Introduction
Spinal cord injury (SCI) is a clinically common
serious trauma. The occurrence rate has the tendency to rise with
increases in annual traffic accidents (1,2).
Furthermore, SCI has a characteristically high disability rate and
low mortality, which burden society and individuals (2). Pathophysiological changes of SCI are
divided into primary mechanical damage and secondary damage that
occurs subsequent to primary damage (3). When primary mechanical damage occurs
in SCI, the degree of damage is directly associated with the degree
of damaged spinal cord tissue. Clinical intervention in SCI is
currently difficult (4). At
present, SCI treatment primarily aims to cure secondary damage,
prevent further intensification of secondary damage and recover
spinal cord functions (5).
Secondary SCI is further damage that is caused by
various factors following primary damage of spinal cord. The
mechanism is extremely complicated (6). A series of neurobiochemical,
microcirculatory and inflammatory responses continue to occur
within the damage zone of SCI, which results in local vasospasm,
coagulation and thrombogenesis (7). A series of pathological reactions
damage persistent nerve cells and also cause damage to spinal cord
tissue (8). Currently, it is
considered that the mechanisms implicated in secondary SCI
primarily include blood vessels, oxidative stress, inflammatory
responses and apoptosis (3,9).
The membrane structure of spinal cord tissues
contains abundant lipids. Following damage, the generation and
release of oxygen radicals is increased. Oxygen radicals act on the
polyunsaturated fatty acids of the cytomembrane to generate lipid
peroxidase, change membrane permeability, disintegrate lysosomes
and cause necrocytosis, which results in secondary damage of the
spinal cord. Free radical scavenging in cells primarily involves
two antioxidant systems, including enzymatic defense reactions
superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px)
(10).
SCI is the continuous destruction of the spinal
canal caused by external force, fracture or instant damage of the
spinal cord caused by dislocation upon trauma and is the opposite
to physical trauma (11).
Secondary damage is further injury to the spinal cord, which is
caused by a series of biochemical reaction events, including
bleeding, edema, local ischemia reperfusion, inflammatory response,
Ca2+ overflow and reactive oxide species after the
primary damage has occurred (11).
The degree of damage in secondary SCI far exceeds primary damage
and is the principal factor for resulting neuronal death and
neurofunction deficit (12). SCI
is associated with local microcirculation disturbance, damage
induced by neuroinflammation and reactive oxygen species, and toxic
effects induced by excitatory amino acids, electrolyte imbalance
and nerve cell apoptosis (13).
Spinal nerve cell apoptosis caused by a series of
immune-inflammatory responses is considered to be a major factor
involved in the aggravation of secondary damage of the spinal cord.
Therefore, the development of methods to preventing secondary
damage in the spinal cord after primary damage has occurred is
required (13).
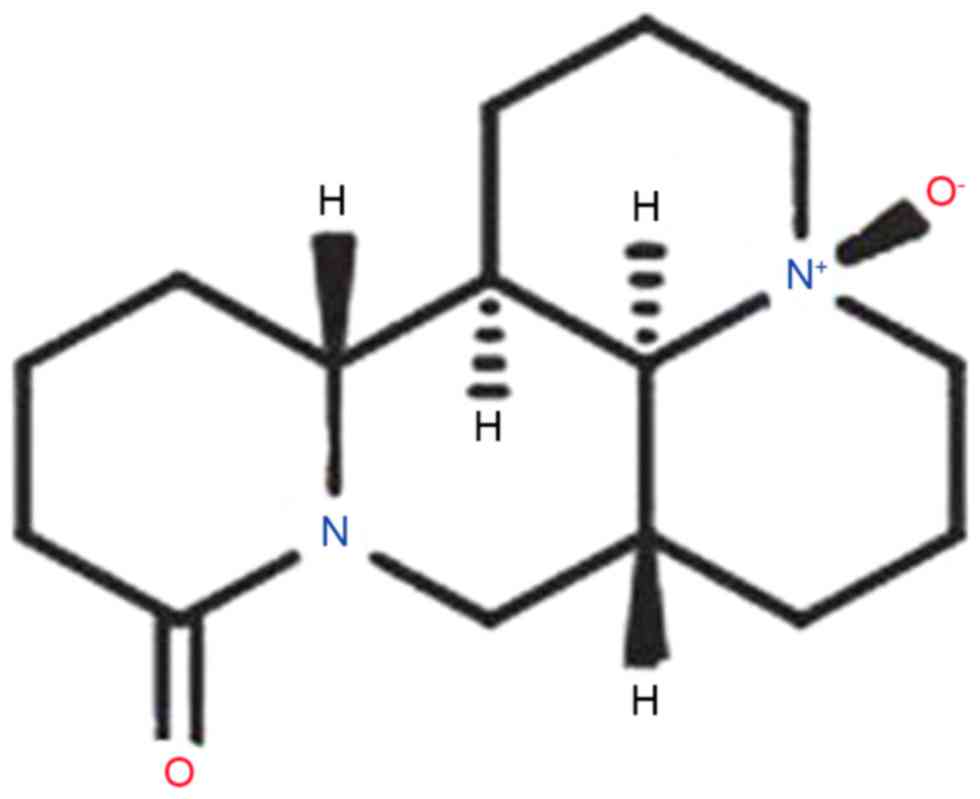
The pharmacological action of oxysophoridine (OSR),
whose chemical structure is presented in Fig. 1 (14). OSR belongs to the quinolizidine
alkaloid group as do other alkaloids of Sophora
alopecuroides (14). A
previous study demonstrated that OSR has various pharmacological
actions, including antiarrhythmic, protection of cardiac muscle,
antiviral, antineoplastic effects (15). Furthermore, OSR has antiviral
pharmacological actions, which is similar to sophoridine. OSR
exhibits anti-inflammatory action and inhibits the biosynthesis of
leukotriene B4 (16). The present
study hypothesizes that the anti-inflammatory effect of OSR rescues
SCI via anti-inflammatory, anti-oxidative stress and anti-apoptosis
effects.
