Introduction
A decrease in the number of spiral ganglion neurons
(SGNs), abnormal dendritic growth and impairments in polarity have
been associated with sensorineural hearing loss, neural tinnitus
and unsatisfying electronic cochlear implant (CI) results (1). Strategies to improve the effects of
CI include promoting the survival of SGNs, as well as potentiating
the growth of functional fibers from residual SGNs (1,2).
Nerve fiber growth is regulated by neuritogenesis, polarity
development and synapse formation. Therefore, exploring the
mechanisms underlying these events in SGNs may yield potential
therapeutic strategies to improve the outcome for patients
undergoing CI treatment.
Notch signaling is implicated in the mechanisms
underlying the development of several tissues, including the inner
ear. Previous studies have reported roles for Notch signaling
within multiple steps of the generation and differentiation of
inner ear structures through various pathways (3–6). The
Delta/Notch-like epidermal growth factor (EGF)-related receptor
(DNER) is a single-pass transmembrane protein with 10 EGF-like
repeats in its extracellular domain. The EGF-like repeats in the
DNER structure are similar to those found in the structures of
Notch and its ligands Delta and Jagged (7,8).
DNER expression has been reported in the olfactory bulb (8), the developing and mature central
nervous system (CNS) (7–12), and the inner ear (13–15).
DNER has been implicated in neural progenitor development, neural
proliferation, and neuronal and glial differentiation via
Notch-dependent and Notch-independent pathways (9,10,16).
Notably, Hartman et al (13) reported a robust expression pattern
for DNER in developing and mature neurons and hair cells in the
inner ear, and also demonstrated that supporting cells and glia
appeared normal in the inner ear of adult DNER−/− mice.
However, a study by Kowalik and Hudspeth (14) suggested that DNER and
protein-tyrosine-phosphatase (PTP) ζ may control hair-bundle
morphology in order to establish the tonotropic gradient between
the high- and low-frequency regions of the chick cochlea. Further
physiological and comprehensive morphological studies of auditory
and vestibular function are required to elucidate the putative
roles of DNER in neurons and hair cells of the inner ear.
Pleiotrophin-PTP ζ signaling has been implicated in
the control of the subcellular localization of DNER in cerebellar
Purkinje cells and in the Neuro-2a cell line, and has therefore
been suggested to regulate neuritogenesis (16). Furthermore, DNER has been reported
to induce Bergmann glial differentiation during astrocytogenesis in
the CNS, and to inhibit myotube differentiation in C2C12 myoblasts
in vitro (10). Therefore,
the roles of DNER in the development and maturation of the spiral
ganglion may be complex and require further investigation. Previous
studies (13,14) have revealed that DNER was robustly
expressed in the inner ear, including inSGNs. The results of the
present study suggested that DNER may participate in polarization
and neurite extension processes in SGNs and may exert its actions
via the Notch signaling pathway.
Materials and methods
Mouse husbandry
A total of 20 female and 10 male wild-type C57BL/6J
mice were propagated and housed in the Experimental Animal Center
of Sun Yat-sen University (Guangzhou, China) under specific
pathogen-free condition and allowed free access to sterile water
and food with a 12:12-h light/dark cycle (lights on at 6:00 a.m.
and off at 6:00 p.m.) at 22°C. The mice were time-mated, and
embryonic day 0.5 (E0.5) was defined as noon on the day of the
observation of the vaginal plug. The embryos were staged according
to the EMAP eMouse Atlas Project (http://www.emouseatlas.org) (17). The Institutional Animal Care and
Use Committee of the Sun Yat-sen University approved the
experimental methods and animal care procedures.
Primary SGN culture
Primary cells from the cochlear modiolus were
cultured as previously reported (18). Briefly, on postnatal day 1 (P1)
C57BL/6 mice were sacrificed and cochlear modioli were collected
and dissected in Hank's balanced salt solution (pH 7.4; HBSS;
Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA). The
cochlear sensory epithelium was removed from the modiolus and the
entire modiolus was placed in 0.05% trypsin/EDTA in HBSS that had
been prewarmed to 37°C for 5 min. Subsequently, 20% fetal bovine
serum (Gibco; Thermo Fisher Scientific Inc.) was added to terminate
the trypsin digestion and a homogeneous single cell suspension was
obtained via gentle pipetting. The suspension was centrifuged for 6
min at 200 x g at 22°C and resuspended in differentiation
medium at a concentration of 106 cells/ml. The
differentiation medium contained serum-free Dulbecco's modified
Eagle's medium-F12 (1:1) (Gibco; Thermo Fisher Scientific Inc.),
supplemented with 20 ng/ml neurotrophin-3 (PeproTech China, Suzhou,
China), 20 ng/ml brain-derived neurotrophic factor (PeproTech,
Suzhou, China), 2 mM L-glutamine (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 10 ng/ml leukemia inhibitory factor
(R&D Systems, Inc., Minneapolis, MN, USA) and 3 mM KCl (Merck
KGaA, Darmstadt, Germany). The suspension was passed through a
70-µm cell filter and the cells were plated on collagen-coated
coverslips in 6-well plates at the density of 2×105/ml
for use in subsequent experiments.
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT) treatment
Primary cells from the cochlear modiolus prepared as
above, were divided into two groups, one as a control group, and
the other as the DAPT treated group. DAPT (D5942; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at a final concentration of 5 µM as
in a previous study (19) was
added to the culture medium and the cells treated for 24 h to 48 h
to the time of sample preparation.
