Introduction
Obstructive cholestasis often arises as an
incidental surgical symptom, and is caused by malignant biliary
obstruction due to gallbladder carcinoma, intrahepatic
cholangiocarcinoma and particularly perihilar cholangiocarcinoma
(PHCC) (1,2). Extra-cholestasis status has been
associated with the enrichment of hepatocellular hydrophobic bile
acid and severe liver injury (3,4).
Most patients with PHCC require ~50% hepatectomy to achieve radical
resection (1); therefore,
investigations into the effect of obstructive jaundice on liver
mass and functional restoration following partial hepatectomy (PH)
is of surgical importance (5,6).
Previous studies have reported that high total bilirubin (TB) serum
levels (>170 µmol/l) cause post-hepatectomy liver failure and
mortality (7,8). Therefore, biliary drainage is
performed to reduce obstructive cholestasis-induced liver injury,
which can also increase in-hospital morbidity and tract seeding
(9,10). However, a meta-analysis did not
report any benefits associated with preoperative biliary drainage
(11). Research based on
obstruction of various durations, the extent of liver sectioning
and the option of biliary drainage has not provided any significant
innovations into perioperative management of patients with PHCC,
without surgical treatment. However, contradicting conclusions
regarding impaired liver regeneration due to obstructive
cholestasis post-PH have been reported (12–15).
The effects of obstructive cholestasis on liver regeneration
require further investigation and clinical confirmation.
Hepatocellular triglyceride (TG) accumulation has
been observed in the early phase of normal liver regeneration
(16) and serves a crucial role in
the maintenance of increasing energy demands via β-oxidation
(17); metabolic factors involved
in liver regeneration within extra-cholestatic liver tissue require
further investigation. A previous study indicated that cholestasis
decreased hepatic energy charge and increased hepatic lipoperoxide
levels (18); alterations in TG
metabolism may also mediate cholestatic liver inflammation and
fibrotic processes in turn (19).
Therefore, whether alterations in TG metabolism within
extra-cholestatic conditions involve impairment of liver
regeneration following PH remains to be determined.
In the present study, alterations in TG metabolism
during liver regeneration within extra-cholestatic liver tissue
were investigated. The results suggested that overactivation of
farnesoid X receptor (FXR) signaling-induced reduction in TG may
contribute to the impairment of liver regeneration in post-PH
extra-cholestatic livers.
Materials and methods
Patients
A total of 10 paraffin-embedded specimens of liver
tissues from patients with PHCC were obtained from the Pathology
Department of Nanjing Drum Tower Hospital (Nanjing, China) between
April 2001 and May 2006. The diagnosis of PHCC was confirmed by
histology or cytology. Patients did not have any history of biliary
drainage. Preoperative serum TB levels within these patients were
>170 µmol/l (mean ± standard deviation, 216.5±45.08 µmol/l;
range, 171.4–304.5 µmol/l).
Animals and surgeries
Animals
A total of 140 male Sprague-Dawley rats (230–250 g,
8 weeks-old) were purchased from the Laboratory Animal Centre of
the Affiliated Drum Tower Hospital of Nanjing University Medical
School (Nanjing, China). Rats were housed in a specific
pathogen-free environment under a 12 h light/dark cycle, maintained
in a temperature-(22°C), air pressure- and humidity
(60%)-controlled environment and fed ad libitum. All animal
procedures were carried out in accordance with the Animal Care and
Use Committee at the Model Animal Research Center of Nanjing
University, (Nanjing, China). The present study was approved by the
Ethics Committee of Nanjing Drum Tower Hospital, the Affiliated
Hospital of Nanjing University Medical School.
Ligation of the common bile duct (BDL)
and sham operations
Rats were injected intraperitoneally with sodium
pentobarbital (70 mg/kg body weight) for anesthetization prior to
operations. Within the BDL group, two ligations of the distal
common bile duct (CBD) were performed using 5–0 chinlon, and the
CBD was cut between the ligations. Within the control (CTL) group,
the same abdominal incision was made; however, the CBD and adjacent
tissue were only touched with swabs; 3–0 chinlon was used to suture
the abdomen.
PH and internal biliary drainage
PH was performed according to Nagai et al
(20). Epidural catheters with 0.7
mm outer diameter were used to carry out internal biliary drainage.
Following 50% PH, the two ends of the epidural catheter were
inserted into the duodenum and the dilated CBD; purse-string
sutures were carried out for reinforcement.
Rats were randomly and equally separated into the
CTL (n=5), 7 day post-BDL (BDL-7 days, n=5) and 14 day post-BDL
(BDL-14 days, n=5) groups; sham and BDL operations were performed
as aforementioned. Rats were sacrificed on day 7 or 14 post-BDL and
liver samples were obtained. Following comparisons of alterations
within liver tissues from patients with PHCC, rats in the BDL-7
days group were selected for subsequent analyses. PH with internal
biliary drainage was conducted 7 days following BDL operations in
the BDL group and PH was performed following the sham operation
within the CTL group. Rats were sacrificed on day 7 post-BDL and
serum and liver samples were obtained (0 day).
For overall survival analysis, survival curves were
calculated based on survival within 7 days post-PH in the CTL and
BDL-7 days group using a novel group of rats (n=16).
For studying the liver regeneration, a separate
group of rats were sacrificed at day 1 (n=6), day 3 (n=8) and day 7
(n=8) post-PH in the CTL and BDL-7 days group to obtain liver and
plasma samples to determine liver regeneration status.
Serum data analysis
Blood samples were collected from the orbital venous
plexus of rats and were subsequently centrifuged at 3,000 × g for
15 min (room temperature). Serum was obtained and analyzed in the
Clinical Laboratory of the Affiliated Drum Tower Hospital of
Nanjing University Medical School.
Morphological analysis
Liver tissue was fixed in 4% paraformaldehyde or 10%
buffered formalin at 4°C for 24 h, followed by paraffin embedding
and sectioned to 5 µm. Subsequently, hematoxylin and eosin
(H&E) staining was performed at room temperature (3 min for
hematoxylin staining and 5 sec for eosin staining), and Sirius red
staining was used to measure the hepatic collagen content in liver
fibrosis. In brief, deparaffinised liver sections were incubated
with Sirius red for 2 h at room temperature. Areas stained with
Sirius Red were analyzed with ImageJ software (version 1.50;
National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
In brief, deparaffinised liver sections were blocked
with 1% bovine serum albumin (Applygen Technologies, Inc., Beijing,
China) for 1 h at room temperature and then incubated with a
primary antibody against proliferating cell nuclear antigen (PCNA;
1:200) at 4°C overnight. Subsequently, they were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody (1:100)
for 3 h at room temperature. The localization and expression of
PCNA within rat liver tissues were detected with DAB (Applygen
Technologies, Inc.) as previously described (21). Localization and expression of PCNA
were assessed and images were captured under an Olympus BX51
microscope (Olympus Corporation, Tokyo, Japan). The positive
stained cells were assessed by Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). Primary antibodies against
PCNA (cat. no. sc-7907) and horseradish peroxidase-tagged secondary
antibody (cat. no. sc-2004) were purchased from Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling
(TUNEL)
In situ DNA fragmentation within rat liver
was assessed using the DeadEnd Colorimetric TUNEL system (Promega
Corporation, Madison, WI, USA). In brief, deparaffinised and
rehydrated liver sections were incubated with 1% proteinase K
(Promega Corporation), then the in situ DNA fragmentation
was assessed according to the manufacturer's protocol. At least 5
fields of view/slices were assessed and images were captured under
an Olympus BX51 microscope (Olympus Corporation). The positive
staining hepatocytes were assessed by Image-Pro Plus 6.0 (Media
Cybernetics, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract rat liver
RNA. RT was performed using PrimeScript™ RT reagent
(Takara Bio Inc., Otsu, Japan) according to the manufacturer's
protocol. Subsequent RT-qPCR was carried out on an ABI-7300
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using
SYBR®−Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) as previously described (22). The thermocycling conditions were:
95°C for 1 min; 40 cycles of 95°C for 15 sec, 58°C for 15 sec and
72°C for 30 sec; with final heating at 72°C for 10 min. All
quantification was performed in triplicate and normalized to
β-actin. The relative expression level was calculated using the
2−ΔΔCq method (23).