Materials and methods
Animals
The experimental protocol was approved by Shandong
Provincial Hospital Affiliated to Shandong University Animal Care
and Ethics Committee. Female adult Sprague-Dawley rats (weight,
200–230 g, n=50) were purchased from the Experimental Animal Center
of Shandong University, and maintained in standard cages (22–24°C
and 55–60% humidity) with water and food ad libitum and a
12-h light/dark cycle. All rats were randomly assigned into five
groups; sham-operation group, SCI model group, 60 mg/kg OSR group,
120 mg/kg OSR group and 180 mg/kg OSR group. Anesthetized
Sprague-Dawley rats received a midline 150 kdyne contusion injury
in spinal level T10 using an Infinite Horizon impactor device,
which was considered to be the SCI model. The establishment of the
SCI model was confirmed by analysis of the Basso, Beatie and
Bresnahan (BBB) Locomotor Rating Scale (17) and spinal cord tissue water content.
In the 60, 120 and 180 mg/kg OSR groups, SCI rats were administered
intragastrically with 60, 120 and 180 mg/kg OSR once per day for 10
days. OSR was purchased from Jinghua Pharmaceutical Group Co., Ltd.
(Yanchi, China). In sham-operation group and SCI model group, rats
were administered normal saline intragastrically.
Behavioral assessments
Functional recovery was assessed following treatment
with OSR using the BBB Locomotor Rating Scale to ensure consistency
of the lesion (17). Following 10
days treatment with OSR, the rats were narcotized with 35 mg/kg of
pentobarbital and then sacrificed using decollation. Subsequently,
abdomen of rats was cut open, spinal level T10 was peeled and
spinal cord tissues were collected and washed with PBS. Spinal cord
tissues were weighed as wet weight and heated at 80°C for 48 h, and
subsequently weighed as dry weight. Spinal cord tissue water
content was calculated by (wet weight/dry weight) ×100.
Inflammatory activation as measured by
ELISA
Whole blood (500 µl) was centrifuged at 2,000 × g
for 10 min at 4°C and serum was collected in every rat to determine
the levels of tumor necrosis factor-α (TNF-α; H052), interleukin
(IL)-1β (H002), IL-6 (H007), IL-8 (H008), malondialdehyde (MDA;
A003-1), SOD (A001-1) and GSH-Px (A005) using commercial ELISA kits
from Nanjing Jiancheng Biology Engineering Institute (Nanjing,
China) according to the manufacturer's protocol.
Western blotting
Spinal cord tissues were isolated from every rat and
homogenized in RIPA assay (Beyotime Institute of Biotechnology,
Haimen, China). Protein concentrations were measured using a BCA
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Proteins (50–80 µg) were fractionated by 12% SDS-PAGE and
transferred to a nitrocellulose membrane. The membranes were
blocked in 5% skim milk in TBS-Tween-20 (TBS-T; 0.05%) at room
temperature for 1 h on a shaker and incubated with the following
primary antibodies: Prostaglandin E2 (PGE2; sc-20771; 1:500),
intercellular adhesion molecule-1 (ICAM-1; sc-7891; 1:500),
cyclooxygenase-2 (COX-2; sc-7951; 1:500), nuclear factor-κB (NF-κB;
sc-109; 1:500), Bcl-2-associated X (Bax; sc-6236; 1:500), Bcl-2
(sc-783; 1:500) and GAPDH (sc-367714; 1:500; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight. The
membrane was washed with TBS-T and treated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:3,000), visualized with a BeyoECL Star (Beyotime Institute of
Biotechnology) and quantified using Bio-Rad Laboratories Quantity
One software version 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
differences were determined using one-way ANOVA followed by a Tukey
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
OSR rescues BBB scores and reduces
spinal cord tissue water content in SCI rats
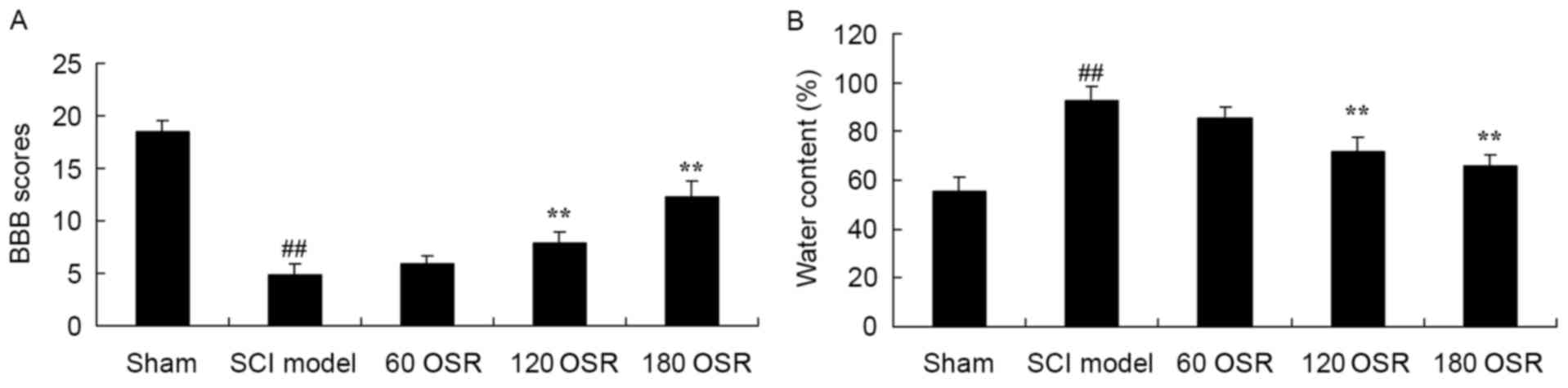
The BBB scores in the SCI model group were
significantly reduced, compared with the sham group (Fig. 2A). Furthermore, treatment with OSR
(120 or 180 mg/kg) significantly increased the BBB scores in SCI
rats, compared with the SCI model group (Fig. 2A). Inversely, the spinal cord
tissue water content of the SCI model group was significantly
higher compared with the sham group (Fig. 2B), and 120 or 180 mg/kg of OSR
significantly inhibited the increased water content of SCI model
rats, compared with the SCI model group (Fig. 2B).
