Introduction
Diabetic nephropathy (DN) is one of the major
complications of diabetes, and is a major cause of end-stage renal
disease worldwide (1). Since the
rise in the incidence of obesity and type 2 diabetes, high
morbidity and mortality associated with DN (2,3).
Over the past few decades, even though big strides have been made
in improving diagnosis and treatment of DN patients, we are still
unable to dramatically reduce the mortality of diabetic patients
worldwide. Nevertheless, if therapeutic interventions start in the
early stages of DN, its pathophysiological progression can be
slowed down considerably (4–6).
Therefore, there is urgent need to identify key biomarkers to
correctly classify the clinical stage of DN. Recent study found
Hydrogen sulfide possessed important therapeutic characteristics
that prevented the development of DN (7). However, many studies are conflicting
regarding the specificity and sensitivity of biomarkers currently
used in clinical practice for DN diagnosis (8,9).
Recent studies suggest that the membrane attack complex and levels
of mannose-binding lectin might contribute to renal damage in the
hyperglycaemic mileu (10) and
reticulum stress response in renal cells is a key factor of
progression of DN (11).
As a diabetic microangiopathy, the pathogenesis of
DN begins with thickening of the glomerular basement membrane (GBM)
and glomerulosclerosis (12). The
damage to the capillaries in the kidneys' glomeruli by the GBM
thickening and increased mesangial matrix accompanies irreversible
loss of kidney function and is associated with excessive deposition
of extracellular matrix (ECM) (13). The functional integrity of the
basement membrane largely depends on the appropriate cross-linking
of collagen. Cross-linked collagen fibers show progressively
increased tensile strength and become insoluble, in a process that
is essential for normal tissue function (14). This natural vascular ECM
cross-linking stabilizes the fibrous ECM proteins in a beneficial
manner, but the over-abundance of collagen cross-linking renders
collagen resistant to proteinase degradation (15). Thus, cross-linking of ECM molecules
is an initial and important step for matrix maturation and
stabilization that is mediated by extracellular enzymes, including
transglutaminases (16), matrix
metalloproteinases-2 and −9 (17),
and lysyl oxidases (LOXs).
The group of LOXs includes five members, LOX and
lysyl oxidase-like 1, 2, 3, 4 (LOXL1, LOXL2, LOXL3, and LOXL4). It
was reported that LOX and LOXL may be associated with several
abnormalities related to an imbalance in ECM synthesis and
degradation (18,19). Study from Kiemer et al also
indicated that LOXL was upregulated in differentiated kidney
epithelial cells undergoing the epithelial-mesenchymal transition,
suggesting LOXL may have a role in remodeling the extracellular
environment during dynamic processes such as tissue injury
(20). As to LOXL4, a member of
the LOX family, has been reported that its expression levels are
much lower than levels of other members in various normal tissues
(21) and might have some
correlation to tumor progression, such as gastric cancer (22), bladder cancer (23) and head/neck cancer (24,25).
However, no studies have analyzed the correlation between the
expression of LOXs and DN, and most have focused on renal fibrosis
(26,27), which only appears in the advanced
stages of DN.
So we hypothesized that there is a relationship
between LOXs and classical lesions of early DN and LOXs may be a
key biomarker in the early stage of DN. Both glomerulosclerosis and
thickening of the GBM can develop as classical lesions of early DN
and often are harbingers of progressive glomerular destruction.
Although glomerular damage is believed to cause early kidney damage
in DN, tubular injury also causes damage in DN (28). To determine whether LOX and LOXL1-3
associate to the initial stage of the DN, we separately examined
its expression of the glomerulus and renal tubules in the kidney of
type 2 diabetes model rats.
Materials and methods
Diabetic model of rats
In the preliminary study, the 8-week-old male
Sprague-Dawley (SD) rats were purchased from the Experimental
Animal Center of Sichuan University and randomly divided into
normal control group (n=5) and diabetes group (n=5). To induce type
2 diabetes, diabetes rats were maintained on a high-fat diet (38%
fat, 12% protein, and 50% carbohydrate), whereas control rats
received a standard rat chow. After 8 weeks of dietary
manipulation, diabetes rats were received a single intraperitoneal
injection of streptozocin (STZ; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at a dose of 40 mg/kg diluted in citrate buffer
(pH 4.0), while the control rats were injected citrate buffer with
vehicle in an equivalent dose (29). Seventy-two hours after STZ
injection, the diabetes rats developed hyperglycemia with blood
glucose levels over 16.7 mmol/l. In our current experiment, the
8-week-old obese male Zucker diabetic fatty (ZDF) rats
(ZDF/Crl-Leprfa), a model of type 2 diabetes (T2D), were
purchased from the Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China) and maintained on high-fat diet (Purina
5008; Harlan Teklad, Indianapolis, IN, USA). According to time of
collecting samples, ZDF rats were assigned to two groups: 9 weeks
groups and 16 weeks groups (n=10 in each group). ZDF rats (fa/fa)
(diabetes group) and ZDF lean rats (fa/+) (control group) were
examined for blood glucose and body weight at intervals of at least
one week. All rats were housed at a temperature of 20–25°C,
humidity of 65–69%, and were subjected to a 12-h light/dark cycle
with free access to food and tap water. Nine and sixteen weeks
after the induction of diabetes, rats were euthanized separately
and kidney samples were collected. The study was performed in
accordance with the guidelines issued by the Ethics Committee of
the West China College of Stomatology of Sichuan University
(WCCSIRB-D-2015-135). The experiment was repeated three times on
three different occasions. All experiments were repeated in 3
independent occasions which make the total rat number 60.
Morphological analysis of the
kidney
The renal tissue specimens were fixed in 10%
formalin and embedded in paraffin. For assessment of injury,
sections of 3-µm thickness were stained with hematoxylin and eosin
(H&E), Masson's trichrome staining, and periodic acid-schiff
silver methenamine (PASM). Histological changes in all 3 anatomic
compartments of the renal tissue (glomeruli, renal tubule, and
tubulointerstitium) were assessed and scored.
Glomerular lesions
Glomerulosclerosis was defined as mesangial
expansion and ECM deposition. The mesangial expansion was evaluated
by the assessment of PASM-positive and nucleus-free areas in the
glomeruli. According to the percentage of glomerular involvement,
the degree of glomerulosclerosis was graded from 0 to 4 as
previously described (30).
Briefly, a score of 0 indicated no sclerosis; 1, <25% sclerotic
changes in glomeruli; 2, 25–50% sclerotic areas in glomeruli; 3,
50–75% and 4, >75% sclerotic areas. In each round of experiment,
10 glomeruli were randomly selected in cortical fields and
evaluated at 20X power in each kidney section, and an average score
was calculated.
The index of GBM expansion was determined by a semi
quantitative estimate of the width of basement membranes in each
glomeruli as observed with PASM dyeing (31). The scoring was 0 as normal, 1.0 as
twice the normal thickness, 2.0 as three times the normal
thickness, and so on. Half grades were assigned where appropriate.
In each round of experiment, 10 glomeruli were randomly selected in
cortical fields and evaluated at 20X power in each kidney section,
and an average score was calculated.
Tubular lesions
Histological injury of renal tubule was evaluated as
the percentage of tubules that showed tubular dilation, tubular
atrophy, tubular epithelial cell necrosis, and cast formation as
follows: 0, normal; 1, <10%; 2, 10 to 25%; 3, 26 to 50%; 4, 51
to 75%; and 5, >75% (32).
H&E stained sections were used to evaluate the renal tubular
injury score. At 20X power in each kidney section, 10 areas of
renal tubule were randomly chosen per kidney for the assessment and
an average score was calculated in each round of experiment.
Tubulointerstitial lesions
We evaluated the tubulointerstitial damage with
observation at 20X power-field according to the scoring system
(33). The injury of
tubulointerstitial was scored according to the degree of tubular
dilation, tubular atrophy, cast formation, ECM accumulation, the
number of inflammatory cells, and extent of tubulointerstitial
fibrosis. A score of 0 was assigned when the section shows no
damage, a score of 1 was assigned when less than 25% was present, a
score of 2 was assigned when there was at least 25% but less than
50%, a score of 3 was assigned when there was at least 51% but less
than 75%, a score of 4 was assigned when there was at least 76% but
less than 95%, and finally, a score of 5 was assigned when there
was at least 95%. Glomeruli, arterioles, and blood vessels of the
cortex were excluded. At 20X power, the severity of
tubulointerstitial injury was evaluated by examining 10 randomly
selected fields in each kidney section stained with Masson's
trichrome staining in each round of experiment.
