Introduction
Levodopa (L-DOPA), the precursor of dopamine (DA),
has provided obvious effective treatment for Parkinson's disease
(PD) by replacing DA neurotransmission following the death of
substantia nigra neurons (1). Its
high efficacy is due to the ability to restore synaptic DA levels,
an effect thought to be mediated by the spared DA neurons.
Following chronic administration of L-DOPA, however, leads to motor
side effects, L-DOPA-induced dyskinesias (LIDs), limiting the
utility of the drug (2). LIDs are
perceived as debilitating by affected patients and continue to be a
major problem to the clinical management of PD patients. For this
reason, there is a great interest in developing non-dopaminergic
treatments that can be added to L-DOPA to reduce these untoward
effects (3).
Together with previous findings in LID models, this
led to the overall impression that the development of LID is caused
by a complex interaction of both pre- and postsynaptic changes,
taking place not only in the DA system, but also involving a
variety of other mechanisms (4).
There are literature reports indicating that especially long-time
L-DOPA therapy may cause side effects in the form of increased
toxicity and inflammatory response, as well as disturbances in
biothiol metabolism (5). Spencer
et al (6) reported that the
augmented oxidative stress in patients treated with L-DOPA may have
resulted from lowered levels of antioxidants, disturbed
mitochondrial transport, and from excessive oxidation of DA.
Moreover, some previous studies indicated that, in PD patients
treated with L-DOPA, increased plasma levels of neuroinflammation
markers, such as oxidized-low density lipoproteins and soluble
intracellular adhesion molecule (7). Therefore, in PD patients treated with
L-DOPA, monitoring of oxidative stress markers and inflammatory
factors, as well as biothiol compounds is recommended.
α-lipoic acid (ALA), is an antioxidant naturally
synthesized in human body with potential therapeutic value against
a range of pathophysiological insults (8). Emerging evidence has indicated that
ALA has effective antioxidative activities by scavenging reactive
oxygen species (ROS) and inhibits free radical formation by
chelating various metal types, indicating that it can exert
beneficial effects on various disorders correlated with oxidative
stress (9). In addition, ALA is
reported to have anti-inflammatory properties and to increase
intracellular glutathione (GSH) formation in a range of cell types
and tissues, which may be beneficial in neurodegenerative
conditions (10). It has been
reported that ALA protected DA neurons against MPPT-induced
apoptosis by attenuating ROS formation (11). Moreover, a previous study (12) demonstrated that ALA prevented the
damage induced by 6-OHDA or by chronic use of L-DOPA in
dopaminergic neurons, suggesting that ALA could be a new
therapeutic target for PD prevention and treatment (13). Indeed, emerging evidences in
vivo and in vitro have come to further support the
crucial position of ALA in the neuroprotective of PD, especially in
the field of anti-oxidative stress and anti-inflammation. However,
there is still limited data on ALA in animal models of LID.
Therefore, the aim of the current study was to investigate
neurochemical and behavioral effects of ALA in combination with
L-DOPA using an animal model of LID in 6-OHDA-lesioned parkinsonian
rats.
Materials and methods
Animals
Experiments were conducted on 48 female
Sprague-Dawley (SD) rats (age, 3–4 months; weight, 180–220 g),
which were purchased from the Experimental Animal Center of China
Medical University (Beijing, China). Upon their arrival, the
animals were housed in clean cages with a maximum of four rats per
cage under a 12 h light:12 h dark cycle, relative humidity of
55±10% and temperature 22.0±2.0°C. Animals had unrestricted access
to standard chow and water, which were supplemented daily, and
animal care was supervised by skilled veterinarians in the health
care center (Medical School of Shanghai Jiaotong University,
Shanghai, China). All experimental protocols involving the animals
were reviewed and approved by the Ethical Committee of the Medical
School of Shanghai Jiaotong University (Shanghai, China). Efforts
were made to reduce to a minimum the number of animals required for
statistically valid analyses and to minimize their suffering. The
methods were carried out in accordance with the approved guidelines
and regulations of the National Institutes of Health for the Care
and Use of Laboratory Animals (Bethesda, MD, USA).
Induction of parkinsonism and
L-DOPA-induced dyskinesia
6-OHDA-lesioned PD rats were induced by the methods
mentioned above (14). Briefly,
rats were deeply anesthetized by 10% chloral hydrate (0.35 ml/100
g; Beyotime Institute of Biotechnology, Shanghai, China) and
mounted in a stereotaxic apparatus equipped with a rat adaptor.
Using a syringe, 4 µl 6-OHDA (4 µg/µl) in 0.2% ascorbic acid were
injected into the right medial forebrain bundle (MFB) of rats in
two deposits at the following stereotaxic coordinates as follows:
1) anterior-posterior (AP), −4.4 mm, medial-lateral (ML), −1.2 mm,
dorsal-ventral (DV), −7.8 mm; 2) AP, −3.7 mm, ML, −1.7, DV, −7.8.
The tooth bar was set to −2.4 mm. At 3 weeks following surgery, the
lesioned rats were screened behaviorally using an apomorphine
hydrochloride-induced [0.5 mg/kg, intraperitoneally (i.p.)]
rotation test and all animals exhibited >7 full body turns/min
toward the side of the unlesioned side were selected for the next
experiment. Once parkinsonism was stable, they were then treated
with twice-daily administration of L-DOPA (25 mg/kg/d, i.p.) plus
benserazide (6.25 mg/kg/d, i.p.) for 3 weeks to induce a rat model
of dyskinesia.
Drugs and treatment
Validated PD rats received vehicle or levodopa
injection for 21 d. Apomorphine hydrochloride (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was administered (0.5 mg/kg). L-DOPA
(Sigma-Aldrich; Merck KGaA, 25 mg/kg) with a fixed dose of the
peripheral DOPA-decarboxylase inhibitor benserazide (Sigma-Aldrich;
Merck KGaA, 6.25 mg/kg) were administered twice-daily (9:00 and
16:00). ALA was dissolved in normal saline and was administered
i.p. (ALA-L group, 31.5 mg/kg; ALA-H group, 63 mg/kg, respectively)
30 min prior to L-DOPA intake for 3 weeks.