OSR suppresses inflammation in SCI
rats
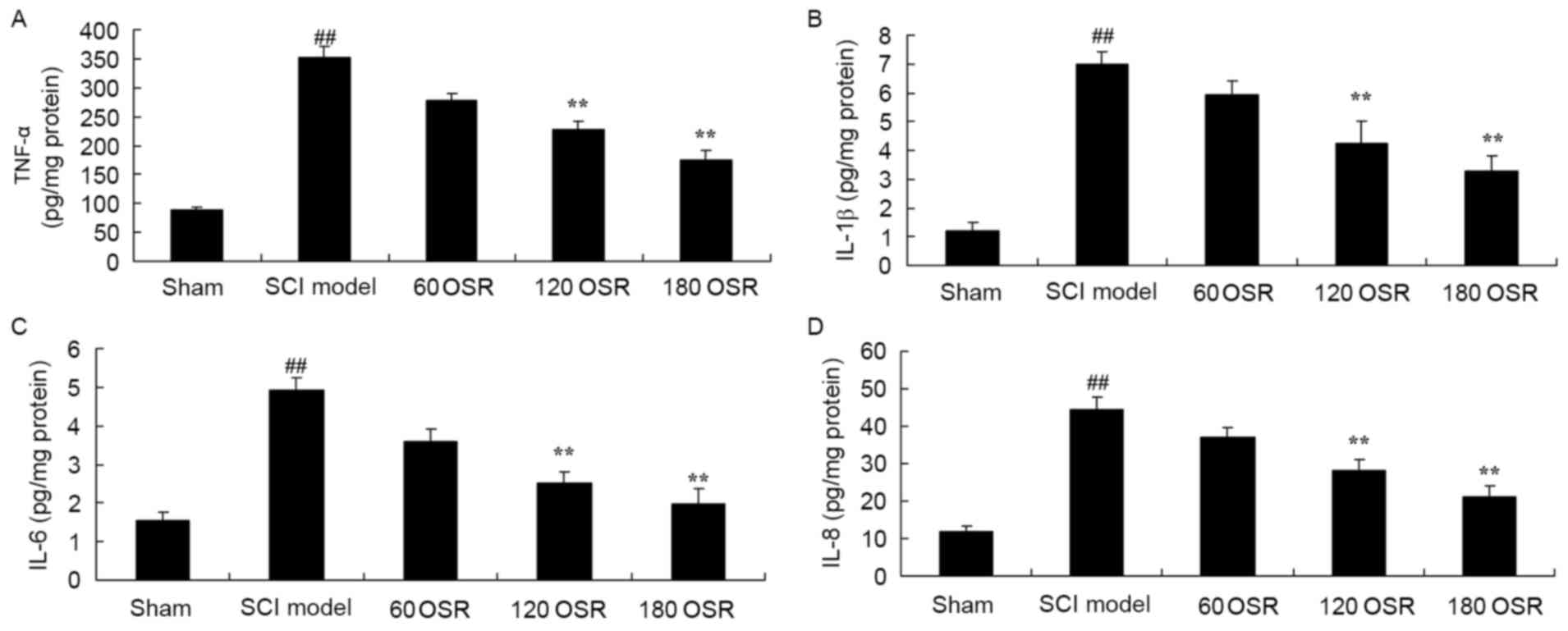
The present study confirmed the anti-inflammatory
effect of OSR on inflammation in SCI rats by measuring the levels
of TNF-α, IL-1β, IL-6 and IL-8 in the serum using ELISA kits. The
results indicate that TNF-α, IL-1β, IL-6 and IL-8 levels in SCI
model rats were significantly higher compared with levels in the
sham group (Fig. 3). After 10 days
of treatment with 120 or 180 mg/kg OSR, serum TNF-α, IL-1β, IL-6
and IL-8 levels in SCI rats were significantly reduced compared
with levels in the SCI model group (Fig. 3).
