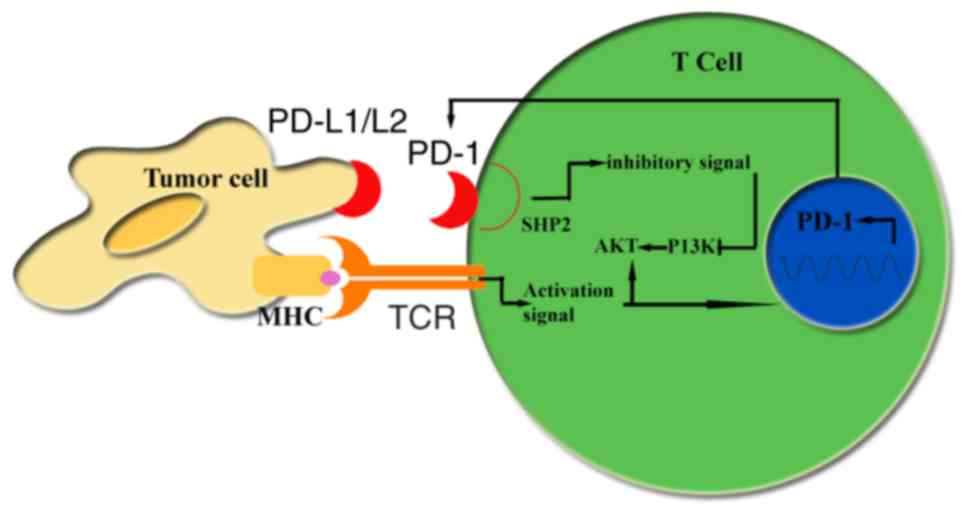
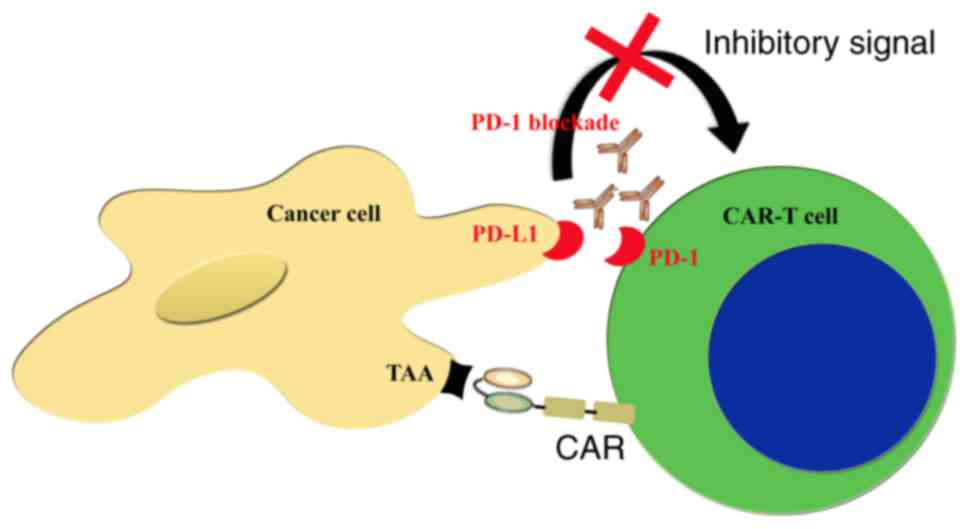
|
1
|
Yang JC and Rosenberg SA: Adoptive T-Cell
therapy for cancer. Adv Immunol. 130:279–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maus MV and June CH: Making better
chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer
Res. 22:1875–1884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whilding LM and Maher J: CA0R T-cell
immunotherapy: The path from the by-road to the freeway? Mol Oncol.
9:1994–2018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haji-Fatahaliha M, Hosseini M, Akbarian A,
Sadreddini S, Jadidi-Niaragh F and Yousefi M: CAR-modified T-cell
therapy for cancer: An updated review. Artif Cells Nanomed
Biotechnol. 44:1339–1349. 2016.PubMed/NCBI
|
|
5
|
Sadelain M, Brentjens R and Rivière I: The
basic principles of chimeric antigen receptor design. Cancer
Discov. 3:388–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: Integrating immunity's roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T-cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davenport AJ, Jenkins MR, Cross RS, Yong
CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK and
Neeson PJ: CAR-T-cells inflict sequential killing of multiple tumor
targeT-cells. Cancer Immunol Res. 3:483–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruella M and Kalos M: Adoptive
immunotherapy for cancer. Immunol Rev. 257:14–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Riaz IB, Zahid U, Kamal MU, Husnain M,
McBride A, Hua A, Hamadani AA, George L, Zeeshan A, Sipra QR, et
al: Anti-CD 19 and anti-CD 20 CAR-modified T cells for B-cell
malignancies: A systematic review and meta-analysis. Immunotherapy.
9:979–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taylor A, Rothstein D and Rudd C: Small
molecule inhibition of PD-1 transcription is an effective
alternative to antibody blockade in cancer therapy. Cancer Res.
