Introduction
Post-traumatic stress disorder (PTSD) is a
long-lasting mental disorder that develops after exposure to a
traumatic event such as traffic collisions, sexual assault, natural
disaster, or other threats. Symptoms that often last for more than
a month or even years after the event may include continuous
disturbing re-experience of the traumatic event, avoidance of
trauma-related cues, hypervigilance and numbing of general
responsiveness (1,2). However, the pathological mechanisms
of PTSD is not well clarified, although recent studies indicate
that calcium ion disturbances, apoptosis, dysfunction of
mitochondria or endoplasmic reticulum (ER) are involved in PTSD
(3–6).
As a multifunctional organelle, the ER participated
in multiple cellular functions, including production of glycogen
and steroids, folding and transport of various proteins,
sequestration of calcium and cell apoptosis (7–9).
Physiological and pathological stimuli that disrupt ER homeostasis
are responsible for dysfunction of ER or ER stress, including
perturbation of calcium homeostasis, accumulative unfolded or
misfolded proteins and viral infection. (10–12).
Cells cope with ER stress via an adaptive unfolding protein
response (UPR) (13,14).
Both calreticulin (CRT) and calnexin (CNX) are ER
resident calcium-binding chaperones (15–17)
and play a vital role in the folding of newly synthesized proteins
and quality control pathways in the ER (16,18).
ERp57 belongs to the protein disulfide isomerses family (PDIs),
participating in the folding of newly synthesized glycoproteins, in
collaboration with CRT and CNX (19–21).
As folding proteins and chaperones, CRT, CNX and ERp57 also
participate in dealing with misfolded proteins from the ER via its
own unique mechanism (13). These
folding proteins are important, since fluctuations of intraluminal
Ca2+ level could affect ER function and induce cell
death (22).
As part of the limbic system in brain, amygdala has
long been considered as a pivotal brain structure responsible for
anxiety, fear, learning and memory modulation associated with
emotional events (23–25). Amygdala nuclei encompass several
structures: Basolateral complex, cortical nucleus, medial nucleus,
central nucleus, and the intercalated cell clusters (26). Among these nuclei subgroups, the
basolateral nuclei play a major role in mediating anxiety,
emotional arousal and memory (27), as indicated by many studies.
Therefore, the present study focused on observing Single-prolonged
stress (SPS)-induced changes of the basolateral nuclei.
Lower plasma cortisol level and enhanced inhibition
of the hypothalamo-pituitary-adrenal (HPA) axis are
neuroendocrinological mark of PTSD (28). SPS paradigms were shown to induce
these changes and widely used in the studies of PTSD (29–31).
In this study, we also created animal models by exposure to SPS.
The purpose of this work was to investigate whether CRT, CNX and
ERp57 were involved in dysfunction of amygdala on rats exposed to
SPS using immunofluorescence, western blot and qPCR to assess the
changes, which might provide novel insights into the pathogenesis
of PTSD and experimental basis for new treatments.
Materials and methods
Animals
Eighty male Wistar rats weighing 150–160 g at the
start of the study, were supplied by Changsheng Biotechnology Co.,
Ltd. (Liaoning, China). Rats were housed 2–3 animals per plastic
cage on a 12 h light-dark schedule at 22±2°C with free access to
water and food for 7 days. All procedures were conducted in
conformity with Guidance for the Care and Use of Laboratory
Animals, the National Institute of Health. The study was approved
by the Ethics Committee of Laboratory Animal Welfare and Ethics
(China Medical University) All efforts were made to reduce the
number of animals used and to minimize animal suffering during the
experiment.
Grouping and model establishment
Animals were divided randomly into four groups: 1)
the control group (Cont); 2) SPS 1 day group; 3) SPS 7 days group;
4) SPS 14 days group. Control animals remained in their home cages
with no handling for 7 or 14 days and were sacrificed at the same
time as the SPS groups. The SPS-rats underwent the SPS procedure on
the first day and remained in their cages until sacrifice. The SPS
protocol was based on a combined plural stress paradigm (29,30):
Immobilization (compression with plastic bags) for 2 h, followed by
forced swimming for 20 min in a plexiglass cylinder (40 cm depth;
23±2°C), and then rest for 15 min, ether anesthesia until loss of
consciousness at last. Cervical dislocation was used as the method
of sacrifice.
Measurement of animal body weight
The body weights of both the control and SPS groups'
rats were recorded every other day, and then the body weight growth
curve was drawn based on the average weight in each group of
rats.