AIM ratings and forelimb functional
test
On testing days, rats were placed individually in
transparent plastic cages 10 min prior to drug treatment. As
described previously (15),
abnormal involuntary movements (AIM) were classified into four
subtypes: 1) axial AIM: dystonic posturing or choreiform twisting
of the neck and upper body towards the side contralateral to the
lesion; 2) limb AIM: abnormal, purposeless movements of the
forelimb and digits contralateral to the lesion; 3) orolingual AIM:
empty jaw movements and contralateral tongue protrusion; and 4)
locomotion AIM: increased locomotion with contralateral side bias.
The AIM scores were tested at 2, 7, 14 and 21 days during levodopa
treatment. Each of these subtypes was scored on a severity scale
from 0 to 4. Forelimb functional test was performed five times at
5, 9, 13, 16 and 20 day during L-DOPA treatment, which could as an
index of parkinsonion disability score. The rats were placed in a
glass cylinder (22×35 cm) to record forelimb use during vertical
exploration for 60 min. During a period of 60 min following L-DOPA
treatment, forelimb functional test was assessed every 20 min (3
min monitoring period for each). The final value was expressed in
terms of the percentage use of the impaired forelimb
(contralateral) compared with the total number of limb use
movements.
Measurement of GSH and lipid
peroxide
To assess the enzymatic activity of GSH and lipid
peroxide in striatum, the tissues were homogenized in 0.1 mol/l PBS
containing 0.05 mmol/l EDTA. The homogenate was centrifuged at
12,000 × g for 15 min at 4°C. The supernatants were kept for the
measurement. Total GSH was assayed by 5,5-dithiobis
(2-nitrobenzoic) acid (DTNB)-GSSG reductase recycling. GSSG was
obtained by determining the absorbance of 5-thio-2-nitrobenzoic
acid produced from the reaction of the reduced GSH with DTNB
according to the manufacturer's protocols. The reduced GSH was
obtained by subtracting GSSG from the total GSH. Absorbance was
determined at 412 nm by using the microplate reader. GSH activity
was assessed with GSH Assay kit (Beyotime Institute of
Biotechnology, Haimen, China) by the mays of GSH/GSSG. The level of
MDA, a product of lipid peroxidation, was measured with MDA Assay
kit (Beyotime Institute of Biotechnology) based on modified
thiobarbituric acid method.
Immunofluorescence (IFC)
IFC was carried out in free-floating sections using
a standard avidin-biotin immunocytochemical protocol. Rats were
rapidly anesthetized with 10% chloral hydrate (350 mg/kg, i.p.) and
transcardially perfused with 4% paraformaldehyde. Whole brains were
post-fixed overnight in the 4% paraformaldehyde, stored at 4°C and
then stored in a solution containing 30% sucrose. Sections (30 µm)
were cut with a slicing machine and blocked for 10 min at room
temperature in 5% normal donkey serum (Beyotime Institute of
Biotechnology), and then incubated overnight at 4°C in the primary
antibody solution (monoclonal rabbit anti-IBA; 1:200; cat. no.
ab178680; Abcam, Cambridge, UK). Sections were rinsed in PBS and
incubated with fluorescein isothiocyanate-conjugated donkey
anti-rabbit antibody (cat. no. A0453; 1:200; Beyotime Institute of
Biotechnology) for 1 h at room temperature. Subsequently, sections
were again rinsed in PBS, mounted on slides, cover slipped, and
examined with confocal microscopy. Digitized images were analyzed
for distribution of immunoreactive cells in the lesioned hemisphere
striatum and substantia nigra of rats.
Western blot analysis
Striatal tissues and substantia nigra were
homogenized in 20 mM Tris-HCl (pH 7.4), containing 1 mM NaF, 150 mM
NaCl, 1% Triton X-100 and freshly-added protease inhibitor cocktail
(Roche Diagnostics, Basel, Switzerland), and 100 µM
phenylmethylsulfonyl fluoride. Cytosols were prepared by
centrifugation at 12,000 × g for 10 min at 4°C. Proteins were
separated by SDS-PAGE electrophoresis, using different percentages
of gels based on the different protein weights (range, 6–12%) and
transferred overnight to polyvinylidene difluoride membranes. Then,
the membrane was incubated with polyclonal rabbit anti-caspase-3
(cat. no. 9661S; 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA) and polyclonal rabbit anti-poly (ADP-ribose) polymerase
(PARP; cat. no. 9542S; 1:1,000; Cell Signaling Technology)
overnight at 4°C, respectively, and then incubated in horseradish
peroxidase conjugated secondary anti-rabbit β-actin IgG (cat. no.
A0208; 1:1,000; Beyotime Institute of Biotechnology). The signal
was visualized by enhanced chemiluminescence reagent (EMD
Millipore, Billerica, MA, USA) and quantified using Quantity One
software (Image Lab).
Statistical analysis
The scores assigned for AIM and parkinsonian
disability were non-parametric and were analyzed using a Kruskal
Wallis followed by Dunn's test for multiple comparisons in the case
of comparing data over multiple days. The western blot analysis and
IHC conformed to normal distribution were performed using a one-way
analysis of variance (ANOVA) followed by LSD post-hoc comparisons
when appropriate as indicated in the figure legends. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were conducted using SPSS software (version, 16.0; SPSS
Inc., Chicago, IL, USA).