The primer sequences were: Tumor necrosis factor-α (TNF-α) forward,
5′-CATCCGTTCTCTACCCAGCC-3′ and reverse, 5′-AATTCTGAGCCCGGAGTTGG-3′;
interleukin (IL)-6 forward, 5′-CACTTCACAAGTCGGAGGCT-3′ and reverse,
5′-TCTGACAGTGCATCATCGCT-3′; hepatocyte growth factor (HGF) forward,
5′-CCTTCGAGCTATCGCGGTAAA-3′ and reverse,
5′-GAATTTGTGCCGGTGTGGTG-3′; transforming growth factor-β1 (TGF-β1)
forward, 5′-AGGGCTACCATGCCAACTTC-3′ and reverse,
5′-CCACGTAGTAGACGATGGGC-3′; sterol-regulatory element-binding
protein-1c (SREBP-1c) forward, 5′-CCCGGTTTCCCAGGAACTTT-3′ and
reverse, 5′-CTGTCTCACCCCCAGCATAG-3′; peroxisome proliferator
activated receptor α (PPARα) forward, 5′-TTCGTGGAGTCCTGGAACTGA-3′
and reverse, 5′-CCACAGAGCACCAATCTGTGA-3′; acetyl-coA carboxylase 1
(ACC1) forward, 5′-CTTGGGGTGATGCTCCCATT-3′ and reverse,
5′-GCTGGGCTTAAACCCCTCAT-3′; fatty acid synthase (FASN) forward,
5′-GCATTTCCACAACCCCAACC-3′ and reverse, 5′-AACGAGTTGATGCCCACGAT-3′;
carnitine palmitoyltransferase 1A (CPT1A) forward,
5′-CCTACCACGGCTGGATGTTT-3′ and reverse, 5′-TACAACATGGGCTTCCGACC-3′
and β-actin forward, 5′-GCAGGAGTACGATGAGTCCG-3′ and reverse,
5′-ACGCAGCTCAGTAACAGTCC-3′.
Tissue TG content
Tissue TG reagents (Applygen Technologies, Inc.,
Beijing, China) were used to examine rat liver tissue TG content,
according to the manufacturer's protocol.
Western blot analysis
Hepatocytes were isolated a described previously by
Chen et al (24). Proteins
were isolated from rat hepatocytes using radioimmunoprecipitation
assay buffer containing protease inhibitors (Roche Diagnostics
GmbH, Mannheim, Germany) and quantified with bicinchoninc acid
assay kits (Applygen Technologies, Inc.); proteins were then boiled
in loading buffer. Proteins were separated by 12% SDS-PAGE with 50
µg total protein per lane and were then transferred onto
polyvinylidene difluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), then the membranes were blocked with 5% skim
milk for 1 h at room temperature. Membranes were incubated with
primary antibodies for at least 8 h at 4°C, Protein levels were
detected by incubation with HRP-conjugated secondary antibody for 1
h at room temperature and the signal was developed with ECL (EMD
Millipore, Billerica, MA, USA) and visualized using a Tanon 5200
imaging system (Tanon Science and Technology Co., Ltd., Shanghai,
China). Densitometric analysis was performed with ImageJ software
(version 1.50; National Institutes of Health, Bethesda, MD, USA).
Primary antibodies against small heterodimer partner (SHP; 1:100;
cat. no. sc-30169), FXR (1:200; cat. no. sc-13063), β-actin
(1:1,000, cat. no. sc-376421), goat anti-rabbit immunoglobulin
(Ig)G-HRP and goat anti-mouse IgG-HRP (1:10,000; cat. nos. sc-2004
and sc-2005) were purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
Experiments were repeated ≥3 times; data are
presented as the mean ± standard deviation. Statistical
calculations were performed with GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA) or SPSS statistical software 19
(IBM Corp., Armonk, NY, USA). Statistical significance was assessed
by an unpaired two-tailed Student's t-test or one-way analysis of
variance and Tukey's Highest significant difference test using,
Differences in overall survival were evaluated by log-rank test.
P<0.05 was considered to indicate a statistically significant
difference and P<0.01 was considered to indicate a highly
statistically significant difference.
Results
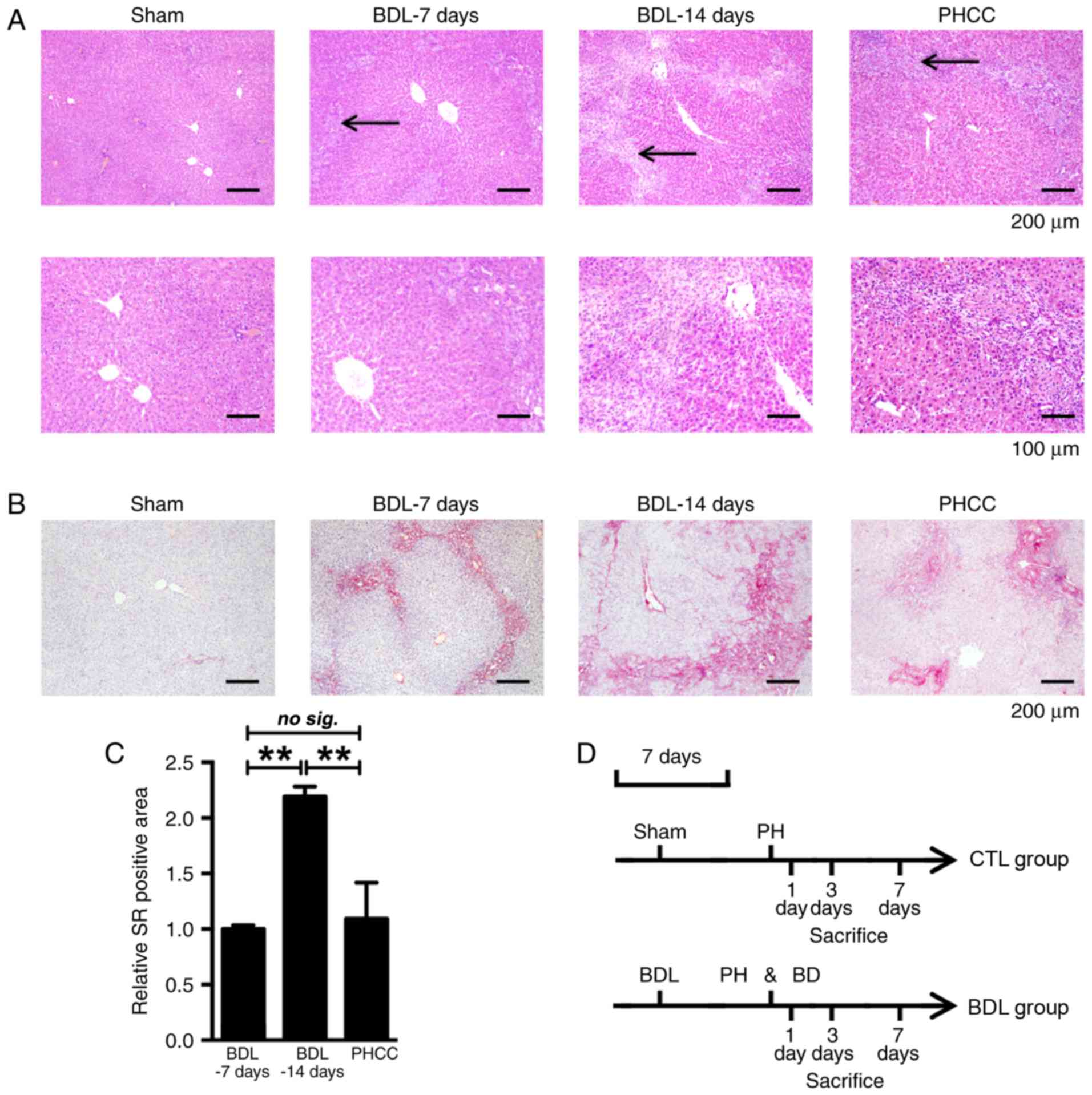
Hepatic morphological alterations
within patients with PHCC are similar to those in BDL-7 days group
rats
To determine the appropriate duration for
obstructive cholestatic development, morphological alterations
within hepatic PHCC samples were analyzed with H&E and Sirius
Red staining (Fig. 1). Moderate
biliary hyperplasia and inflammatory cell infiltration within the
portal area were observed within the BDL-7 days group and were more
severe within the BDL-14 days group, compared with the sham group.