Perfusion based sections
Rats of each group were perfused via left ventricle
and fixed with 250 ml of pre-cold heparinized 0.9% saline, followed
by 300 ml of 4% paraformaldehyde in 0.01 M phosphate buffer (pH
7.2). Then the brain tissues were rapidly separated and fixed in 4%
PFA for 6 h at 4°C, and were immersed in a 30% sucrose solution in
0.01 M phosphate-buffered saline (PBS; pH 7.2) at 4°C after then.
Tissues were fast frozen in liquid nitrogen and sectioned
coronally. Frozen sections (12 µm) were prepared for
immunohistochemistry or immunofluorescence analysis using a
cryostat (CM 3050; Leica, Mannheim, Germany).
Immunofluorescence analysis of
CRT
After being washed with 0.01 M PBS for three times,
the sections of each group were incubated with 5% bovine serum
albumin (BSA) for 30 min to block non-specific staining at room
temperature (RT). The sections were then incubated with mouse
monoclonal anti-CRT antibody (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) in 0.01 M PBS overnight at 4°C. After
being washed with PBS for three times, the sections were incubated
with Cy3-conjugated goat anti-mouse IgG (1:50; Boster, Wuhan,
China) for 2 h at RT. After being washed with PBS, slices were then
mounted with glycerin and observated by fluorescence
microscope.
Five slides were randomly selected from each group
and five visual fields in the basolateral amygdala were randomly
selected from each slide (magnification, ×400). We recorded the
fluorescent intensity of CRT-immunopositive cells to evaluate the
average fluorescent intensity using a Meta Morph/DPIO/BX41
morphology image analysis system.
Immunohistochemical analysis of CNX
and ERp57
After being washed with PBS three times, the
sections of each group were incubated with 5% BSA for 30 min to
block non-specific staining at RT. The sections were then incubated
with goat polyclonal anti-CNX antibody (1:200; Santa Cruz
Biotechnology, Inc.) or mouse monoclonal anti-ERp57 antibody
(1:200; Santa Cruz, USA) overnight at 4°C. The sections were
incubated with rabbit anti-goat IgG (1:50; Boster) or goat
anti-mouse IgG (1:50; Boster) for 2 h at 37°C and then with
streptomycin-avidin-biotin-peroxidase complex (SABC) for 20 min at
37°C. The sections were washed three times with PBS after each of
incubation and subsequently incubated with 3,3′-diaminobenzidine
(DAB).
Five slides were randomly selected from each group
and five visual fields in the basolateral amygdala were randomly
selected from each slide (magnification, ×400). We recorded the
optical density (OD) of positive cells in each field to evaluate
the average OD value. The OD of immunoreactivity of CNX or
ERp57-immunopositive cells were analyzed using a
MetaMorph/DPIO/BX41 morphology image analysis system.
Western blot analysis to detect CRT,
CNX and ERp57
Rats of each group were decapitated, and the brains
were removed rapidly and the basolateral amygdala was separated
immediately on ice. After being washed twice with cold 0.01 M PBS,
the tissues were homogenized with RIPA Lysis buffer respectively.
The supernatant liquor was collected, and then concentration of
protein was measured respectively via BCA kit. Equal amounts of
protein (50 µg/lane) prepared from each tissue was separated by 10%
(w/v) SDS-PAGE (110 V) and transferred onto a PVDF membrane via
electroblotting for 70 min at 350 mA. After being blocked with 5%
dried skim milk in 0.05% Tween-20-TBST at RT for 3 h, the membrane
was incubated with mouse anti-CRT (1:500), goat anti-CNX (1:500) or
mouse anti-ERp57 (1:200; all Santa Cruz Biotechnology, Inc.)
overnight at 4°C.
The membrane were washed three times with 0.01 M
TBST and incubated with the HRP-conjugated secondary antibody for 2
h at RT. Then the blots were visualized by enhanced
chemiluminescence (ECL; Beyotime Biotechnology, Jiangsu, China). To
confirm equal protein loading the same blots were reincubated with
antibodies against GAPDH (1:1,000; Boster). Immunoreaction for
GAPDH was also detected by the ECL. The OD were analyzed on the Gel
Image Analysis System. The levels of proteins were evaluated by
calculating the OD ratio of CRT/GAPDH, CNX/GAPDH and
ERp57/GAPDH.
