Introduction
Tyrosine-protein phosphatase non-receptor type 11
(Shp2) is a ubiquitously expressed intracellular enzyme which has
critical cellular functions in a variety of processes, including
survival, proliferation and differentiation (1,2);
however, systemic Shp2 loss has been associated with embryonic
lethality (3).
Shp2 has been demonstrated to regulate numerous
signaling events, among which the mitogen-activated protein kinase
(MAPK) signaling pathway was identified to be commonly facilitated
by Shp2. Mutations in the protein tyrosine phosphatase,
non-receptor type 11 (PTPN11) gene, which encodes Shp2 protein,
have been reported to inhibit MAPK signaling (4,5). Due
to its pivotal role, investigations into the effects exhibited by
Shp2 have been conducted using Shp2 conditional knockout (cKO)
mouse models in a variety of neural tissues and cell types using
distinct Cre recombinase drivers. These models revealed that Shp2
may have roles within numerous areas and cell types during brain
developmental processes, including forebrain neuron development
(6), cerebellum foliation
(7) and oligodendrocyte generation
(8). Radial glia are neural
progenitors with long radial processes, which serve key roles in
the development of the central nervous system. The properties of
radial glia were identified due to similarities with astrocytes,
which contain glycogen granules and express glial fibrillary acidic
protein (GFAP). In addition, radial glia have been considered to
support the migration of nascent neurons (9); however, in the previous decade,
investigations have focused on the progenitor capacity of radial
glia within the cerebral cortex (10,11)
and cerebellum (11,12).
In the present study, transgenic mice with hGFAP-Cre
under the control of a glial-specific promoter were used to ablate
Shp2 expression within radial glia (13,14).
In contrast to the GFAP-Cre system, which is commonly used to
generate astrocyte-specific gene knockout model, the hGFAP-Cre
recombinase is expressed within cerebral cortical radial glia at
E13.5-E14 and cerebellar radial glia at E13.5-E16.5 (15,16).
Shp2 knockout within radial glia was associated with postnatal
growth failure, cerebral cortical dysplasia with decreased
extracellular signal-regulated kinase (ERK) signaling, glial
defects of cerebellum and impaired sensory-motor functions.
Collectively, the findings of the present study revealed the
critical function of Shp2 in radial glia that contributes to
cerebral cortical and cerebellar development in newborn mice.
Materials and methods
Mice
A total of 290 newborn mice were used in this study.
The newborn mice as well as their parents had continuous access to
food and water and were housed in cages in a room maintained at a
temperature of 20–22°C with a 12-h light/dark cycle. The present
study was approved by the Ethics Committee the First Affiliated
Hospital, Zhejiang University School of Medicine (Hangzhou, China).
All mice received humane care, in accordance with the guide
prepared by the Committee of Care and Use of Laboratory Animals.
The hGFAP-Cre line and floxed Shp2 line were both purchased from
the Jackson Laboratory (Ben Harbor, ME, USA). hGFAP-Cre line
(17) were interbred with the
floxed Shp2 line (18) to generate
Shp2f/f; hGAFP Cre/+ mice, in which the Shp2 gene was
conditionally knocked out (Shp2 CKO).
Western blot analysis
Whole brain and microdissected cortices lysates
obtained from 14-day-old male newborn mice were extracted with
radioimmunoprecipitation assay buffer (Biyuntian Biotechnology Co.,
Ltd., Haimen, China) containing 1 mM phenylmethylsulfonyl fluoride
(Haoxin Biotechnology, Hangzhou, China) (http://www.hzhxbio.com). Protein concentration was
measured using a bicinchoninic acid protein assay kit (CW Biotech,
Beijing, China). A total of 40 µg/lane protein was separated by 10%
SDS-PAGE, transferred onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA), and then incubated with 10% bovine serum
albumin solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at
37°C for 1 h. Blots were probed with antibodies specific for Shp2
(sc-7384, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), phosphorylated ERK (pERK, 4370S, 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), total ERK (tERK, 9102S,
1:1,000), pAKT serine/threonine kinase (pAKT, 4060S, 1:2,000) and
total AKT (tAKT, 4685S, 1:1,000) (all from Cell Signaling
Technology, Inc.) and β-tubulin (sc-365791, 1:500; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Membranes were then probed
with goat anti-rabbit 800 (SA5-35571, 1:10,000; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) or anti-mouse antibodies
(A-11001, 1:5,000; Invitrogen; Thermo Fisher Scientific, Inc.) for
2 h at room temperature. Immunoreactive bands were visualized using
a two-color infrared imaging system (LI-COR Biosciences, Lincoln,
NE, USA).