Immunohistochemistry
All specimens were fixed with 4% formaldehyde,
dehydrated, embedded and cut into 3 µm serial sections. Briefly,
the sections were heated in a microwave oven in citrate buffer
(pH=6.0) for 15 min at 95°C and was then cooled to room
temperature. Endogenous peroxidase activity was blocked by
incubation in 3% hydrogen peroxide for 20 min at room temperature.
After washing with PBS, non-specific binding sites were blocked
with normal goat serum for 30 min at room temperature. The sections
were then incubated overnight at 4°C with primary antibody (rabbit
monoclonal anti-LOX (1:400), anti-LOXL1 (1:400), anti-LOXL2 (1:400)
and anti-LOXL3 (1:400). After washing with PBS, sections were
incubated with secondary antibodies for 30 min at 37°C. The
sections were then washed three times with PBS and the sections
were visualized with diaminnobenzidine-tetrahydrochloride (DAB kit;
Zhonshan Goldenbridge Biological Technology Co., Ltd., Beijing,
China). Finally, the sections were counterstained with hematoxylin
and dehydrated. The semiquantification assessment of the
immunohistochemical staining was performed at 20X power in each
kidney section. In each round of experiment, 10 glomeruli and 10
fields of tubules were randomly chosen in the kidney section. The
means of integrated option density (MD) were determined for LOX and
LOXL1-3 for each glomeruli and tubule using Image J software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All of the scores and index values, including the
glomerulosclerosis score, the index of GBM expansion, the score of
tubular injury, and the MD of LOX and LOXL1-3 were expressed as the
mean ± standard deviation. Data were analyzed with the two-tailed
Student's t-test for parametric test. Mann-Whitney U test was used
for non-parametric test. P<0.05 or U>1.96 were considered to
indicate a statistically significant difference.
Results
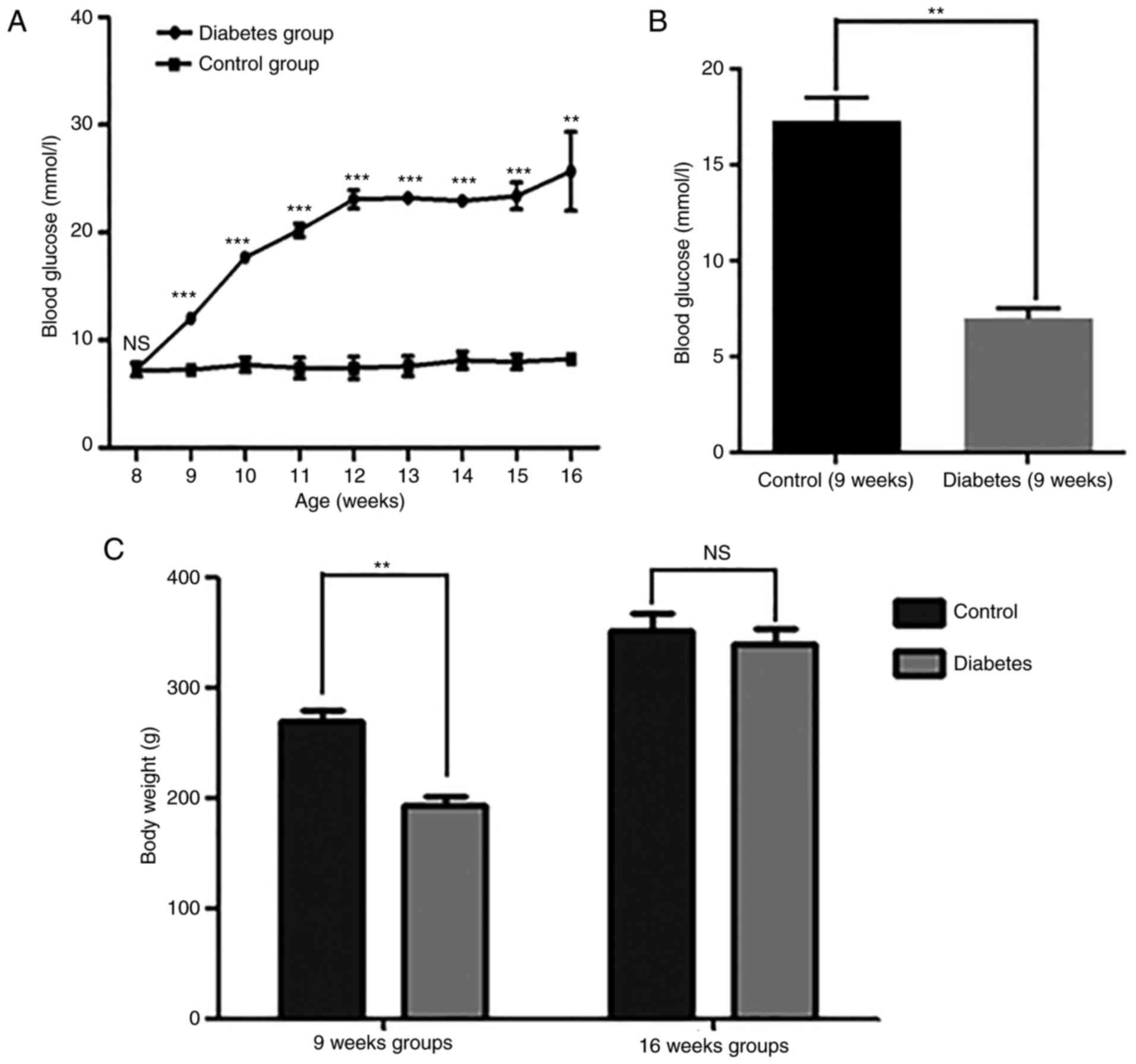
Blood glucose levels and body weight
in the two groups
Both at 9 and 16 weeks, the diabetic group showed
marked increases in blood glucose levels compared with the control
group (Fig. 1A and B). At 9 weeks,
the body weight was significantly increased in the diabetic group,
but at 16 weeks, there was no significant difference in body weight
between the two groups (Fig.
1C).
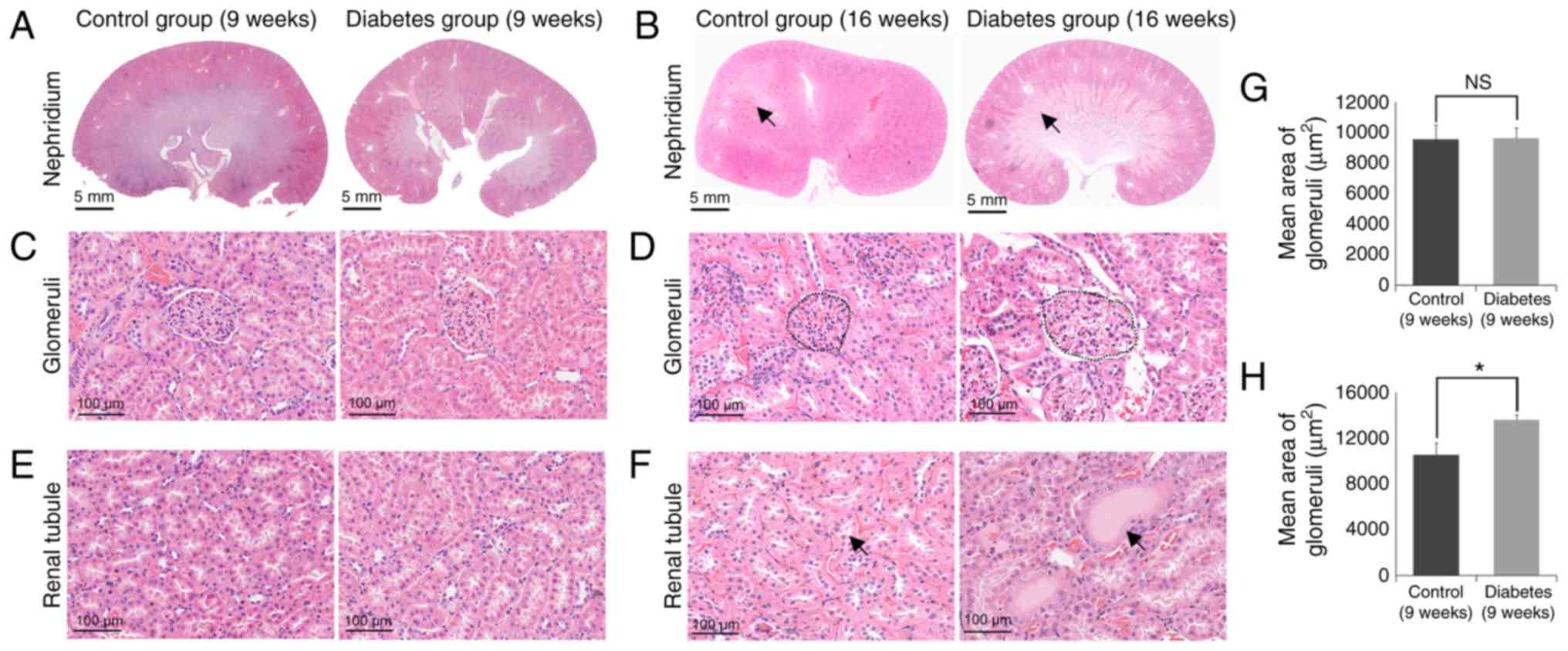
Histopathological examination showed
increased damage of kidney tissue in the diabetes group
Diabetes is typically accompanied by progressive
glomerular and tubular damage (Fig.