OSR suppresses oxidative stress in SCI
rats
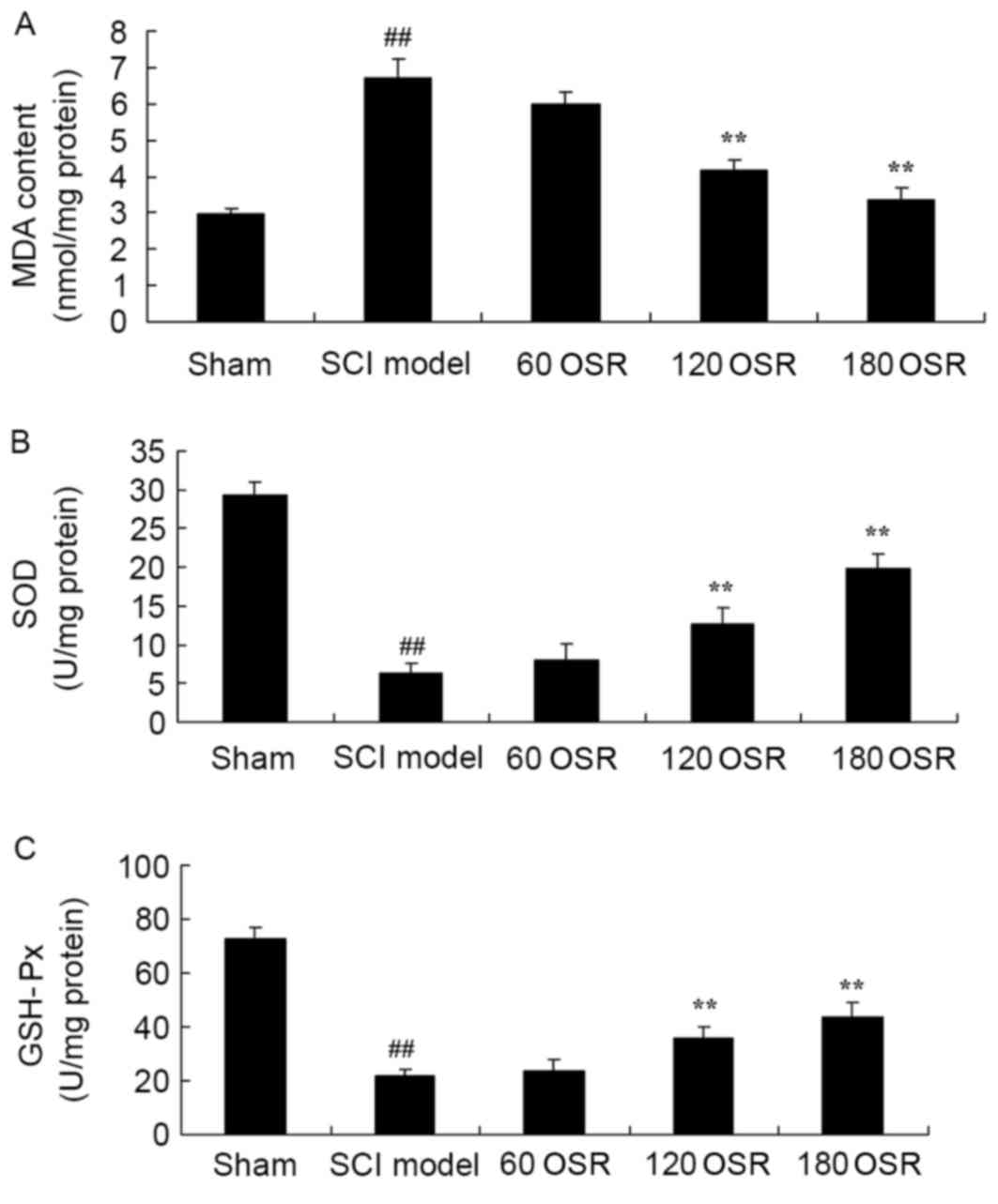
To confirm the anti-oxidative stress effect of OSR
in SCI rats, the levels of MDA, SOD and GSH-Px in the serum were
measured using ELISA kits. Compared with the sham group, MDA levels
were significantly increased, and SOD and GSH-Px activities were
significantly reduced in SCI model rats (Fig. 4). Treatment with 120 or 180 mg/kg
OSR significantly reduced MDA levels, and increased SOD and GSH-Px
levels in SCI rats compared with the SCI model group (Fig. 4).
Anti-inflammatory effect of OSR
suppresses PGE2 protein expression in SCI rats
PGE2 protein expression in the spinal cord tissue of
SCI model rats was significantly higher compared with the sham
group (Fig. 5A and B). Treatment
with 120 or 180 mg/kg OSR significantly suppressed PGE2 protein
expression in SCI rats compared with the SCI model group (Fig. 5A and B).
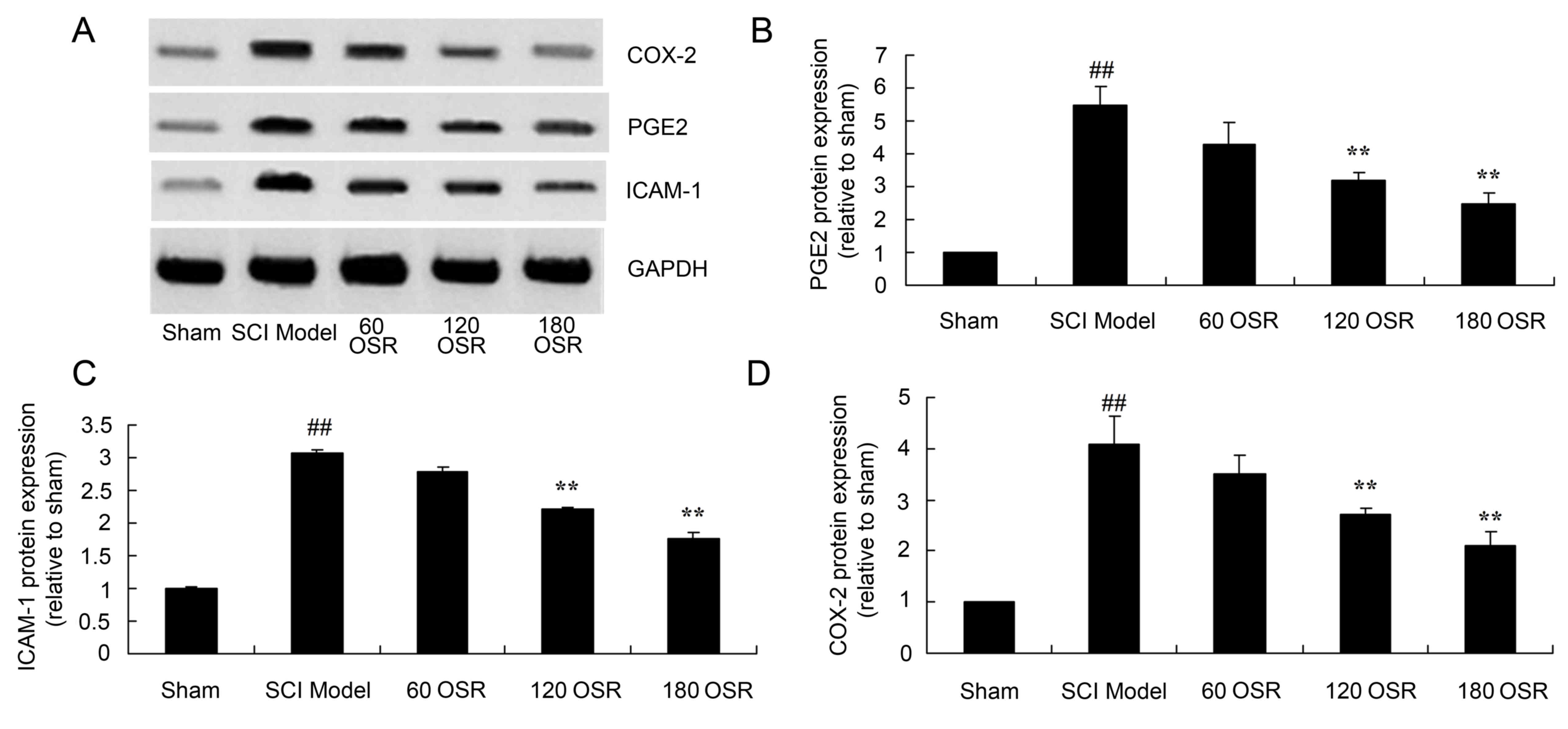 | Figure 5.OSR suppresses the protein expression
of PGE2, ICAM-1 and COX-2 in SCI model rats. (A) Representative
image of western blot analysis of PGE2, ICAM-1 and COX-2 protein
expression. Densitometric analysis indicated that OSR suppressed
the protein expression of (B) PGE2, (C) ICAM-1 and (D) COX-2 in SCI
model rats. ##P<0.01 vs. sham group and **P<0.01
vs. SCI model group. OSR, oxysophoridine; PGE2, prostaglandin E2;
ICAM-1, intercellular adhesion molecule-1; COX-2, cyclooxygenase-2;
SCI, spinal cord injury; 60 OSR, 60 mg/kg OSR; 120 OSR, 120 mg/kg
OSR; 180 OSR, 180 mg/kg OSR. |
Anti-inflammatory effect of OSR
suppresses ICAM-1 protein expression in SCI rats
The ICAM-1 protein expression in the spinal cord
tissue of SCI model rats was significantly increased compared with
the sham group (Fig. 5A and C).
Administration of 120 or 180 mg/kg OSR significantly reduced the
induction of ICAM-1 protein expression in SCI rats compared with
the SCI model group (Fig. 5A and
C).
Anti-inflammatory effect of OSR
suppresses COX-2 protein expression in SCI rats
As demonstrated in Fig.
5A and D, the COX-2 protein expression in the spinal cord
tissue of SCI model rats was significantly increased compared with