Morphological alterations exhibited by the BDL-7 days group were
similar to those within patients with PHCC (Fig. 1A). The aforementioned observations
were confirmed via Sirius Red staining. Collagen deposition within
the BDL-7 days group was similar to that of patients with PHCC
(Fig. 1B). A ~2.2-fold increase in
collagen deposition was observed in the BDL-14 days group compared
with in the BDL-7 days group. Rats in the BDL-7 days group were
selected for subsequent analysis of clinical obstructive
cholestasis via PH with internal biliary drainage; PH alone was
conducted within rats of the CTL group (Fig. 1D).
Obstructive jaundice leads to
hepatocyte proliferation and increased liver weight (LW)
Analysis of LW and body weight (BW) prior to PH
revealed that obstructive cholestasis was associated with reduced
body weight (BW) and malnutrition (12). Therefore, the LW/BW ratio was
inapplicable to the evaluation of liver regeneration following PH
as body weight was influenced by obstructive cholestasis status.
Conversely, LW was increased, as was the LW/BW ratio (LW/BW;
Fig. 2A); In addition to collagen
deposition, biliary hyperplasia and inflammatory infiltration,
hepatocellular behavior, including proliferation and apoptosis may
also contribute to alterations in LW. Hepatocyte proliferation was
determined by analyzing the number of PCNA-positive hepatocytes;
21% hepatocytes replicated pre-PH within the BDL-7 days group
(Fig. 2B). Apoptotic rate was
investigated using a TUNEL assay. As presented in Fig. 2C, apoptosis was increased within
the BDL group compared with in the CTL group (2.1 vs. 8.2%). The
findings of the present study indicated that obstructive jaundice
was associated with hepatocyte proliferation, biliary hyperplasia,
collagen deposition and inflammatory infiltration; therefore, LW
and LW/BW increased.
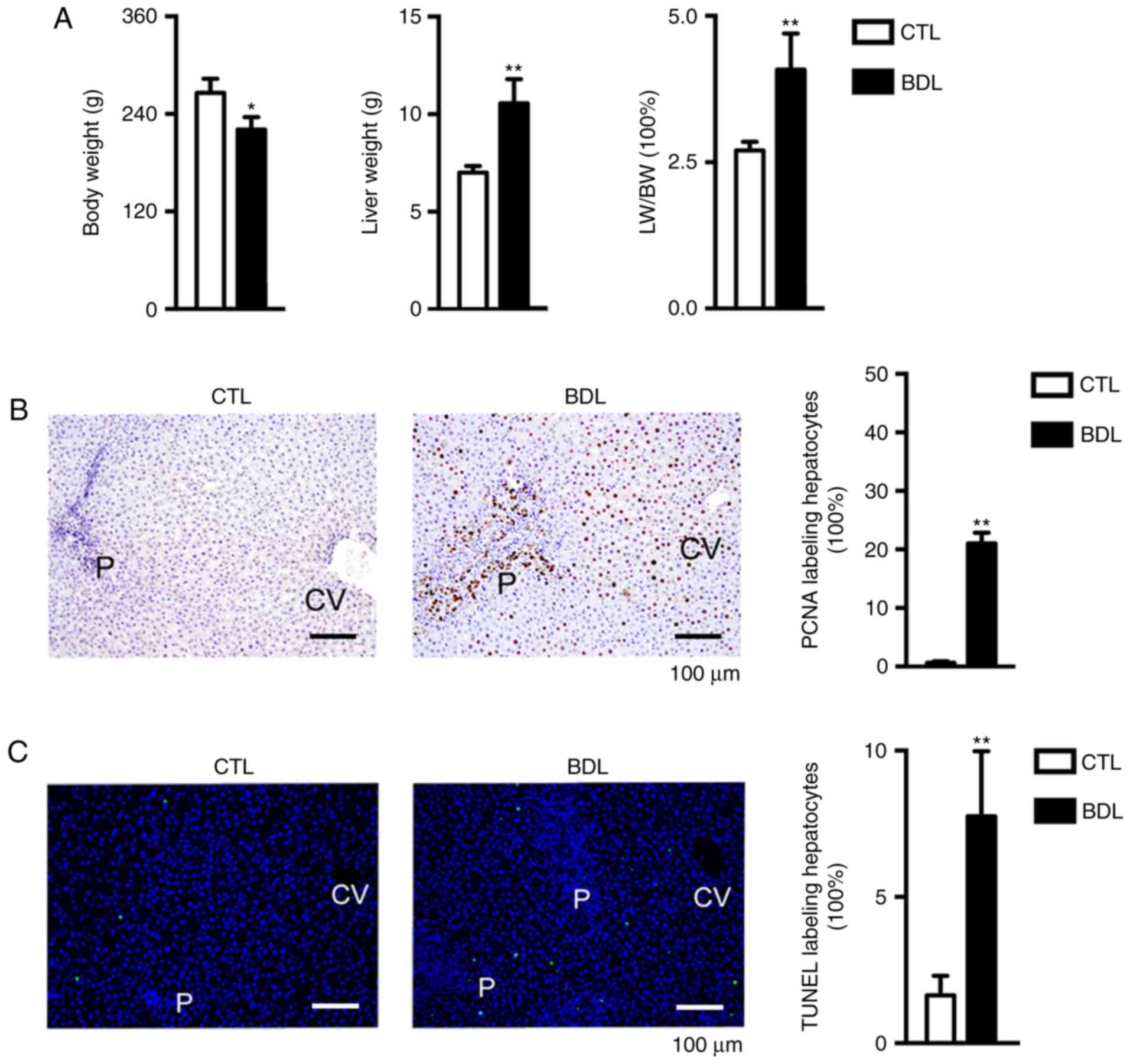 | Figure 2.LW, BW and hepatocyte behavior in CTL
and BDL groups pre-PH. (A) LW was significantly increased and BW
was markedly decreased within the BDL group compared with in the
CTL group; the LW/BW ratio pre-PH was significantly increased
within the BDL group. *P<0.05; **P<0.01. (B) Hepatocyte
proliferation was detected, as determined by immunohistochemistry.