Immunofluorescence
Immunostaining was performed as previously described
(20) using the following primary
antibodies: Goat polyclonal anti-DNER (AF2254; 1:300; R&D
Systems, Inc., Minneapolis, MN, USA), mouse monoclonal
anti-neuron-specific class III β-tubulin (Tuj1; 1:200; MO15013;
Neuromics, Inc., Edina, MN, USA), mouse monoclonal anti-activated
Notch1 (1:200; ab8925; Abcam, Cambridge, MA, USA), rabbit
polyclonal anti-α-synuclein (1:200; 2628; Cell Signaling
Technology, Inc., Danvers, MA, USA) and rabbit polyclonal
anti-GluR2/3 (1:300; AB1506; Chemicon®; EMD Millipore,
Billerica, MA, USA). Hoechst 33342 (1:1,000; H1399; Invitrogen;
Thermo Fisher Scientific, Inc.) was used to stain the nucleus. The
following secondary antibodies were used: Alexa Fluor®
488-conjugated and Alexa Fluor® 594-conjugated donkey
anti-mouse, Alexa Fluor®488-conjugated and Alexa
Fluor® 594-conjugated donkey anti-rabbit, and Alexa
Fluor® 594-conjugated donkey anti-goat antibodies
(1:400; R37114, R37115, R37118, R37119, A-11055 and A-11058
respctively; Invitrogen; Thermo Fisher Scientific, Inc.).
Time-mated pregnant mice were sacrificed under isoflurane (4%)
anesthesia by cervical dislocation at E10.5–18.5 and embryonic
otocysts were carefully dissected under a stereomicroscope.
Embryonic otocysts (E12.5, E14.5, E16.5 and E18.5) and postnatal
day 1 and 28, and weeks 6 and 35 inner ear samples were collected
and immediately fixed in 4% paraformaldehyde (PFA)/PBS at 4°C
overnight. Subsequently, samples were washed three times with PBS
and dehydration was performed in graded sucrose. Tissue samples
were sliced into 7 µm sections using a cryostat and air-dried for
30 min at room temperature, followed by long-term storage at −20°C.
Following rehydration in PBS, the sections were permeabilized in
0.1% Triton X-100 in PBS for 15 min and washed three times for 5
min in PBS. Sections were blocked in 5% bovine serum albumin
(AR1006; Boster Biological Technology, Ltd., Wuhan, China)/0.05%
Triton X-100 in PBS for 1 h prior to incubation with the primary
antibodies overnight at 4°C. Incubation with the secondary
antibodies (1:400) was performed at 4°C overnight or at room
temperature for 2 h. Nuclei were stained with Hoechst 33342
(1:1,000) at room temperature for 15 min. Samples were mounted and
observed under a fluorescence microscope (Olympus IX71; Olympus
Corporation, Tokyo, Japan; or Leica Microsystems, Inc., Buffalo
Grove, IL, USA) and a FluoView FV1000 confocal microscope (Olympus
Corporation). Cultured SGNs were prepared for staining and fixed on
coverslips in 4% PFA/PBS for 20 min at room temperature and then
washed in PBS three times. Subsequently, permeabilization, blocking
and antibody incubation were performed as described for tissue
samples.
Reverse transcription
semi-quantitative polymerase chain reaction (RT-semi-quantitative
PCR)
Early-stage otocysts (E14.5, 16.5 and 19) and
late-stage cochlear modioli (P1) were carefully dissected and
collected in Eppendorf tubes. The cells were collected in Eppendorf
tubes at different time points (E14.5, E16.5, E19 and P1). In
addition, cultured SGNs treated with DAPT or not at 72 h were
collected to extract total RNA. Total RNA was extracted using the
RNeasy purification kit (Qiagen GmbH, Hilden, Germany) according to
the manufacturer's protocol. Total RNA was treated with RNase-free
DNase (Roche Diagnostics, Indianapolis, IN, USA) and reverse
transcribed into cDNA using SuperScript III Reverse Transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
protocol provided by manufacturer. The qPCR process consists of 1
cycle at 94°C for 3 min followed by 35 cycles at 94°C for 30 sec,
35 cycles at 55°C for 30 sec and 72°C for 1 min, followed by 72°C
for 5 min with PCR Reagent (KT207-01; Tiangen Biotech Co., Ltd.,
Beijing, China). The following primers were used: DNER, forward
5′-GCCGCCTTTGTGCTTCTGTTC-3′, reverse 5′-CCGGTGGTCTGTCTGGTCGTC−3′;
glial fibrillary acidic protein (GFAP), forward
5′-GGGGCAAAAGCACCAAAGAAG-3′, reverse 5′-GGGACAACTTGTATTGTGAGCC-3′;
and GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′, reverse
5′-GAAGATGGTGATGGGATTTC-3′. The PCR products were resolved by 1%
agarose gel electrophoresis containing 0.4 µg/ml of ethidium
bromide. The level of gene expression was semi-quantified by grey
scale ratio of DNER vs. GAPDH by Quantity One (version 462; Bio-Rad
Laboratories, Inc. Hercules, CA, USA). The experiment was
independently performed at least three times with at least four
otocysts or cochleae from each group.
Western blot analysis
Dissected cochlear modioli from E14.5, E18.5 and P1
mice and the left hemisphere of the brains from P1 neonatal mice
were washed twice in ice-cold PBS, resuspended and homogenized in
lysis buffer containing 50 mM Tris-HCl, pH 7.4, 1% NP-40, 10%
glycerol, 150 mM NaCl, 11 mM sodium orthovanadate, 1 mM
phenylmethylsulfonyl fluoride and a protease inhibitor cocktail
(Roche Diagnostics). Homogenates were centrifuged at 1,000 ×
g for 5 min at 4°C, and supernatants were collected. Protein
concentration was determined by Eppendorf
BioSpectrometer® basic at UV 280 nm (Eppendorf, Hamburg,
Germany). Equal amounts of extracted protein (20 µg) were separated
by 8–15% SDS-PAGE, transferred onto polyvinylidene fluoride
membranes which were immersed into methanol for 10 sec at room
temperature and incubated with the following antibodies: Anti-DNER
(1:1,000; AF2254, R&D Systems, Inc., Minneapolis, MN, USA),
anti-Notch1 (ab8925; 1:2,000; Abcam, Cambridge, MA, USA) and
anti-GAPDH (1:3,000; sc-47724, Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight, according to the manufacturer's
protocol. After washing 3 times in TBST each for 5 min, the
membranes were incubated for 2 h at room temperature with secondary
antibodies goat anti-rabbit and mouse immunoglobulin G/horseradish
peroxidase (bs-0295G-HRP and bs-0296G-HRP; Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). After extensive washes,
chemiluminescence was used to visualize the expression of protein
with a Novex® ECL Chemiluminescent Substrate Reagent kit
(WP20005; Invitrogen, Thermo Fisher Scientific, Inc.) as according
to the manufacturer's protocols.