2017. View Article : Google Scholar
|
|
14
|
Sathyanarayanan V and Neelapu SS: Cancer
immunotherapy: Strategies for personalization and combinatorial
approaches. Mol Oncol. 9:2043–2053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang WL: CAR T-cell therapy:
Opportunities and challenges. Immunotherapy. 8:245–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim MG, Kim D, Suh SK, Park Z, Choi MJ and
Oh YK: Current status and regulatory perspective of chimeric
antigen receptor-modified T-cell therapeutics. Arch Pharm Res.
39:437–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Restifo NP, Dudley ME and Rosenberg SA:
Adoptive immunotherapy for cancer: Harnessing the T-cell response.
Nat Rev Immunol. 12:269–281. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
June CH: Principles of adoptive T-cell
cancer therapy. J Clin Invest. 117:1204–1212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
June CH: Adoptive T-cell therapy for
cancer in the clinic. J Clin Invest. 117:1466–1476. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Till BG, Jensen MC, Wang J, Chen EY, Wood
BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, et al:
Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle
cell lymphoma using genetically modified autologous CD20-specific
T-cells. Blood. 112:2261–2271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al: Antitumor
activity and long-term fate of chimeric antigen receptor-positive
T-cells in patients with neuroblastoma. Blood. 118:6050–6056. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brentjens RJ, Riviere I, Park JH, Davila
ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda
O, et al: Safety and persistence of adoptively transferred
autologous CD19-targeted T-cells in patients with relapsed or
chemotherapy refractory B-cell leukemias. Blood. 118:4817–4828.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kakarla S and Gottschalk S: CAR T-cells
for solid tumors: Armed and ready to go? Cancer J. 20:151–155.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Figueroa JA, Reidy A, Mirandola L, Trotter
K, Suvorava N, Figueroa A, Konala V, Aulakh A, Littlefield L,
Grizzi F, et al: Chimeric antigen receptor engineering: A right
step in the evolution of adoptive cellular immunotherapy. Int Rev
Immunol. 34:154–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedognetti D, Maccalli C, Bader SB,
Marincola FM and Seliger B: Checkpoint inhibitors and their
application in breast cancer. Breast care (Basel). 11:108–115.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thommen DS, Schreiner J, Muller P, Herzig
P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M, et al:
Progression of lung cancer is associated with increased dysfunction
of T-cells defined by coexpression of multiple inhibitory
receptors. Cancer Immunol Res. 3:1344–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blank C and Mackensen A: Contribution of
the PD-L1/PD-1 pathway to T-cell exhaustion: An update on
implications for chronic infections and tumor evasion. Cancer
Immunol Immunother. 56:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kedmi M, Avigdor A and Nagler A:
Anti-PD-1-targeted therapies focusing on lymphatic malignancies:
Biological rationale, clinical challenges and opportunities. Acta
Haematol. 133:129–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Keir ME, Butte MJ, Freeman GJ and Sharpel
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao S and Chen L: PD-1 as an immune
modulatory receptor. Cancer J. 20:262–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen DS, Irving BA and Hodi FS: Molecular
pathways: Next-generation immunotherapy-inhibiting programmed
death-ligand 1 and programmed death-1. Clin Cancer Res.
18:6580–6587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okazaki T, Chikuma S, Iwai Y, Fagarasan S
and Honjo T: A rheostat for immune responses: The unique properties
of PD-1 and their advantages for clinical application. Nat Immunol.
14:1212–1218. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Massari F, Santoni M, Ciccarese C, Santini
D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R,
Montironi R, et al: PD-1 blockade therapy in renal cell carcinoma:
Current studies and future promises. Cancer Treat Rev. 41:114–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity and immune correlates
of anti-PD-1 antibody in cancer. N Engl J Med. 366:2443–2454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ratta R, Zappasodi R, Raggi D, Grassi P,
Verzoni E, Necchi A, Di Nicola M, Salvioni R, de Braud F and
Procopio G: Immunotherapy advances in uro-genital malignancies.
Crit Rev Oncol Hematol. 105:52–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bracarda S, Altavilla A, Hamzaj A, Sisani
M, Marrocolo F, Del Buono S and Danielli R: Immunologic checkpoints
blockade in renal cell, prostate and urothelial malignancies. Semin
Oncol. 42:495–505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Joshi M, Pal SK and Drabick JJ: Novel
approaches in cancer immunotherapy-a light at the end of the
tunnel. Discov Med. 21:479–487. 2016.PubMed/NCBI
|
|
39
|
Thanarajasingam G, Thanarajasingam U and
Ansell SM: Immune checkpoint blockade in lymphoid malignancies.
FEBS J. 283:2233–2244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weber JS: Practical management of
immune-related adverse events from immune checkpoint protein
antibodies for the oncologist. Am Soc Clin Oncol Educ Book. 1–177.
2012.
|
|
41
|
Zhu Y, Tan Y, Ou R, Zhong Q, Zheng L, Du
Y, Zhang Q and Huang J: Anti-CD19 chimeric antigen
receptor-modified T cells for B-cell malignancies: A systematic
review of efficacy and safety in clinical trials. Eur J Haematol.