the sham group. Furthermore, treatment with 120 or 180 mg/kg OSR
significantly suppressed COX-2 protein expression compared with the
SCI model group (Fig. 5A and
D).
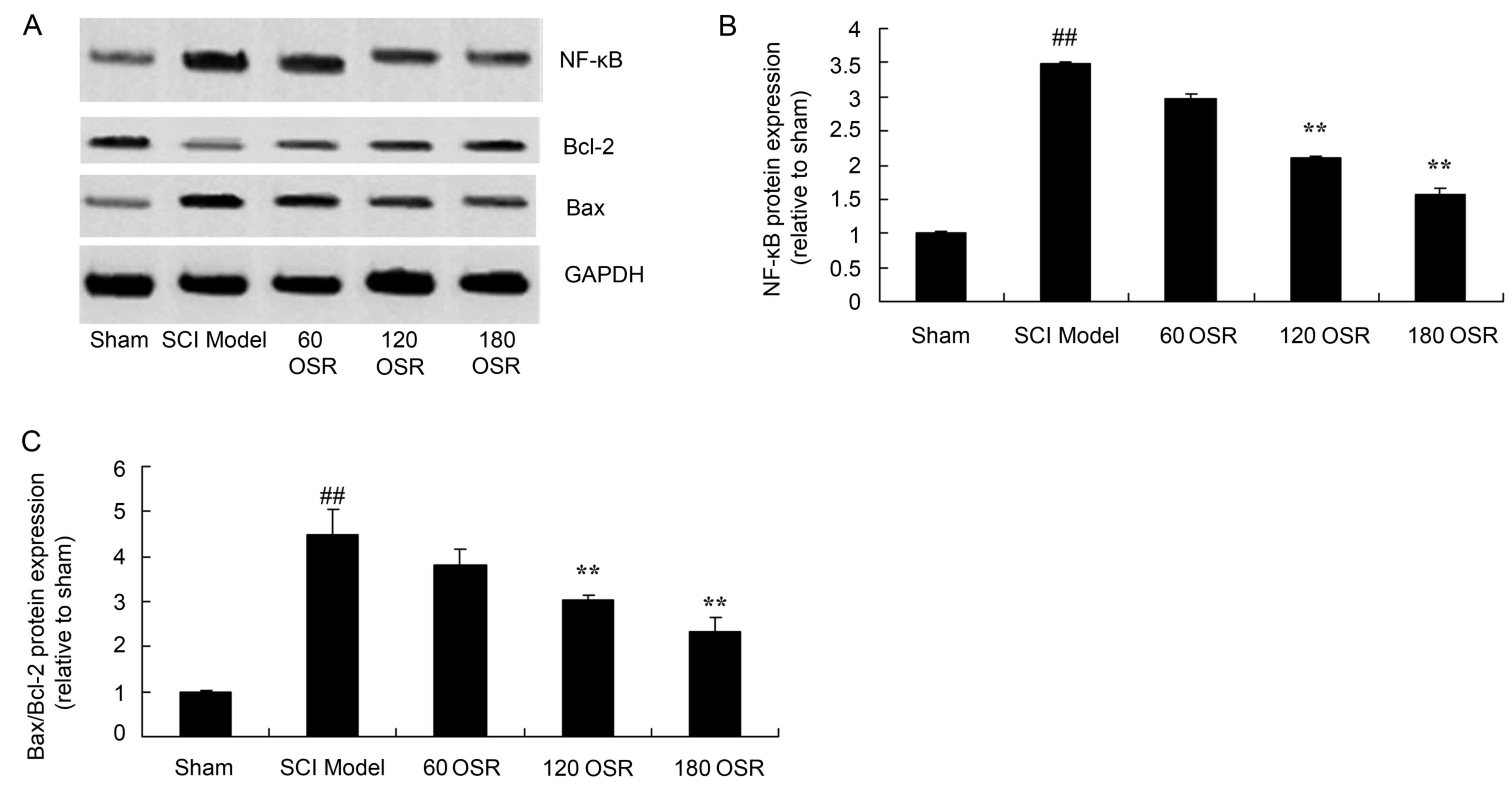
Anti-inflammatory effect of OSR
suppresses NF-κB protein expression in SCI rats
To investigate the involvement of the NF-κB pathway
in the anti-inflammatory effect of OSR in SCI rats, NF-κB protein
expression in the spinal cord tissue was measured using western
blotting. SCI significantly induced NF-κB protein expression
compared with the sham group (Fig. 6A
and B). Treatment with 120 or 180 mg/kg OSR significantly
inhibited the NF-κB protein expression induced by SCI, compared
with the SCI model group (Fig. 6A and
B).
OSR suppresses the Bax/Bcl-2 protein
expression ratio in SCI rats
To investigate the effect of OSR on apoptosis in the
SCI rat model, the Bax/Bcl-2 protein expression ratio in the spinal
cord tissue of rats was measured by western blotting. The ratio of
Bax/Bcl-2 protein expression in SCI model rats was significantly
higher compared with the sham group (Fig. 6A and C). However, treatment with
120 or 180 mg/kg OSR significantly reduced the ratio of Bax/Bcl-2
protein expression in SCI model rats, compared with the SCI model
group (Fig. 6A and C).
Discussion
Pathophysiological changes in SCI are divided into
primary mechanical damage and secondary damage that occurs
subsequent to primary damage (1).
When primary damage occurs in SCI, it is impossible to intervene
clinically, and, currently, treatment of SCI only aims to cure
secondary damage (18). Secondary
damage of the spinal cord is further injury to the spinal cord that
is caused by various factors following primary damage of the spinal
cord (19). Spinal damage that
results from secondary damage may greatly exceed primary damage. A
recent study has investigated the mechanism of SCI, and the
mechanisms that are thought to be involved include oxidative
stress, inflammatory responses and apoptosis (8). The results of the present study
demonstrated that OSR increased BBB scores and reduced spinal cord