Construction of DNER recombinant
lentivirus and cell transfection
According to the mRNA sequence of DNER available on
GenBank (NM_139072; https://www.ncbi.nlm.nih.gov/genbank/), the following
pair of target sequences of short hairpin (sh)RNA met the
requirements of the BLOCK-iT Lentiviral RNA interference (RNAi)
expression system (Invitrogen; Thermo Fisher Scientific, Inc.):
shRNA1, 745′-CGACGATTGTCCAGGAAACAA-3′ 794; shRNA2, 2241
5′-GACCAACTGTGACATCAACAA-3′ 2261. The following two pairs of
complementary stem-loop structures and one pair of negative control
structures encoding the shRNA target sequences were designed and
synthesized: DNER shRNA1, top strand
5′-caccGTTGTTTCCTGGACAATCGTCGcgaaCGACGATTGTCCAGGAAACAA-3′, bottom
strand 5′-aaaaTTGTTTCCTGGACAATCGTCGttcgCGACGATTGTCCAGGAAACAAC-3′;
DNER shRNA2, top strand
5′-caccGTTGTTGATGTCACAGTTGGTCcgaaGACCAACTGTGACATCAACAA-3′, bottom
strand 5′-aaaaTTGTTGATGTCACAGTTGGTCttcgGACCAACTGTGACATCAACAAC-3′;
negative control shRNA, top strand
5′-caccGTTCTCCGAACGTGTCACGTcgaaACGTGACACGTTCGGAGAA-3′, bottom
strand 5′-aaaaTTCTCCGAACGTGTCACGTttcgACGTGACACGTTCGGAGAAC-3′. (The
lower case letters in the top strand oligo, ‘cacct’, at the 5′ end
of the oligo is complementary to the overhang sequence, GTGG, in
the pENTR™/U6 vector and constitutes the last 4 bases of
the U6 promoter. The lower case letters in bottom strand oligo
‘aaaa’ at the 5′ end of the oligo is complementary to the overhang
sequence, TTT T, in the pENTR™/U6 vector and constitutes
the first 4 bases of the Pol III terminator. While the lower-case
letters in the middle of oligos indicated the loop structure.) The
single-stranded oligonucleotides were annealed to create
double-stranded oligonucleotides, which were cloned into the
pENTR™/U6 vector (BLOCK-iT™ U6 RNAi Entry
Vector Kit; Invitrogen; Thermo Fisher Scientific, Inc.) using an
optimized 5-min ligation procedure. Competent E. coli cells
(kept and propagated in the Laboratory of Otorhinolaryngology, the
First Affiliated Hospital of Sun Yat-sen University, Guangzhou,
China) were transformed and selected for entry clones. A
pLenti6/BLOCK-iT™-DEST vector (Thermo Fisher Scientific,
Inc.) and a pENTR™/U6 entry clone with the ds oligo
encoding the shRNA of DNER were used in a Gateway LR recombination
reaction according to the manufacturer's protocols to generate an
expression clone containing the U6 RNAi cassette of interest. The
pLenti6/BLOCK-iT™-DEST expression construct and the
ViraPower™ Lentiviral Packaging Mix (Thermo Fisher
Scientific, Inc.) were co-transfected into the 293FT cell line
(Thermo Fisher Scientific, Inc.) to produce a lentiviral stock.
Viruses were collected from these cells and stored at −80°C. Viral
titers were averaged and typically ranged between
1–5×108 IU/ml. Briefly, 293T cells were cultured in 10
wells of 96-well plate at the density of 1×105/ml for 24
h. Then 10-fold serial dilution virus solution were added to the 10
wells respectively, and incubated until day 5. The number of cells
with green-fluorescent protein (GFP) expression in the last two
wells with observable GFP expression were counted as X and Y under
a fluorescence microscope. The primary virus titer (IU/ml) was
determined by (X+Yx10)x1000/2/volume (original virus solution in
the well with the X value). Lentiviral stocks were used to
transduce the cultured SGNs and perform transient RNAi analysis or
generate a stably transduced cell line. Viral transduction was
performed twice with multiplicity of infection ~50.
Neuronal populations and neurite
length
Transduced SGNs were fixed and immunostained at
various post-differentiation time points (48 and 72 h), and
observed under an Olympus IX71 fluorescent microscope (Olympus
Corporation). Experiments were independently repeated three times
under identical conditions. To assess neurite growth, the entire
length of the longest neurite extending from each differentiated
neuron (as identified by Tuj1) was measured within 30 high-power
randomly selected visual fields (under a 40× objective). Neurites
that were not entirely in the frame were excluded. Neuronal
processes that could not be distinguished from others were
eliminated. Neurite length was measured using Image-Pro Plus
software version 6.0 (Media Cybernetics Inc., Rockville, MD, USA).
The relative proportions of the various neuronal populations were
determined in 10 randomly selected low-power fields (under a 10×
objective). All neurons within the image frame were counted by
Image-Pro Plus software version 6.0 (Media Cybernetics Inc.). Cells
that were located in clumps and could not be clearly observed were
excluded.
Statistical analysis
The statistical significance of the differences
between the control and experimental groups was assessed by
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. The analysis was performed
using SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA).