Histology and
immunohistochemistry
Newborn mice were anesthetized with pentobarbital
sodium salt (Sigma-Aldrich; Merck KGaA) and transcardially perfused
with 0.9% NaCl, followed by 3 or 4% paraformaldehyde/PBS. The
cerebrum and cerebellum were removed, fixed overnight with PBS (pH
7.2) containing 4% paraformaldehyde at 4°C and embedded in paraffin
wax. Samples were cut into 4 µm sections. Hematoxylin and eosin
(H&E) staining (Beyotime Institute of Biotechnology, Haimen,
China) and toluidine blue staining (Wuhan Goodbio Technology Co.,
Ltd., Wuhan, China) (http://www.servicebio.cn) were performed according to
the manufacturer's instructions. Following deparaffinization,
tissues were rehydrated with graded alcohol (100, 95 and 50%) and
blocked with goat serum (Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China) at 37°C for 15 min. Antigen retrieval was performed using
0.01 M citrate buffer (pH 6.0) at 100°C for 2 min, and then washed
with PBS. Permeablization was performed for 10 min with PBS
containing 0.1% Triton X-100 and then washed with PBS. Incubation
of primary and secondary antibodies for immunostaining was
performed according to standard protocols. The primary antibodies
were incubated overnight at 4°C using the following: Anti-GFAP
(HPA056030, 1:100; Sigma-Aldrich; Merck KGaA), anti-neuronal
specific nuclear protein (NeuN, ABN78, 1:200; EMD Millipore),
anti-Ki67 (ab15580, 1:100; Abcam, Cambridge, UK), anti-ERK (4696S,
1:100; Cell Signaling Technology, Inc.), anti-pERK (9101S, 1:250;
Cell Signaling Technology, Inc.), anti-AKT (4685S, 1:100; Cell
Signaling, MA, USA), anti-pAKT (4060S, 1:100; Cell Signaling
Technology, Inc.). Then incubation of secondary antibodies at room
temperature for 2 h was performed with Goat anti-rabbit 800
(SA5-35571, 1:100; Invitrogen; Thermo Fisher Scientific, Inc.) or
anti-mouse antibodies (A-11001, 1:100; Invitrogen; Thermo Fisher
Scientific, Inc.). Terminal deoxynucleotidyl-transferase-mediated
dUTP nick end labeling (TUNEL) staining was performed according to
the manufacturer's protocol (Roche Applied Science, Branford, CT,
USA). Images were collected with a laser confocal microscope
(Olympus IX71; Olympus Corporation, Tokyo Japan), or the Nikon
E600FN (Nikon Corporation, Tokyo, Japan) microscope and 5 randomly
selected fields of view were observed.
Cell counting was performed according to a
previously published method (19).
Quantification of cell numbers was performed using the ImageJ
software version 1.48 (National Institutes of Health, Bethesda, MD,
USA) by a blinded observer.
Newborn mice behavior analyses
Behavior of newborn mice aged 5–15 days was examined
as described in a recent study (20) with modifications. General health of
the newborns was evaluated by measuring body weight. For motor and
sensory reflex development evaluation, the righting reflex test,
sound attraction test, the wire hanging test and nest finding test
were performed as follows.
Righting reflex test
Newborn mice were placed on their backs and the
duration for righting itself on all four limbs was measured.
‘Success’ was defined as the duration of righting in <3 sec.
Sound attraction test
Each newborn was placed at the center of a round
platform with a radius of 15 cm with soft walls to provide
protection against edges. Auditory stimuli were administered by
gentle scratch to sandpaper and the response of the newborn was
measured. A score of 0 indicated no response, 0.5 was given when
the newborn turned his head to the direction of the sound and a
score of 1 was given when the newborn moved towards the sound on
three consecutive accounts.