Results
Treatment with ALA prevents the
development of LID in 6-OHDA-lesioned rat model of PD
A total of 48 SD rats were unilaterally injected
with 6-OHDA in the MFB (n=12 per group). The anti-dyskinetic
potential of ALA against LID was evaluated at two different doses
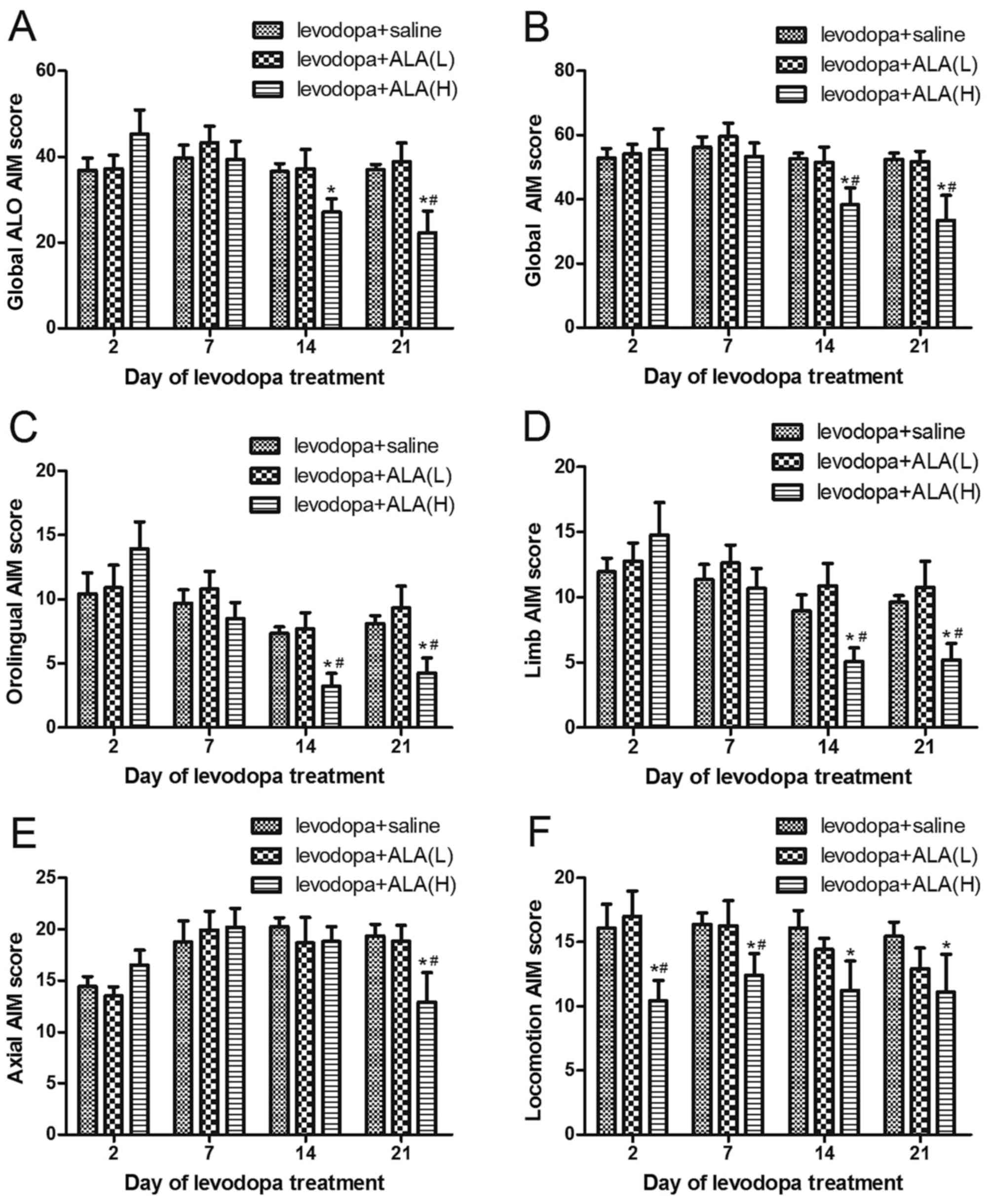
(31.5 and 63 mg/kg). As can be observed in Fig. 1, ALA reduced AIM scores and
observed after L-DOPA (25 mg/kg) in a dose-dependent manner.
6-OHDA-lesioned rats treated with L-DOPA for 21 days developed a
progressive increase in LID (P<0.05 compared with PD and ALA
groups; Fig. 1A and B). The
6-OHDA-lesioned rats received the saline for 21 days did not
develop LID features. Meanwhile, co-administration of ALA with
L-DOPA did not develop severe LID over the 21 day treatment period,
which differed significantly from the LID group in all testing
sessions except at 2 and 7 day time point following administration
(P<0.05; Fig. 1A and B).
Furthermore, the ALA-H (63 mg/kg) group demonstrated a greater
reduction in the AIM scores compared with the rats receiving ALA-L
(31.5 mg/kg), but the rats still presented a mild dyskinesia, this
effect did not reach completely reversal. Similarly, this seemed to
be the same trend in orolingual AIM (Fig. 1C), limb AIM (Fig. 1D), Axial AIM (Fig. 1E), as well as locomotion AIM
(Fig. 1F).