Results
DNER expression in the developing and
mature mouse inner ear
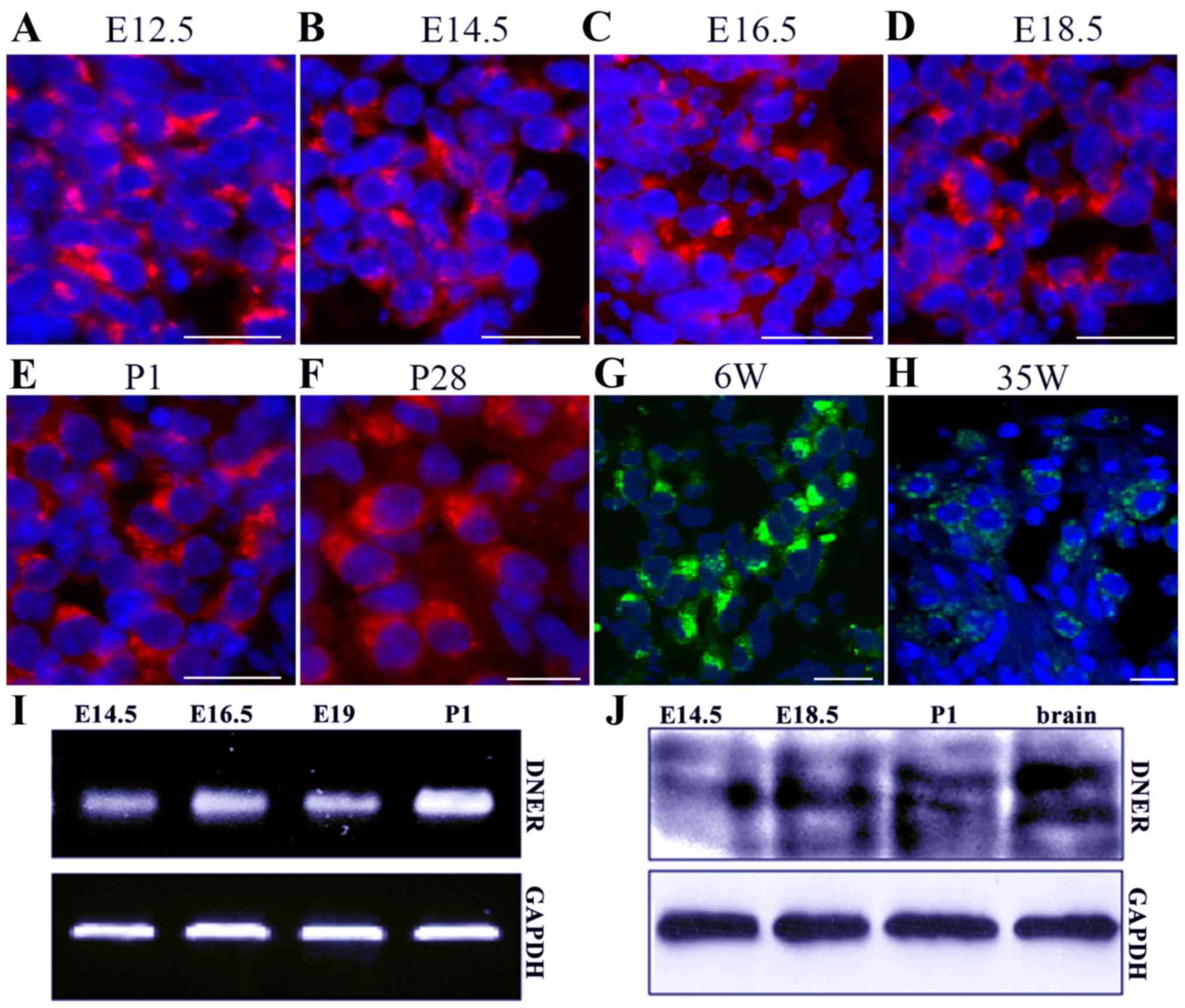
DNER expression has previously been detected in hair
cells and SGNs of the inner ear (13,14).
The results of the present study demonstrated that DNER was first
expressed in developing SGNs from E12.5 to P1, and maintained in
P28 and 35-week-old adult mice (Fig.
1A-H). During the early developmental stages, between E12.5 and
E18.5, neuronal DNER expression was not characterized by
significant polarity. However, in later developmental stages and in
adult mice, between P1 and P28, DNER expression was localized in
one neuronal pole, and significant polarity was apparent in
6-week-old mice. SGN loss and degeneration in the inner ear
appeared on week 35, with a decreasing number of neurons appearing
in the in spiral ganglion, at which time DNER expression in SGNs
also appeared to decrease and lose its polarity (Fig. 1H). Furthermore,
RT-semi-quantitative PCR and western blot analysis revealed that
DNER expression increased with inner ear development and peaked on
P1 (Fig. 1I and J). In accordance
with previous studies (7–10), DNER was also observed to be
markedly expressed in the brain (Fig.
1J).