water content in SCI model rats. These results are similar to the
Yang et al (16) which
demonstrated that OSR presents effective antinociception in the
spinal cord of mice.
A previous study demonstrated that inflammatory
responses have an important role on the pathogenesis of secondary
damage (20). Another previous
study indicated that there is a high number of various types of
inflammation-associated proteins in the tissue following spinal
cord damage, including TNF-α, IL-1β, IL-6, IL-8, ICAM-1 and
vascular cell adhesion molecule 1 (VCAM-1), suggesting that they
may have an important role in the pathophysiological mechanism of
secondary damage following SCI (10). The results of the current study
demonstrate that treatment with OSR suppressed TNF-α, IL-1β, IL-6
and IL-8 levels in SCI model rats. Previously, Meng et al
(15) demonstrated that OSR
attenuated acute myocardial infarction through anti-oxidative,
anti-inflammatory and anti-apoptotic pathways.
SOD has an important role in the oxidation and
antioxidant balance, and scavenges free radicals and protect cells
from damage (21). MDA is the
lipolysis product of lipid peroxidase. The body can generate oxygen
radicals through the enzyme system (12), which can attach polyunsaturated
fatty acids to the biological membrane, causing lipid peroxidation,
forming MDA from lipid peroxide, and causing cell damage.
Measurement of MDA is often coordinated with measurement of SOD
(21). The level of activity of
SOD indicates the ability of the body to scavenge free radicals
directly. GSH-Px is an important peroxidase enzyme that is
expressed throughout the body (12). GSH-Px eliminates harmful peroxide
metabolites in cells and intercepts lipid peroxidation chain
reactions, which protects cytomembrane structure (21). The results of the present study
indicate that OSR significantly reduced MDA levels, and increased
SOD and GSH-Px levels in SCI model rats. This anti-oxidative stress
effect of OSR was also reported in a study by Meng et al
(15), which demonstrated that OSR
attenuated acute myocardial infarction through anti-oxidative,
anti-inflammatory and anti-apoptotic pathways.