Effects of LA on forelimb functional
test
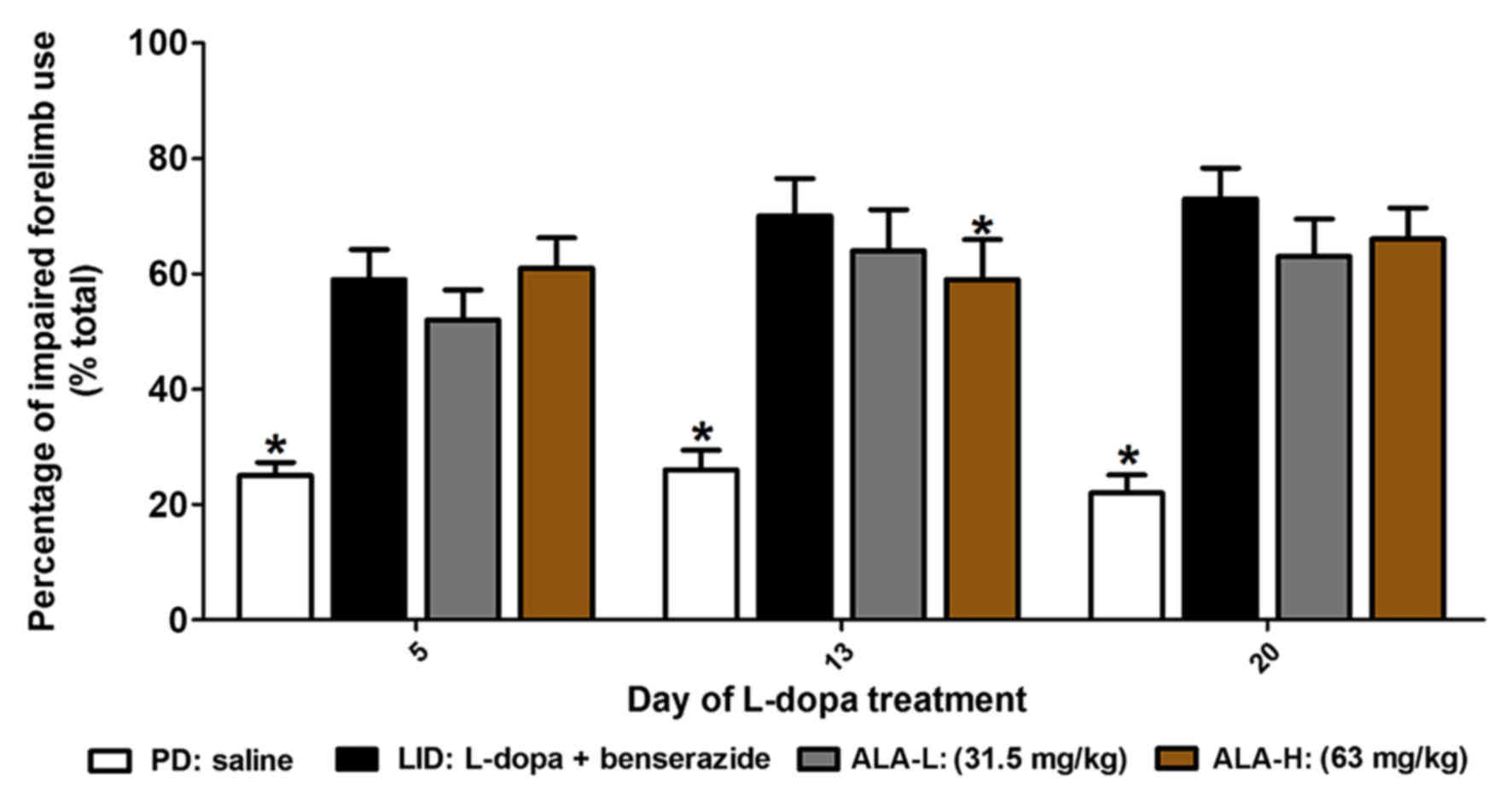
Following this, the authors sought to determine
whether ALA improvement of LID without ablation of the therapeutic
response to L-DOPA. The authors observed that PD rats treated with
L-DOPA prefer to use the contralateral forelimb to touch the inner
wall of the cylinder compared with the PD group (P<0.05;
Fig. 2). If the animals were
co-injected with ALA-L (31.5 mg/kg) or ALA-H (63 mg/kg) for 21
days, they also demonstrated preferential to touch the wall with
contralateral forelimb at 5, 13 and 20 day time points following
administration (P>0.05 vs. LID group, P<0.05 vs. PD group,
respectively; Fig. 2). When they
continued to measure forelimb preference between ALA-L and ALA-H
groups, no significant difference was identified between two groups
(P>0.05; Fig. 2).
Effects of LA on MDA and GSH
activity
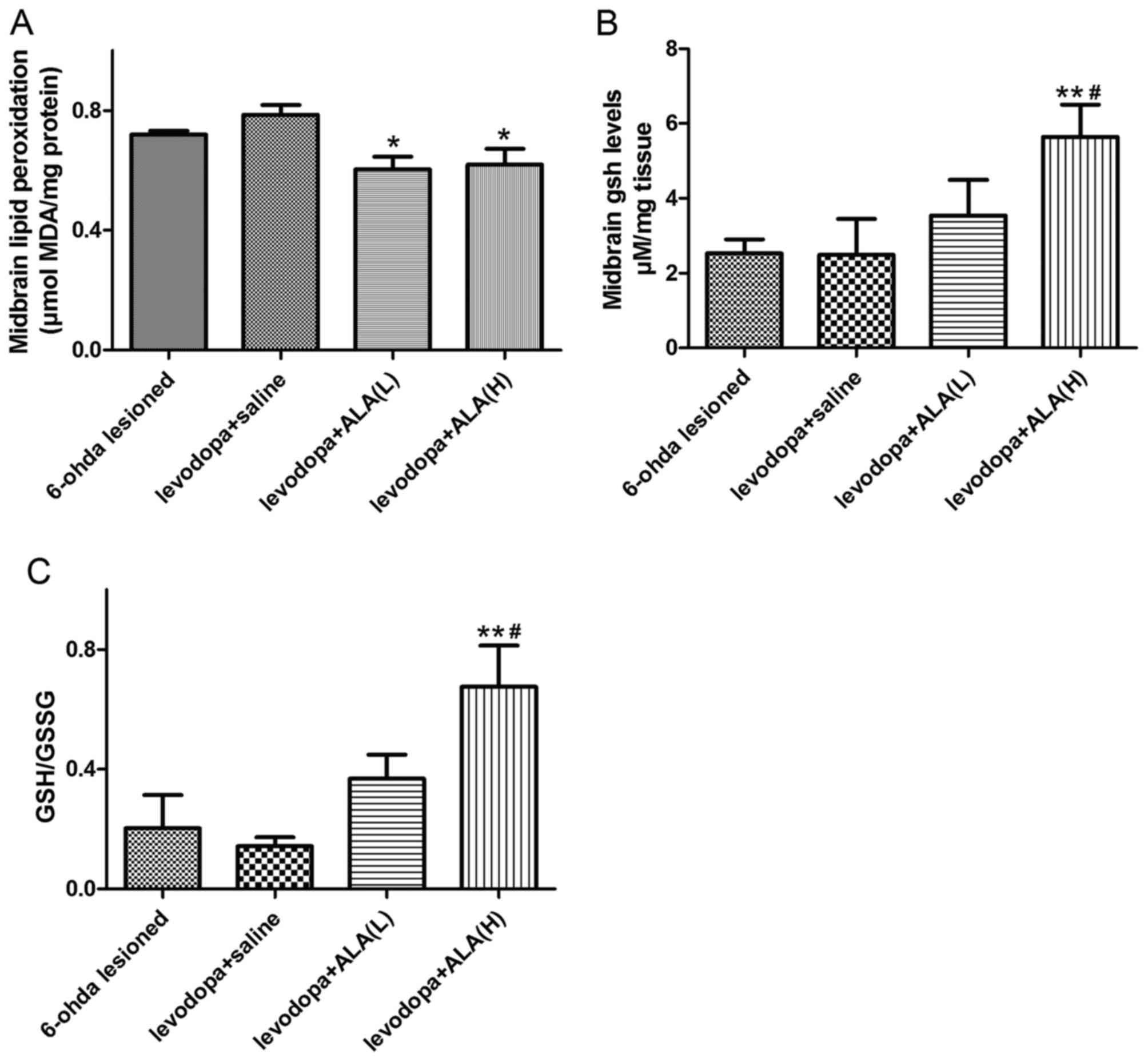
In the current study, the MDA level as a measure of
lipid peroxidation was remarkably increased in the LID group
compared with the PD group (P<0.05; Fig. 3A). Either the ALA-L group (31.5
mg/kg) or the ALA-H group (31.5 mg/kg) treatment significantly
reduced the MDA levels compared to the LID group (P<0.05,
Fig. 3A). Similarly, no
significant difference was observed between the ALA-L and ALA-H
groups in terms of reducing MDA level (P>0.05; Fig. 3A). To investigate the effect of ALA
on anti-oxidative stress in an LID model, the authors assessed the