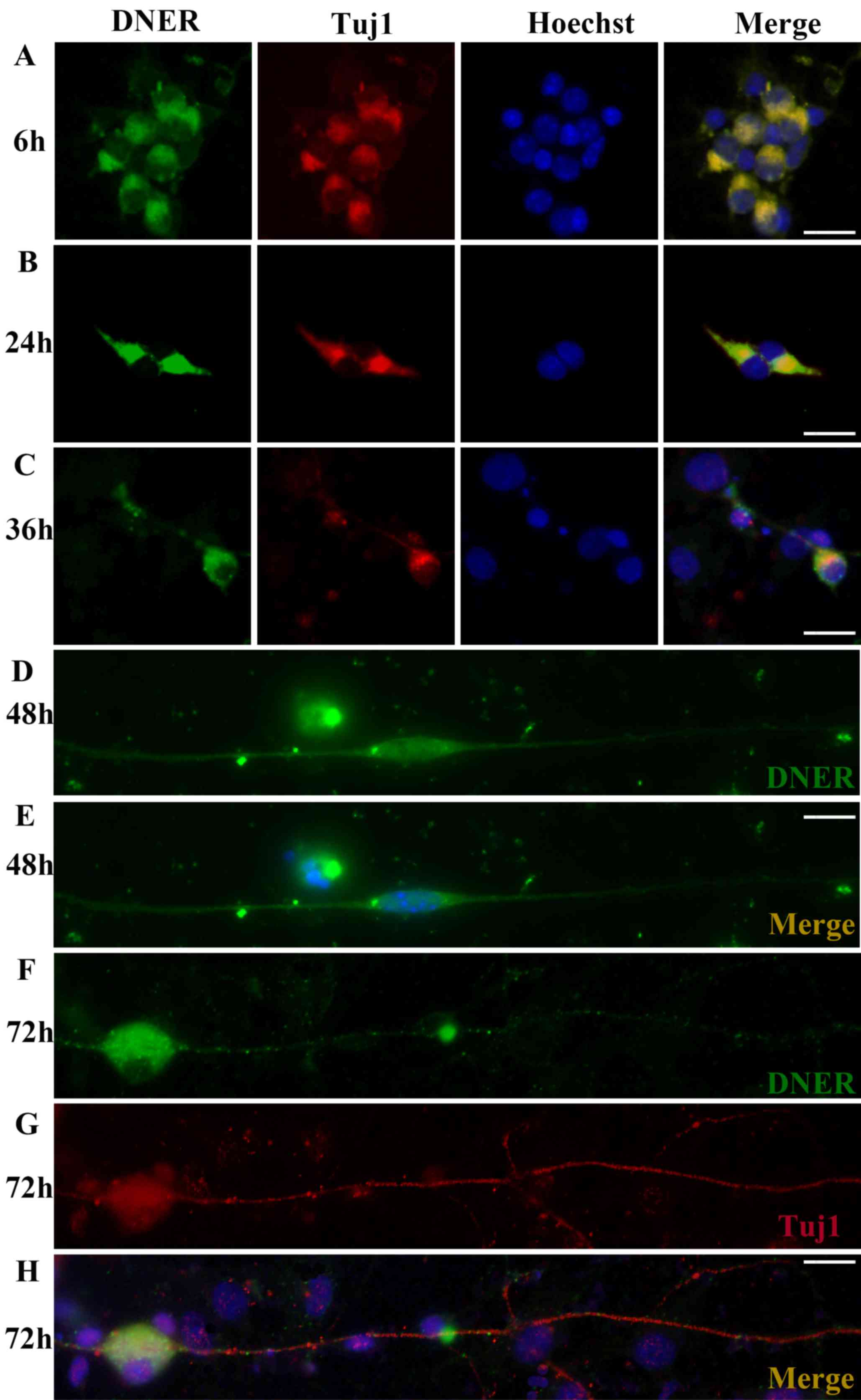
DNER expression in cultured SGNs
Inner ear modioli isolated from P1 mice were used to
generate primary neural progenitor cultures. DNER expression was
observed in undifferentiated neurons and was characterized by an
apparently polarized distribution. Following in vitro
culture for 6 h, protrusions formed on one pole of the SGNs, and
DNER appeared robustly expressed in the somatoplasm and in small
processes (Fig. 2A). As culture
duration increased (24, 36 and 48 h; Fig. 2B-D), SGN protrusions developed into
dendrites or axons, and DNER expression was observed in these
processes. However, at longer culture times (72 h; Fig. 2F-H), dendritic DNER expression
appeared punctate and dispersed, and ultimately lacked
polarity.
DNER silencing affects polarity and
protrusion formation in SGNs
DNER expression was silenced in spiral neuron
precursor cells in vitro. The expression of DNER was almost
completely inhibited following 48 h of SGN culture and the number
of differentiated neurons was significantly decreased. Neurons in
the DNER knockdown group were primarily monopolar or multipolar
(Fig. 3), whereas the control
group was dominated by bipolar neurons (Fig. 2). Furthermore, neurons not
expressing DNER sprouted markedly shorter dendrites (79.69±19.6 µm)
compared with control neurons (123.8±9.1 µm; Fig. 3I-K). In addition, DNER knockdown
resulted in reduced expression of α-synuclein and GluR2/3 in SGNs
compared with in the control group (Fig. 4).
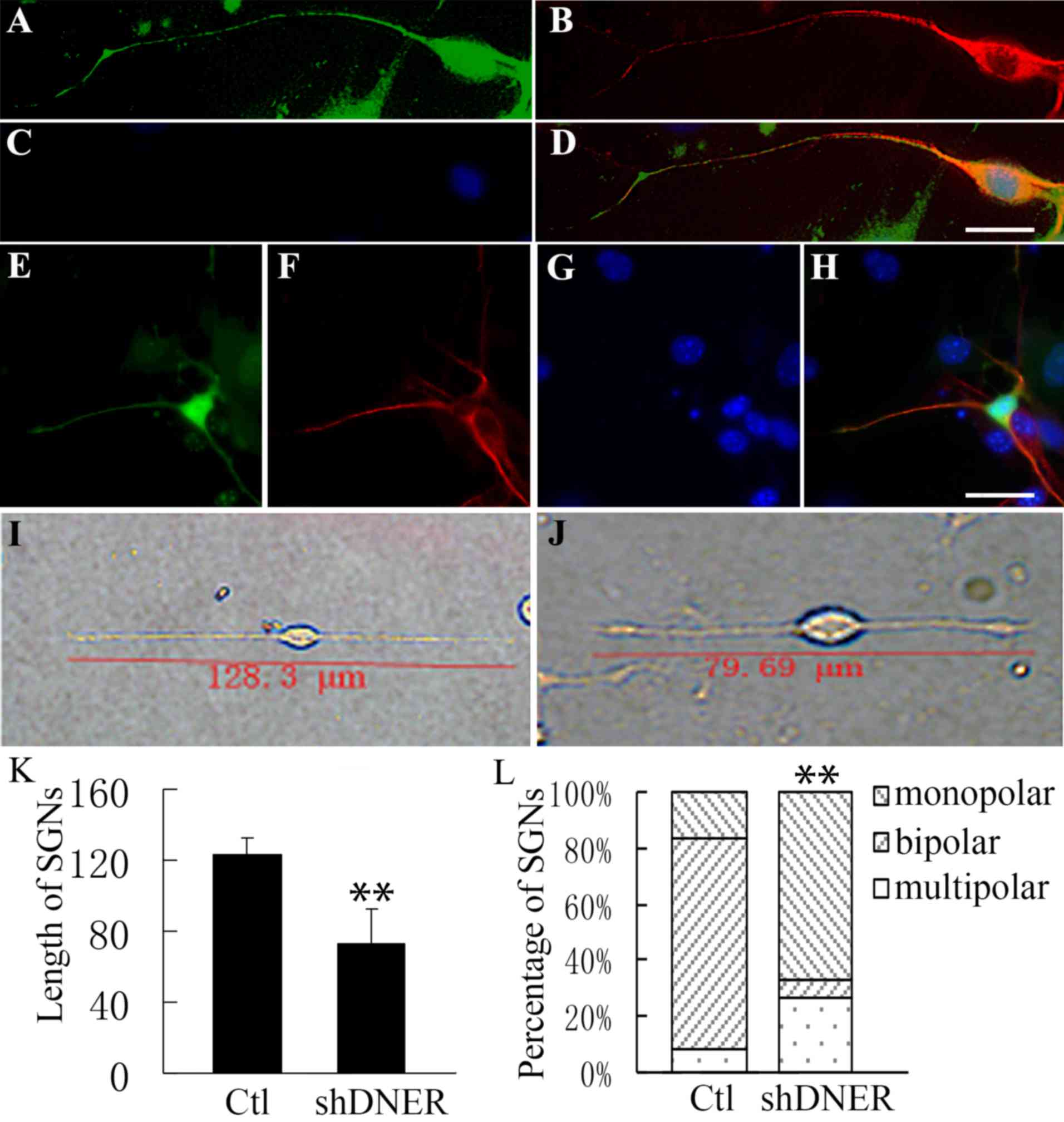 | Figure 3.Neuronal polarity was affected during
neural progenitor development following DNER knockdown in
vitro. Neural progenitors developed into (A-D) unipolar or
(E-H) multipolar neurons following DNER knockdown. (A and E) Green
indicates GFP expression accompanied by the DNER RNAi via the Block
iT™ Lentiviral RNAi expression system. GFP indicates the
knockdown of DNER. (B and F) Neuron-specific class III β-tubulin is
presented in red. (C and G) Nuclei stained with Hoechst 33342 are