Quantitative real-time reverse
transcription-PCR to detect CRT, CNX and ERp57
Total mRNA from the basolateral amygdala of each
group was extracted according to the protocol of Trizol (Takara
Biotech, Otsu, Japan) and 1 µg of total RNA was reverse transcribed
into cDNA. Then the cDNA was used as a template in RT-PCR
amplifications performed via a SYBR Real-Time PCR kit (Takara
Biotech, Dalian, China). The following primers were used: CRT
(upper, 5′-TTCTTGGACGGAGATGCCTG-3′ and lower,
5′-GGTCCCCGTAGAATTTGCCA-3′), CNX (upper, 5′-CCGGGAGGCTCGAGATAGA-3′
and lower, 5′-ATCCACCCTGACAGAGACCC-3′), GAPDH (upper,
5′-GGCACAGTCAAGGCTGAGAATG-3′, and lower,
5′-ATGGTGGTGAAGACGCCAGTA-3′). All primers were synthetized by
Shenggong Biotech Company (Shanghai, China) according to the serial
number from Genbank. The results were analyzed using the Rotor
Genne PCR-3000 (Corbett Research, Sydney, Australia). Relative mRNA
levels were calculated using the 2−∆∆Ct method and
normalized against.
Statistical analysis
All the experiment results were analyzed by one-way
analysis of variance (ANOVA) using SPSS 23.0 software. All data
were expressed as means ± standard error. P<0.05 was considered
to indicate a statistically significant difference.
Results
Decreased animal body weight after SPS
stimuli
Compared with the normal control, rats after SPS
stimuli presented loss of appetite. Accordingly, the body weight
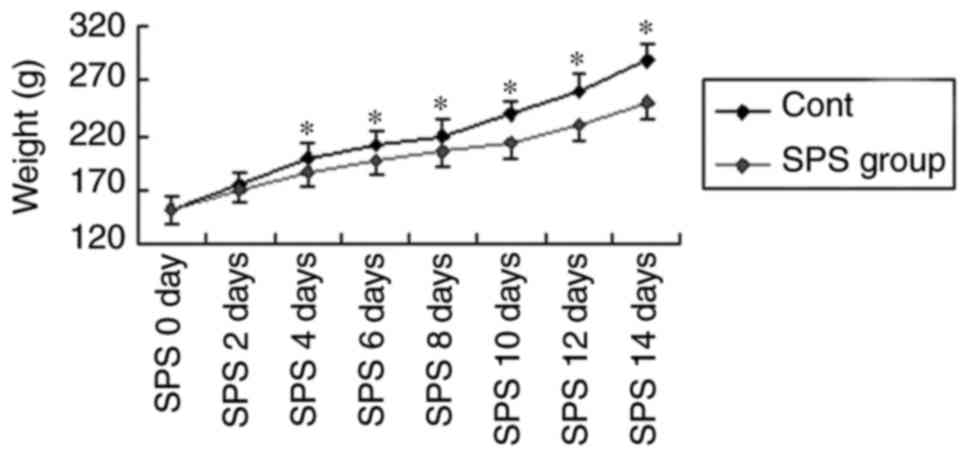
growth curve reflected this difference. As shown in Fig. 1, rats in the control group showed a
normal increase in body weight over time, rats in the model group
presented lighter weight after stimulation (P<0.05).
Immunofluorescence staining analysis
results of CRT
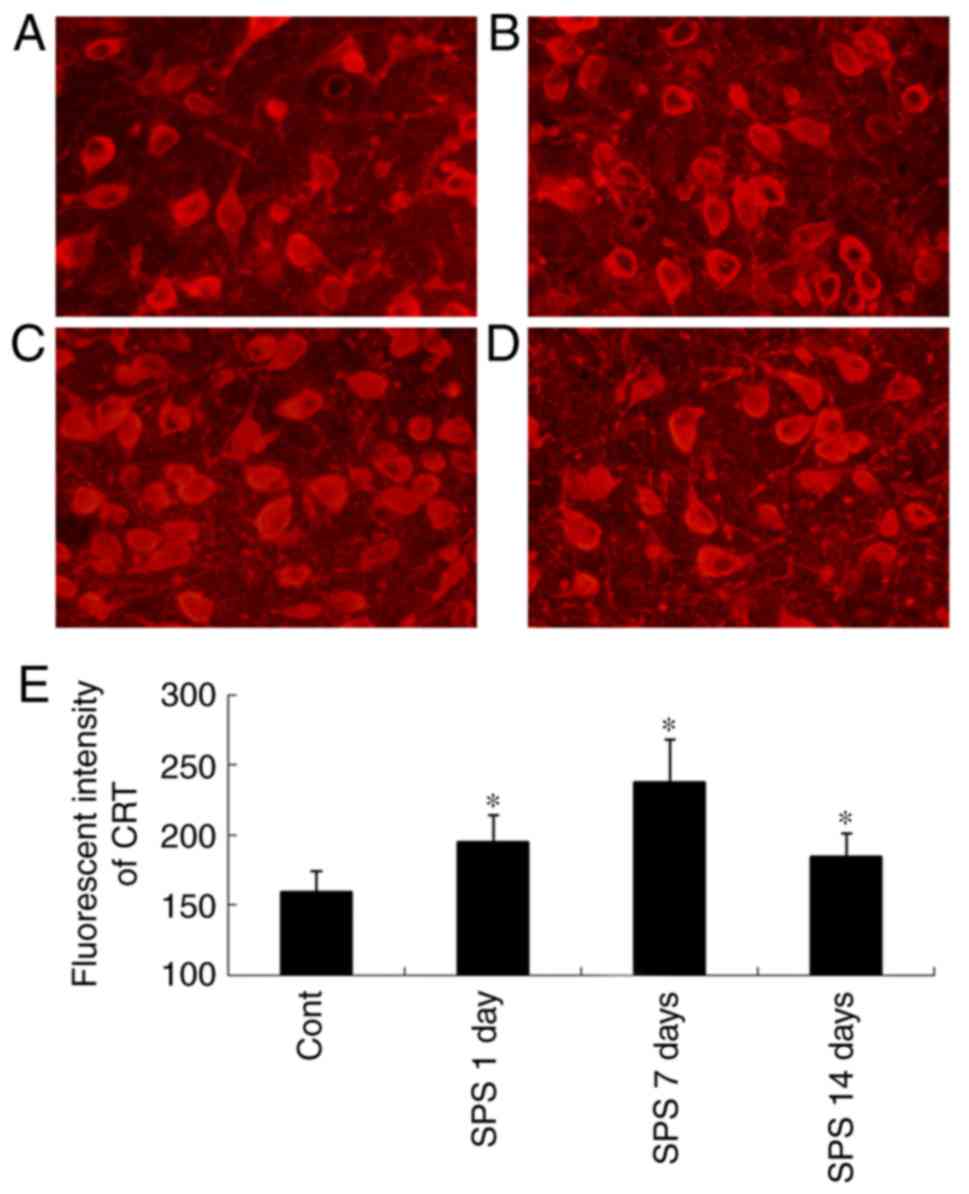
CRT-ir was were shown in Fig. 2. via immunofluorescence staining.
The CRT-ir was located in cytoplasm (Fig. 2A-D). In the Cont group, weak