biomarkers of oxidative stress, including GSH and GSSG. The level
of GSH activity by the means of GSH/GSSG in the LID group was
significantly decreased compared with the PD group (P<0.05;
Fig. 3B and C). Meanwhile,
treatment with ALA remarkably alleviated the GSH activity compared
with the LID group (P<0.05; Fig. 3B
and C). Furthermore, the ALA-H group demonstrated more effects
in the GSH activity compared with the rats receiving ALA-L
(P<0.05; Fig. 3B and C).
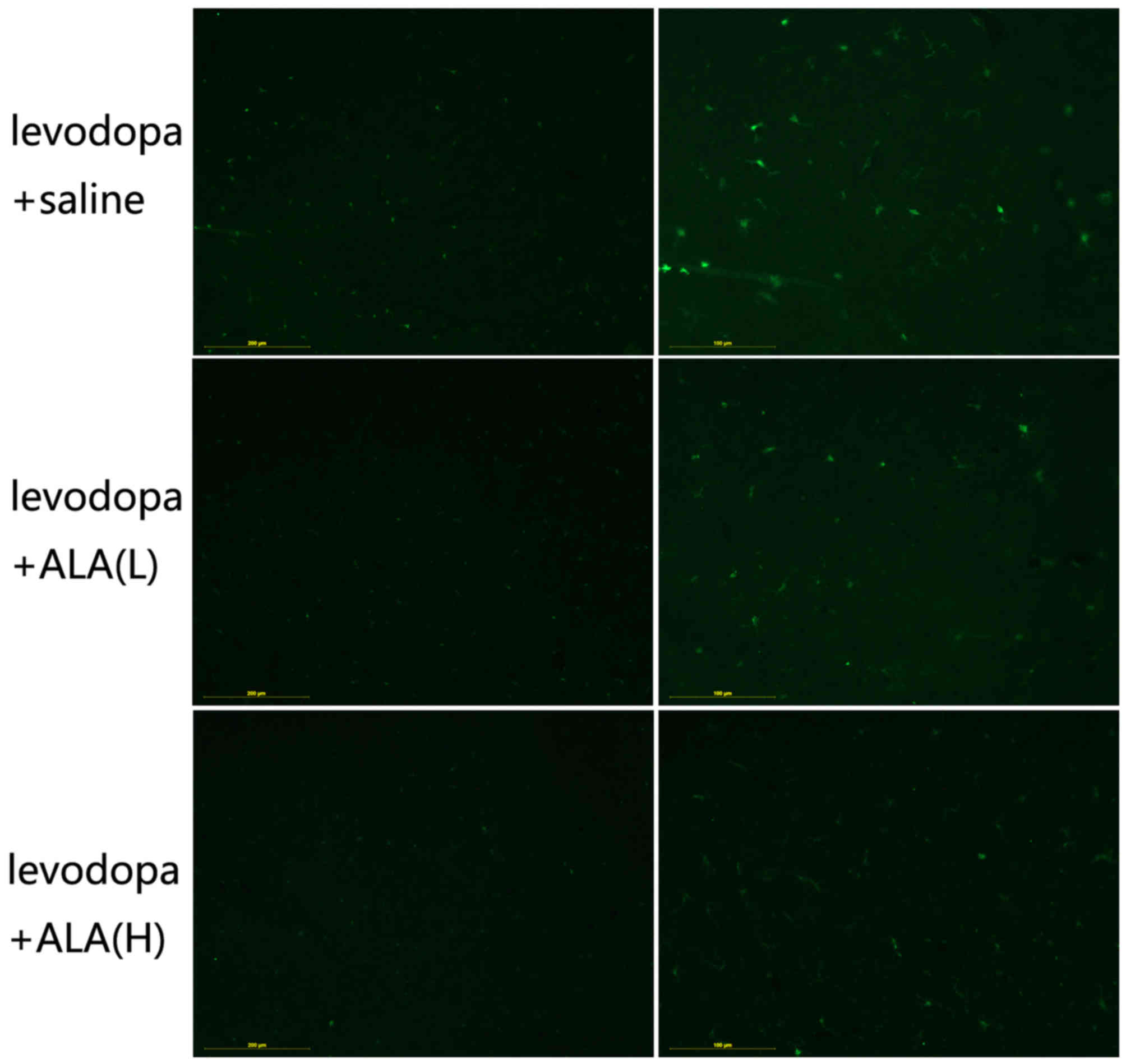
LA treatment ameliorates IBa-1
positive neurons in substantia nigra
IBa-1 is a protein that is specifically expressed in
macrophages/microglia and is upregulated during the activation of
these cells (16). Activated
macrophages are reported in tissues with inflammation. Therefore,
IBa-1 levels have been found to positively correlate with chronic
inflammation indicators. IBa-1 positive neurons are induced about
two-fold increase in the substantia nigra by chronic L-DOPA
treatment compared with other two groups (P<0.05; Fig. 4), the inductive effect of L-DOPA on
this marker can be seen in all substantia nigra regions. Meanwhile,
animals treated with ALA tended to present lower levels of cellular
immunostaining for IBa-1 positive neurons than LID group
(P<0.05; Fig. 4). Note that the
ALA-H entailed significantly more effective than the treatment with
ALA-L in terms of lower the levels of IBa-1 positive neurons in the
substantia nigra (P<0.05; Fig.
