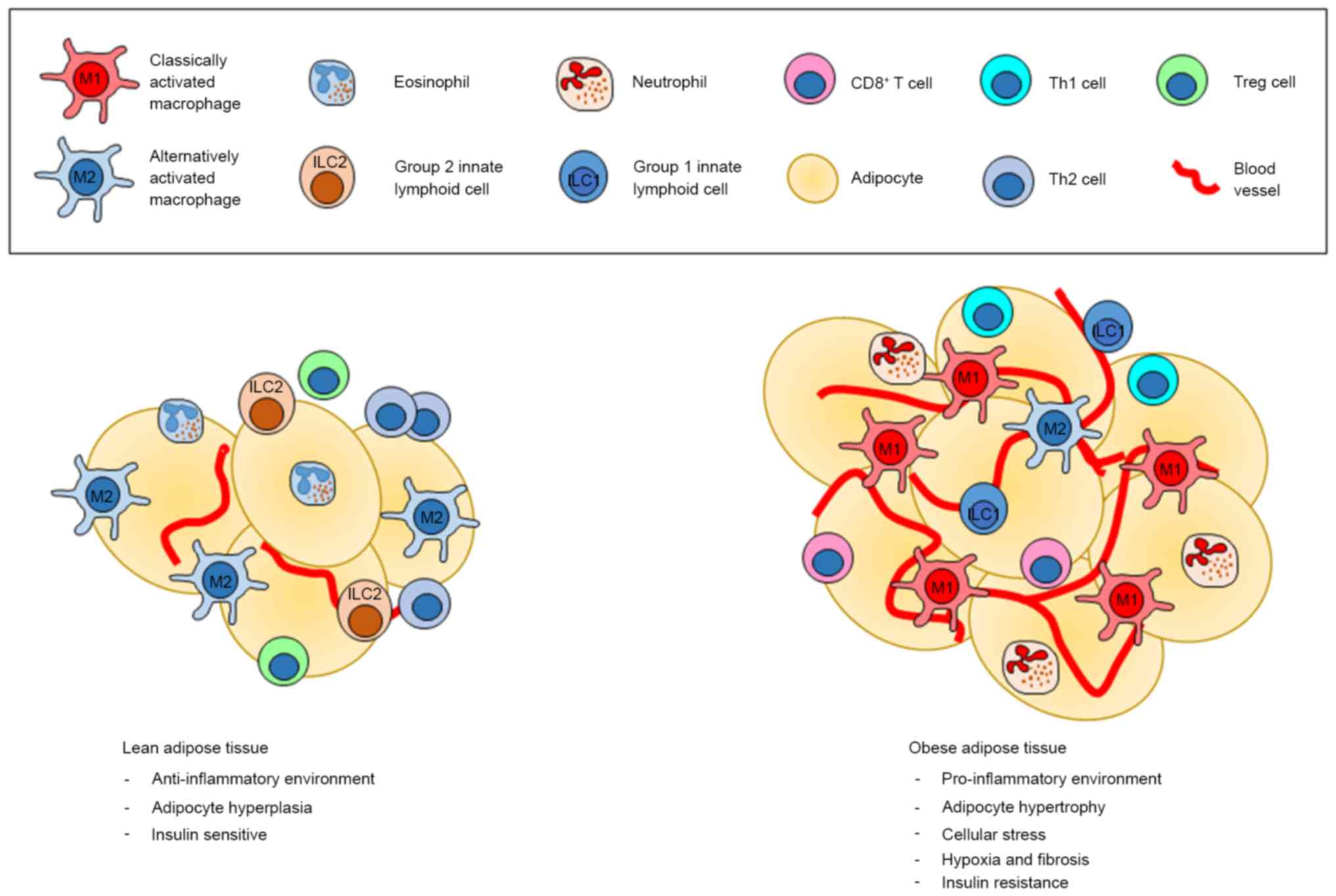
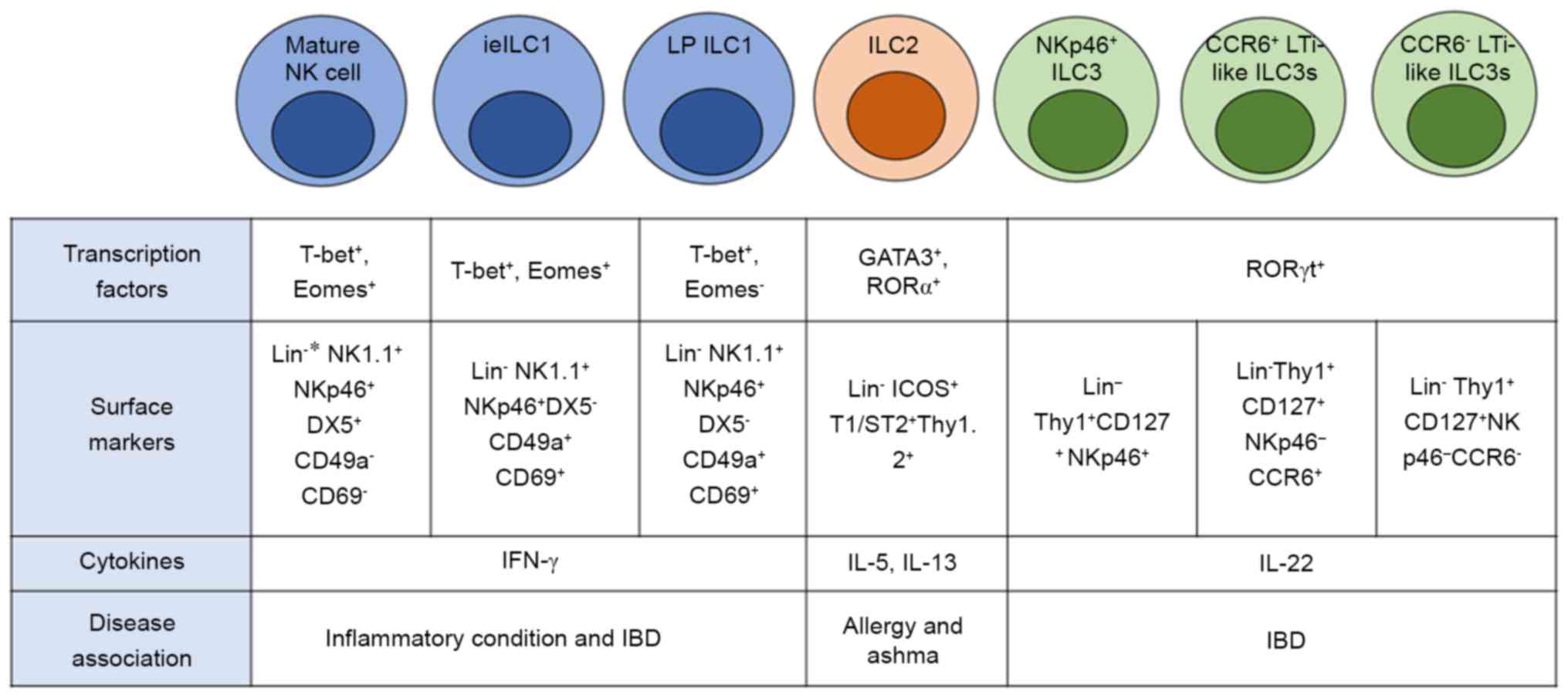
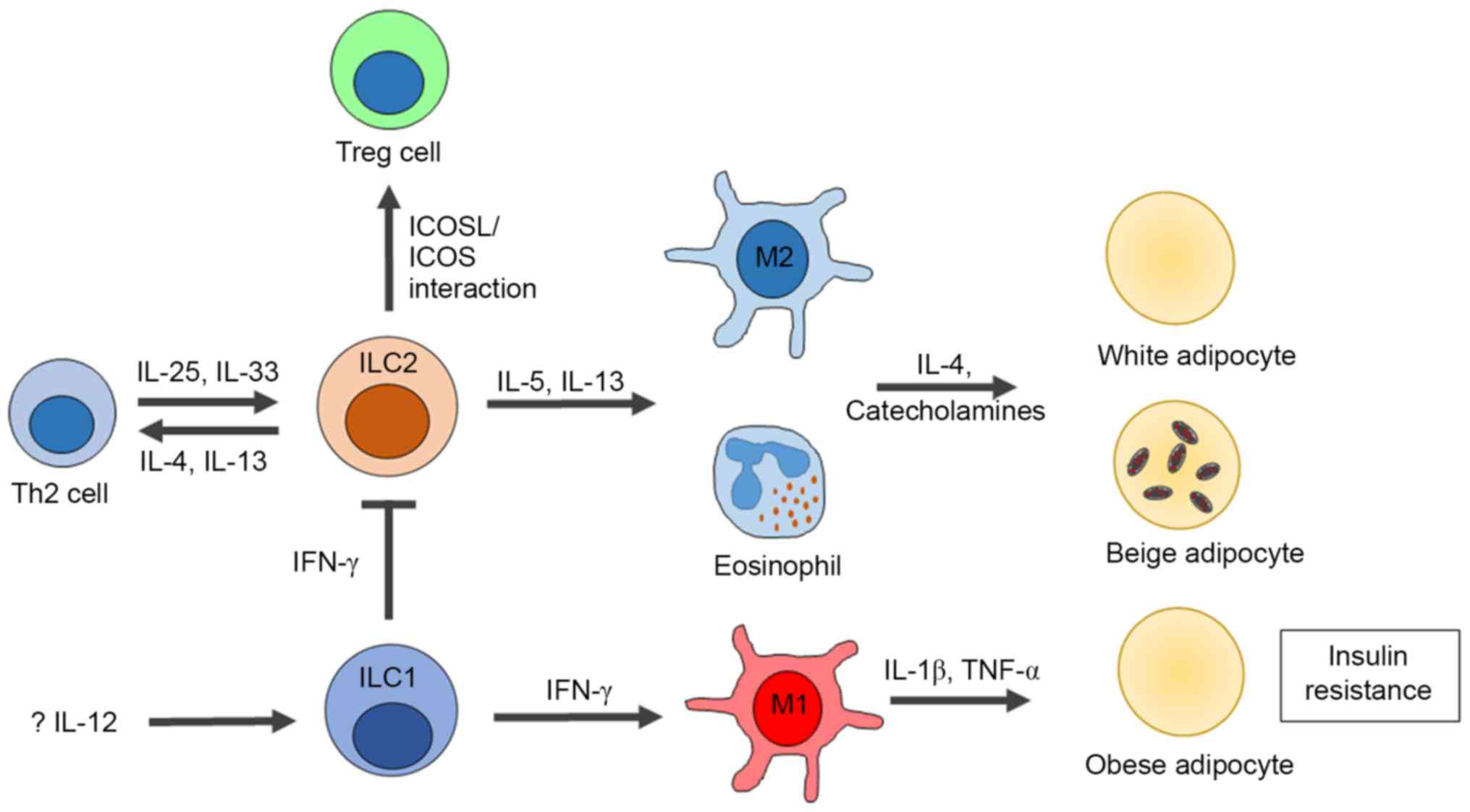
|
1
|
Boulangé CL, Neves AL, Chilloux J,
Nicholson JK and Dumas ME: Impact of the gut microbiota on
inflammation, obesity and metabolic disease. Genome Med. 8:422016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu Y and Loos RJ: Obesity genomics:
Assessing the transferability of susceptibility loci across diverse
populations. Genome Med. 5:552013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westerterp KR and Plasqui G: Physically
active lifestyle does not decrease the risk of fattening. PLoS One.
4:e47452009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiNicolantonio JJ, O'Keefe JH and Lucan
SC: Added fructose: A principal driver of type 2 diabetes mellitus