fluorescent intensity of CRT-positive cells was shown in Fig. 2A, and that of SPS rats were
significantly strong compare to the Cont group (P<0.01)
(Fig. 2E).
Immunohistochemical staining analysis
results of CNX and ERp57
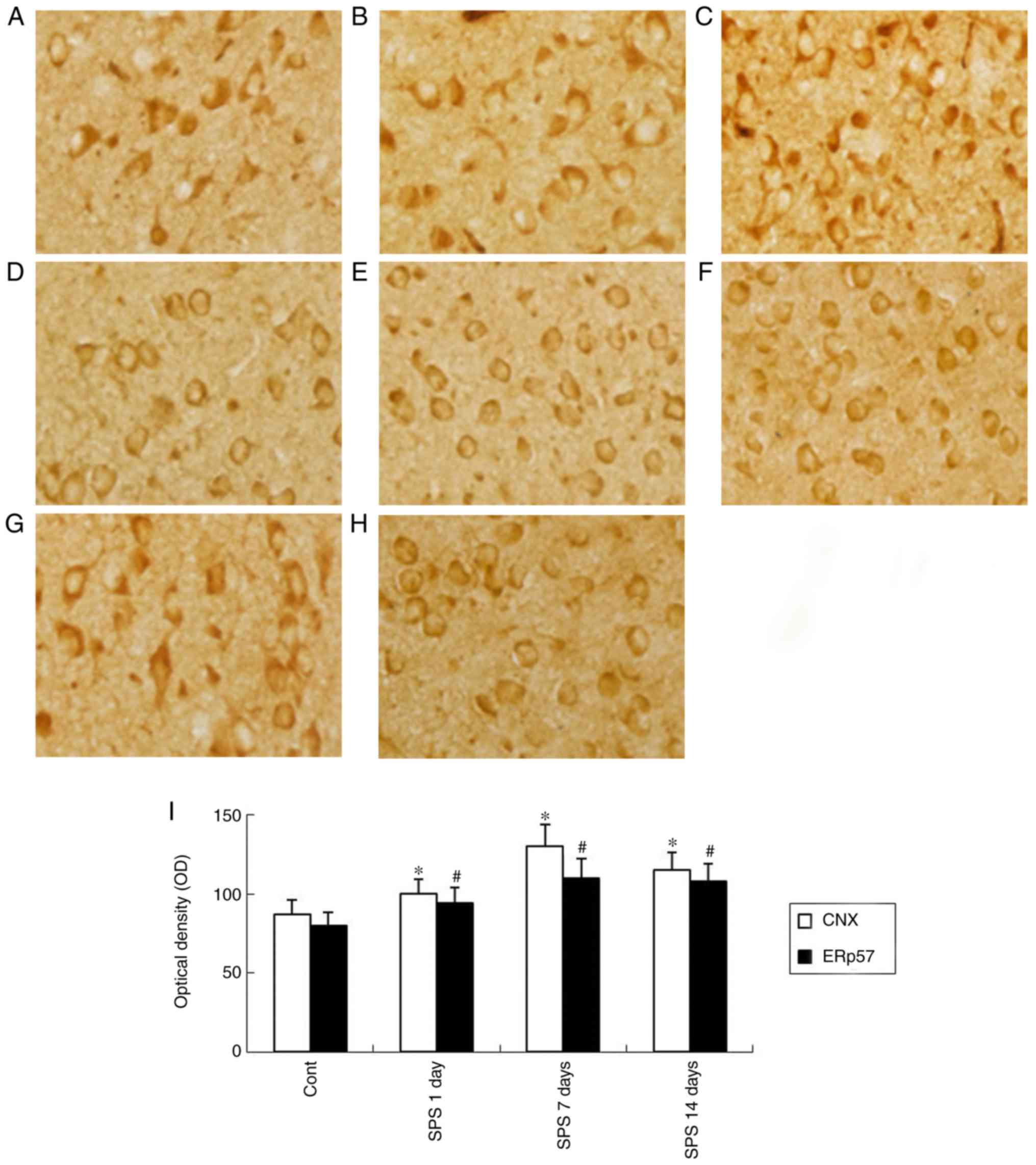
As was shown in Fig.
3, CNX and ERp57 widely distributed in the cytoplasm, and also
around the nucleus of cells in immunohistochemical staining.
Evaluation of CNX and ERp57 by immunohistochemical indicated a
stronger positive immunoreaction in the SPS model groups compared
with the Cont group. As shown in Fig.
3I, the histogram indicated this change.
Western bloting analysis protein
expression levels
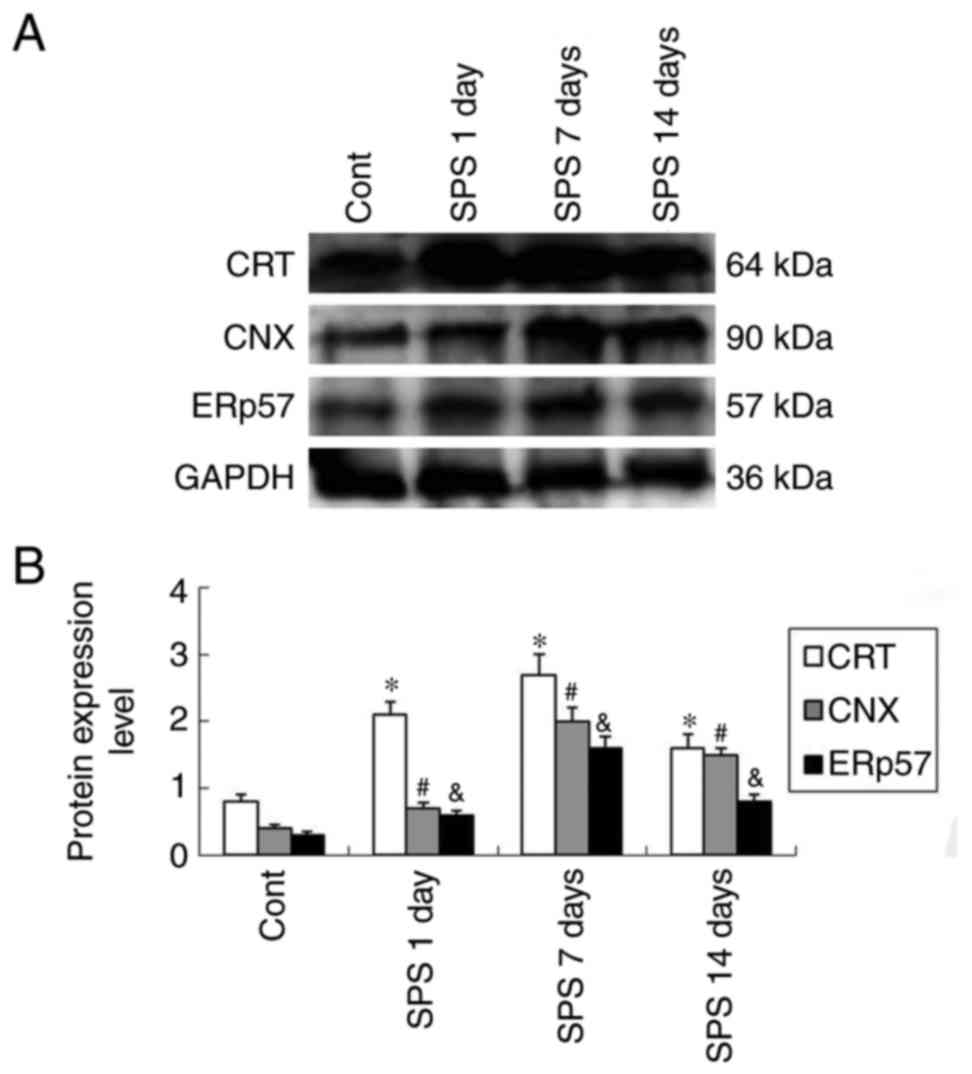
Molecular weights of CRT, CNX and ERp 57 were 64,
90, and 57 kDa, respectively, showing clear bands detected by
western blot. Evaluated by calculating the OD ratio of CRT/GAPDH,
CNX/GAPDH and ERp57/GAPDH, the level of protein expression