NF-κB is a transcription factor that has an
important regulatory role in inflammatory responses, immune
responses, cell growth, differentiation and apoptosis (22). NF-κB consists of p65 and p50
subunits, which combine to form a heterodimer. Activated NF-κB
participates in multiple inflammatory cell factors individually or
cooperates with other transcription factors, including TNF-α,
IL-1β, IL-6, IL-8, ICAM-1 and VACM-1 (13). The gene products induced by NF-κB
action further participate in inflammatory and immune responses,
and have important functions in physiological and pathological
conditions of the body (23). The
current study demonstrated that OSR significantly suppressed PGE2,
ICAM-1 and COX-2 protein expression in the spinal cord tissue of
SCI model rats. Wang et al (24) also reported that OSR protected
against ischemia-induced injury via PGE2, COX-2 and ICAM-1
expression, and the NF-κB pathway.
The inflammatory response is one of primary
mechanisms of aggravating secondary SCI. The process is primarily
induced by the toll-like receptor (TLR)-NF-κB signaling pathways
(25). Knowledge of the signal
transduction process associated with TLRs-NF-κB signaling is
largely based on the investigation of TLR2 and TLR4 (26). Activated TLRs not only induce
inflammatory responses, but also promote the differentiation and
maturity of antigen-specificity acquired immune responses (26). The present study demonstrated that
OSR significantly inhibited NF-κB protein expression in SCI model
rats. Wang et al (24) also
reported that effects on the NF-κB pathway were associated with the
anti-inflammatory effects of OSR, which protected against
ischemia-induced injury.
Bcl-2 is a cytoplasmic protein that has a higher
level of expression during development of the central nervous
system (27). Once development of
the nervous system is complete, Bcl-2 expression is maintained at a
low level. Bcl-2 is an anti-apoptotic gene and can prevent various
apoptosis pathways following spinal damage (21). Previous research has demonstrated
that overexpression of Bcl-2 in experimental animals alleviated
nerve injury, improved the anti-injury function of the tissue,
promoted outward growth of axons, repaired damaged central nervous
system tissue and prevented nerve cell apoptosis (28). Bax is located in the cytoplasm.
After receiving an apoptosis signal, it is activated to alter the
membrane permeability of the mitochondrial membrane and promote the
apoptosis of cells (28). The
current study demonstrated that OSR significantly reduced the
Bax/Bcl-2 protein expression ratio in SCI model rats. Wang et
al (29) previously reported
that OSR also protects against focal cerebral ischemic injury via
Bax/Bcl-2 expression.
In summary, OSR rescues SCI as treatment with OSR
increased BBB scores, reduced spinal cord tissue water content,
reduced the production of pro-inflammatory cytokines, increased the
levels of anti-oxidant enzymes and reduced the ratio of Bax/Bcl-2
protein expression in SCI model rats, which revealed
anti-inflammatory, anti-oxidative stress and anti-apoptosis effects
of OSR that may be mediated via NF-κB and the Bax/Bcl-2 pathway. In
conclusion, OSR may exert a protective effect on SCI by
anti-inflammatory, anti-oxidative stress and anti-apoptosis
effects, which indicates that OSR may be a potential therapeutic
agent for SCI.
References
|
1
|
Jones ML, Evans N, Tefertiller C, Backus
D, Sweatman M, Tansey K and Morrison S: Activity-based therapy for
recovery of walking in individuals with chronic spinal cord injury:
Results from a randomized clinical trial. Arch Phys Med Rehabil.
95:2239–2246 e2. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richards JS, Bombardier CH, Wilson CS,
Chiodo AE, Brooks L, Tate DG, Temkin NR, Barber JK, Heinemann AW,
McCullumsmith C and Fann JR: Efficacy of venlafaxine XR for the
treatment of pain in patients with spinal cord injury and major
depression: A randomized, controlled trial. Arch Phys Med Rehabil.
96:680–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spinu A, Onose G, Daia C, Panţu C,
Anghelescu A, Onose L and Mihăescu A: Intermittent catheterization
in the management of post spinal cord injury (SCI) neurogenic
bladder using new hydrophilic, with lubrication in close circuit
devices-our own preliminary results. J Med Life. 5:21–28.
2012.PubMed/NCBI
|
|
4
|
D'Amico JM, Li Y, Bennett DJ and Gorassini
MA: Reduction of spinal sensory transmission by facilitation of
5-HT1B/D receptors in noninjured and spinal cord-injured humans. J
Neurophysiol. 109:1485–1493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allison DJ and Ditor DS: Targeting
inflammation to influence mood following spinal cord injury: A
randomized clinical trial. J Neuroinflammation. 12:2042015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li XQ, Lv HW, Wang ZL, Tan WF, Fang B and
Ma H: MiR-27a ameliorates inflammatory damage to the blood-spinal
cord barrier after spinal cord ischemia: Reperfusion injury in rats
by downregulating TICAM-2 of the TLR4 signaling pathway. J
Neuroinflammation. 12:252015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang W, Li M, He F, Bian Z, Liu J, He Q,
Wang X, Sun T and Zhu L: Dopamine D1 receptor agonist A-68930
inhibits NLRP3 inflammasome activation and protects rats from
spinal cord injury-induced acute lung injury. Spinal Cord.