and its consequences. Mayo Clin Proc. 90:372–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DiNicolantonio JJ, Lucan SC and O'Keefe
JH: The evidence for saturated fat and for sugar related to
coronary heart disease. Prog Cardiovasc Dis. 58:464–472. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gregor MF and Hotamisligil GS:
Inflammatory mechanisms in obesity. Annu Rev Immunol. 29:415–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sell H, Habich C and Eckel J: Adaptive
immunity in obesity and insulin resistance. Nat Rev Endocrinol.
8:709–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Purkayastha S and Cai D: Neuroinflammatory
basis of metabolic syndrome. Mol Metab. 2:356–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cani PD, Amar J, Iglesias MA, Poggi M,
Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et
al: Metabolic endotoxemia initiates obesity and insulin resistance.
Diabetes. 56:1761–1772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang D, Yang W, Tian Z, van Velkinburgh
JC, Song J, Wu Y and Ni B: Innate lymphoid cells as novel
regulators of obesity and its-associated metabolic dysfunction.
Obes Rev. 17:485–498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi H, Kokoeva MV, Inouye K, Tzameli I,
Yin H and Flier JS: TLR4 links innate immunity and fatty
acid-induced insulin resistance. J Clin Invest. 116:3015–3025.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ley RE, Bäckhed F, Turnbaugh P, Lozupone
CA, Knight RD and Gordon JI: Obesity alters gut microbial ecology.