2). At 9 weeks, there was no significant morphological change
in the kidneys of the rats (Fig. 2A, C
and E), by contrast, at 16 weeks, the kidneys of the rats in
the diabetes group showed significant morphological changes
compared with the control group (Fig.
2B, D and F). Compared to the control group, the glomerular
volume was significantly increased in the diabetic group (Fig. 2D). Our data showed that mean area
of glomeruli was remarkably increased in the diabetes group (16
weeks) (Fig. 2H), but there is no
different between the in the diabetes group (9 weeks) the control
group (9 weeks) (Fig. 2G).
Additionally, cast formation was observed in the tubular area of
the diabetic kidney that was not evident in the control group
(Fig. 2F).
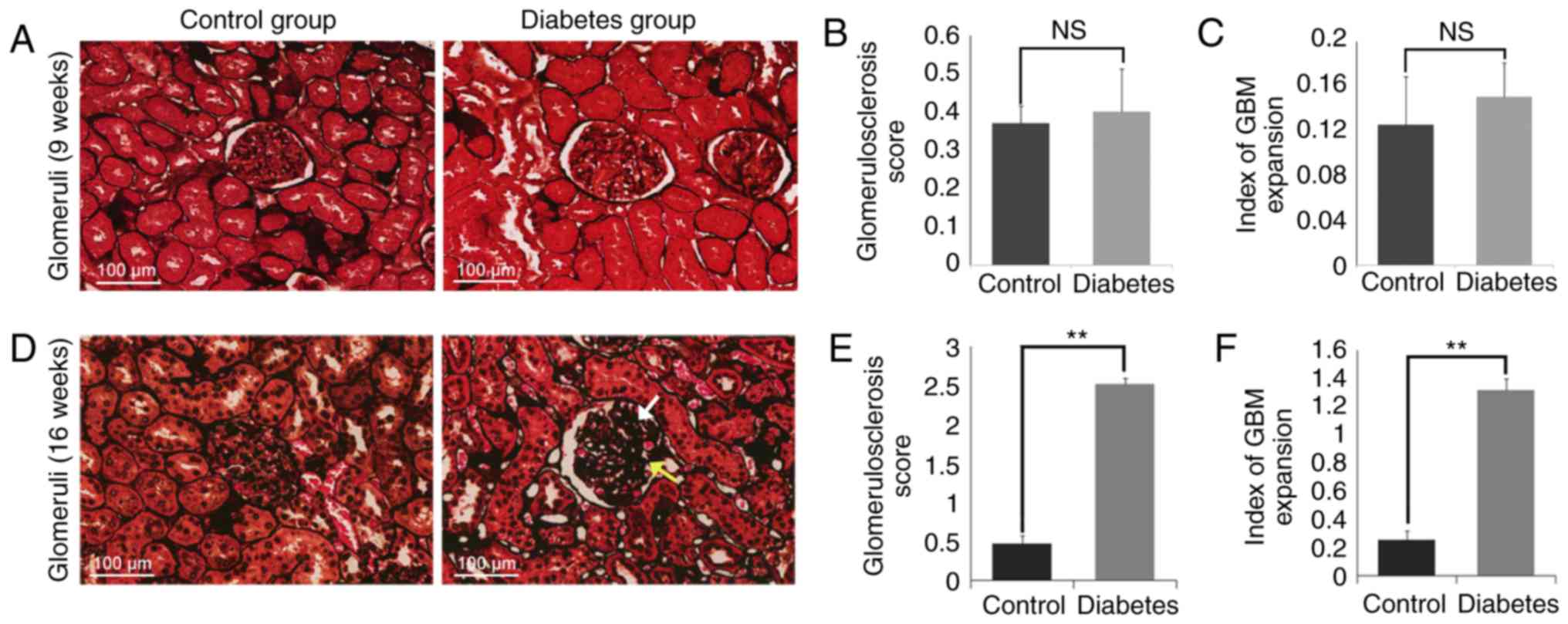
PASM staining of kidney tissues showed
glomerular damage in the diabetes group
PASM staining on the kidney tissues was then
performed as presented in Fig. 3.
GBM thickening and mesangial matrix expansion were observed in the
diabetes group (16 weeks), but the control group (9 weeks, 16
weeks) and the diabetes group (9 weeks) all showed nearly normal
glomeruli with only few fields of mesangial matrix expansion
(Fig. 3A and D). Enlarged
glomeruli in diabetes group were observed compared with those in
the control group. There was a mild increase in the mesangial
matrix in the glomeruli in the diabetic rats, and a few glomerulus
appeared to be undergoing sclerosis. We consistently observed GBM
thickening and a range of mild to moderate mesangial matrix
expansion causing capillary luminal narrowing. No
Kimmelstiel-Wilson lesions or fibrosis were observed in either
group. Light photomicrographs revealed the early developmental
stages of glomerular lesions in DN. The degree of
glomerulosclerosis, defined as ECM deposition and mesangial matrix
expansion, was assessed using the scoring system as previously
described (30).
Glomerulosclerosis was defined with an average score of 2.52±0.104
in the diabetes group (16 weeks), significantly higher than the
average score of the control group (16 weeks) (P<0.01; Fig. 3E). Additionally, the GBM index in
the diabetes group (16 weeks) was significantly higher (1.33±0.095)
compared to that of the control group (16 weeks) (0.175±0.066;
P<0.01; Fig. 3F). But there was
no diffidence in 9 weeks groups. (Fig.
3B and C).
Masson's trichrome and H&E
staining of kidney tissues showed tubulointerstitial and tubular
tissue damage in the diabetes group
Next, Masson's trichrome and H&E staining was
performed (Fig. 4). By 16 weeks,
the control group showed no obvious tubular damage in the
tubulointerstitial areas, but the diabetic group showed remarkable
changes (Fig. 4C and E). Few areas
of interstitial fibrosis surrounding the tubules were observed, but
there was scattered tubular dilation and atrophy (Fig. 4C). There was a statistically
difference in damage between the control group (16 weeks)
(0.82±0.225) and the diabetes group (16 weeks) (3.48±0.076;
P<0.01; Fig. 4D). Additionally,
tubular atrophy, tubular dilation, and tubular epithelial cell
necrosis was observed in the diabetes group (16 weeks) (Fig. 4E). There was scattered tubular
dilatation that affected the cortical and juxtamedullary tubules,
but was more prominent in the juxtamedullary zone. The renal tubule
score in the control group (16 weeks) was 0.38±0.189 compared to
2.35±0.1 in the diabetes group (16 weeks), significantly higher
than the control group (16 weeks) (P<0.01; Fig. 4F). On the contrary,
tubulointerstitial and tubular tissue appeared normal in the 9
weeks groups (Fig. 4A and B).