presented in blue. Merged images (D) from A, B and C or (H) from E,
F and G. (I-J) Compared with (I) the control group, neurons (J) not
expressing DNER exhibited markedly shorter dendrites. (K) SGN
length following DNER knockdown was significantly shorter compared
with the control group. **P<0.01. Bars indicate mean ± standard
deviation. (L) Following DNER knockdown, spiral ganglia neural
progenitors gave rise to a significantly lower proportion of
bipolar neurons compared with the control group. **P<0.01.
Stacked bars indicated the mean proportion of neuron types. Scale
bar, 20 µm. DNER, Delta/Notch-like epidermal growth factor-related
receptor; GFP, green fluorescent protein; SGN, spiral ganglion
neuron; sh, short hairpin; Ctl, control. |
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT)affects SGN polarity and protrusion formation similar
to DNER knockdown
During the maturation of spiral ganglion
progenitors, Notch1 is cleaved by γ-secretase to produce its active
form, active-Notch1 (3), which was
revealed to be co-expressed with DNER in SGNs from P1 mice
(Fig. 5A). Treatment with the
γ-secretase inhibitor DAPT (5 µM) suppressed the activation of
Notch in cultured neurons. Compared with neurons where DNER was
knocked down, spiral ganglion progenitors treated with DAPT
exhibited impaired differentiation, and the proportion of monopolar
neurons was greater among DAPT-treated cells compared with controls
(Fig. 5D). Furthermore, DAPT
treatment reduced the expression of DNER and GFAP during the
development of spiral ganglion progenitors (Fig. 5E).
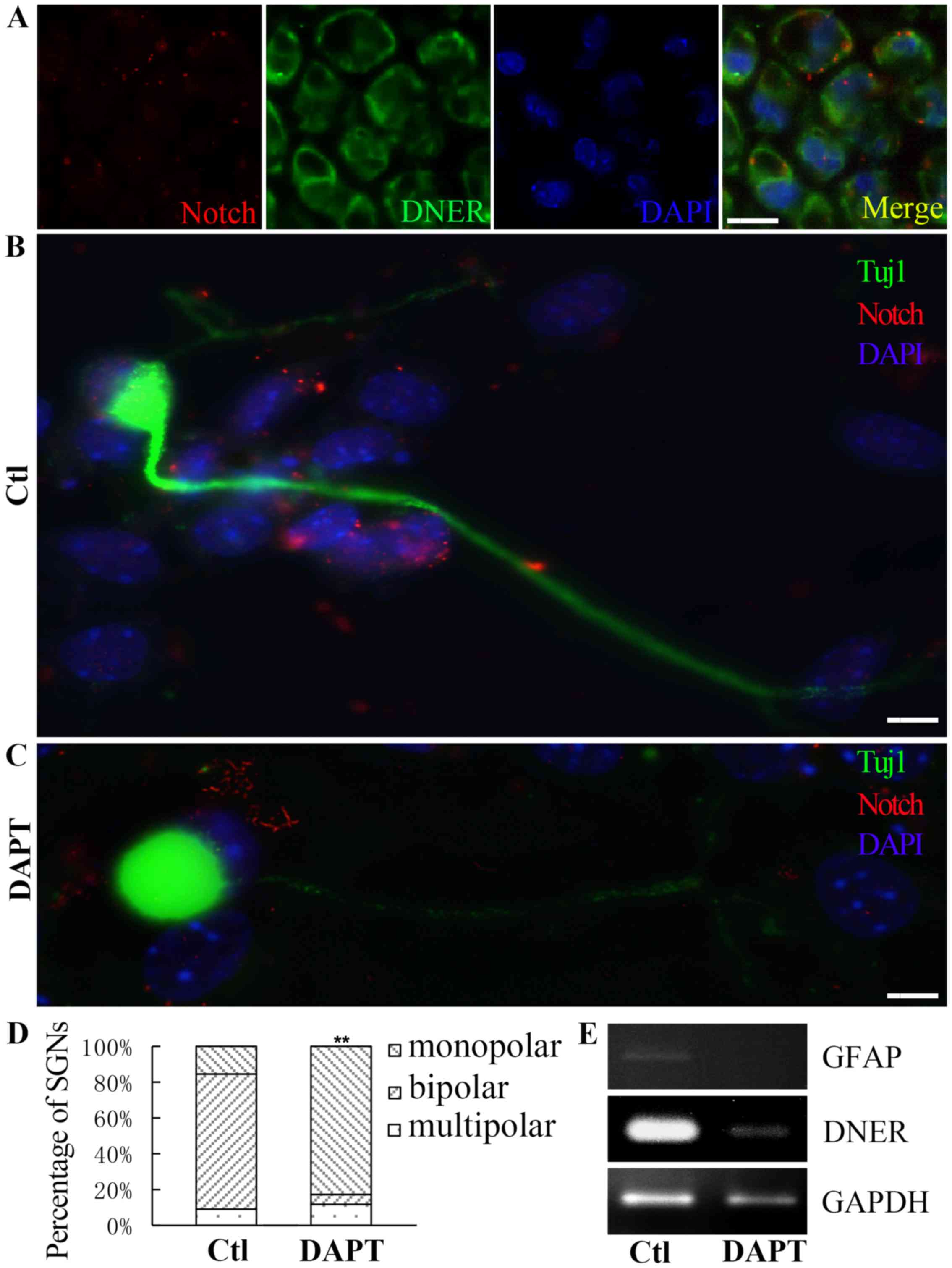 | Figure 5.DAPT affected the polarity and the
formation of protrusions in cultured neurons. (A) Notch expressed
in SGNs from a P1 mouse. Scale bar, 40 µm. (B) In the absence of
DAPT, the proportion of neural progenitors differentiating into
active-Notch expressing bipolar neurons was greater. Scale bar, 5
µm. (C) Following DAPT treatment, the proportion of spiral ganglion
progenitors differentiating into monopolar neurons was greater.