The percentage of PCNA-positive hepatocytes was significantly
increased within the BDL group compared with in the CTL group.
**P<0.01. (C) Hepatocyte apoptosis was analyzed via TUNEL assay;
apoptosis was significantly increased within the BDL group compared
with in the CTL group. **P<0.01. Data are presented as the mean
± standard deviation. BDL, ligation of the common bile duct; BW,
body weight; CTL, control; CV, central vein; LW, liver weight P,
portal area; PCNA, proliferating cell nuclear antigen; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick-end
labeling. |
Obstructive cholestasis causes severe
liver injury pre- and post-PH
Serum data was collected to examine liver injury.
Increased alanine transferase (ALT), aspartate transaminase (AST),
TB and total bile acids (TBA) levels were detected within the BDL
group prior to PH (Fig. 3A-D),
which was associated with severe liver injury. Following PH with
internal biliary drainage, serum ALT levels were significantly
decreased within the BDL group compared with in the CTL group;
however, significant alterations in serum AST levels were not
observed (Fig. 3A and B). Elevated
TB and TBA serum levels rapidly decreased post-PH within the BDL
group (Fig. 3C and D), which
indicated that biliary drainage was successful. The findings of the
present study suggested that obstructive cholestasis led to liver
injury prior to and post-PH.
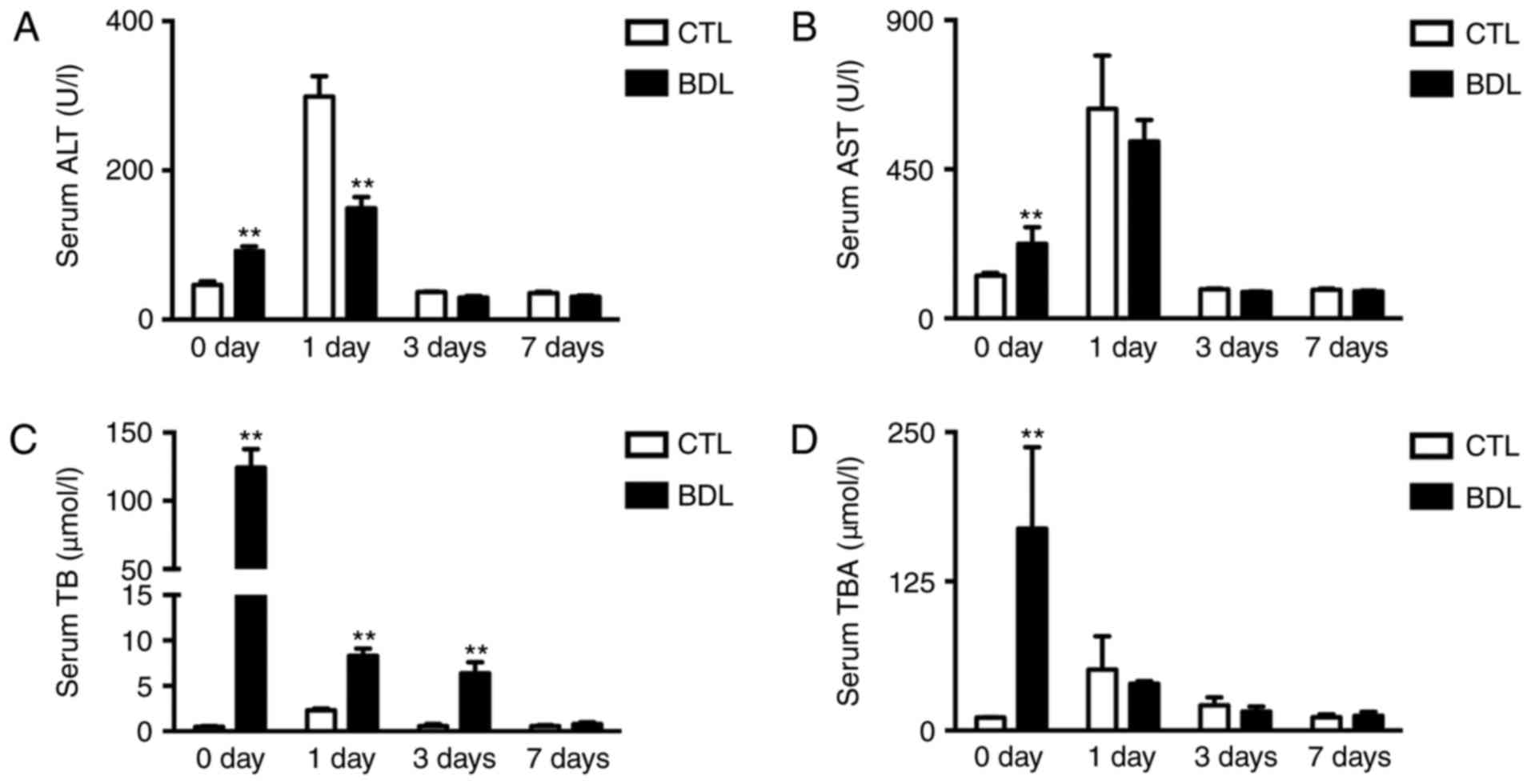 | Figure 3.Obstructive cholestasis causes liver
injury pre- and post-PH. Serum (A) ALT, (B) AST, (C) TB and (D) TBA
levels within BDL and CTL groups pre- and post-PH. Data are
expressed as the mean ± standard deviation, n≥6; **P<0.01. ALT,
alanine transaminase; AST, aspartate aminotransferase; BDL,
ligation of the common bile duct; CTL, control; PH, partial
hepatectomy; TB, total bilirubin; TBA, total bile acids. |
Obstructive cholestasis inhibits liver
mass restoration via hepatocyte proliferation suppression
post-PH
A decrease in overall survival was reported post-PH,
which was associated with extra-cholestasis; ~56% BDL rats survived
post-PH, whereas the CTL group exhibited 100% overall survival
(P<0.01; Fig. 4A).
Surgical-associated factors, including hemorrhage, re-obstruction,
bile leakage and organ injury, were excluded, thus indicating that
extra-cholestasis independently decreased overall survival rate
post-PH. In the present study, rat mortality was reported 2 days
post-PH within the BDL group; acute liver failure following PH may
have accounted for observed mortalities. The capacity of liver mass
restoration was significantly impaired post-PH (Fig. 4B); therefore, post-PH liver failure
may result from an impaired capacity for liver regeneration. LW of
each time point post-PH was normalized to the pre-PH value.