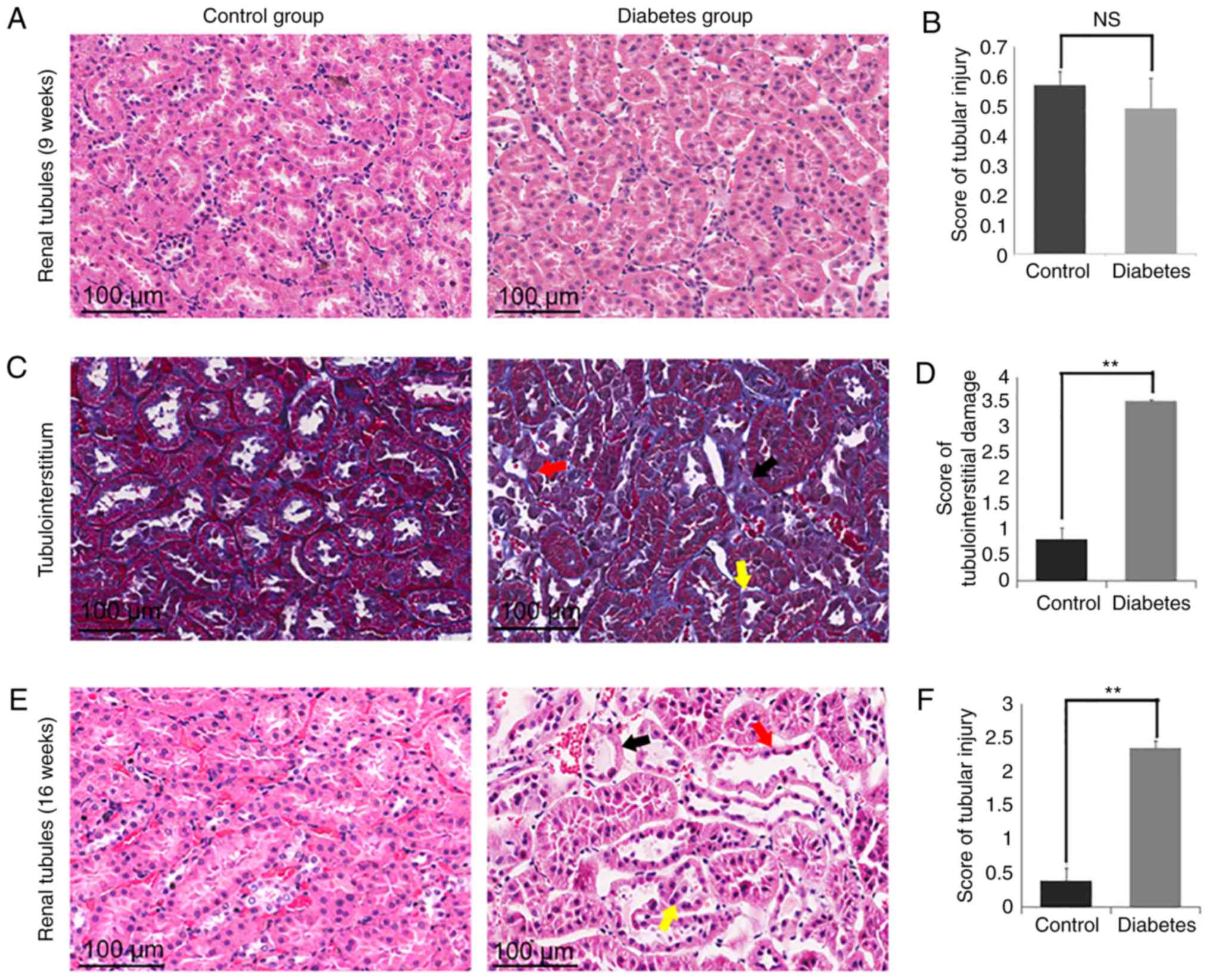 | Figure 4.Representative images of Masson's
trichrome and H&E staining of kidney tissues showed
tubulointerstitial and tubular tissue damage in the diabetes group.
(A) H&E staining showed no histologic abnormalities in the
renal tubules in the two groups (9 weeks). (B) The severity of the
tubular injury was scored from 0 to 5, according to the degree of
tubular dilation, tubular atrophy, tubular epithelial cell
necrosis, and cast formation. The score of tubular injury showed no
significant difference between the two groups (9 weeks). (C)
Masson's trichrome staining showed histologic abnormalities in the
tubulointerstitial in the diabetes group (16 weeks). Tubular
dilation (red arrow), atrophy (yellow arrow), and
tubulointerstitial fibrosis (black arrow) were detected in the
diabetes group (16 weeks). (D) The severity of the
tubulointerstitial damage injury was scored from 0 to 5, according
to the degree of tubular dilation, tubular atrophy, cast formation,
extracellular matrix accumulation, the number of inflammatory
cells, and the presence of tubulointerstitial fibrosis. The scores
of tubulointerstitial damage were significantly different between
the control group (16 weeks) and the diabetes group (16 weeks). (E)
H&E staining showed histologic abnormalities in the tubules in
the diabetes group (16 weeks). Tubular dilation (red arrow),
tubular atrophy (yellow arrow), and tubular epithelial cell
necrosis (black arrow) were detected in kidney tissue from rats in
the diabetes group (16 weeks). (F) The severity of the tubular
injury was scored from 0 to 5, according to the degree of tubular
dilation, tubular atrophy, tubular epithelial cell necrosis, and
cast formation. The score of tubular injury showed a significant
difference between the control group (16 weeks) and diabetes group
(16 weeks). **P<0.01; NS, no significant difference. n=5,
repeated 3 times. |
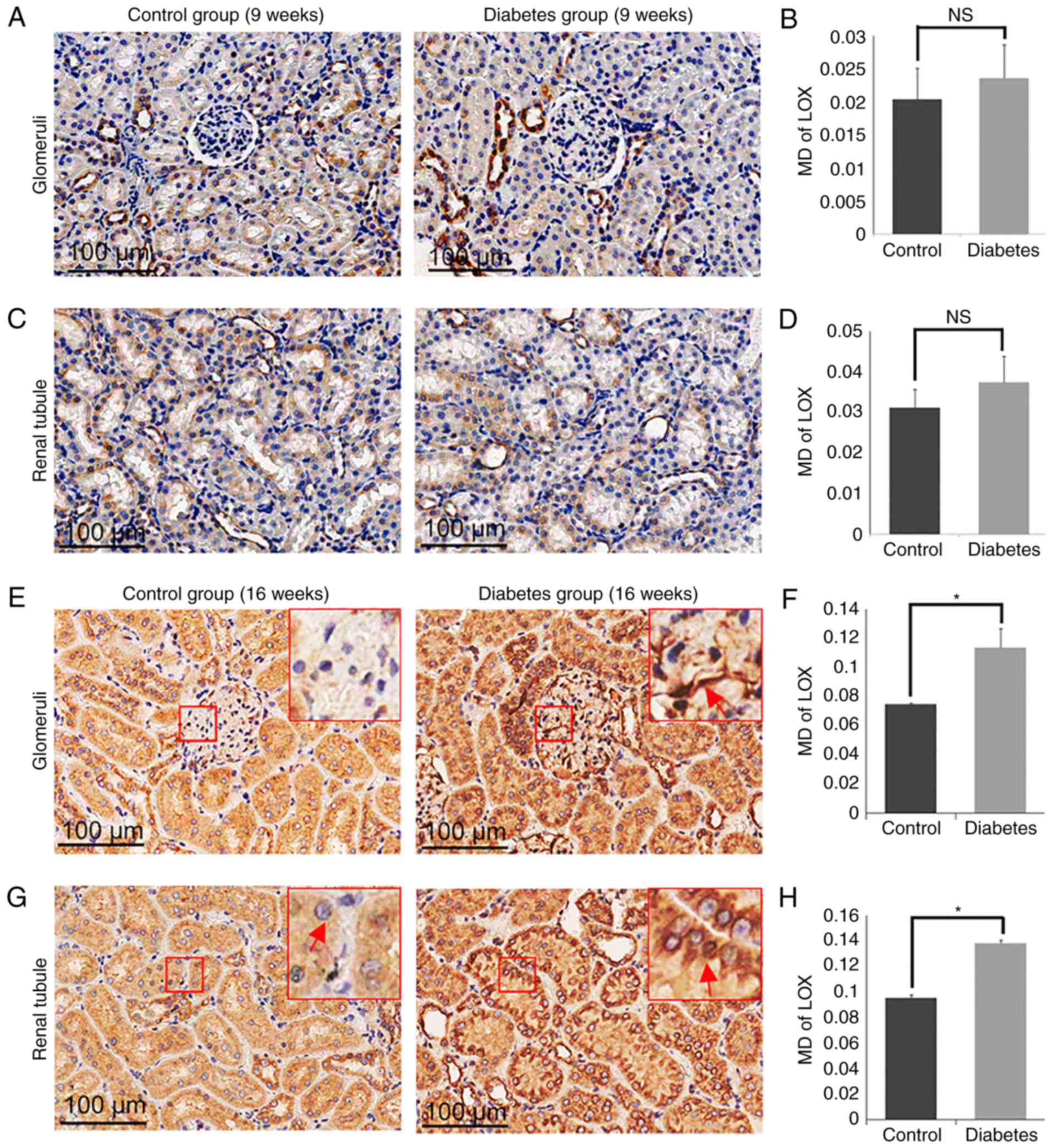
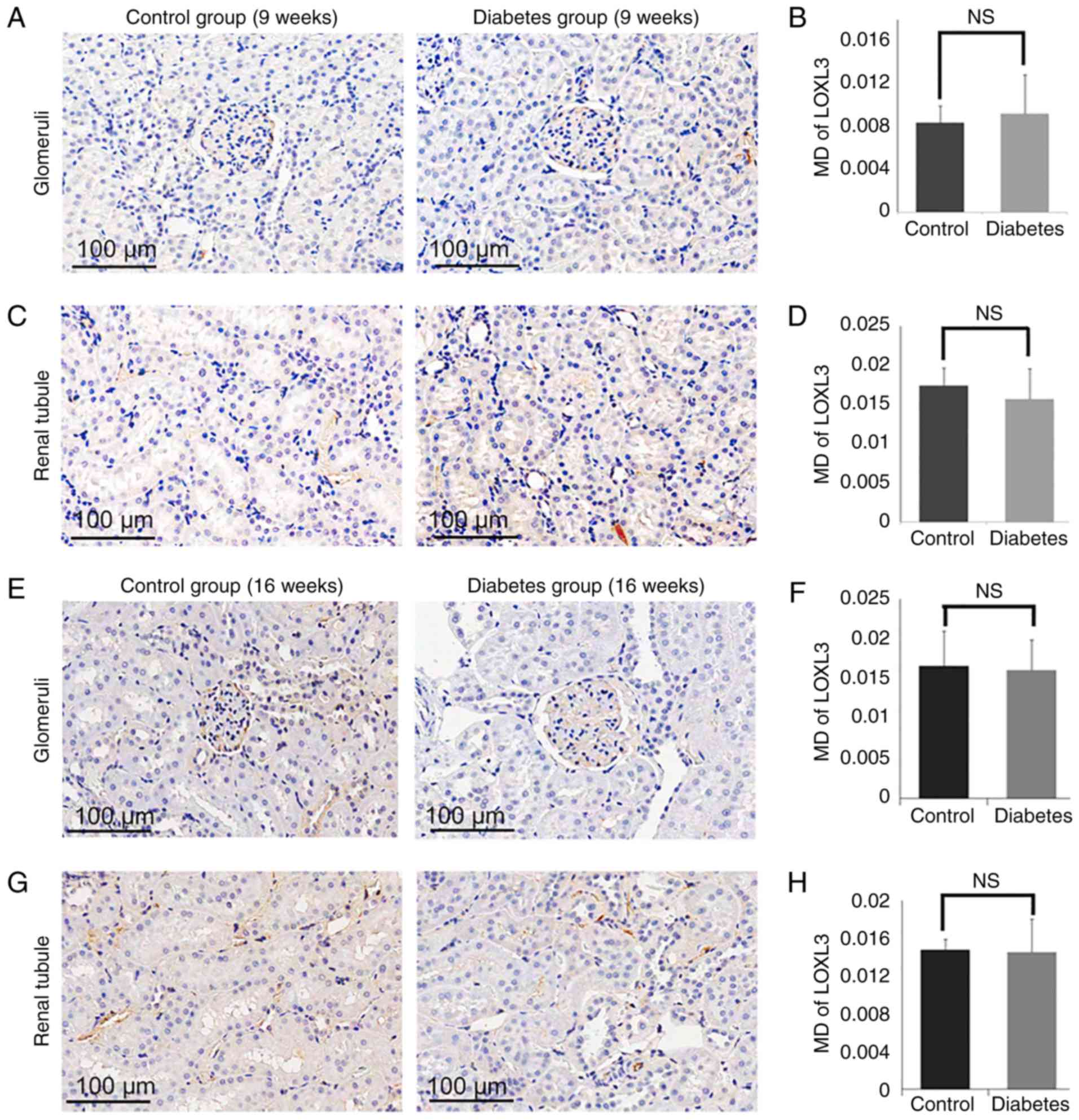
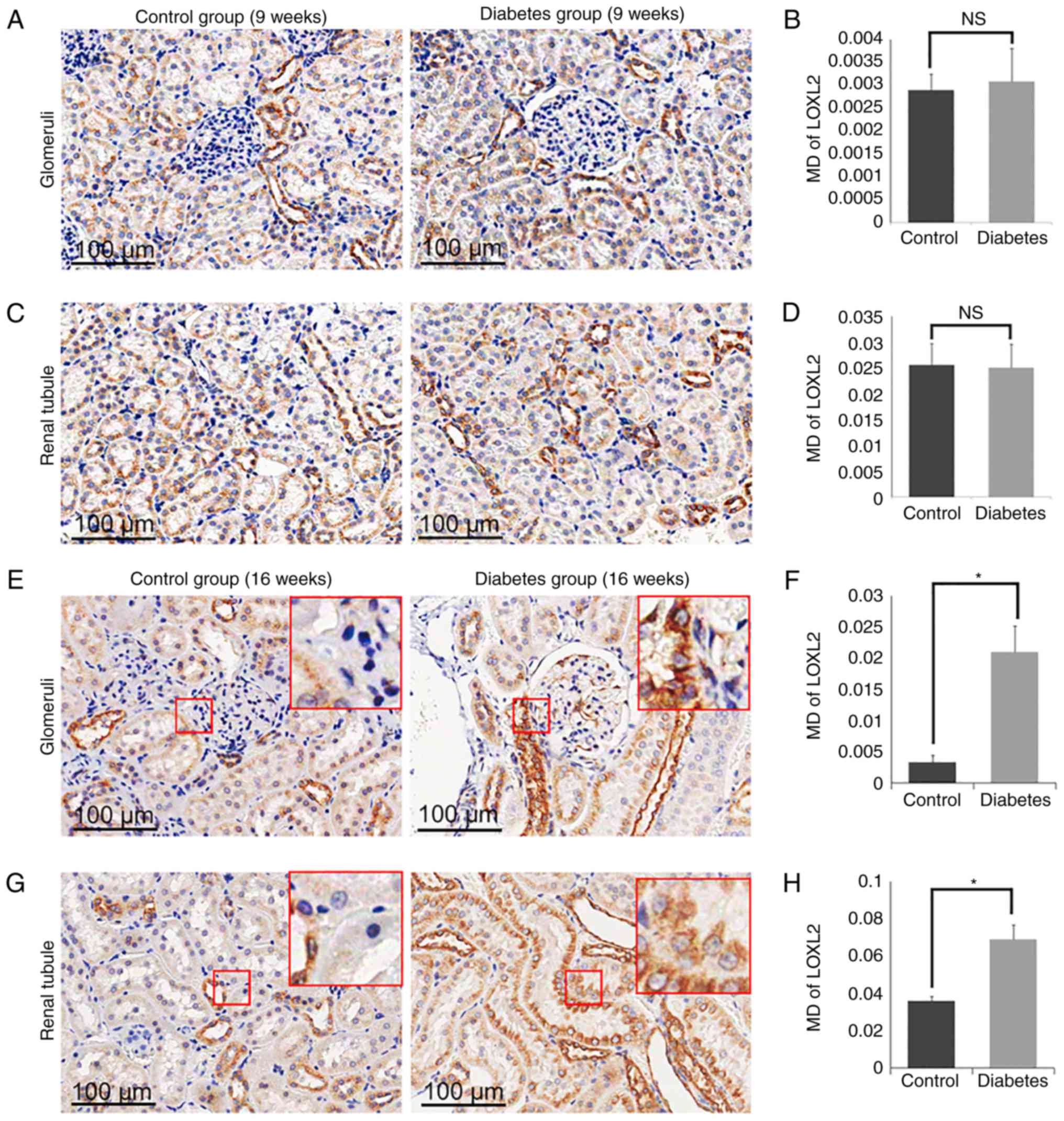
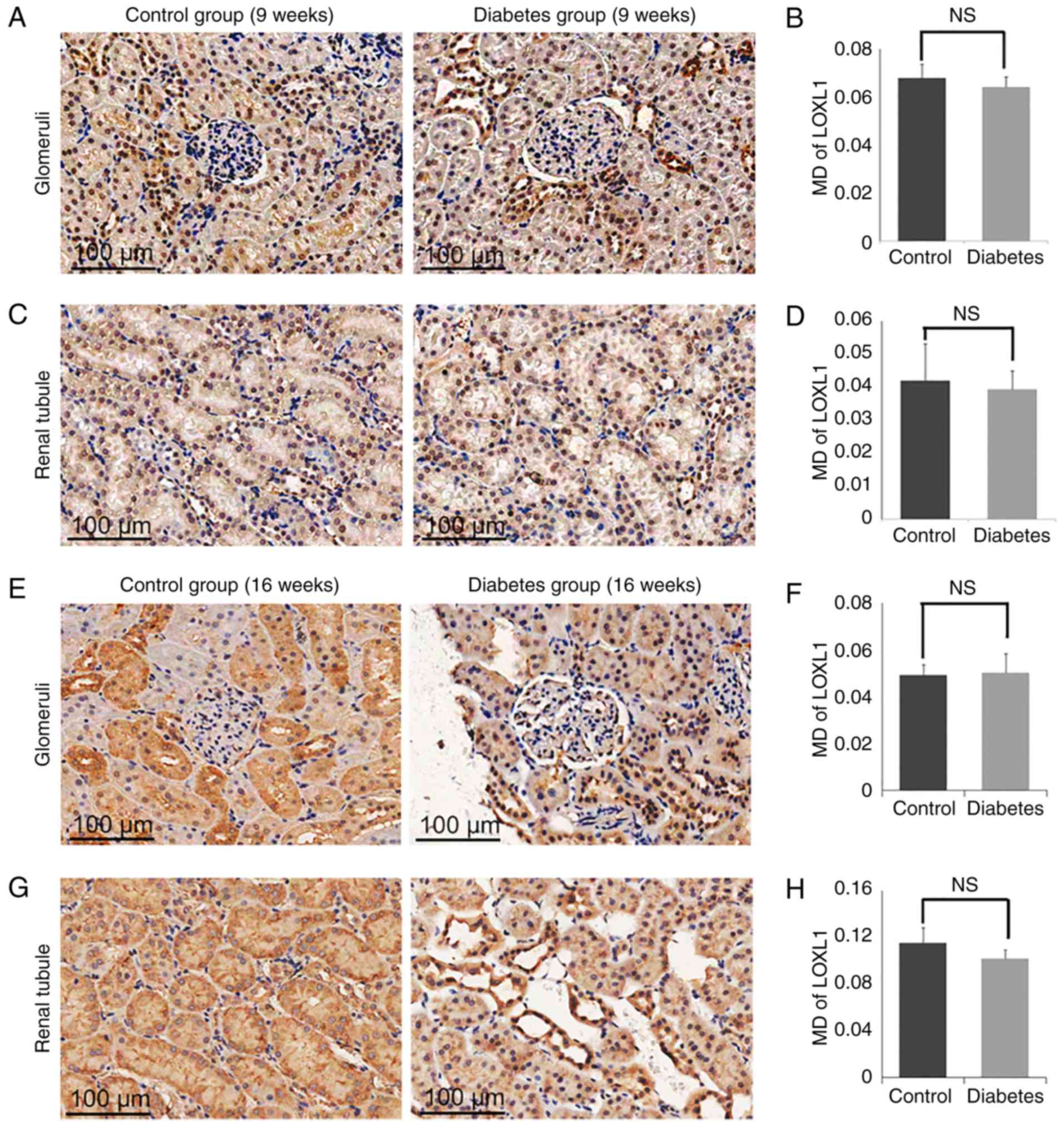
Expression of LOX and LOXL1-3 in
control and diabetes group
To determine the role of LOXs in the processes of