4).
Treatment with LA prevents activation
cleaved-caspase-3 and PARP
Fig. 5 indicated
that chronic L-DOPA treatment of hemi-parkinsonian rats increased
cleaved-caspase-3 and PARP levels in the lesioned substantia nigra.
ALA at 31.5 mg/kg or 63 mg/kg both reduced the induction of
cleaved-caspase-3 and PARP levels following chronic L-DOPA
treatment in parkinsonian animals. Furthermore, ALA-H demonstrated
more reduction in terms of cleaved-caspase-3 and PARP levels
compared with ALA-L administration (P<0.05; Fig. 5A-F). The degree of dopamine
depletion was verified by western blotting with an antibody raised
against tyrosine hydroxylase (TH). Significant changes were
observed in TH levels in the substantia nigra between the PD group
and other four groups (P<0.05; Fig.
5G and H), indicating >90% depletion of nigral dopamine cell
bodies in PD, LID and ALA groups.
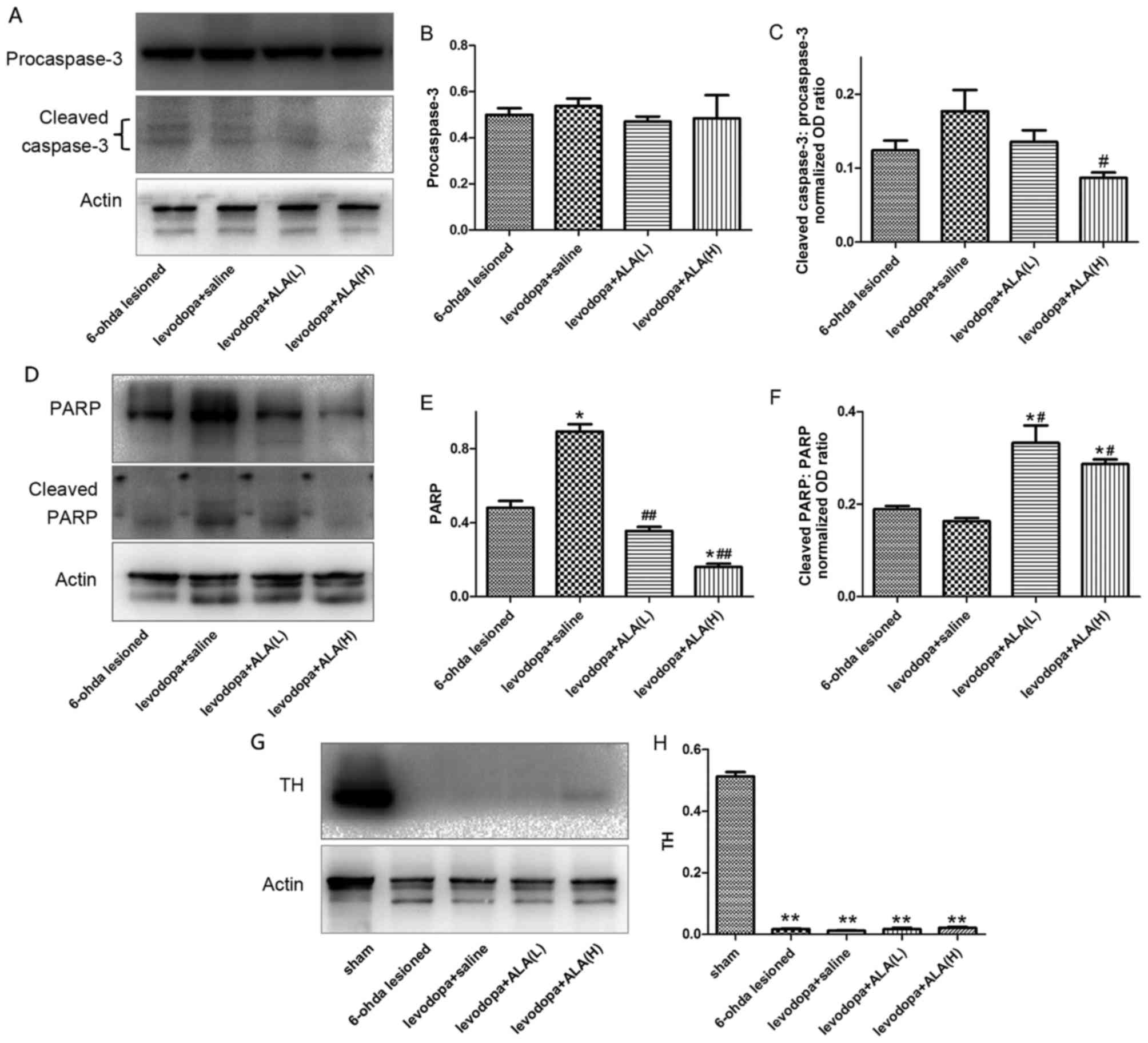 | Figure 5.Protein levels were evaluated by
western blotting of proteins extracted from the ipsilateral
striatum of the rat brain. They were assessed in extracts from
6-OHDA-lesioned rats treated with vehicle, levodopa, ALA-L and
ALA-H. (A) Representative image and quantification of (B)
pro-caspase-3, and (C) cleaved-caspase-3 level relative to actin
level. (D) Representative image and quantification of (E) PARP, and
(F) cleaved-PARP relative to actin level. (G) Representative image
and (H) quantification of TH level relative to actin level. The
data represent the mean of relative optical density ± standard
deviation; *P<0.05, **P<0.01 vs. 6-OHDA group;
#P<0.05, ##P<0.01 vs. LID group
(one-way analysis of variance followed by least significant
difference post-hoc analysis). LID, levodopa-induced dyskinesia;
ALA, α-lipoic acid; TH, tyrosine hydroxylase; PARP, poly
(ADP-ribose) polymerase. |
Discussion
L-DOPA is the most successful approach to manage
motor symptoms in PD patients. However, the emergence of LID with
long-term use is a severe challenge for PD. The present study has
demonstrated that chronic challenges of ALA (31.5 and 63 mg/kg) in
combination with L-DOPA significantly alleviates dyskinesia. The
major findings from this study were: (1) ALA ameliorated L-DOPA induced
dyskinesia; (2) the effective ALA
dose (31.5 mg/kg or 63 mg/kg) did not interfere with the
therapeutic motor effects of L-DOPA; (3) ALA reduced L-DOPA-induced MDA
over-expression in substantia nigra and upregulated the level of
GSH; (4) ALA reduced
L-DOPA-induced cleaved-caspase-3 and PARP overexpression in the
substantia nigra; (5) ALA reduced
L-DOPA-induced IBa-1 positive neurons in the substantia nigra. The
anti-dyskinetic effect of the 31.5 or 63 mg/kg dose of ALA was
observed 7 days following L-DOPA administration, when dyskinesias
were occurring. Moreover, this effect of ALA was maintained over
the entire 21 days without development of tolerance. The current
study revealed, for the first time, that the ALA could alleviate
LID in 6-OHDA parkinsonian rats via anti-oxidative stress in terms
of reduced MDA level and upregulated GSH activity. Moreover, these
results further indicate that co-administration with ALA reduced
the percentage of apoptotic cells in the substantia nigra observed
following chronic L-DOPA treatment. Using western blotting for the
distinction of apoptotic event, the authors demonstrated that ALA
could attenuate the apoptotic event induced by L-DOPA. The findings
of anti-apoptotic effects of ALA against L-DOPA-induced apoptosis
are consistent with those obtained with this compound in previous
studies (11,17).