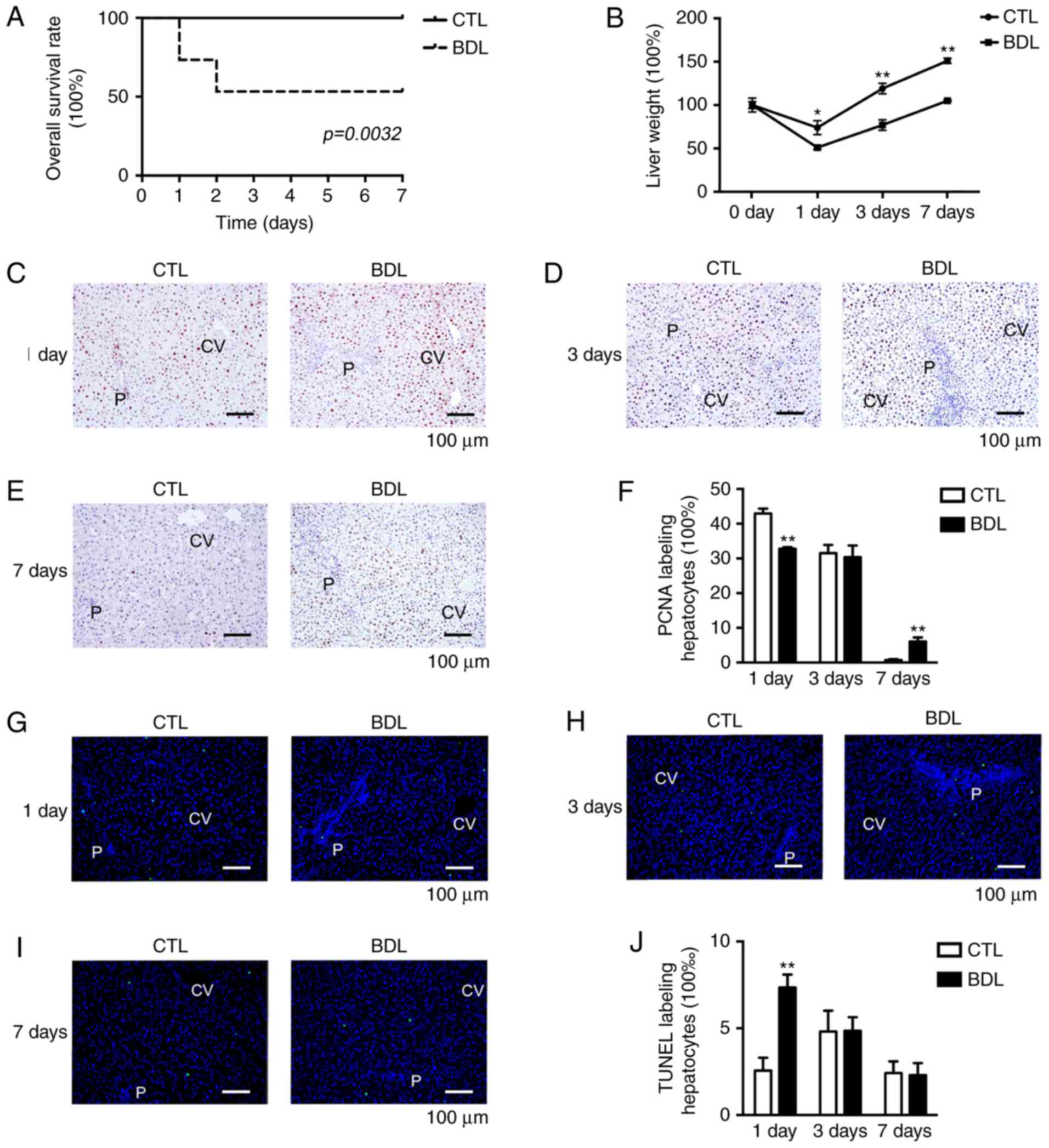 | Figure 4.Obstructive cholestasis leads to
impaired liver regeneration and mortality. (A) Decreased overall
survival was observed in BDL group (56 vs. 100% within the CTL
group). (B) Restoration of liver weight within BDL and CTL groups
following PH. Hepatocyte proliferation (C) 1 day, (D) 3 days and
(E) 7 days post-PH was detected by immunohistochemistry. (F)
Percentage of PCNA-positive hepatocytes. Apoptosis of hepatocytes
was measured (G) 1 day, (H) 3 days and (I) 7 days post-PH by TUNEL
assay. (J) Percentage of TUNEL-positive hepatocytes. Data are
expressed as the mean ± standard deviation, n=16. *P<0.01 and
**P<0.05. BDL, ligation of common bile duct; CTL, control; CV,
central vein; P, portal area; PCNA, proliferating cell nuclear
antigen; PH, partial hepatectomy; TUNEL, terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling. |
Suppression of liver mass restoration may be
associated with abnormal hepatocellular proliferative and apoptotic
activities following PH. Compared with in the CTL group, the PCNA
labeling index was markedly decreased at 1 day within the BDL group
(43.2 vs. 33.7%; Fig. 4C-F).
Analysis of hepatocyte apoptotic ability demonstrated a significant
increase within the BDL group on day 1 compared with in the CTL
group (2.5 vs. 7.2%; Fig. 4G-J).
However, as apoptotic ability was weak between the groups, other
factors may contribute to the inhibition of liver mass regeneration
following PH. Therefore, extra-cholestasis may result in a
reduction in overall survival and impaired liver mass restoration
in response to early stage hepatocyte proliferation inhibition.
Reduced accumulation of TG and altered
cytokine and growth factor expression inhibits early stage
post-PH-hepatocyte proliferation
The process of liver regeneration is tightly
regulated by cytokines, growth factors and metabolic factors
(25). The expression levels of
TNF-α, IL-6, HGF and TGF-β1 were analyzed by RT-qPCR. The results
of the present study revealed a decrease in TNF-α, IL-6 and HGF
expression levels on day 1 within the BDL group (Fig. 5A-C). Significant decreases in
TGF-β1 expression levels were observed on days 3 and 7 within the
BDL group (Fig. 5D), which may be
associated with increased hepatocyte proliferative ability at day 7
in the BDL group (Fig. 4E and F).
Inflammation infiltration was decreased on days 1, 3 and 7 in the
BDL group. In addition, compared with the CTL group, TG
accumulation was inhibited on days 1 and 3 within the BDL group
(Fig. 5E-G). The findings of the
present study were confirmed by hepatic TG analysis (Fig. 5H), which indicated that energy
supplies were insufficient during the liver regeneration process.
Therefore, an insufficient energy supply and reduced TNF-α, IL-6
and HGF expression may be associated with impaired capacity of
early stage liver regeneration.