96:389–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Srivastava S and Riddell SR: Engineering
CAR-T-cells: Design concepts. Trends Immunol. 36:494–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation car T-cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Adachi K and Tamada K: Immune checkpoint
blockade opens an avenue of cancer immunotherapy with a potent
clinical efficacy. Cancer Sci. 106:945–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chicaybam L, Sodré AL and Bonamino M:
Chimeric antigen receptors in cancer immuno-gene therapy: Current
status and future directions. Int Rev Immunol. 30:294–311. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morales-Kastresana A, Labiano S, Quetglas
JI and Melero I: Better performance of CARs deprived of the PD-1
brake. Clin Cancer Res. 19:5546–5548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shirasu N and Kuroki M: Functional design
of chimeric T-Cell antigen receptors for adoptive immunotherapy of
cancer: Architecture and outcomes. Anticancer Res. 32:2377–2383.
2012.PubMed/NCBI
|
|
49
|
Cheadle EJ, Gornall H, Baldan V, Hanson V,
Hawkins RE and Gilham DE: CAR T-cells: Driving the road from the
laboratory to the clinic. Immunol Rev. 257:91–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Blank C, Gajewski TF and Mackensen A:
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific
T-cells as a mechanism of immune evasion: Implications for tumor
immunotherapy. Cancer Immunol Immunother. 54:307–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
John LB, Devaud C, Duong CP, Yong CS,
Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH and Darcy PK:
Anti-PD-1 antibody therapy potently enhances the eradication of
established tumors by gene-modified T-cells. Clin Cancer Res.
19:5636–5646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu J, Wang Y, Yan F, Li H and Ren X:
Response to comment on ‘Myeloid-derived suppressor cells suppress
antitumor immune responses through IDO expression and correlate
with lymph node metastasis in patients with breast cancer’. J
Immunol. 190:5341–5342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mazzoni A, Bronte V, Visintin A, Spitzer
JH, Apolloni E, Serafini P, Zanovello P and Segal DM: Myeloid
suppressor lines inhibit T-cell responses by an NO-dependent
mechanism. J Immunol. 168:689–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mauldin IS, Tung KS and Lorenz UM: The
tyrosine phosphatase SHP-1 dampens murine Th17 development. Blood.
119:4419–4429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Moon EK, Wang LC, Dolfi DV, Wilson CB,
Ranganathan R, Sun J, Kapoor V, Scholler J, Puré E, Milone MC, et
al: Multifactorial T-cell hypofunction that is reversible can limit
the efficacy of chimeric antigen receptor-transduced human T-cells
in solid tumors. Clin Cancer Res. 20:4262–4273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Burga RA, Thorn M, Point GR, Guha P,
Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Joseph Espat N,
Junghans RP and Katz SC: Liver myeloid-derived suppressor cells
expand in response to liver metastases in mice and inhibit the
anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother.
64:817–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suarez ER, Chang de K, Sun J, Sui J,
Freeman GJ, Signoretti S, Zhu Q and Marasco WA: Chimeric antigen
receptor T-cells secreting anti-PD-L1 antibodies more effectively
regress renal cell carcinoma in a humanized mouse model.
Oncotarget. 7:34341–34355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gargett T, Yu W, Dotti G, Yvon ES, Christo
SN, Hayball JD, Lewis ID, Brenner MK and Brown MP: GD2-specific CAR
T-cells undergo potent activation and deletion following antigen
encounter but can be protected from activation-induced cell death
by PD-1 blockade. Mol Ther. 24:1135–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Prosser ME, Brown CE, Shami AF, Forman SJ
and Jensen MC: Tumor PD-L1 co-stimulates primary human CD8(+)
cytotoxic T-cells modified to express a PD1: CD28 chimeric
receptor. Mol Immunol. 51:263–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chang ZL, Silver PA and Chen YY:
Identification and selective expansion of functionally superior
T-cells expressing chimeric antigen receptors. J Transl Med.
13:1612015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Terakura S, Yamamoto TN, Gardner RA,
Turtle CJ, Jensen MC and Riddell SR: Generation of CD19-chimeric
antigen receptor modified CD8+ T-cells derived from virus-specific
central memory T-cells. Blood. 119:72–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cruz CR, Micklethwaite KP, Savoldo B,
Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, et al:
Infusion of donor-derived CD19-redirected virus-specific T-cells
for B-cell malignancies relapsed after allogeneic stem cell
transplant: A phase 1 study. Blood. 122:2965–2973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pegram HJ, Park JH and Brentjens RJ: CD28z
CARs and Armored CARs. Cancer J. 20:127–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shi H, Liu L and Wang Z: Improving the
efficacy and safety of engineered T-cell therapy for cancer. Cancer
Lett. 328:191–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jones BS, Lamb LS, Goldman F and Di Stasi
A: Improving the safety of cell therapy products by suicide gene
transfer. Front Pharmacol. 5:2542014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang X, Chang WC, Wong CW, Colcher D,
Sherman M, Ostberg JR, Forman SJ, Riddell SR and Jensen MC: A
transgene-encoded cell surface polypeptide for selection, in vivo
tracking, and ablation of engineered cells. Blood. 118:1255–1263.
2011. View Article : Google Scholar : PubMed/NCBI
|