the DN, we measured the expression of LOX family members (LOX and
LOXL1-3) in the diabetic group and the control group (Fig. 5–8). By 16 weeks, the expression of LOX was
increased significantly in the glomerular area of the rats in the
diabetic group (MD=0.1135±0.0130), compared with the levels of rats
in the control group (MD=0.0747±0.0008; P<0.05; Fig. 5E and F). Similarly, the expression
of LOX in the tubular zones was also increased significantly in the
diabetes group (MD=0.1384±0.0024) compared to the control group
(MD=0.0953±0.0026; P<0.05; Fig. 5G
and H). The expression of LOXL2 was also increased markedly in
the glomerulus in the diabetes group (MD=0.0210±0.0042) compared to
the control group (MD=0.0033±0.0011; P<0.05; Fig. 6E and F), as well as in the tubular
zones MD=0.0689±0.0079 for the diabetic model compared to
MD=0.0360±0.0023 for the control group (P<0.05; Fig. 6G and H). However, there was no
significant difference in the levels of LOXL1 (Fig. 7) or LOXL3 (Fig. 8) between the diabetes and control
group. In addition, the expression of LOXs showed no significant
difference in 9 weeks groups (Figs.
5A-D, 6A-D, 7A-D and 8A-D).
Discussion
The accumulation of ECM is considered an indication
of pathological alterations in DN. This excessive accumulation may
lead to mesangial matrix expansion and thickening of GBM (34–36),
which signal subsequent progressive glomerular destruction. As the
leading causes of end-stage renal disease, the identification of
relevant biomarkers of early stages of DN is thus necessary. Our
study focuses on presenting LOXs involved in DN pathogenesis and
progression, with a hope to develop new biomarkers, especially to
classify and to broaden the therapeutic window for patients who are
at different stages of DN.
ZDF rats were chosen as model of type 2 diabetes as
they exhibit the pertinent clinical signs of DN including early
stage and disease progression. So we can better assess the effects
of diabetes with and without renal dysfunction, as well as with
concomitant obesity. Distinguish from traditional T2D model which
used STZ to produce diabetes, around the ages of 8–16 weeks, male
obese ZDF rats appear obese, insulin resistant, and progress to
non-insulin dependent diabetes. Consequently, ZDF rats develop