Oxidative stress is a central event in a range of
pathological conditions. Such a pathway appears to underlie the
pathological processes of PD, in which the inhibition of the
mitochondrial complex I elicited by the neuron toxicant increases
the formation of ROS that cause the mitochondrial dysfunction
finally result in PD occur (18).
6-OHDA has been widely used to study the pathogenesis of PD and is
thought to selectively kill dopaminergic neurons and to elicit
severe parkinsonism-like symptoms in humans and animals (19). Moreover, the previous data
indicated that L-DOPA therapy of PD patients may induce oxidative
stress by different mechanisms, and increase the levels of
inflammatory markers and leaded to apoptotic event (7). All of these events induced by L-DOPA
probably serve a vital role in the development of LID. Namely,
long-term follow-up of PD therapy with L-DOPA improves the
parkinsonian symptoms but may lead to fluctuations and dyskinesias
and on-off phenomena. Motor fluctuations and LID are common
sequelae of PD that may limit function and quality of life.
Meanwhile, in the present study, results indicated that treatment
with chronic L-DOPA significantly increased level of MDA and
reduced the GSH activity. In addition, the present findings
demonstrated that ALA co-administration with L-DOPA could reverse
the effect by L-DOPA in the LID models.
Another related issue in the exploration of
microglial activation phases is the reliance on Iba-1
immunoreactivity to report on their activation state (20). Based on a previous study, IBa-1
levels were revealed to positively correlate with chronic
inflammation indicators (20). The
results demonstrated that IBa-1 positive neurons are induced
~two-fold more in the substantia nigra by chronic L-DOPA treatment;
the inductive effect of L-DOPA on this marker can be seen in all
substantia nigra regions. Meanwhile, co-administration with ALA
tended to report lower levels of cellular immunostaining for IBa-1
positive neurons than the LID group. However, the limitation of
present study is that only Iba-1 dyeing was used to reflect
inflammatory states of glia. Indeed, in the future, studies should
show the importance of using multiple approaches when reporting on
the level of microglial activation. Moreover, caspase-3 has been
identified as a key mediator of neuronal programmed cell death.
Caspase-3 activation, a crucial event of neuronal cell death
program, is also a feature of many chronic neurodegenerative
diseases (21). One previous study
demonstrated that PD patients treated with L-DOPA had significantly
increased levels of caspase-3 and PARP protein (22). It seems that pharmacological
treatment of PD patients with L-DOPA has a major role in modulating
the levels of some apoptotic proteins in lymphocytes, which are
important for this process, which were consistent with that present
results that state that chronic L-DOPA treatment could upregulate
the levels of caspase-3 and PARP. In resent research, chronic
L-DOPA treatment of hemi-parkinsonian rats increased
cleaved-caspase-3 and PARP in the lesioned substantia nigra. ALA at
31.5 mg/kg or 63 mg/kg both reduced the induction of
cleaved-caspase-3 and PARP levels following chronic L-DOPA
treatment in parkinsonian animals. In conclusion, these data
demonstrated that ALA prevents molecular changes from occurring and
lends support to the hypothesis that ALA alleviates L-DOPA-induced
dyskinesia in 6-OHDA parkinsonian rats via anti-oxidative stress;
this may be a promising mode of administration to avoid LID.