Notch is presented in red, DNER is presented in green and nuclei
are presented in blue. Scale bar, 5 µm. (D) The control group, in
the absence of DAPT, was dominated by bipolar neurons, whereas,
following DAPT treatment, the proportions of monopolar and
multipolar neurons were higher. Mean ± standard deviation;
**P<0.01. (E) DAPT treatment resulted in reduced DNER and GFAP
expression during neural progenitor differentiation. DAPT,
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester; SGN, spiral ganglion neuron; DNER, Delta/Notch-like
epidermal growth factor-related receptor; GFAP, glial fibrillary
acidic protein; Ctl, control. |
Discussion
Previous studies have demonstrated that DNER is
specifically expressed during CNS development and maturation
(7,8) and important roles have been suggested
for it in neural development (9,10,12,18).
In the developing cerebellum, DNER and Notch1 have been suggested
to interact at Purkinje cell-Bergmann glia contacts (10). Notch signaling may be implicated in
the control of the generation and differentiation of neuronal and
glial cells through various pathways. It has been suggested that
DNER-Notch signaling may be involved in the morphological
differentiation of Bergmann glial cells (10). Treatment with DNER or activation of
Notch signaling in Bergmann glia in DNER−/− mice has
been reported to attenuate impairments in Bergmann fiber formation
(10). In vitro, DNER has
been demonstrated to specifically bind to Notch-expressing fusiform
glial cells and induce process elongation to promote radial fiber
formation in Bergmann glia, via a γ-secretase- and Deltex-dependent
but cerebellar soluble lectin-independent Notch signaling pathway
(10). Notably, the
Deltex-dependent pathway was activated by either F3 neuronal
adhesion molecule or DNER and was reported to induce terminal
differentiation of glia after fate determination, whereas the
canonical pathway was demonstrated to maintain an undifferentiated
state. After binding to ligands such as Delta or Jagged, Notch
undergoes two proteolytic cleavages by tumor necrosis
factor-α-converting enzyme (TACE) and γ-secretase-like proteinases
that release its intracellular domain (NICD). The cleaved NICD
translocates to the nucleus and forms a complex with CBF1
(RBP-J)/Su(H)/LAG1 (CSL) family transcription factors, which in
turn activates transcription of Notch effector genes, including Hes
genes. Therefore, DNER has been suggested to activate various
pathways and responses depending on cell type and state, via
Notch-dependent and Notch-independent mechanisms (9).
The present results, in accordance with previous
studies, demonstrated that in the peripheral nervous system DNER
was expressed in the somatoplasm of embryonic and adult SGNs,
including their afferent and efferent nerve endings to cochlear
hair cells. Furthermore, in the vestibule and cochlea, DNER
expression has been reported in developing and mature hair cells
and vestibular neurons (13).
These findings suggested that DNER, which is widely expressed in
the peripheral neurosensory epithelium, may serve important roles
in its development and differentiation, similar to its roles in the
CNS (9,10). However, the role of DNER in spiral
ganglion development and differentiation has yet to be elucidated.
The present study demonstrated that DNER was expressed in the mouse
inner ear, and its expression was temporospatially specific and
polarized. During cochlear development, DNER expression was
demonstrated to gradually increase, and marked polarity was
sustained in adult neurons; however, DNER expression appeared
evanescent in aged mice. Neuronal precursors from spiral ganglia
cultured in vitro also exhibited similar characteristics and
polar DNER distribution during neuronal differentiation, whereas
the polarity disappeared during further culture. Therefore, it may
be hypothesized that DNER is related to SGN polarity, as well as
protrusion formation, dendritic growth and neuronal survival.
When the expression of DNER was silenced in neural
progenitors isolated from neonatal mouse cochlea in vitro,
the number of differentiated neurons significantly decreased, and
fewer bipolar neurons with shorter neurites were present compared
with the control group. Furthermore, the expression of α-synuclein,
which is involved in synaptic vesicle transport and exocytosis, and
the GluR2/3 subunits of the synaptic
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid glutamate
receptor were markedly reduced following DNER knockdown. These
findings suggested that DNER may be involved in synapse formation.
Similar findings were observed following treatment with the
γ-secretase inhibitor DAPT, as it inhibitedNotch1 activation, and
resulted in reduced DNER expression, as well as reduced neuronal
differentiation and neurite growth. In accordance with previous
studies (9,10,13),
the present results suggested that DNER may be able to induce
neuronal and glial differentiation, and regulate neurite extension
and growth (9,21–24).