progressive DN causally linked to diabetes and show a
well-characterized animal model of DN (37,38).
Using type 2 diabetes model of male ZDF rats, we provided evidence
that diabetic rats display significant histologic changes,
including mesangial matrix expansion and GBM thickening. Of these
pathological events, the two histologic changes are considered the
earliest indicator of renal failure in the diabetic kidney. By
analysis of kidney photomicrograph images, we confirmed that the
rat model of diabetes reflects the early stage of DN.
Recent study has confirmed a key factor of heart
failure is adverse ECM remodeling, which is associated with LOX,
the change in LOX expression relates to alterations in cardiac
function and LOX inhibition could prevented the cardiac dysfunction
(39). Moreover, a previous study
found that HG-induced increased LOX expression is associated with
retinal endothelial cell excesspermeability (40). Therefore, our immunohistochemical
data suggests that increased LOX expression in the glomerulus
tissue of diabetic rats correlate with increased mesangial matrix
expansion and GBM thickening, which contributed to increased
glomerular filtration rate. In retinal capillary endothelial cells,
HG-induced up-regulation of LOX could improve stiffness of
subendothelial basement membrane (41), besides abnormity of the levels of
LOX could impair the function of the vascular endothelial barrier,
resulting in vascular endothelial dysfunction (42). Because each glomerulus consists of
a complex branching system of capillaries, our study suggests that
increased LOX expression may compromise capillary endothelial cells
and impair GBM functional integrity. We also found that LOX
expression was increased in the tubular compartment of kidneys from
diabetic rats. Most studies have examined tubulointerstitial
fibrosis in the advanced stages of DN, but the mechanisms of
tubular injury have not been thoroughly explored during the early
stage of DN. Therefore, we infer that increased LOX expression in
renal tubular epithelial cells may promote injury by excess ECM
accumulation. All the results in our current study indicate that
LOX should be considered in the development of DN.