Proc Natl Acad Sci USA. 102:11070–11075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Venkatesh M, Mukherjee S, Wang H, Li H,
Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al:
Symbiotic bacterial metabolites regulate gastrointestinal barrier
function via the xenobiotic sensor PXR and Toll-like receptor 4.
Immunity. 41:296–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Zhang M, Wang S, Han R, Cao Y,
Hua W, Mao Y, Zhang X, Pang X, Wei C, et al: Interactions between
gut microbiota, host genetics and diet relevant to development of
metabolic syndromes in mice. ISME J. 4:232–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sekirov I, Russell SL, Antunes LC and
Finlay BB: Gut microbiota in health and disease. Physiol Rev.
90:859–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Turnbaugh PJ, Hamady M, Yatsunenko T,
Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA,
Affourtit JP, et al: A core gut microbiome in obese and lean twins.
Nature. 457:480–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trompette A, Gollwitzer ES, Yadava K,
Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod
LP, Harris NL and Marsland BJ: Gut microbiota metabolism of dietary
fiber influences allergic airway disease and hematopoiesis. Nat
Med. 20:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ley RE, Turnbaugh PJ, Klein S and Gordon
JI: Microbial ecology: Human gut microbes associated with obesity.
Nature. 444:1022–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwiertz A, Taras D, Schäfer K, Beijer S,
Bos NA, Donus C and Hardt PD: Microbiota and SCFA in lean and
overweight healthy subjects. Obesity (Silver Spring). 18:190–195.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duncan SH, Lobley GE, Holtrop G, Ince J,
Johnstone AM, Louis P and Flint HJ: Human colonic microbiota
associated with diet, obesity and weight loss. Int J Obes (Lond).
32:1720–1724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montiel-Castro AJ, González-Cervantes RM,
Bravo-Ruiseco G and Pacheco-López G: The microbiota-gut-brain axis:
Neurobehavioral correlates, health and sociality. Front Integr
Neurosci. 7:702013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harris K, Kassis A, Major G and Chou CJ:
Is the gut microbiota a new factor contributing to obesity and its
metabolic disorders? J Obes. 2012:8791512012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amar J, Chabo C, Waget A, Klopp P, Vachoux
C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S,
et al: Intestinal mucosal adherence and translocation of commensal
bacteria at the early onset of type 2 diabetes: Molecular
mechanisms and probiotic treatment. EMBO Mol Med. 3:559–572. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cox LM and Blaser MJ: Pathways in
microbe-induced obesity. Cell Metab. 17:883–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burcelin R, Garidou L and Pomié C:
Immuno-microbiota cross and talk: The new paradigm of metabolic
diseases. Semin Immunol. 24:67–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eagle Red A and Chawla A: In obesity and
weight loss, all roads lead to the mighty macrophage. J Clin
Invest. 120:3437–3440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kratz M, Coats BR, Hisert KB, Hagman D,
Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS,
et al: Metabolic dysfunction drives a mechanistically distinct
proinflammatory phenotype in adipose tissue macrophages. Cell
Metab. 20:614–625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu X, Grijalva A, Skowronski A, van Eijk
M, Serlie MJ and Ferrante AW Jr: Obesity activates a program of
lysosomal-dependent lipid metabolism in adipose tissue macrophages
independently of classic activation. Cell Metab. 18:816–830. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feuerer M, Herrero L, Cipolletta D, Naaz
A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S and
Mathis D: Lean, but not obese, fat is enriched for a unique
population of regulatory T cells that affect metabolic parameters.
Nat Med. 15:930–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strissel KJ, DeFuria J, Shaul ME, Bennett
G, Greenberg AS and Obin MS: T-cell recruitment and Th1
polarization in adipose tissue during diet-induced obesity in
C57BL/6 mice. Obesity (Silver Spring). 18:1918–1925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells – a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spits H and Cupedo T: Innate lymphoid
cells: Emerging insights in development, lineage relationships and
function. Annu Rev Immunol. 30:647–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Artis D and Spits H: The biology of innate
lymphoid cells. Nature. 517:293–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Villanova F, Flutter B, Tosi I, Grys K,
Sreeneebus H, Perera GK, Chapman A, Smith CH, Di Meglio P and
Nestle FO: Characterization of innate lymphoid cells in human skin
and blood demonstrates increase of NKp44+ ILC3 in
psoriasis. J Invest Dermatol. 134:984–991. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fuchs A and Colonna M: Innate lymphoid
cells in homeostasis, infection, chronic inflammation and tumors of
the gastrointestinal tract. Curr Opin Gastroenterol. 29:581–587.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cortez VS, Robinette ML and Colonna M:
Innate lymphoid cells: New insights into function and development.
