionals in Shanghai University
of Traditional Chinese Medicine (grant no. B-X-78), and Shanghai
Municipal Health and Family Planning Commission (grant no.
201540056).
References
|
1
|
Galichon P and Hertig A: Epithelial to
mesenchymal transition as a biomarker in renal fibrosis: Are we
ready for the bedside? Fibrogenesis Tissue Repair. 4:112011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
He J, Xu Y, Koya D and Kanasaki K: Role of
the endothelial-to-mesenchymal transition in renal fibrosis of
chronic kidney disease. Clin Exp Nephrol. 17:488–497. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carew RM, Wang B and Kantharidis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allison SJ: Fibrosis: Targeting EMT to
reverse renal fibrosis. Nat Rev Nephrol. 11:5652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zha D, Cheng H, Li W, Wu Y, Li X, Zhang L,
Feng YH and Wu X: High glucose instigates tubulointerstitial injury
by stimulating hetero-dimerization of adiponectin and angiotensin
II receptors. Biochem Biophys Res Commun: pii:
S0006-291X(17)31600-5. 2017. View Article : Google Scholar
|
|
7
|
Wahl P, Ducasa GM and Fornoni A: Systemic
and renal lipids in kidney disease development and progression. Am
J Physiol Renal Physiol. 310:F433–F445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cuenca-Sánchez M, Navas-Carrillo D and
Orenes-Piñero E: Controversies surrounding high-protein diet
intake: Satiating effect and kidney and bone health. Adv Nutr.
6:260–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noone D and Licht C: Chronic kidney
disease: A new look at pathogenetic mechanisms and treatment
options. Pediatr Nephrol. 29:779–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee HS and Song CY: Oxidized low-density
lipoprotein and oxidative stress in the development of
glomerulosclerosis. Am J Nephrol. 29:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee HS, Kim BC, Kim YS, Choi KH and Chung
HK: Involvement of oxidation in LDL-induced collagen gene
regulation in mesangial cells. Kidney Int. 50:1582–1590. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurukulasuriya Romayne L, Athappan G, Saab
G, Connell Whaley A and Sowers JR: HMG CoA reductase inhibitors and
renoprotection: The weight of the evidence. Ther Adv Cardiovasc
Dis. 1:49–59. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sawamura T, Kume N, Aoyama T, Moriwaki H,
Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T and Masaki
T: An endothelial receptor for oxidized low-density lipoprotein.
Nature. 386:73–77. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu C, Kang BY, Megyesi J, Kaushal GP,
Safirstein RL and Mehta JL: Deletion of LOX-1 attenuates renal
injury following angiotensin II infusion. Kidney Int. 76:521–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly KJ, Wu P, Patterson CE, Temm C and
Dominguez JH: LOX-1 and inflammation: A new mechanism for renal
injury in obesity and diabetes. Am J Physiol Renal Physiol.
294:F1136–F1145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Wu L, Du S, Hu Y, Fan Y and Ma J:
1,25(OH)2D3 inhibits high glucose-induced apoptosis and ROS
production in human peritoneal mesothelial cells via the MAPK/P38
pathway. Mol Med Rep. 14:839–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pinto M, Pickrell AM, Wang X, Bacman SR,
Yu A, Hida A, Dillon LM, Morton PD, Malek TR, Williams SL and
Moraes CT: Transient mitochondrial DNA double strand breaks in mice
cause accelerated aging phenotypes in a ROS-dependent but
p53/p21-independent manner. Cell Death Differ. 24:288–299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan X, Lederman R, Eng JM, Shoshtari SS,
Saleem MA, Malhotra A and Singhal PC: Nicotine induces podocyte
apoptosis through increasing oxidative stress. PLoS One.
11:e01670712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Y, Han B, Luo K, Ren Z, Cai L and Sun
L: NOX2-ROS-HIF-1a signaling is critical for the inhibitory effect
of oleanolic acid on rectal cancer cell proliferation. Biomed
Pharmacother. 85:733–739. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh
ST and Lee HB: Role of reactive oxygen species in TGF-beta1-induced
mitogen-activated protein kinase activation and
epithelial-mesenchymal transition in renal tubular epithelial
cells. J Am Soc Nephrol. 16:667–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He T, Guan X, Wang S, Xiao T, Yang K, Xu
X, Wang J and Zhao J: Resveratrol prevents high glucose-induced
epithelial-mesenchymal transition in renal tubular epithelial cells
by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol.
402:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jha JC, Thallas-Bonke V, Banal C, Gray SP,
Chow BS, Ramm G, Quaggin SE, Cooper ME, Schmidt HH and
Jandeleit-Dahm KA: Podocyte-specific Nox4 deletion affords
renoprotection in a mouse model of diabetic nephropathy.
Diabetologia. 59:379–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duan SB, Liu GL, Wang YH and Zhang JJ:
Epithelial-to-mesenchymal transdifferentiation of renal tubular
epithelial cell mediated by oxidative stress and intervention
effect of probucol in diabetic nephropathy rats. Ren Fail.