Disorder of the ECM increases LOXL2 expression and
contributes to several pathological lesion such as invasive cancer
and fibrosis (43–45). In contrast, LOXL2 is increased in
invasive cancer and fibrosis as a consequence of abnormal signaling
pathways and inflammatory mediators leading to remodeling of ECM
(46), furthermore, our data
reveal that increased LOXL2 expression may be associated with renal
injury, such as glomeruluar damage, by increasing the levels of
ECM-modifying factors during the early stage of DN. Thus, we
speculate that LOXL2 could have role in the process of DN. Also
because LOXL2 mediates ECM remodeling by upregulation of the tissue
inhibitor of metalloproteinase-1 (TIMP-1) and matrix
metalloproteinase-9 (MMP-9) (47),
so we propose that the inhibition of LOXL2 may effectively delay
glomerular damage in DN, which needed to be studied deeply in
future.
LOXL1 appears to play a key role in glaucoma
(48,49) and may be also a critical factor for
cardiovascular complications (50–52).
In contrast, cleft palate and spinal deformities were observed in
the LOXL3 null mice (53) and
LOXL3 was likely associated with Stickler Syndrome (54) and high myopia (55) in human beings. But In our study we
found the expression of LOXL1and LOXL3 in diabetes groups was
similar to control groups as well, so we speculated that LOXL1 and
LOXL3 could have no role in the process of DN.
Previous studies suggested that LOXs are important
contributors to the pathogenesis of renal fibrosis (27,56).
Consistent with those results, increased LOXL2 expression was also
observed in renal biopsy tissues from patients with chronic renal
disease (27). However, to date,
nothing has been reported about the relationship of the expression
of LOXs and the histologic changes of early stages of DN. In the
present study, by using type 2 diabetes model of male ZDF rats in
the early stage of DN, our data indicated that the LOX and LOXL2
expression of the diabetic groups were significantly higher than
that of the control groups. This suggests that LOX and LOXL2 may be
a novel well-validated biomarker in early stage of DN. When used in
combination with conventional biomarkers, LOX and LOXL2 can improve
the level for diagnosing the stage of DN and accurately stratify DN
patients according to their disease stage, providing targeted
personalized treatment for them even.
Our report indicates LOX and LOXL2 increased
expression levels were observed in diabetic rat kidneys, so we
presume that the level of LOX and LOXL2 may be required for the
stability of renal tubular epithelial cells, glomerular capillary
epithelial cells, and mesangial cells during early DN. And we
speculate that there is a balance between ECM formation and the
level of expression of the LOXs, and factors that disrupt this
balance can lead to mesangial expansion, GBM thickening, or even
renal tubule injury. However, the molecular events are likely
complex and require further study.
In conclusion, we establish a link between LOX,
LOXL2 and the early stage of DN. This suggests that LOX and LOX2
may be helpful to accurately classify disease stage of DN patients
and design treatment strategies accordingly, as novel potential
therapeutic targets and biomarker for DN. Further study is needed
to determine if the pharmacological inhibition of LOXs is feasible
in clinical practice to slow the progression of DN.
Acknowledgements
This study was supported by medical pathological
laboratory of the Core Facility of West China Hospital, and by the
State Key Laboratory of Oral Diseases of West China College of
Stomatology. We would like to thank Dr Lin Yang and Professor Yi
Zhang for their kind support for experimental pathological
technique and pathological image analysis. We thank Mr. Rachel
Mooney for her assistance with the manuscript. This study was
supported by the National Natural Science Foundation of China
(grant nos. 81400523 and 81570987); the Sichuan provincial Science
and Technology Foundation (grant no. 2016FZ0074); and the Sichuan
University Foundation for Young Teacher (grant no.
2082604194255).
References
|
1
|
Fineberg D, Jandeleit-Dahm KA and Cooper
ME: Diabetic nephropathy: Diagnosis and treatment. Nat Rev
Endocrinol. 9:713–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bloomgarden Z: Questioning glucose
measurements used in the International Diabetes Federation (IDF)
Atlas. J Diabetes. 8:746–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mendis S, Davis S and Norrving B:
Organizational update: The world health organization global status
report on noncommunicable diseases 2014; one more landmark step in
the combat against stroke and vascular disease. Stroke.
46:e121–e122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenner BM, Cooper ME, de Zeeuw D, Keane
WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z and
Shahinfar S; RENAAL Study Investigators, : Effects of losartan on
renal and cardiovascular outcomes in patients with type 2 diabetes
and nephropathy. N Engl J Med. 345:861–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haller H, Ito S, Izzo JL Jr, Januszewicz
A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM,
et al: Olmesartan for the delay or prevention of microalbuminuria
in type 2 diabetes. N Engl J Med. 364:907–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jermendy G and Ruggenenti P: Preventing
microalbuminuria in patients with type 2 diabetes. Diabetes Metab
Res Rev. 23:100–110. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dugbartey GJ: Diabetic nephropathy: A
potential savior with ‘rotten-egg’ smell. Pharmacol Rep.
69:331–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fioretto P, Steffes MW and Mauer M:
Glomerular structure in nonproteinuric IDDM patients with various
levels of albuminuria. Diabetes. 43:1358–1364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perkins BA, Ficociello LH, Roshan B,
Warram JH and Krolewski AS: In patients with type 1 diabetes and
new-onset microalbuminuria the development of advanced chronic
kidney disease may not require progression to proteinuria. Kidney
Int. 77:57–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flyvbjerg A: The role of the complement
system in diabetic nephropathy. Nat Rev Nephrol. 13:311–318. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan Y, Lee K, Wang N and He JC: The role
of endoplasmic reticulum stress in diabetic nephropathy. Curr Diab
Rep. 17:172017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schena FP and Gesualdo L: Pathogenetic
mechanisms of diabetic nephropathy. J Am Soc Nephrol. 16 Suppl
1:S30–S33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fioretto P and Mauer M: Diabetic
nephropathy: Diabetic nephropathy-challenges in pathologic
classification. Nat Rev Nephrol. 6:508–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagenseil JE and Mecham RP: Vascular
extracellular matrix and arterial mechanics. Physiol Rev.
89:957–989. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van der Slot-Verhoeven AJ, van Dura EA,
Attema J, Blauw B, Degroot J, Huizinga TW, Zuurmond AM and Bank RA:
The type of collagen cross-link determines the reversibility of
experimental skin fibrosis. Biochim Biophys Acta. 1740:60–67. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aeschlimann D and Thomazy V: Protein
crosslinking in assembly and remodelling of extracellular matrices:
The role of transglutaminases. Connect Tissue Res. 41:1–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dimas GG, Didangelos TP and Grekas DM:
Matrix gelatinases in atherosclerosis and diabetic nephropathy:
Progress and challenges. Curr Vasc Pharmacol. 15:557–565. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Csiszar K: Lysyl oxidases: A novel
multifunctional amine oxidase family. Prog Nucleic Acid Res Mol
Biol. 70:1–32. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kagan HM and Li W: Lysyl oxidase:
Properties, specificity, and biological roles inside and outside of
the cell. J Cell Biochem. 88:660–672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kiemer AK, Takeuchi K and Quinlan MP:
Identification of genes involved in epithelial-mesenchymal
transition and tumor progression. Oncogene. 20:6679–6688. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mäki JM, Tikkanen H and Kivirikko KI:
Cloning and characterization of a fifth human lysyl oxidase
isoenzyme: The third member of the lysyl oxidase-related subfamily
with four scavenger receptor cysteine-rich domains. Matrix Biol.
20:493–496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li RK, Zhao WY, Fang F, Zhuang C, Zhang
XX, Yang XM, Jiang SH, Kong FZ, Tu L, Zhang WM, et al: Lysyl
oxidase-like 4 (LOXL4) promotes proliferation and metastasis of
gastric cancer via FAK/Src pathway. J Cancer Res Clin Oncol.