Curr Opin Immunol. 32:71–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cortez VS and Colonna M: Diversity and
function of group 1 innate lymphoid cells. Immunol Lett. 179:19–24.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun JC and Lanier LL: NK cell development,
homeostasis and function: Parallels with CD8+ T cells.
Nat Rev Immunol. 11:645–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Robinette ML, Fuchs A, Cortez VS, Lee JS,
Wang Y, Durum SK, Gilfillan S and Colonna M: Immunological Genome
Consortium: Transcriptional programs define molecular
characteristics of innate lymphoid cell classes and subsets. Nat
Immunol. 16:306–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fuchs A, Vermi W, Lee JS, Lonardi S,
Gilfillan S, Newberry RD, Cella M and Colonna M: Intraepithelial
type 1 innate lymphoid cells are a unique subset of IL-12- and
IL-15-responsive IFN-γ-producing cells. Immunity. 38:769–781. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cichicki F, Schlums H, Theorell J, Tesi B,
Miller JS, Ljunggren HG and Bryceson YT: Diversification and
functional specialization of human NK cell subsets. Curr Top
Microbiol Immunol. 395:63–94. 2016.PubMed/NCBI
|
|
45
|
Fuchs A: ILC1s in tissue inflammation and
infection. Front Immunol. 7:1042016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gasteiger G, Fan X, Dikiy S, Lee SY and
Rudensky AY: Tissue residency of innate lymphoid cells in lymphoid
and nonlymphoid organs. Science. 350:981–985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peng H, Jiang X, Chen Y, Sojka DK, Wei H,
Gao X, Sun R, Yokoyama WM and Tian Z: Liver-resident NK cells
confer adaptive immunity in skin-contact inflammation. J Clin
Invest. 123:1444–1456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klose CSN, Flach M, Möhle L, Rogell L,
Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira
D, et al: Differentiation of type 1 ILCs from a common progenitor
to all helper-like innate lymphoid cell lineages. Cell.
157:340–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Daussy C, Faure F, Mayol K, Viel S,
Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA,
et al: T-bet and Eomes instruct the development of two distinct
natural killer cell lineages in the liver and in the bone marrow. J
Exp Med. 211:563–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Spits H, Bernink JH and Lanier L: NK cells
and type 1 innate lymphoid cells: Partners in host defense. Nat
Immunol. 17:758–764. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sonnenberg GF and Artis D: Innate lymphoid
cells in the initiation, regulation and resolution of inflammation.
Nat Med. 21:698–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
53
|
Karta MR, Broide DH and Doherty TA:
Insights into group 2 innate lymphoid cells in human airway
disease. Curr Allergy Asthma Rep. 16:82016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Walker JA, Barlow JL and McKenzie AN:
Innate lymphoid cells – how did we miss them? Nat Rev Immunol.
13:75–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hoyler T, Klose CS, Souabni A,
Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M
and Diefenbach A: The transcription factor GATA-3 controls cell
fate and maintenance of type 2 innate lymphoid cells. Immunity.
37:634–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wong SH, Walker JA, Jolin HE, Drynan LF,
Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, et al:
Transcription factor RORα is critical for nuocyte development. Nat
Immunol. 13:229–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Moro K, Yamada T, Tanabe M, Takeuchi T,
Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H and Koyasu S:
Innate production of T(H)2 cytokines by adipose tissue-associated
c-Kit(+)Sca-1(+) lymphoid cells. Nature. 463:540–544. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Saenz SA, Siracusa MC, Perrigoue JG,
Spencer SP, Urban JF Jr, Tocker JE, Budelsky AL, Kleinschek MA,
Kastelein RA, Kambayashi T, et al: IL25 elicits a multipotent
progenitor cell population that promotes T(H)2 cytokine responses.
Nature. 464:1362–1366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gentek R, Munneke JM, Helbig C, Blom B,
Hazenberg MD, Spits H and Amsen D: Modulation of signal strength
switches notch from an inducer of T cells to an inducer of ILC2.