indicated a marked upregulation after SPS stimuli and peaked at SPS
7 days group compared with that of the Cont group (P<0.05)
(Fig. 4).
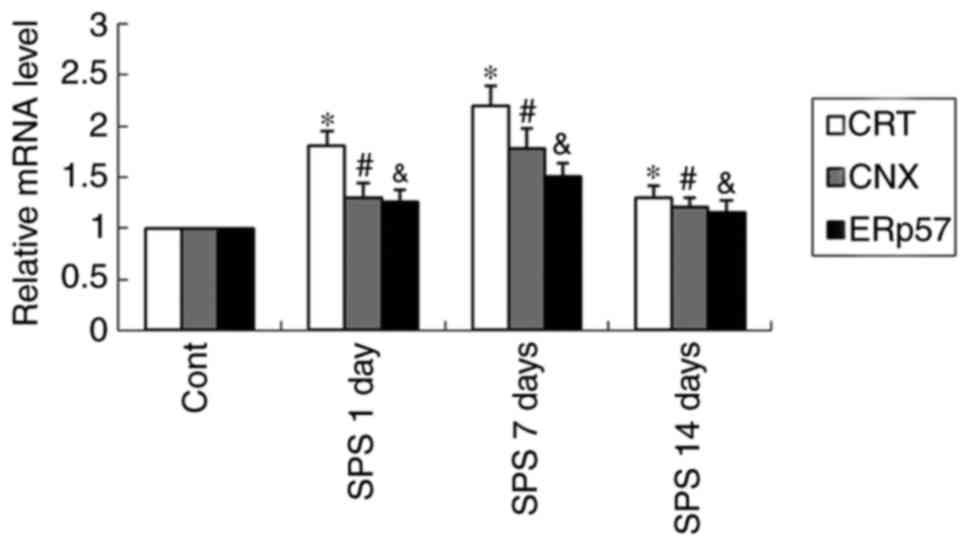
Quantitative real-time PCR analysis
results of CRT, CNX and ERp57
Certain expression of CRT, CNX and ERp 57 mRNA
presented in amygdala neurons of normal control rats (Fig. 5). The levels of CRT, CNX and ERp 57
mRNA were normalized with GAPDH mRNA. The expression of CRT, CNX
and ERp 57- mRNA appeared an obvious increase after exposure to SPS
and began decline on SPS 14-day (P<0.05) (Fig. 5).
Discussion
PTSD is an emotional illness and has long been
thought to involve a dysfunction in reaction to fear-related
stimulation. Many lines of evidence confirmed the crucial role of
amygdala in the processing of fear-related expression based on
investigations on animals and humans (24,25),
and multiple studies have indicated the basolateral amygdala as a
key area for regulating stress-related memory (27,32).