141:269–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu G, Guo Z, Chang X, Kim MS, Nagpal JK,
Liu J, Maki JM, Kivirikko KI, Ethier SP, Trink B and Sidransky D:
LOXL1 and LOXL4 are epigenetically silenced and can inhibit
ras/extracellular signal-regulated kinase signaling pathway in
human bladder cancer. Cancer Res. 67:4123–4129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang WP, Sima ZH, Wang HC, Zhang JY, Sun
LS, Chen F and Li TJ: Identification of the involvement of LOXL4 in
generation of keratocystic odontogenic tumors by RNA-Seq analysis.
Int J Oral Sci. 6:31–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weise JB, Rudolph P, Heiser A, Kruse ML,
Hedderich J, Cordes C, Hoffmann M, Brant O, Ambrosch P, Csiszar K
and Görögh T: LOXL4 is a selectively expressed candidate diagnostic
antigen in head and neck cancer. Eur J Cancer. 44:1323–1331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goto Y, Uchio-Yamada K, Anan S, Yamamoto
Y, Ogura A and Manabe N: Transforming growth factor-beta1 mediated
up-regulation of lysyl oxidase in the kidneys of hereditary
nephrotic mouse with chronic renal fibrosis. Virchows Arch.
447:859–868. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higgins DF, Kimura K, Bernhardt WM,
Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler
M, Cohen CD, et al: Hypoxia promotes fibrogenesis in vivo via HIF-1
stimulation of epithelial-to-mesenchymal transition. J Clin Invest.
117:3810–3820. 2007.PubMed/NCBI
|
|
28
|
Magri CJ and Fava S: The role of tubular
injury in diabetic nephropathy. Eur J Intern Med. 20:551–555. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reed MJ, Meszaros K, Entes LJ, Claypool
MD, Pinkett JG, Gadbois TM and Reaven GM: A new rat model of type 2
diabetes: The fat-fed, streptozotocin-treated rat. Metabolism.
49:1390–1394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tervaert TW, Mooyaart AL, Amann K, Cohen
AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer
E, et al: Pathologic classification of diabetic nephropathy. J Am
Soc Nephrol. 21:556–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Giunti S, Calkin AC, Forbes JM, Allen TJ,
Thomas MC, Cooper ME and Jandeleit-Dahm KA: The pleiotropic actions
of rosuvastatin confer renal benefits in the diabetic Apo-E
knockout mouse. Am J Physiol Renal Physiol. 299:F528–F535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim J, Jang HS and Park KM: Reactive
oxygen species generated by renal ischemia and reperfusion trigger
protection against subsequent renal ischemia and reperfusion injury
in mice. Am J Physiol Renal Physiol. 298:F158–F166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Racusen LC, Solez K, Colvin RB, Bonsib SM,
Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo
AB, et al: The Banff 97 working classification of renal allograft
pathology. Kidney Int. 55:713–723. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bangstad HJ, Osterby R, Hartmann A, Berg
TJ and Hanssen KF: Severity of glomerulopathy predicts long-term
urinary albumin excretion rate in patients with type 1 diabetes and
microalbuminuria. Diabetes Care. 22:314–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drummond K and Mauer M; International
Diabetic Nephropathy Study Group, : The early natural history of
nephropathy in type 1 diabetes: II. Early renal structural changes
in type 1 diabetes. Diabetes. 51:1580–1587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsumae T, Jimi S, Uesugi N, Takebayashi
S and Naito S: Clinical and morphometrical interrelationships in
patients with overt nephropathy induced by non-insulin-dependent
diabetes mellitus. A light- and electron-microscopy study. Nephron.
81:41–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Griffen SC, Wang J and German MS: A
genetic defect in beta-cell gene expression segregates
independently from the fa locus in the ZDF rat. Diabetes. 50:63–68.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hempe J, Elvert R, Schmidts HL, Kramer W
and Herling AW: Appropriateness of the Zucker Diabetic Fatty rat as
a model for diabetic microvascular late complications. Lab Anim.
46:32–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El Hajj EC, El Hajj MC, Ninh VK, Bradley
JM, Claudino MA and Gardner JD: Detrimental role of lysyl oxidase
in cardiac remodeling. J Mol Cell Cardiol. 109:17–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chronopoulos A, Tang A, Beglova E,
Trackman PC and Roy S: High glucose increases lysyl oxidase
expression and activity in retinal endothelial cells: Mechanism for
compromised extracellular matrix barrier function. Diabetes.
59:3159–3166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang X, Scott HA, Monickaraj F, Xu J,
Ardekani S, Nitta CF, Cabrera A, McGuire PG, Mohideen U, Das A and
Ghosh K: Basement membrane stiffening promotes retinal endothelial
activation associated with diabetes. FASEB J. 30:601–611. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Raposo B, Rodríguez C, Martínez-González J
and Badimon L: High levels of homocysteine inhibit lysyl oxidase
(LOX) and downregulate LOX expression in vascular endothelial
cells. Atherosclerosis. 177:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akiri G, Sabo E, Dafni H, Vadasz Z,
Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M
and Neufeld G: Lysyl oxidase-related protein-1 promotes tumor
fibrosis and tumor progression in vivo. Cancer Res. 63:1657–1666.
2003.PubMed/NCBI
|
|
44
|
Barker HE, Cox TR and Erler JT: The
rationale for targeting the LOX family in cancer. Nat Rev Cancer.
12:540–552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bonnans C, Chou J and Werb Z: Remodelling
the extracellular matrix in development and disease. Nat Rev Mol
Cell Biol. 15:786–801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Torres S, Garcia-Palmero I, Herrera M,
Bartolomé RA, Peña C, Fernandez-Aceñero MJ, Padilla G,
Peláez-García A, Lopez-Lucendo M, Rodriguez-Merlo R, et al: LOXL2
is highly expressed in cancer-associated fibroblasts and associates
to poor colon cancer survival. Clin Cancer Res. 21:4892–4902. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barker HE, Chang J, Cox TR, Lang G, Bird
D, Nicolau M, Evans HR, Gartland A and Erler JT: LOXL2-mediated
matrix remodeling in metastasis and mammary gland involution.
Cancer Res. 71:1561–1572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schlötzer-Schrehardt U, Hammer CM, Krysta
AW, Hofmann-Rummelt C, Pasutto F, Sasaki T, Kruse FE and Zenkel M:
LOXL1 deficiency in the lamina cribrosa as candidate susceptibility
factor for a pseudoexfoliation-specific risk of glaucoma.
Ophthalmology. 119:1832–1843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Braunsmann C, Hammer CM, Rheinlaender J,
Kruse FE, Schäffer TE and Schlötzer-Schrehardt U: Evaluation of
lamina cribrosa and peripapillary sclera stiffness in
pseudoexfoliation and normal eyes by atomic force microscopy.
Invest Ophthalmol Vis Sci. 53:2960–2967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schumacher S, Schlötzer-Schrehardt U,
Martus P, Lang W and Naumann GO: Pseudoexfoliation syndrome and
aneurysms of the abdominal aorta. Lancet. 357:359–360. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
French DD, Margo CE and Harman LE: Ocular
pseudoexfoliation and cardiovascular disease: A national
cross-section comparison study. N Am J Med Sci. 4:468–473. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang W, He M, Zhou M and Zhang X: Ocular
pseudoexfoliation syndrome and vascular disease: A systematic
review and meta-analysis. PLoS One. 9:e927672014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Yang R, Liu Z, Hou C, Zong W,
Zhang A, Sun X and Gao J: Loss of lysyl oxidase-like 3 causes cleft
palate and spinal deformity in mice. Hum Mol Genet. 24:6174–6185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Alzahrani F, Al Hazzaa SA, Tayeb H and
Alkuraya FS: LOXL3, encoding lysyl oxidase-like 3, is mutated in a
family with autosomal recessive Stickler syndrome. Hum Genet.
134:451–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li J, Gao B, Xiao X, Li S, Jia X, Sun W,
Guo X and Zhang Q: Exome sequencing identified null mutations in
LOXL3 associated with early-onset high myopia. Mol Vis. 22:161–167.
2016.PubMed/NCBI
|
|
56
|
Haase VH: Pathophysiological consequences
of HIF activation: HIF as a modulator of fibrosis. Ann N Y Acad
Sci. 1177:57–65. 2009. View Article : Google Scholar : PubMed/NCBI
|