Front Immunol. 4:3342013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Price AE, Liang HE, Sullivan BM, Reinhardt
RL, Eisley CJ, Erle DJ and Locksley RM: Systemically dispersed
innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad
Sci USA. 107:11489–11494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tait Wojno ED and Artis D: Innate lymphoid
cells: Balancing immunity, inflammation and tissue repair in the
intestine. Cell Host Microbe. 12:445–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Neill DR, Wong SH, Bellosi A, Flynn RJ,
Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al:
Nuocytes represent a new innate effector leukocyte that mediates
type-2 immunity. Nature. 464:1367–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wilhelm C, Turner JE, Van Snick J and
Stockinger B: The many lives of IL-9: A question of survival? Nat
Immunol. 13:637–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Doherty TA and Broide DH: Group 2 innate
lymphoid cells: New players in human allergic diseases. J Investig
Allergol Clin Immunol. 25:1–11; quiz 2p following 11.
2015.PubMed/NCBI
|
|
65
|
Oliphant CJ, Hwang YY, Walker JA, Salimi
M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST,
et al: MHCII-mediated dialog between group 2 innate lymphoid cells
and CD4(+) T cells potentiates type 2 immunity and promotes
parasitic helminth expulsion. Immunity. 41:283–295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Drake LY, Iijima K and Kita H: Group 2
innate lymphoid cells and CD4+ T cells cooperate to
mediate type 2 immune response in mice. Allergy. 69:1300–1307.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fajt ML, Gelhaus SL, Freeman B, Uvalle CE,
Trudeau JB, Holguin F and Wenzel SE: Prostaglandin D2
pathway upregulation: Relation to asthma severity, control and TH2
inflammation. J Allergy Clin Immunol. 131:1504–1512. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yu X, Pappu R, Ramirez-Carrozzi V, Ota N,
Caplazi P, Zhang J, Yan D, Xu M, Lee WP and Grogan JL: TNF
superfamily member TL1A elicits type 2 innate lymphoid cells at
mucosal barriers. Mucosal Immunol. 7:730–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Motomura Y, Morita H, Moro K, Nakae S,
Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S and Kubo M:
Basophil-derived interleukin-4 controls the function of natural
helper cells, a member of ILC2s, in lung inflammation. Immunity.
40:758–771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Maazi H, Patel N, Sankaranarayanan I,
Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH and Akbari O:
ICOS: ICOS-ligand interaction is required for type 2 innate
lymphoid cell function, homeostasis and induction of airway
hyperreactivity. Immunity. 42:538–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Salimi M, Barlow JL, Saunders SP, Xue L,
Gutowska-Owsiak D, Wang X, Huang LC, Johnson D, Scanlon ST,
McKenzie AN, et al: A role for IL-25 and IL-33-driven type-2 innate
lymphoid cells in atopic dermatitis. J Exp Med. 210:2939–2950.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Molofsky AB, Van Gool F, Liang HE, Van
Dyken SJ, Nussbaum JC, Lee J, Bluestone JA and Locksley RM:
Interleukin-33 and interferon-γ counter-regulate group 2 innate
lymphoid cell activation during immune perturbation. Immunity.
43:161–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Walford HH and Doherty TA: Diagnosis and
management of eosinophilic asthma: A US perspective. J Asthma
Allergy. 7:53–65. 2014.PubMed/NCBI
|
|
74
|
Oboki K, Ohno T, Kajiwara N, Arae K,
Morita H, Ishii A, Nambu A, Abe T, Kiyonari H, Matsumoto K, et al:
IL-33 is a crucial amplifier of innate rather than acquired
immunity. Proc Natl Acad Sci USA. 107:18581–18586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chang YJ, Kim HY, Albacker LA, Baumgarth
N, McKenzie AN, Smith DE, Dekruyff RH and Umetsu DT: Innate
lymphoid cells mediate influenza-induced airway hyper-reactivity
independently of adaptive immunity. Nat Immunol. 12:631–638. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Brestoff JR, Kim BS, Saenz SA, Stine RR,
Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P
and Artis D: Group 2 innate lymphoid cells promote beiging of white
adipose tissue and limit obesity. Nature. 519:242–246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Molofsky AB, Nussbaum JC, Liang HE, Van
Dyken SJ, Cheng LE, Mohapatra A, Chawla A and Locksley RM: Innate
lymphoid type 2 cells sustain visceral adipose tissue eosinophils
and alternatively activated macrophages. J Exp Med. 210:535–549.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hams E, Locksley RM, McKenzie AN and
Fallon PG: Cutting edge: IL-25 elicits innate lymphoid type 2 and
type II NKT cells that regulate obesity in mice. J Immunol.