Wire hanging test
The duration the newborn can hold on a vertical wire
before landing on a soft material was measured. The mean time was
recorded for three sequential times.
Nest finding test
The ability for the newborn to return to the nest
was measured by placing the newborn 2 cm from the nest and observed
for 60 sec. Mice behavior was scored 0 when no attempt to return to
the nest was observed. A score of 0.5 was given when the newborn
successfully made to the nest or moved in the correct direction
towards the nest at least once and a score of 1 was given when the
newborn successfully arrived the nest on three consecutive
accounts.
Statistical analysis
Statistical analyses were conducted using either
two-way analysis of variance with the Bonferroni's post hoc test or
the Student's t-test to compare groups. All experiments were
repeated ≤4 times. Data are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Deletion of Shp2 in radial glia leads
to postnatal growth failure and lethality in newborn mice
Shp2f/f mice were crossed with hGFAP-Cre
mice to produce litters of +/+ Shp2 wild-type (WT), -/+
heterozygous Shp2 KO and -/- Shp2 CKO mice. Successful deletion of
Shp2 in the radial glia was validated on both mRNA and protein
levels (data not shown). Retardation in postnatal growth and
reductions in body weight were observed in the Shp2 CKO group
compared with the WT mice in the first two weeks after birth;
alterations in postnatal weight of heterozygous mice were not
observed (Fig. 1A and B). The
results of the present study suggested that heterozygous Shp2 KO
was not associated with a change in postnatal weight. In addition,
postnatal lethality exhibited by Shp2 CKO mice appeared to
be associated with sex, Shp2 CKO male mice were affected more
severely compared with in Shp2 CKO female mice (Fig. 1C; average lifetimes: Male 19.36
days, female 21.63 days; P=0.042, Student's t-test).
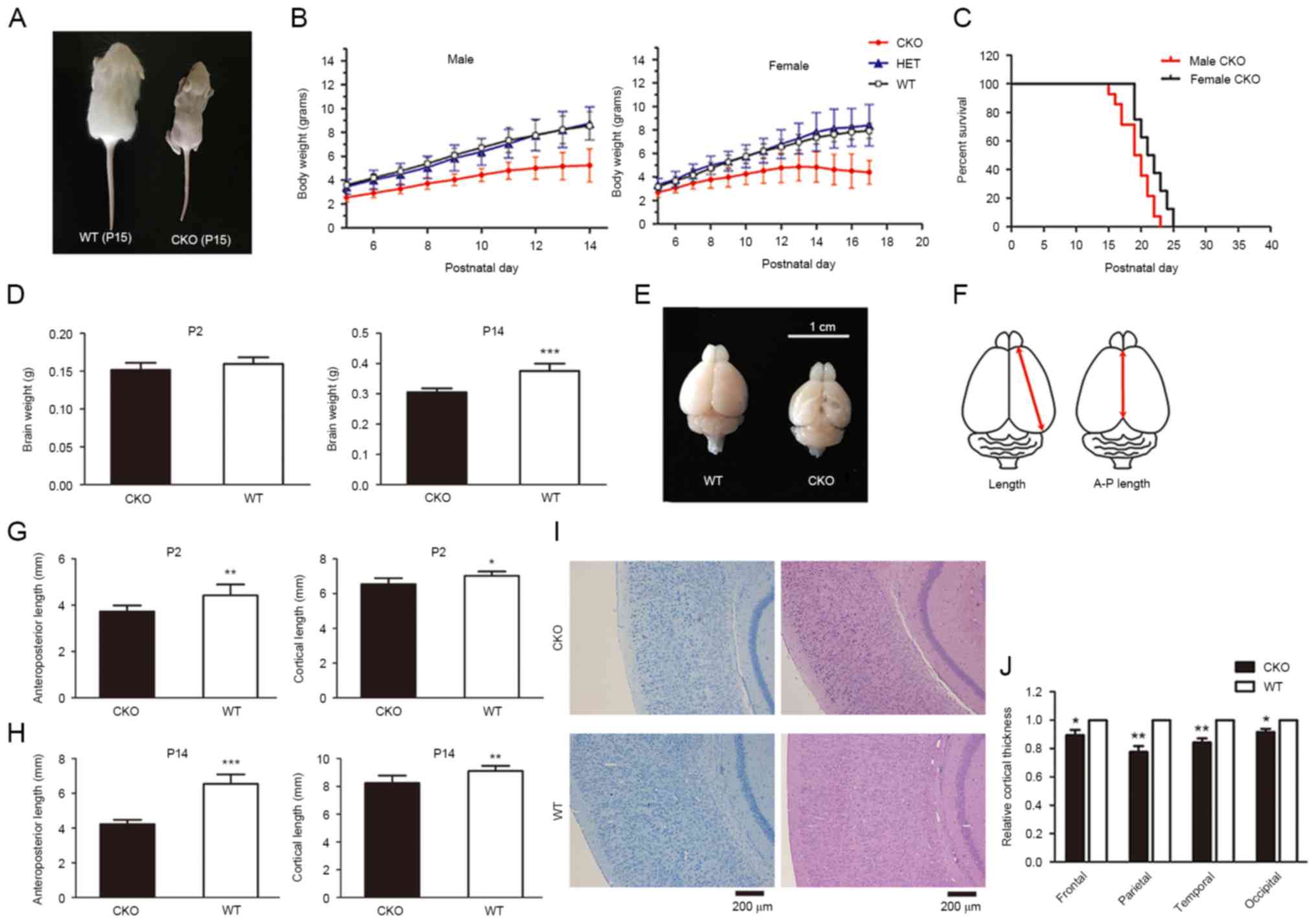 | Figure 1.Loss of Shp2 in radial glia leads to
postnatal growth retardation, defects in cerebral development and