Based on the present findings, ALA could be
recommended as a promising disease-modifying therapy when
administered with L-DOPA early in the course of PD. The exact
mechanism for this action, although incompletely understood,
appears to relate to anti-oxidative stress and anti-apoptosis.
Acknowledgements
The present study was supported by the Projects of
National Science Foundation of China (grant nos. 81171203,
81471148, 81400925, 81171204 and 81200871), and the Projects of the
Shanghai Committee of Science and Technology (grant nos.
11nm0503300 and 12XD1403800).
References
|
1
|
Mosharov EV, Borgkvist A and Sulzer D:
Presynaptic effects of levodopa and their possible role in
dyskinesia. Mov Disord. 30:45–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hely MA, Morris JG, Reid WG and
Trafficante R: Sydney multicenter study of Parkinson's disease:
Non-L-DOPA-responsive problems dominate at 15 years. Mov Disord.
20:190–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Finlay C and Duty S: Therapeutic potential
of targeting glutamate receptors in Parkinson's disease. J Neural
Transm (Vienna). 121:861–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schaeffer E, Pilotto A and Berg D:
Pharmacological strategies for the management of levodopa-induced
dyskinesia in patients with Parkinson's disease. CNS Drugs.
28:1155–1184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dorszewska J, Prendecki M, Lianeri M and
Kozubski W: Molecular effects of L-dopa therapy in parkinson's
disease. Curr Genomics. 15:11–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spencer JP, Jenner P and Halliwell B:
Superoxide-dependent depletion of reduced glutathione by L-DOPA and
dopamine. Relevance to Parkinson's disease. Neuroreport.
6:1480–1484. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andican G, Konukoglu D, Bozluolcay M,
Bayulkem K, Firtiina S and Burcak G: Plasma oxidative and
inflammatory markers in patients with idiopathic Parkinson's
disease. Acta Neurol Belg. 112:155–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferreira PM, Militão GC and Freitas RM:
Lipoic acid effects on lipid peroxidation level, superoxide
dismutase activity and monoamines concentration in rat hippocampus.
Neurosci Lett. 464:131–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith AR, Shenvi SV, Widlansky M, Suh JH
and Hagen TM: Lipoic acid as a potential therapy for chronic
diseases associated with oxidative stress. Curr Med Chem.
11:1135–1146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Araújo DP, Lobato Rde F, Cavalcanti JR,
Sampaio LR, Araújo PV, Silva MC, Neves KR, Fonteles MM, Sousa FC
and Vasconcelos SM: The contributions of antioxidant activity of
lipoic acid in reducing neurogenerative progression of Parkinson's
disease: A review. Int J Neurosci. 121:51–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li DW, Li GR, Lu Y, Liu ZQ, Chang M, Yao
M, Cheng W and Hu LS: α-lipoic acid protects dopaminergic neurons
against MPP+-induced apoptosis by attenuating reactive oxygen
species formation. Int J Mol Med. 32:108–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Araújo DP, Lobato Rde F, Cavalcanti JR,
Sampaio LR, Araújo PV, Silva MC, Neves KR, Fonteles MM, Sousa FC
and Vasconcelos SM: The contributions of antioxidant activity of
lipoic acid in reducing neurogenerative progression of Parkinson's
disease: A review. Int J Neurosci. 121:51–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Araújo DP, De Sousa CN, Araújo PV,
Menezes CE, Rodrigues Sousa FT, Escudeiro SS, Lima NB, Patrocínio
MC, Aguiar LM, Viana GS and Vasconcelos SM: Behavioral and
neurochemical effects of alpha-lipoic Acid in the model of
Parkinson's disease induced by unilateral stereotaxic injection of
6-ohda in rat. Evid Based Complement Alternat Med. 2013:5713782013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie CL, Wang WW, Zhang SF, Yuan ML, Che
JY, Gan J, Song L, Yuan WE and Liu ZG: Levodopa/benserazide
microsphere (LBM) prevents L-dopa induced dyskinesia by
inactivation of the DR1/PKA/P-tau pathway in 6-OHDA-lesioned
Parkinson's rats. Sci Rep. 4:75062014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Xie C, Wang Q and Liu Z:
Interactions of CaMKII with dopamine D2 receptors: Roles in
levodopa-induced dyskinesia in 6-hydroxydopamine lesioned
Parkinson's rats. Sci Rep. 4:68112014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deininger MH, Meyermann R and Schluesener
HJ: The allograft inflammatory factor-1 family of proteins. FEBS
Lett. 514:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jalali-Nadoushan M and Roghani M:
Alpha-lipoic acid protects against 6-hydroxydopamine-induced
neurotoxicity in a rat model of hemi-parkinsonism. Brain Res.
1505:68–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perfeito R, Cunha-Oliveira T and Rego AC:
Reprint of: Revisiting oxidative stress and mitochondrial
dysfunction in the pathogenesis of Parkinson disease-resemblance to
the effect of amphetamine drugs of abuse. Free Radic Biol Med.
62:186–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schober A: Classic toxin-induced animal
models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res.
318:215–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Norden DM, Trojanowski PJ, Villanueva E,
Navarro E and Godbout JP: Sequential activation of microglia and
astrocyte cytokine expression precedes increased Iba-1 or GFAP
immunoreactivity following systemic immune challenge. Glia.
64:300–316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Amelio M, Sheng M and Cecconi F:
Caspase-3 in the central nervous system: Beyond apoptosis. Trends
Neurosci. 35:700–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hald A and Lotharius J: Oxidative stress
and inflammation in Parkinson's disease: Is there a causal link?
Exp Neurol. 193:279–290. 2005. View Article : Google Scholar : PubMed/NCBI
|