54:951–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang Y, Gong FL, Zhao GB and Li J:
Chrysin suppressed inflammatory responses and the inducible nitric
oxide synthase pathway after spinal cord injury in rats. Int J Mol
Sci. 15:12270–12279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellenbroek D, Kressler J, Cowan RE, Burns
PA, Mendez AJ and Nash MS: Effects of prandial challenge on
triglyceridemia, glycemia, and pro-inflammatory activity in persons
with chronic paraplegia. J Spinal Cord Med. 38:468–475. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gökce EC, Kahveci R, Gökce A, Cemil B,
Aksoy N, Sargon MF, Kısa Ü, Erdoğan B, Güvenç Y, Alagöz F and
Kahveci O: Neuroprotective effects of thymoquinone against spinal
cord ischemia-reperfusion injury by attenuation of inflammation,
oxidative stress, and apoptosis. J Neurosurg Spine. 24:949–959.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu G, Zhao J, Chang Z and Guo G: CaMKII
activates ASK1 to induce apoptosis of spinal astrocytes under
oxygen-glucose deprivation. Cell Mol Neurobiol. 33:543–549. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan LH, Wang KZ, Cheng B, Wang CS and Dang
XQ: Anti-apoptotic and neuroprotective effects of
Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC
Neurosci. 7:482006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yarar-Fisher C, Bickel CS, Kelly NA, Stec
MJ, Windham ST, McLain AB, Oster RA and Bamman MM: Heightened
TWEAK-NF-kB signaling and inflammation-associated fibrosis in
paralyzed muscles of men with chronic spinal cord injury. Am J
Physiol Endocrinol Metab. 310:E754–E761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rui C, Yuxiang L, Ning J, Ningtian M,
Qingluan Z, Yinju H, Ru Z, Lin M, Tao S and Jianqiang Y:
Anti-apoptotic and neuroprotective effects of oxysophoridine on
cerebral ischemia both in vivo and in vitro. Planta Med.
79:916–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng C, Liu C, Liu Y and Wu F:
Oxysophoridine attenuates the injury caused by acute myocardial
infarction in rats through anti-oxidative, anti-inflammatory and
anti-apoptotic pathways. Mol Med Rep. 11:527–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang G, Gao J, Jia Y, Yan L, Yu J and
Jiang Y: Oxysophoridine through intrathecal injection induces
antinociception and increases the expression of the GABAAα1
receptor in the spinal cord of mice. Planta Med. 78:874–880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han D, Chen S, Fang S, Liu S, Jin M, Guo
Z, Yuan Y, Wang Y, Liu C and Mei X: The Neuroprotective effects of
muscle-derived stem cells via brain-derived neurotrophic factor in
spinal cord injury model. Biomed Res Int. 2017:19726082017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jan YK, Liao F, Jones MA, Rice LA and
Tisdell T: Effect of durations of wheelchair tilt-in-space and
recline on skin perfusion over the ischial tuberosity in people
with spinal cord injury. Arch Phys Med Rehabil. 94:667–672. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leitner L, Walter M, Freund P, Mehnert U,
Michels L, Kollias S and Kessler TM: Protocol for a prospective
magnetic resonance imaging study on supraspinal lower urinary tract
control in healthy subjects and spinal cord injury patients
undergoing intradetrusor onabotulinumtoxinA injections for treating
neurogenic detrusor overactivity. BMC Urol. 14:682014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wanner IB, Anderson MA, Song B, Levine J,
Fernandez A, Gray-Thompson Z, Ao Y and Sofroniew MV: Glial scar
borders are formed by newly proliferated, elongated astrocytes that
interact to corral inflammatory and fibrotic cells via
STAT3-dependent mechanisms after spinal cord injury. J Neurosci.
33:12870–12886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Mangiferin attenuates contusive spinal cord injury in rats
through the regulation of oxidative stress, inflammation and the
Bcl2 and Bax pathway. Mol Med Rep. 12:7132–7138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S, Liu K, Seneviratne CJ, Li X,
Cheung GS, Jin L, Chu CH and Zhang C: Lipoteichoic acid from an
Enterococcus faecalis clinical strain promotes TNF-α expression
through the NF-κB and p38 MAPK signaling pathways in differentiated
THP-1 macrophages. Biomed Rep. 3:697–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Machova Urdzikova L, Karova K, Ruzicka J,
Kloudova A, Shannon C, Dubisova J, Murali R, Kubinova S, Sykova E,
Jhanwar-Uniyal M and Jendelova P: The anti-inflammatory compound
curcumin enhances locomotor and sensory recovery after spinal cord
injury in rats by immunomodulation. Int J Mol Sci. 17:pii: E492015.
View Article : Google Scholar
|
|
24
|
Wang YS, Li YX, Zhao P, Wang HB, Zhou R,
Hao YJ, Wang J, Wang SJ, Du J, Ma L, et al: Anti-inflammation
effects of oxysophoridine on cerebral ischemia-reperfusion injury
in mice. Inflammation. 38:2259–2268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XQ, Lv HW, Tan WF, Fang B, Wang H and
Ma H: Role of the TLR4 pathway in blood-spinal cord barrier
dysfunction during the bimodal stage after ischemia/reperfusion
injury in rats. J Neuroinflammation. 11:622014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YK, Liu JT, Peng ZW, Fan H, Yao AH,
Cheng P, Liu L, Ju G and Kuang F: Different TLR4 expression and
microglia/macrophage activation induced by hemorrhage in the rat
spinal cord after compressive injury. J Neuroinflammation.
10:1122013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibata M, Murray M, Tessler A, Ljubetic
C, Connors T and Saavedra RA: Single injections of a DNA plasmid
that contains the human Bcl-2 gene prevent loss and atrophy of
distinct neuronal populations after spinal cord injury in adult
rats. Neurorehabil Neural Repair. 14:319–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen MH, Ren QX, Yang WF, Chen XL, Lu C
and Sun J: Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions
in rats with spinal cord injury. Int J Clin Exp Pathol.
6:2312–2322. 2013.PubMed/NCBI
|
|
29
|
Wang TF, Lei Z, Li YX, Wang YS, Wang J,
Wang SJ, Hao YJ, Zhou R, Jin SJ, Du J, et al: Oxysophoridine
protects against focal cerebral ischemic injury by inhibiting
oxidative stress and apoptosis in mice. Neurochem Res.
38:2408–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|