lethality within newborn mice. (A) Gross appearance of a
representative pair of WT and CKO male littermates at P15. (B) Male
and female body weight growth of newborn mice. (C) Postnatal
lethality of Shp2 CKO male mice, mean survival days=19.4 and
median survival days=19.5. Female mice, mean survival days=21.6 and
median survival days=21.5. (D) Whole brain weights of Shp2 CKO and
Shp2 WT mice at P2 and P14. ***P<0.001 vs. CKO. (E) Whole brains
of Shp2 CKO and WT mice at P14. (F) Measurements of cortical
parameters. Shp2 CKO mice revealed a decrease in A-P length and
cortical length at (G) P2 and (H) P14 compared with in Shp2 WT
mice. *P<0.05, **P<0.01, ***P<0.001 vs. CKO. (I)
Hematoxylin and eosin and toluidine blue staining of coronal
sections of Shp2 CKO and WT brains at P14. Scale bar, 200 µm. (J)
Quantitative analysis of cortical thickness. P<0.05,
**P<0.01. Data are expressed as the mean ± standard deviation.
Panel B, n=4; panel C, n=14; panel D, n=6; panel E, n=6; panel F,
P=6; panel J, n=5. A-P length, anteroposterior length; CKO,
conditional knockout; Shp2, tyrosine-protein phosphatase
non-receptor type 11; WT, wild-type; HET, heterozygous. |
Shp2 CKO mice exhibit cerebral
cortical development defects
A previous study in which Erk2 was conditionally
deleted in radial glia resulted in smaller brain size and defective
cortical cytoarchitecture (19).
As Shp2 is an important modulator of the MAPK signaling (21), the effects of Shp2 knockout may be
associated to the effects observed with in Erk2 CKO mice. The total
brain weight was similar in Shp2 CKO group and the WT group at P2,
but was significantly lower in Shp2 CKO group compared with WT mice
at P14 (Fig. 1D). Shp2 CKO mice
also exhibited a decrease in brain size (Fig. 1E), mainly caused by significant
decrease in cortical area measured by anteroposterior length and
cortical length compared with WT at P2 and P14 (Fig. 1F-H). Further analysis of cortical
thickness within the Shp2 CKO mice indicated a significant
reduction in cortical thickness (Fig.