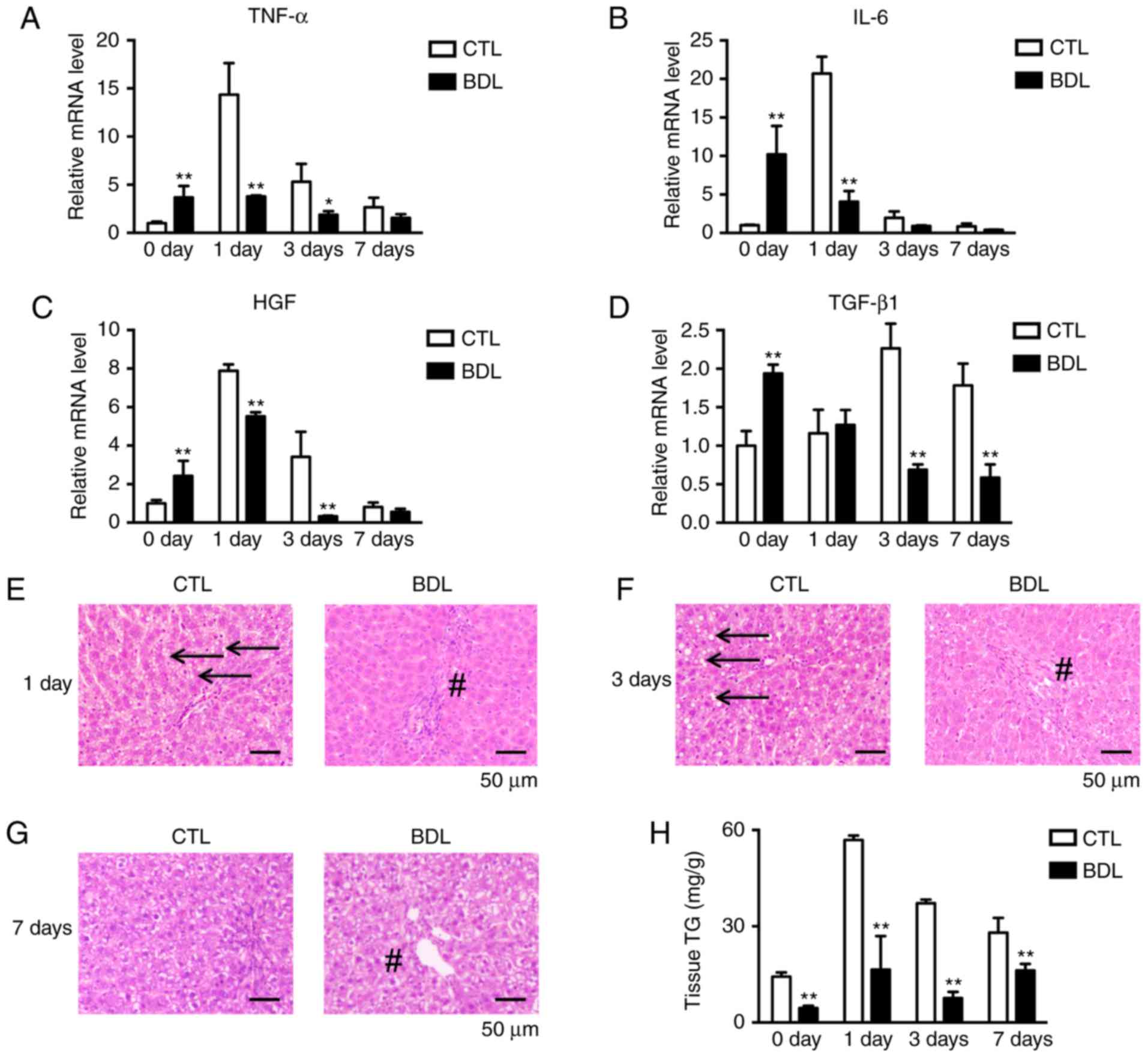 | Figure 5.Cytokine, growth factor and metabolic
factor expression levels associated with hepatocyte proliferation
were evaluated pre- and post-PH. Quantification of hepatic (A)
TNF-α, (B) IL-6, (C) HGF and (D) TGF-β expression levels at the
indicated time points pre- and post-PH. Expression was normalized
to β-actin expression and calculated as the fold change compared
with in the CTL group pre-PH. *P<0.05 and **P<0.01.
Morphological analysis of the CTL and BDL groups at (E) 1 day, (F)
3 days and (G) 7 days. Normal TG deposition was decreased post-PH
(arrows) within the BDL group; biliary hyperplasia and inflammatory
infiltration (#) in the portal area were decreased post-PH. (H) TG
quantification. **P<0.01. Data are expressed as the mean ±
standard deviation. BDL, ligation of common bile duct, CTL,
control; IL-6, interleukin-6; HGF, hepatocyte growth factor; PH,
partial hepatectomy; TG, triglycerides; TGF-β1, transforming growth
factor-β1; TNFα, tumor necrosis factor-α. |
Overactivation of FXR signaling is
associated with reduced TG accumulation post-PH within the BDL
group
TG metabolism is mediated by fatty acid metabolism,
which mainly constitutes fatty acid synthesis and β-oxidation. The
present study aimed to investigate the effect of TG metabolism in
extra-cholestasis on impaired liver regeneration. Fatty acid
β-oxidation is regulated by PPARα and its target gene, CPT1A.
RT-qPCR analysis revealed a significant decrease in PPARα and CPT1A
at 3 and 1 day post-PH, respectively (Fig. 6A and B), which was associated with
reduced fatty acid utilization in early stage liver regeneration.
In addition, the expression levels of fatty acid-synthesis
regulators, including SREBP-1c, FASN and ACC1 were analyzed.
SREBP-1c expression levels were inhibited at 0 and 1 day post-PH
(Fig. 6C), which was associated
with impaired de novo lipogenesis within the BDL group.
Expression levels of FASN and ACC1 were reduced during early stage
regeneration following PH (Fig. 6D and
E). Decreased SREBP-1c activity was associated with reduced
tissue TG content and therefore reduced fatty acid utilization
within the BDL group.
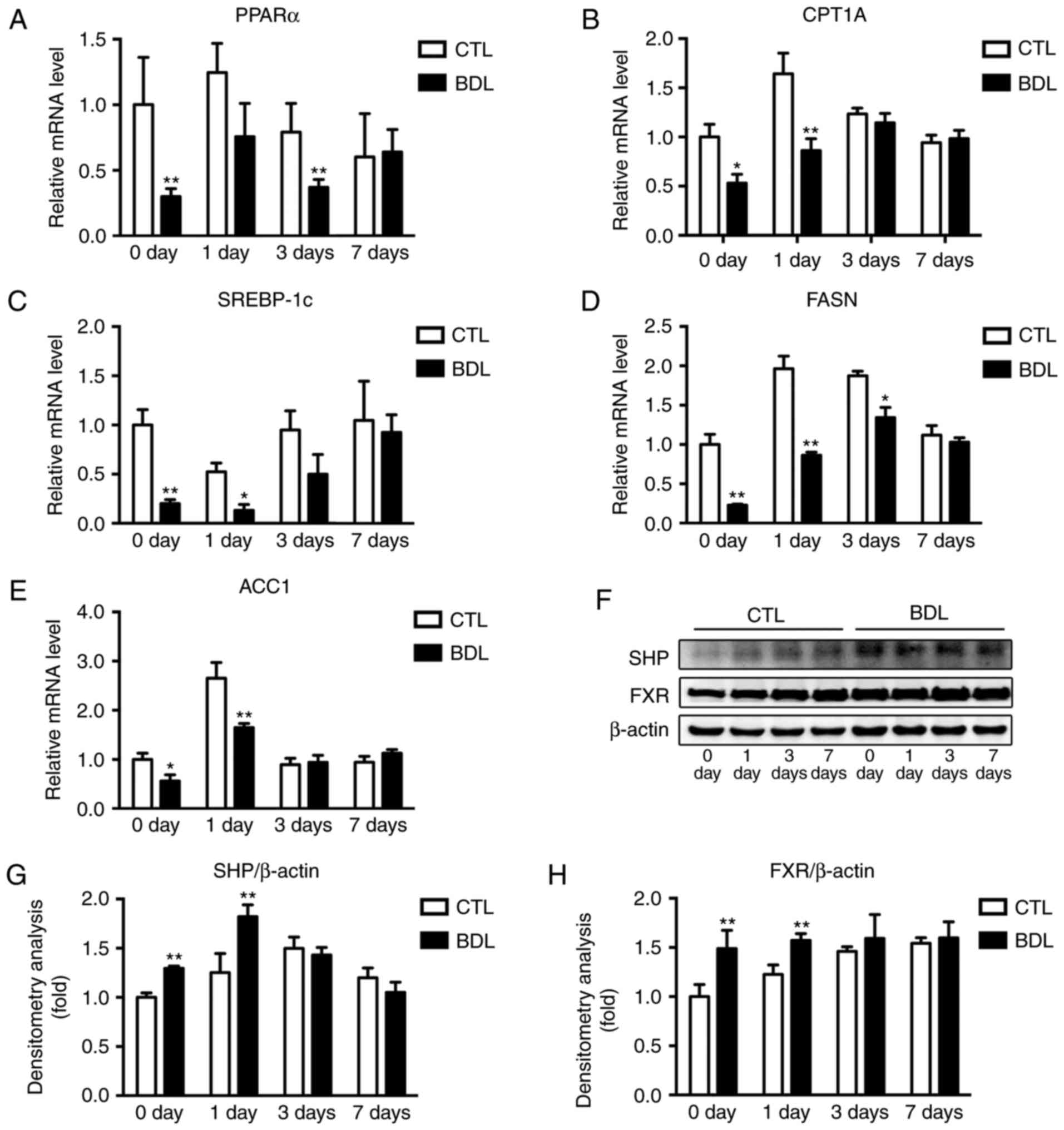 | Figure 6.Fatty acid synthesis and β-oxidation,
and FXR signaling were detected pre- and post-PH. Quantification of
hepatic (A) PPARα, (B) CPT1A, (C) SREBP-1c, (D) FASN and (E) ACC1
expression levels pre- and post-PH within the BDL and CTL groups.