As a second messenger molecule in the cell, calcium
ion plays an important role in development and participating in
many cellular processes. The majority of intracellular calcium ion
is stored in the lumen of the ER. Calcium ion homeostasis play a
vital role in functionating of ER, and disturbance of calcium
homeostasis caused by various factors disrupt correct protein
folding, which could induce an accumulation of misfolded proteins,
or ER stress (13). Our previous
work has indicated that SPS induces Ca2+ overload in the
amygdala neurons of rats after SPS stimuli (33). In this study, we detected changes
of Ca2+ buffering protein. As the master regulator of
protein quality-control system and molecular chaperones in the ER,
CRT and CNX appeared significant upregulation in the amygdala
neurons after SPS stimuli, and peaked at SPS 7 days. It appears
resonable to suppose that the changes of CRT and CNX are
compensatory up-regulation to provide cytoprotection in response to
Ca2+ overload. ERp57 participates in the folding of
newly synthesized glycoproteins and dealing with misfolded proteins
from the ER via its own unique mechanism, in concert with CRT and
CNX (13,19–21).
We also detected changes of ERp57 in the amygdala neurons in this
study. Similarly, the results showed that ERp57 upregulated
significantly after SPS stimulation. It appears resonable to
suppose that all these changes are compensatory in order to
alleviate cell damage, however, this compensatory capacity is
limited. Then downregulation of these proteins appeared at SPS 14
days when it beyond its own compensatory capacity.
In this study, we investigated changes of CRT, CNX
and ERp57 in the amygdala of rats to find these ER-resident
molecular chaperone whether or not participate in PTSD, using
immunofluorescence, western blot and real-time PCR to measure the
protein and mRNA levels. Taken together, we found CRT, CNX and
ERp57 upregulated significantly in the amygdala of rats after
exposure to SPS. The results of qPCR are consistent with western
blot. It appears resonable to suppose that that Ca2+
overload after SPS stimuli induced accumulation of misfolded
protein, which lead to upregulation of CRT, CNX and ERp57 in order
to deal with accumulation of misfolded protein by folding once
again to ease cell damage. Nevertheless, excessive misfolded or
unfolded proteins resulted in dysfunction of ER in amygdala
neurons, which might be involved in pathogenesis for abnormality of
affect and behavior induced by PTSD. This findings provide new
insight into the pathogenesis of PTSD. However, it remains unclear
as to whether the changes of chaperones proteins serve as a trigger
or a consequnce of amygdala neuron dysfunction now.
Up till now, the pathological mechanisms of PTSD are
not yet understood in spite of extensive investigations. PTSD may
induce series of biological and functional abnormalities of the
amygdala and other brain regions, which results in dysfuction of
brain finally. The present study shed some light on the effects of
ER-resident molecular chaperone participating in PTSD, which might
provide experimental basis and a mechanism for the pathophysiology
of PTSD. Further studies on the regulatary mechanisms of molecular
chaperone on neuronal function in PTSD also should be included. So,
there is a need for more in-depth research on PTSD.
Acknowledgements
The present study was funded by a grant from the
National Natural Science Foundation of China (no. 31200772) and
Shenyang Science and Technology Project (no. F16-2-5-1-35).
References
|
1
|
American Psychiatric Association:
Diagnostic and Statistical Manual of Mental Disorders. 5th edition.
Arlington, VA: American Psychiatric Publishing; pp. 271–280.
2013
|
|
2
|
Mol SS, Arntz A, Metsemakers JF, Dinant
GJ, Vilters-van Monfort PA and Knottnerus JA: Symptoms of
post-traumatic stress disorder after non-traumatic events: Evidence
from an open population study. Br J Psychiatry. 186:494–499. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen Y, Li B, Han F, Wang E and Shi Y:
Dysfunction of calcium/calmodulin/CaM kinase IIα cascades in the
medial prefrontal cortex in post-traumatic stress disorder. Mol Med
Rep. 6:1140–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao D, Han F and Shi Y: Effect of
glucose-regulated protein 94 and endoplasmic reticulum modulator
caspase-12 in medial prefrontal cortex in a rat model of
posttraumatic stress disorder. J Mol Neurosci. 54:147–155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie J, Han F and Shi Y: The unfolded
protein response is triggered in rat neurons of the dorsal raphe
nucleus after single-pronged stress. Neurochem Res. 39:741–747.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao W, Han F and Shi YX: IRE1α pathway of
endoplasmic reticulum stress induces neuronal apoptosis in the
locus coeruleus of rats under single prolonged stress. Prog
Neuropsychopharmacol Biol Psychiatry. 69:11–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groenendyk J and Michalak M: Endoplasmic
reticulum quality control and apoptosis. Acta Biochim Pol.