1I) compared with in the Shp2 WT group. In addition, a
reduction in thickness within primary motor, somatosensory,
auditory and visual cortices was observed in CKO mice compared with
WT (Fig. 1J). The results of the
present study indicate that deletion of Shp2 in radial glia led to
defective cortical development.
Loss of Shp2 within radial glia leads
to altered cellular composition of the cortex and impaired
corticogenesis
To investigate the cellular basis for the reduction
in cortical thickness within Shp2 CKO mice, the total number of
DAPI+ and NeuN+ cells per cortical region
were quantified. The results revealed a >40% reduction in total
cell density within the Shp2 CKO cortex (Fig. 2A and B). In addition, a significant
decrease, >60%, of NeuN+ cells per cortical region
and a >2-fold increase in non-neuronal cells was detected in
Shp2 CKO mice compared with WT mice (Fig. 2C and D). The results of the present
study indicated that reduced cortical thickness due to Shp2
deletion within radial glia was associated with the generation of
fewer neurons and more non-neuronal cells. Immunohistochemistry
analysis of glial cell markers led to the identification of
non-neuronal cells; a marked increase in GFAP+ glial
cells was observed throughout the cortex in CKO mice compared with
WT mice (Fig. 2E and F). A
previous study reported that radial glia may regulate gliogenesis
and neurogenesis within the mammalian cerebral cortex (11); the present study suggested that
Shp2 may serve a role in radial glial cell differentiation within
the process of gliogenesis, but inhibits neurogenesis.
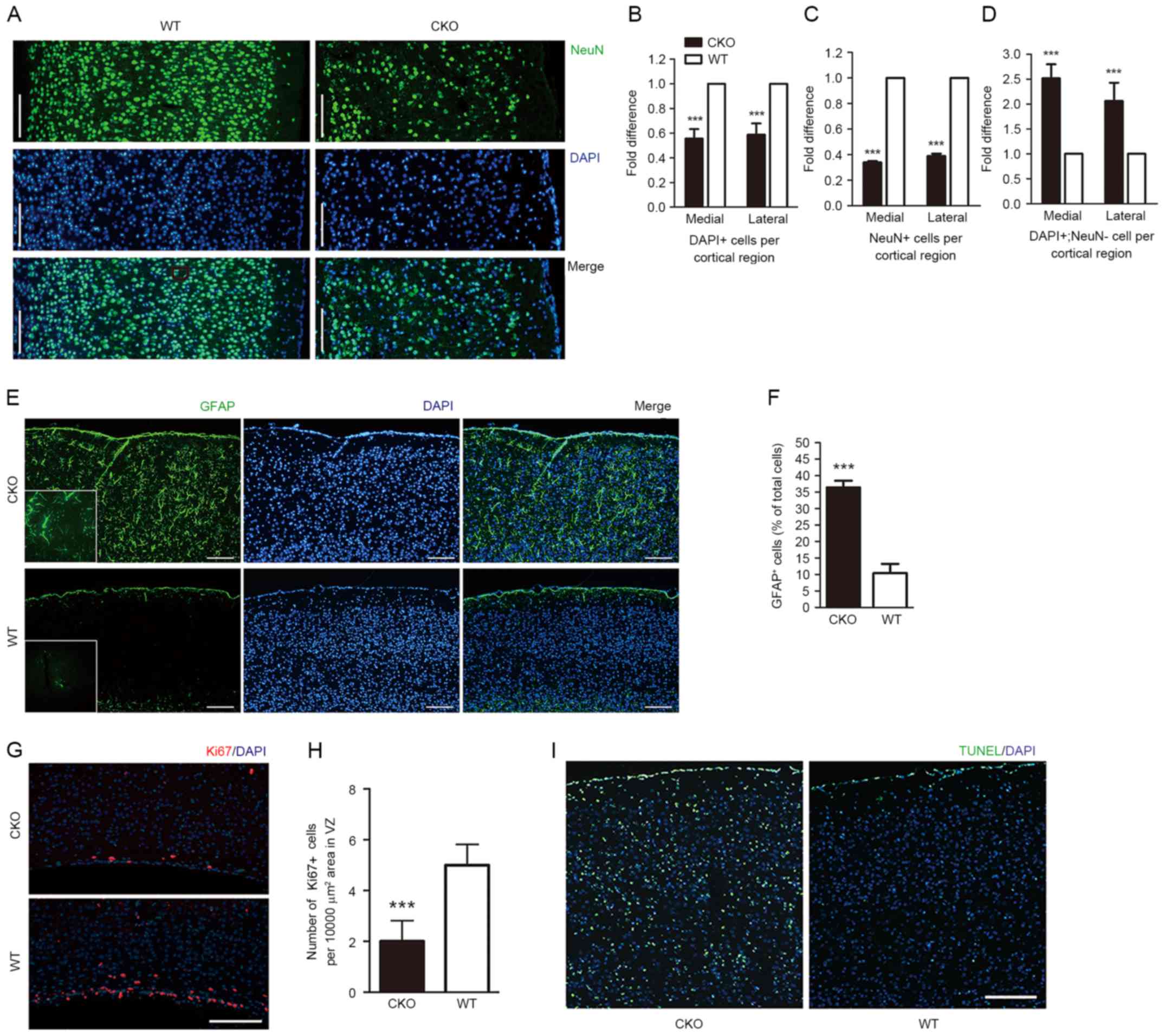 | Figure 2.Loss of Shp2 in radial glia leads to
altered cellular composition of the cortex, impaired corticogenesis
and decreased cortical ERK signaling. (A) Coronal sections of Shp2
CKO and Shp2 WT cortices at P14 were immunostained with anti-NeuN
and counterstained with DAPI. Total cell number per cortical region
from the VZ to the pial surface was calculated by counting (B)
DAPI+ and (C) NeuN+ cells in the primary
motor cortex and the primary somatosensory cortex, presented as the
medial and lateral sections, respectively. (D) Fold difference of