191:5349–5353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Spencer SP, Wilhelm C, Yang Q, Hall JA,
Bouladoux N, Boyd A, Nutman TB, Urban JF Jr, Wang J, Ramalingam TR,
et al: Adaptation of innate lymphoid cells to a micronutrient
deficiency promotes type 2 barrier immunity. Science. 343:432–437.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Satoh-Takayama N, Vosshenrich CA,
Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam
K, Cerf-Bensussan N, Mandelboim O, et al: Microbial flora drives
interleukin 22 production in intestinal NKp46+ cells
that provide innate mucosal immune defense. Immunity. 29:958–970.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cella M, Fuchs A, Vermi W, Facchetti F,
Otero K, Lennerz JK, Doherty JM, Mills JC and Colonna M: A human
natural killer cell subset provides an innate source of IL-22 for
mucosal immunity. Nature. 457:722–725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Satoh-Takayama N: Heterogeneity and
diversity of group 3 innate lymphoid cells: New cells on the block.
Int Immunol. 28:29–34. 2016.PubMed/NCBI
|
|
83
|
van de Pavert SA and Vivier E:
Differentiation and function of group 3 innate lymphoid cells, from
embryo to adult. Int Immunol. 28:35–42. 2016.PubMed/NCBI
|
|
84
|
Klose CS, Kiss EA, Schwierzeck V, Ebert K,
Hoyler T, d'Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver
Y and Diefenbach A: A T-bet gradient controls the fate and function
of CCR6-RORγt+ innate lymphoid cells. Nature.
494:261–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hepworth MR, Fung TC, Masur SH, Kelsen JR,
McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W,
et al: Immune tolerance. Group 3 innate lymphoid cells mediate
intestinal selection of commensal bacteria-specific CD4+
T cells. Science. 348:1031–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sawa S, Cherrier M, Lochner M,
Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP and Eberl G:
Lineage relationship analysis of RORγt+ innate lymphoid
cells. Science. 330:665–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rankin LC, Groom JR, Chopin M, Herold MJ,
Walker JA, Mielke LA, McKenzie AN, Carotta S, Nutt SL and Belz GT:
The transcription factor T-bet is essential for the development of
NKp46+ innate lymphocytes via the Notch pathway. Nat
Immunol. 14:389–395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Takatori H, Kanno Y, Watford WT, Tato CM,
Weiss G, Ivanov II, Littman DR and O'Shea JJ: Lymphoid tissue
inducer-like cells are an innate source of IL-17 and IL-22. J Exp
Med. 206:35–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Hanash AM, Dudakov JA, Hua G, O'Connor MH,
Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, et
al: Interleukin-22 protects intestinal stem cells from
immune-mediated tissue damage and regulates sensitivity to graft
versus host disease. Immunity. 37:339–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pickard JM, Maurice CF, Kinnebrew MA, Abt
MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG,
Turnbaugh PJ and Chervonsky AV: Rapid fucosylation of intestinal
epithelium sustains host-commensal symbiosis in sickness. Nature.
514:638–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Goto Y, Obata T, Kunisawa J, Sato S,
Ivanov II, Lamichhane A, Takeyama N, Kamioka M, Sakamoto M, Matsuki
T, et al: Innate lymphoid cells regulate intestinal epithelial cell
glycosylation. Science. 345:12540092014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Gladiator A, Wangler N, Trautwein-Weidner
K and LeibundGut-Landmann S: Cutting edge: IL-17-secreting innate
lymphoid cells are essential for host defense against fungal
infection. J Immunol. 190:521–525. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Van Maele L, Carnoy C, Cayet D, Ivanov S,
Porte R, Deruy E, Chabalgoity JA, Renauld JC, Eberl G, Benecke AG,
et al: Activation of type 3 innate lymphoid cells and interleukin
22 secretion in the lungs during Streptococcus pneumoniae
infection. J Infect Dis. 210:493–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kirchberger S, Royston DJ, Boulard O,
Thornton E, Franchini F, Szabady RL, Harrison O and Powrie F:
Innate lymphoid cells sustain colon cancer through production of
interleukin-22 in a mouse model. J Exp Med. 210:917–931. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sawa S, Lochner M, Satoh-Takayama N,
Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP and Eberl G:
RORγt+ innate lymphoid cells regulate intestinal
homeostasis by integrating negative signals from the symbiotic
microbiota. Nat Immunol. 12:320–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Taube C, Tertilt C, Gyülveszi G, Dehzad N,
Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC,
Arnold-Schild D, et al: IL-22 is produced by innate lymphoid cells
and limits inflammation in allergic airway disease. PLoS One.