34:1244–1251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ni J, Ma KL, Wang CX, Liu J, Zhang Y, Lv
LL, Ni HF, Chen YX, Ruan XZ and Liu BC: Activation of
renin-angiotensin system is involved in dyslipidemia-mediated renal
injuries in apolipoprotein E knockout mice and HK-2 cells. Lipids
Health Dis. 12:492013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eddy AA: Progression in chronic kidney
disease. Adv Chronic Kidney Dis. 12:353–365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klahr S, Morrissey J, Hruska K, Wang S and
Chen Q: New approaches to delay the progression of chronic renal
failure. Kidney Int Suppl. S23–S26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Liu R, Duan S, Huang G, Ye Y and
Kong Y: High glucose enhances oxLDL-induced apoptosis in human
renal proximal tubular epithelial cells largely via inducing
lectin-like ox-LDL receptor-1. Pharmacology. 98:20–28. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu Y, Ruan S, Xie H and Lin J: Role of
LOX-1 in Ang II-induced oxidative functional damage in renal
tubular epithelial cells. Int J Mol Med. 26:679–690. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai Y, Palade P, Wang X, Mercanti F, Ding
Z, Dai D and Mehta JL: High fat diet causes renal fibrosis in
LDLr-null mice through MAPK-NF-κB pathway mediated by Ox-LDL. J
Cardiovasc Pharmacol. 63:158–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang CY, Shen CY, Kang CK, Sher YP, Sheu
WH, Chang CC and Lee TH: Taurine protects HK-2 cells from oxidized
LDL-induced cytotoxicity via the ROS-mediated mitochondrial and
p53-related apoptotic pathways. Toxicol Appl Pharmacol.
279:351–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu M and Park J, Wu X, Li Y, Tran Q, Mun
K, Lee Y, Hur GM, Wen A and Park J: Shen-Kang protects 5/6
nephrectomized rats against renal injury by reducing oxidative
stress through the MAPK signaling pathways. Int J Mol Med.
36:975–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wankun X, Wenzhen Y, Min Z, Weiyan Z, Huan
C, Wei D, Lvzhen H, Xu Y and Xiaoxin L: Protective effect of
paeoniflorin against oxidative stress in human retinal pigment
epithelium in vitro. Mol Vis. 17:3512–3522. 2011.PubMed/NCBI
|
|
33
|
Cao Y, Zhang Y, Wang N and He L:
Antioxidant effect of imperatorin from Angelica dahurica in
hypertension via inhibiting NADPH oxidase activation and MAPK
pathway. J Am Soc Hypertens. 8:527–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li ZJ, Li XM, Piao YJ, Choi DK, Kim SJ,
Kim JW, Sohn KC, Kim CD and Lee JH: Genkwadaphnin induces reactive
oxygen species (ROS)-mediated apoptosis of squamous cell carcinoma
(SCC) cells. Biochem Biophys Res Commun. 450:1115–1119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang C, Day ML, Poronnik P, Pollock CA
and Chen XM: Inhibition of KCa3.1 suppresses TGF-b1 induced MCP-1
expression in human proximal tubular cells through Smad3, p38 and
ERK1/2 signaling pathways. Int J Biochem Cell Biol. 47:1–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Liang D, Chi ZH, Chu Q, Zhao C,
Ma RZ, Zhao Y and Li H: Effect of zinc on high glucose-induced
epithelial-to-mesenchymal transition in renal tubular epithelial
cells. Int J Mol Med. 35:1747–1754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bai YH, Wang JP, Yang M, Zeng Y and Jiang
HY: SiRNA-HMGA2 weakened AGEs-induced epithelial-to-mesenchymal
transition in tubular epithelial cells. Biochem Biophys Res Commun.
457:730–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou L, Xue H, Wang Z, Ni J, Yao T, Huang
Y, Yu C and Lu L: Angiotensin-(1–7) attenuates high glucose-induced
proximal tubular epithelial-to-mesenchymal transition via
inhibiting ERK1/2 and p38 phosphorylation. Life Sci. 90:454–462.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen Q, Huang Z, Zhou SF, Li XY, Luo N and
Yu XQ: Urinary proteins from patients with nephrotic syndrome
alters the signalling proteins regulating epithelial-mesenchymal
transition. Nephrology (Carlton). 15:63–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei J, Shi Y, Hou Y, Ren Y, Du C, Zhang L,
Li Y and Duan H: Knockdown of thioredoxin-interacting protein
ameliorates high glucose-induced epithelial to mesenchymal
transition in renal tubular epithelial cells. Cell Signal.
25:2788–2796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou G, Wang Y, He P and Li D: Probucol
inhibited Nox2 expression and attenuated podocyte injury in type 2
diabetic nephropathy of db/db mice. Biol Pharm Bull. 36:1883–1890.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamashita S, Masuda D and Matsuzawa Y: Did
we abandon probucol too soon? Curr Opin Lipidol. 26:304–316. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yamashita S, Hbujo H, Arai H, Harada-Shiba
M, Matsui S, Fukushima M, Saito Y, Kita T and Matsuzawa Y:
Long-term probucol treatment prevents secondary cardiovascular
events: A cohort study of patients with heterozygous familial
hypercholesterolemia in Japan. J Atheroscler Thromb. 15:292–303.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamashita S and Matsuzawa Y: Where are we
with probucol: A new life for an old drug? Atherosclerosis.
207:16–23. 2009. View Article : Google Scholar : PubMed/NCBI
|