Expression levels were normalized to β-actin and calculated as the
fold change compared with in the CTL group pre-PH. *P<0.05 and
**P<0.01. (F) Western blotting and (G and H) densitometry
analyses of SHP and FXR were performed using the indicated
antibodies pre- and post-PH. *P<0.05 and **P<0.01. β-actin
was used as the internal control. Expression levels were normalized
to β-actin expression and calculated as the fold change compared
with in the CTL group pre-PH. Data are expressed as the mean ±
standard deviation and calculated as the fold change. ACC1,
acetyl-CoA carboxylase 1; BDL, ligation of common bile duct, CTL,
control; FASN, fatty acid synthase; FXR, farnesoid X receptor;
PARα, peroxisome proliferator activated receptor α; PH, partial
hepatectomy; SHP, small heterodimer partner; SREBP-1c,
sterol-regulatory element-binding protein-1c. |
FXR signaling is required to mediate bile acid
cytotoxicity induced by BDL (26,27)
and serves as a critical regulator of TG metabolism by regulating
SREB-1c activity. In the present study, FXR signaling was analyzed
via western blot analysis (Fig.
6F). Expression levels of SHP and FXR (Fig. 6G and H) were significantly
increased 0 and 1 day post-PH; therefore, FXR signaling
overactivation post-PH within the BDL group may account for
impaired SREB-1c activity. The findings of the present study
indicated that obstructive cholestasis may impair liver
regeneration due to altered cytokine and growth factor expression,
and an insufficient energy supply. In addition, inhibition of
SREB-1c activity in response to overactivation of the FXR signaling
pathway may be associated with reduced TG accumulation during liver
regeneration and impaired hepatocyte proliferation within
obstructive cholestatic liver tissue.
Discussion
Liver failure is a common cause of postoperative
mortality following major hepatectomy. Such surgical treatments are
performed in cases of PHCC, which is characterized by malignant
obstructive cholestasis and often requires major hepatectomy to
cure (1,28). Extra-cholestasis has been reported
to be a poor prognostic risk factor in postoperative analysis;
however, the resection rate in PHCC is too low to investigate
whether extra-cholestasis or cholangiocarcinoma affects overall
survival (9). In addition,
preoperative biliary drainage as a benefit for patients with PHCC
remains controversial (29).
Further investigation into whether cholestasis independently
affects prognosis is required. In the present study, a decrease in
overall survival rate was observed within the BDL group following
PH; therefore, severe cholestasis status may have independently
affected overall survival. In order to reduce risks associated with
cholestasis, appropriate measures, such as preoperative internal
biliary drainage may be conducted (1). Preliminary studies demonstrated that
biliary drainage itself did not have an effect on 7 day
post-operation survival (data not shown).
Liver failure following major hepatectomy is mainly
caused by impaired liver regeneration. The effect of obstructive
cholestasis on liver regeneration post-PH was analyzed in the
present study based on the BDL rat model; however, clinical
significance based on various obstructive durations, liver
sectioning extents and biliary drainage has not been reported in
previous studies (12–15,30).
The present study compared morphological alterations within
patients with PHCC and BDL rat models. PH and internal biliary
drainage were performed to attain the clinical condition within rat
models. Severe obstructive cholestasis was confirmed to impair
liver regeneration. A previous study revealed that alterations in
cytokine and growth factor expression levels affected liver
regeneration within extra-cholestatic liver tissue (15). However, the role of metabolic
factors in this process remains to be investigated, as previous
studies have reported that cholestasis may influence systematic and
hepatic TG metabolism, and restoration of hepatic energy stores
(31,32). In the present study, the capacity
of liver mass regeneration was inhibited within the BDL group. In
addition to energy supplies, alterations in the expression levels
of cytokines and growth factors, including TNFα, IL-6 and TGF-β1,
suppressed hepatocyte proliferation. To the best of the authors'
knowledge, the present study is the first to demonstrate a
potential mechanism underlying cholestasis-induced impairments in
liver regeneration, besides the altered expression of cytokines and
growth factors (6). A previous
study indicated that the application of ω-3 fatty acids and the
content of enteral nutritional suspensions, such as total
protein-medium chain triglycerides, promoted liver regeneration
within normal rats (33). In the
clinical setting, whether enteral nutritional suspensions improve
severe cholestasis-associated impairments post-PH remains to be
investigated. A previous study indicated that deletion of the FXR
gene within mice may reverse obstructive cholestasis-induced liver
injury (26). In the present
study, overactivated FXR signaling was also observed pre-PH and at
1 day post-PH. Therefore, FXR antagonists may serve to improve
obstructive cholestasis-induced liver injury and promote liver
regeneration following PH within extra-cholestatic liver
tissue.
In conclusion, the results of the present study
established the role of TG metabolism in impaired hepatocyte
proliferation within cholestatic livers; impaired liver
regeneration was induced by obstructive cholestasis due to altered
cytokine and growth factor expression levels, and reduced energy
supplies. Furthermore, a decrease in TG accumulation due to
FXR-signaling overactivation may contribute to impairments in liver
regeneration post-PH within extra-cholestatic liver tissue.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81470866,
Yudong Qiu), the Nature Science Foundation of Jiangsu Province
(grant no. BK20160120, Liang Mao) and the Fundamental Research
Funds for the Central Universities (grant no. 021414380175, Liang
Mao).
References
|
1
|
Nagino M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Takahashi Y and Nimura Y: Evolution of surgical
treatment for perihilar cholangiocarcinoma: A single-center 34-year
review of 574 consecutive resections. Ann Surg. 258:129–140. 2013.
View Article : Google Scholar
|
|
2
|
DeOliveira ML, Cunningham SC, Cameron JL,
Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ and Schulick
RD: Cholangiocarcinoma-Thirty-one-year experience with 564 patients
at a single institution. Ann Surg. 245:755–762. 2007. View Article : Google Scholar :
|
|
3
|
Zhang Y, Hong JY, Rockwell CE, Copple BL,
Jaeschke H and Klaassen CD: Effect of bile duct ligation on bile
acid composition in mouse serum and liver. Liver Int. 32:58–69.
2012. View Article : Google Scholar
|
|
4
|
Marin JJ, Macias RI, Briz O, Banales JM
and Monte MJ: Bile acids in physiology, pathology and pharmacology.
Curr Drug Metab. 17:4–29. 2015. View Article : Google Scholar
|
|
5
|
Kennedy TJ, Yopp A, Qin Y, Zhao B, Guo P,
Liu F, Schwartz LH, Allen P, D'Angelica M, Fong Y, et al: Role of
preoperative biliary drainage of liver remnant prior to extended
liver resection for hilar cholangiocarcinoma. HPB (Oxford).