6:e217992011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Jin C, Henao-Mejia J and Flavell RA:
Innate immune receptors: Key regulators of metabolic disease
progression. Cell Metab. 17:873–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Winer DA, Luck H, Tsai S and Winer S: The
intestinal immune system in obesity and insulin resistance. Cell
Metab. 23:413–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bostick JW and Zhou L: Innate lymphoid
cells in intestinal immunity and inflammation. Cell Mol Life Sci.
73:237–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hashiguchi M, Kashiwakura Y, Kojima H,
Kobayashi A, Kanno Y and Kobata T: IL-33 activates eosinophils of
visceral adipose tissue both directly and via innate lymphoid
cells. Eur J Immunol. 45:876–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Lee MW, Odegaard JI, Mukundan L, Qiu Y,
Molofsky AB, Nussbaum JC, Yun K, Locksley RM and Chawla A:
Activated type 2 innate lymphoid cells regulate beige fat
biogenesis. Cell. 160:74–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wensveen FM, Jelenčić V, Valentić S,
Šestan M, Wensveen TT, Theurich S, Glasner A, Mendrila D, Štimac D,
Wunderlich FT, et al: NK cells link obesity-induced adipose stress
to inflammation and insulin resistance. Nat Immunol. 16:376–385.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberlé
D, Shimada T, Kamei N, Park HS, Sasorith S, Woo JR, et al: Adipose
natural killer cells regulate adipose tissue macrophages to promote
insulin resistance in obesity. Cell Metab. 23:685–698. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
O'Sullivan TE, Rapp M, Fan X, Weizman OE,
Bhardwaj P, Adams NM, Walzer T, Dannenberg AJ and Sun JC:
Adipose-resident group 1 innate lymphoid cells promote
obesity-associated insulin resistance. Immunity. 45:428–441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kim HY, Lee HJ, Chang YJ, Pichavant M,
Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et
al: IL-17 producing innate lymphoid cells and the NLRP3
inflammasome facilitate obesity-associated airway hyperreactivity.
Nat Med. 20:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang X, Ota N, Manzanillo P, Kates L,
Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J,
et al: Interleukin-22 alleviates metabolic disorders and restores
mucosal immunity in diabetes. Nature. 514:237–241. 2014.PubMed/NCBI
|
|
108
|
Hasnain SZ, Borg DJ, Harcourt BE, Tong H,
Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T, et al:
Glycemic control in diabetes is restored by therapeutic
manipulation of cytokines that regulate beta cell stress. Nat Med.
20:1417–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Luck H, Tsai S, Chung J, Clemente-Casares
X, Ghazarian M, Revelo XS, Lei H, Luk CT, Shi SY, Surendra A, et
al: Regulation of obesity-related insulin resistance with gut
anti-inflammatory agents. Cell Metab. 21:527–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Garidou L, Pomié C, Klopp P, Waget A,
Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S,
et al: The gut microbiota regulates intestinal CD4 T cells
expressing RORγt and controls metabolic disease. Cell Metab.
22:100–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ding S, Chi MM, Scull BP, Rigby R,
Schwerbrock NM, Magness S, Jobin C and Lund PK: High-fat diet:
Bacteria interactions promote intestinal inflammation which
precedes and correlates with obesity and insulin resistance in
mouse. PLoS One. 5:e121912010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hashiguchi M, Kashiwakura Y, Kojima H,
Kobayashi A, Kanno Y and Kobata T: Peyer's patch innate lymphoid
cells regulate commensal bacteria expansion. Immunol Lett. 165:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Veilleux A, Mayeur S, Bérubé JC, Beaulieu
JF, Tremblay E, Hould FS, Bossé Y, Richard D and Levy E: Altered
intestinal functions and increased local inflammation in
insulin-resistant obese subjects: A gene-expression profile
analysis. BMC Gastroenterol. 15:1192015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Monteiro-Sepulveda M, Touch S, Mendes-Sá
C, André S, Poitou C, Allatif O, Cotillard A, Fohrer-Ting H, Hubert
EL, Remark R, et al: Jejunal T cell inflammation in human obesity
correlates with decreased enterocyte insulin signaling. Cell Metab.
22:113–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Johnson AM, Costanzo A, Gareau MG, Armando
AM, Quehenberger O, Jameson JM and Olefsky JM: High fat diet causes
depletion of intestinal eosinophils associated with intestinal
permeability. PLoS One. 10:e01221952015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cox LM, Yamanishi S, Sohn J, Alekseyenko
AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, et al: Altering
the intestinal microbiota during a critical developmental window
has lasting metabolic consequences. Cell. 158:705–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|