52:381–395. 2005.PubMed/NCBI
|
|
8
|
Breckenridge DG, Germain M, Mathai JP,
Nguyen M and Shore GC: Regulation of apoptosis by endoplasmic
reticulum pathways. Oncogene. 22:8608–8618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiang J, Gu X, Qian S and Chen Z:
Endoplasmic reticulum stress-mediated apoptosis involved in
indirect recognition pathway blockade induces long-term heart
allograft survival. J Biomed Biotechnol. 2010:7054312010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv S, Sun EC, Xu QY, Zhang JK and Wu DL:
Endoplasmic reticulum stress-mediated autophagy contributes to
bluetongue virus infection via the PERK-eIF2α pathway. Biochem
Biophys Res Commun. 466:406–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao RV, Hermel E, Castro-Obregon S, del
Rio G, Ellerby LM, Ellerby HM and Bredesen DE: Coupling endoplasmic
reticulum stress to the cell death program. Mechanism of caspase
activation. J Biol Chem. 276:33869–33874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prell T, Lautenschläger J and Grosskreutz
J: Calcium-dependent protein folding in amyotrophic lateral
sclerosis. Cell Calcium. 54:132–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernales S, Papa FR and Walter P:
Intracellular signaling by the unfolded protein response. Annu Rev
Cell Dev Biol. 22:487–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung J and Michalak M: Cell surface
targeting of myelin oligodendrocyte glycoprotein (MOG) in the
absence of endoplasmic reticulum molecular chaperones. Biochim
Biophys Acta. 1813:1105–1110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams DB: Beyond lectins: The
calnexin/calreticulin chaperone system of the endoplasmic
reticulum. J Cell Sci. 119:615–623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hebert DN and Molinari M: In and out of
the ER: Protein folding, quality control, degradation, and related
human diseases. Physiol Rev. 87:1377–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itakura M, Tsujimura J, Yamamori S, Ohkido
T and Takahashi M: NMDA receptor-dependent recruitment of calnexin
to the neuronal plasma membrane. Neurosci Lett. 550:173–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ellgaard L, Riek R, Herrmann T, Güntert P,
Braun D, Helenius A and Wüthrich K: NMR structure of the
calreticulin P-domain. Proc Natl Acad Sci USA. 98:3133–3138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coe H and Michalak M: ERp57, a
multifunctional endoplasmic reticulum resident oxidoreductase. Int
J Biochem Cell Biol. 42:796–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ellgaard L and Frickel EM: Calnexin,
calreticulin, and ERp57: Teammates in glycoprotein folding. Cell
Biochem Biophys. 39:223–247. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szydlowska K and Tymianski M: Calcium,
ischemia and excitotoxicity. Cell Calcium. 47:122–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amunts K, Kedo O, Kindler M, Pieperhoff P,
Mohlberg H, Shah NJ, Habel U, Schneider F and Zilles K:
Cytoarchitectonic mapping of the human amygdala, hippocampal region
and entorhinal cortex: Intersubject variability and probability
maps. Anat Embryol (Berl). 210:343–352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis M and Whalen PJ: The amygdala:
Vigilance and emotion. Mol Psychiatry. 6:13–34. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pape HC: Petrified or aroused with fear:
The central amygdala takes the lead. Neuron. 67:527–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solano-Castiella E, Anwander A, Lohmann G,
Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD and Turner R:
Diffusion tensor imaging segments the human amygdala in vivo.
Neuroimage. 49:2958–2965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Young MB and Thomas SA: M1-muscarinic
receptors promote fear memory consolidation via phospholipase C and
the M-current. J Neurosci. 34:1570–1578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mihaljević S, Vuksan-Ćusa B, Marčinko D,
Koić E, Kušević Z and Jakovljević M: Spiritual well-being,
cortisol, and suicidality in croatian war veterans suffering from
PTSD. J Relig Health. 50:464–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto S, Morinobu S, Takei S, Fuchikami
M, Matsuki A, Yamawaki S and Liberzon I: Single prolonged stress:
Toward an animal model of posttraumatic stress disorder. Depress
Anxiety. 26:1110–1117. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Liu Y, Zheng H, Wang HN, Jin X,
Chen YC, Zheng LN, Luo XX and Tan QR: A modified single-prolonged
stress model for post-traumatic stress disorder. Neurosci Lett.
441:237–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan S and Liberzon I: Topiramate
attenuated exaggerated acoustic startle in an animal model of PTSD.
Psychopharmacology (Berl). 172:225–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chavez CM, McGaugh JL and Weinberger NM:
The basolateral amygdala modulates specific sensory memory
representations in the cerebral cortex. Neurobiol Learn Mem.
91:382–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao B, Han F and Shi YX: Dysfunction of
Ca2+/CaM kinase IIalpha cascades in the amygdala in post-traumatic
stress disorder. Int J Mol Med. 24:795–799. 2009.PubMed/NCBI
|