11:445–451. 2009. View Article : Google Scholar :
|
|
6
|
Yokoyama Y, Nagino M and Nimura Y:
Mechanism of impaired hepatic regeneration in cholestatic liver. J
Hepatobiliary Pancreat Surg. 14:159–166. 2007. View Article : Google Scholar
|
|
7
|
Xiong JJ, Nunes QM, Huang W, Pathak S, Wei
AL, Tan CL and Liu XB: Preoperative biliary drainage in patients
with hilar cholangiocarcinoma undergoing major hepatectomy. World J
Gastroenterol. 19:8731–8739. 2013. View Article : Google Scholar :
|
|
8
|
Iacono C, Ruzzenente A, Campagnaro T,
Bortolasi L, Valdegamberi A and Guglielmi A: Role of preoperative
biliary drainage in jaundiced patients who are candidates for
pancreatoduodenectomy or hepatic resection: Highlights and
drawbacks. Ann Surg. 257:191–204. 2013. View Article : Google Scholar
|
|
9
|
Maguchi H, Takahashi K, Katanuma A, Osanai
M, Nakahara K, Matuzaki S, Urata T and Iwano H: Preoperative
biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary
Pancreat Surg. 14:441–446. 2007. View Article : Google Scholar
|
|
10
|
Takahashi Y, Nagino M, Nishio H, Ebata T,
Igami T and Nimura Y: Percutaneous transhepatic biliary drainage
catheter tract recurrence in cholangiocarcinoma. Br J Surg.
97:1860–1866. 2010. View
Article : Google Scholar
|
|
11
|
Liu F, Li Y, Wei Y and Li B: Preoperative
biliary drainage before resection for hilar cholangiocarcinoma:
Whether or not? A systematic review. Dig Dis Sci. 56:663–672. 2011.
View Article : Google Scholar
|
|
12
|
Bird MA, Lange PA, Schrum LW, Grisham JW,
Rippe RA and Behrns KE: Cholestasis induces murine hepatocyte
apoptosis and DNA synthesis with preservation of the
immediate-early gene response. Surgery. 131:556–563. 2002.
View Article : Google Scholar
|
|
13
|
Tracy TF Jr, Bailey PV, Goerke ME,
Sotelo-Avila C and Weber TR: Cholestasis without cirrhosis alters
regulatory liver gene expression and inhibits hepatic regeneration.
Surgery. 110:176–783. 1991.
|
|
14
|
Foss A, Andersson R, Ding JW, Hochbergs P,
Paulsen JE, Bengmark S and Ahrén B: Effect of bile obstruction on
liver regeneration following major hepatectomy: an experimental
study in the rat. Eur Surg Res. 27:127–133. 1995. View Article : Google Scholar
|
|
15
|
Makino H, Shimizu H, Ito H, Kimura F,
Ambiru S, Togawa A, Ohtsuka M, Yoshidome H, Kato A, Yoshitomi H, et
al: Changes in growth factor and cytokine expression in biliary
obstructed rat liver and their relationship with delayed liver
regeneration after partial hepatectomy. World J Gastroenterol.
12:2053–2059. 2006. View Article : Google Scholar :
|
|
16
|
García-Arcos I, González-Kother P,
Aspichueta P, Rueda Y, Ochoa B and Fresnedo O: Lipid analysis
reveals quiescent and regenerating liver-specific populations of
lipid droplets. Lipids. 45:1101–1108. 2010. View Article : Google Scholar
|
|
17
|
Brasaemle DL: Cell biology. A metabolic
push to proliferate. Science. 313:1581–1582. 2006. View Article : Google Scholar
|
|
18
|
Komura M, Chijiiwa K, Naito T, Kameoka N,
Yamashita H, Yamaguchi K, Kuroki S and Tanaka M: Sequential changes
of energy charge, lipoperoxide level, and DNA synthesis rate of the
liver following biliary obstruction in rats. J Surg Res.
61:503–508. 1996. View Article : Google Scholar
|
|
19
|
Moustafa T, Fickert P, Magnes C, Guelly C,
Thueringer A, Frank S, Kratky D, Sattler W, Reicher H, Sinner F, et
al: Alterations in lipid metabolism mediate inflammation, fibrosis
and proliferation in a mouse model of chronic cholestatic liver
injury. Gastroenterology. 142:140–151.e12. 2012. View Article : Google Scholar
|
|
20
|
Nagai K, Yagi S, Uemoto S and Tolba RH:
Surgical procedures for a rat model of partial orthotopic liver
transplantation with hepatic arterial reconstruction. J Vis Exp.
e43762013.
|
|
21
|
Jia WJ, Jiang S, Tang QL, Shen D, Xue B,
Ning W and Li CJ: Geranylgeranyl diphosphate synthase modulates
fetal lung branching morphogenesis possibly through controlling
K-Ras prenylation. Am J Pathol. 186:1454–1465. 2016. View Article : Google Scholar
|
|
22
|
Jiang S, Shen D, Jia WJ, Han X, Shen N,
Tao W, Gao X, Xue B and Li CJ: GGPPS mediated Rab27A
geranylgeranylation regulates beta-cell dysfunction during type 2
diabetes development via affecting insulin granule docked pool
formation. J Pathol. 2015.
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Chen WB, Lai SS, Yu DC, Liu J, Jiang S,
Zhao DD, Ding YT, Li CJ and Xue B: GGPPS deficiency aggravates
CCl4-induced liver injury by inducing hepatocyte apoptosis. FEBS
Lett. 589:1119–1126. 2015. View Article : Google Scholar
|
|
25
|
Fausto N, Campbell JS and Riehle KJ: Liver
regeneration. Hepatology. 43 2 Suppl 1:S45–S53. 2006. View Article : Google Scholar
|
|
26
|
Stedman C, Liddle C, Coulter S, Sonoda J,
Alvarez JG, Evans RM and Downes M: Benefit of farnesoid X receptor
inhibition in obstructive cholestasis. Proc Natl Acad Sci USA.
103:11323–11328. 2006. View Article : Google Scholar :
|
|
27
|
Halilbasic E, Baghdasaryan A and Trauner
M: Nuclear receptors as drug targets in cholestatic liver diseases.
Clin Liver Dis. 17:161–189. 2013. View Article : Google Scholar :
|
|
28
|
Soares KC, Kamel I, Cosgrove DP, Herman JM
and Pawlik TM: Hilar cholangiocarcinoma: Diagnosis, treatment
options, and management. Hepatobiliary Surg Nutr. 3:18–34.
2014.
|
|
29
|
Paik WH, Loganathan N and Hwang JH:
Preoperative biliary drainage in hilar cholangiocarcinoma: When and
how? World J Gastrointest Endosc. 6:68–73. 2014. View Article : Google Scholar :
|
|
30
|
Farges O, Regimbeau JM, Fuks D, Le Treut
YP, Cherqui D, Bachellier P, Mabrut JY, Adham M, Pruvot FR and
Gigot JF: Multicentre European study of preoperative biliary
drainage for hilar cholangiocarcinoma. Br J Surg. 100:274–283.
2013. View
Article : Google Scholar
|
|
31
|
Yazgan Y, Oncu K, Kaplan M, Tanoglu A,
Kucuk I, Dιnc M and Demιrturk L: Malignant biliary obstruction
significantly increases serum lipid levels: A novel biochemical
tumor marker? Hepatogastroenterology. 59:2079–2082. 2012.
|
|
32
|
Fuchs C, Claudel T and Trauner M: Bile
acid-mediated control of liver triglycerides. Semin Liver Dis.
33:330–342. 2013. View Article : Google Scholar
|
|
33
|
Yan XP, Wang S, Yang Y and Qiu YD: Effects
of n-3 polyunsaturated fatty acids on rat livers after partial
hepatectomy via LKB1-AMPK signaling pathway. Transplant Proc.
43:3604–3612. 2011. View Article : Google Scholar
|