In conclusion, the present study demonstrated that
DNER expression in developing SGNs was characterized by
temporospatial specificity. The knockdown of DNER expression
reduced the number of differentiated SGNs and impaired
neuritogenesis. DNER appeared to be exerting its actions via a
Notch-dependent signaling pathway. Therefore, the present results
suggested that the DNER/Notch signaling pathway may serve important
roles in spiral ganglion development and neuritogenesis.
Acknowledgements
The study was supported by grants from China
Postdoctoral Science Foundation (grant no. 2014M562329), the
National Natural Science Foundation of China (grant nos. 81271076,
81200748 and 81500802).
References
|
1
|
Xu HX, Kim GH, Snissarenko EP, Cureoglu S
and Paparella MM: Multi-channel cochlear implant histopathology:
Are fewer spiral ganglion cells really related to better clinical
performance? Acta Otolaryngol. 132:482–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roehm PC and Hansen MR: Strategies to
preserve or regenerate spiral ganglion neurons. Curr Opin
Otolaryngol Head Neck Surg. 13:294–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrovic J, Formosa-Jordan P,
Luna-Escalante JC, Abelló G, Ibañes M, Neves J and Giraldez F:
Ligand-dependent Notch signaling strength orchestrates lateral
induction and lateral inhibition in the developing inner ear.
Development. 141:2313–2324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiernan AE: Notch signaling during cell
fate determination in the inner ear. Semin Cell Dev Biol.
24:470–479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brooker R, Hozumi K and Lewis J: Notch
ligands with contrasting functions: Jagged1 and Delta1 in the mouse
inner ear. Development. 133:1277–1286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haddon C, Jiang YJ, Smithers L and Lewis
J: Delta-Notch signalling and the patterning of sensory cell
differentiation in the zebrafish ear: Evidence from the mind bomb
mutant. Development. 125:4637–4644. 1998.PubMed/NCBI
|
|
7
|
Eiraku M, Hirata Y, Takeshima H, Hirano T
and Kengaku M: Delta/notch-like epidermal growth factor
(EGF)-related receptor, a novel EGF-like repeat-containing protein
targeted to dendrites of developing and adult central nervous
system neurons. J Biol Chem. 277:25400–25407. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishizumi H, Komiyama T, Miyabayashi T,
Sakano S and Sakano H: BET, a novel neuronal transmembrane protein
with multiple EGF-like motifs. Neuroreport. 13:909–915. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh FY, Ma TL, Shih HY, Lin SJ, Huang
CW, Wang HY and Cheng YC: Dner inhibits neural progenitor
proliferation and induces neuronal and glial differentiation in
zebrafish. Dev Biol. 375:1–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eiraku M, Tohgo A, Ono K, Kaneko M,
Fujishima K, Hirano T and Kengaku M: DNER acts as a neuron-specific
Notch ligand during Bergmann glial development. Nat Neurosci.
8:873–880. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito SY and Takeshima H: DNER as key
molecule for cerebellar maturation. Cerebellum. 5:227–231. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tohgo A, Eiraku M, Miyazaki T, Miura E,
Kawaguchi SY, Nishi M, Watanabe M, Hirano T, Kengaku M and
Takeshima H: Impaired cerebellar functions in mutant mice lacking
DNER. Mol Cell Neurosci. 31:326–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartman BH, Nelson BR, Reh TA and
Bermingham-McDonogh O: Delta/notch-like EGF-related receptor (DNER)
is expressed in hair cells and neurons in the developing and adult
mouse inner ear. J Assoc Res Otolaryngol. 11:187–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kowalik L and Hudspeth AJ: A search for
factors specifying tonotopy implicates DNER in hair-cell
development in the chick's cochlea. Dev Biol. 354:221–231. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukazawa N, Yokoyama S, Eiraku M, Kengaku
M and Maeda N: Receptor type protein tyrosine phosphatase
zeta-pleiotrophin signaling controls endocytic trafficking of DNER
that regulates neuritogenesis. Mol Cell Biol. 28:4494–4506. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Richardson L, Venkataraman S, Stevenson P,
Yang Y, Moss J, Graham L, Burton N, Hill B, Rao J, Baldock RA and
Armit C: EMAGE mouse embryo spatial gene expression database: 2014
update. Nucleic Acids Res. 42(Database issue): D835–D844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oshima K, Grimm CM, Corrales CE, Senn P,
Martinez Monedero R, Géléoc GS, Edge A, Holt JR and Heller S:
Differential distribution of stem cells in the auditory and
vestibular organs of the inner ear. J Assoc Res Otolaryngol.
8:18–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu
N, Ren LL, Li XX, Liu HZ, Young WY, et al: Effects of DAPT and
Atoh1 overexpression on hair cell production and hair bundle
orientation in cultured organ of corti from neonatal rats. PLoS
One. 6:e237292011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sage C, Huang M, Karimi K, Gutierrez G,
Vollrath MA, Zhang DS, Garcia-Añoveros J, Hinds PW, Corwin JT,
Corey DP and Chen ZY: Proliferation of functional hair cells in
vivo in the absence of the retinoblastoma protein. Science.
307:1114–1118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Madden TA, Barrow D, McClelland RA, Gee JM
and Nicholson RI: Modulation of oestrogen action by receptor gene
inhibition. Eur J Cancer. 36 Suppl 4:S34–S35. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berezovska O, McLean P, Knowles R, Frosh
M, Lu FM, Lux SE and Hyman BT: Notch1 inhibits neurite outgrowth in
postmitotic primary neurons. Neuroscience. 93:433–439. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Redmond L, Oh SR, Hicks C, Weinmaster G
and Ghosh A: Nuclear Notch1 signaling and the regulation of
dendritic development. Nat Neurosci. 3:30–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Redmond L and Ghosh A: The role of Notch
and Rho GTPase signaling in the control of dendritic development.
Curr Opin Neurobiol. 11:111–117. 2001. View Article : Google Scholar : PubMed/NCBI
|