DAPI+ and NeuN+ cell in the primary motor
cortex and primary somatosensory cortex, presented as the medial
and lateral sections. ***P<0.001 vs. WT. Scale bar, 200 µm. (E)
Coronal sections of cortices from Shp2 CKO and Shp2 WT mice at P14
were immunostained with GFAP and counterstained with DAPI. (F)
Corresponding total GFAP+ cell number from the VZ to the
pial surface. ***P<0.001. Scale bar, 200 µm. (G) Immunostaining
revealed Ki67+ cells within the VZ tissue of Shp2 CKO
and Shp2 WT mice tissue. (H) Compared with in the Shp2 WT group,
the number of Ki67+ cells were significantly reduced.
***P<0.001 vs. WT. (I) Cell apoptosis was analyzed with a TUNEL
assay at P14. Scale bar, 200 µm. CKO, conditional knockout; WT,
wild-type; NeuN, neuronal specific nuclear protein; GFAP, glial
fibrillary acidic protein; Shp2, tyrosine-protein phosphatase
non-receptor type 11; VZ, ventricular zone; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labelling. |
The proliferation marker Ki67 in the ventricular
zone (VZ) of mice was analyzed at P4 in the present study. A
significant reduction of Ki67+ cells was observed in the
Shp2 CKO group compared with in the Shp2 WT group (Fig. 2G and H), indicating that neural
stem cell proliferation in the cerebral cortex was impaired within
Shp2 CKO mice. Cell survival was also evaluated within Shp2 CKO
cortex tissue via TUNEL analysis. An increase in apoptosis was
observed at P14 within the Shp2 CKO mice compared with WT (Fig. 2I). The findings of the present
study indicated that the reduction in cell density exhibited within
the Shp2 CKO group may be due to a decrease in cell proliferation
and an increase in cell apoptosis.
Erk activity is altered in the Shp2
CKO newborn mice
Shp2 has been previously demonstrated to be an
important factor involved in a variety of signaling cascades, among
which ERK and AKT are two effector molecules investigated in
studies where Shp2 is dysregulated (21). Phenotypic alterations within the
cerebral cortex of Shp2 CKO mice were similar to those described in
a previous study of mice with defective ERK signaling within the
radial glia (19). Analyses of
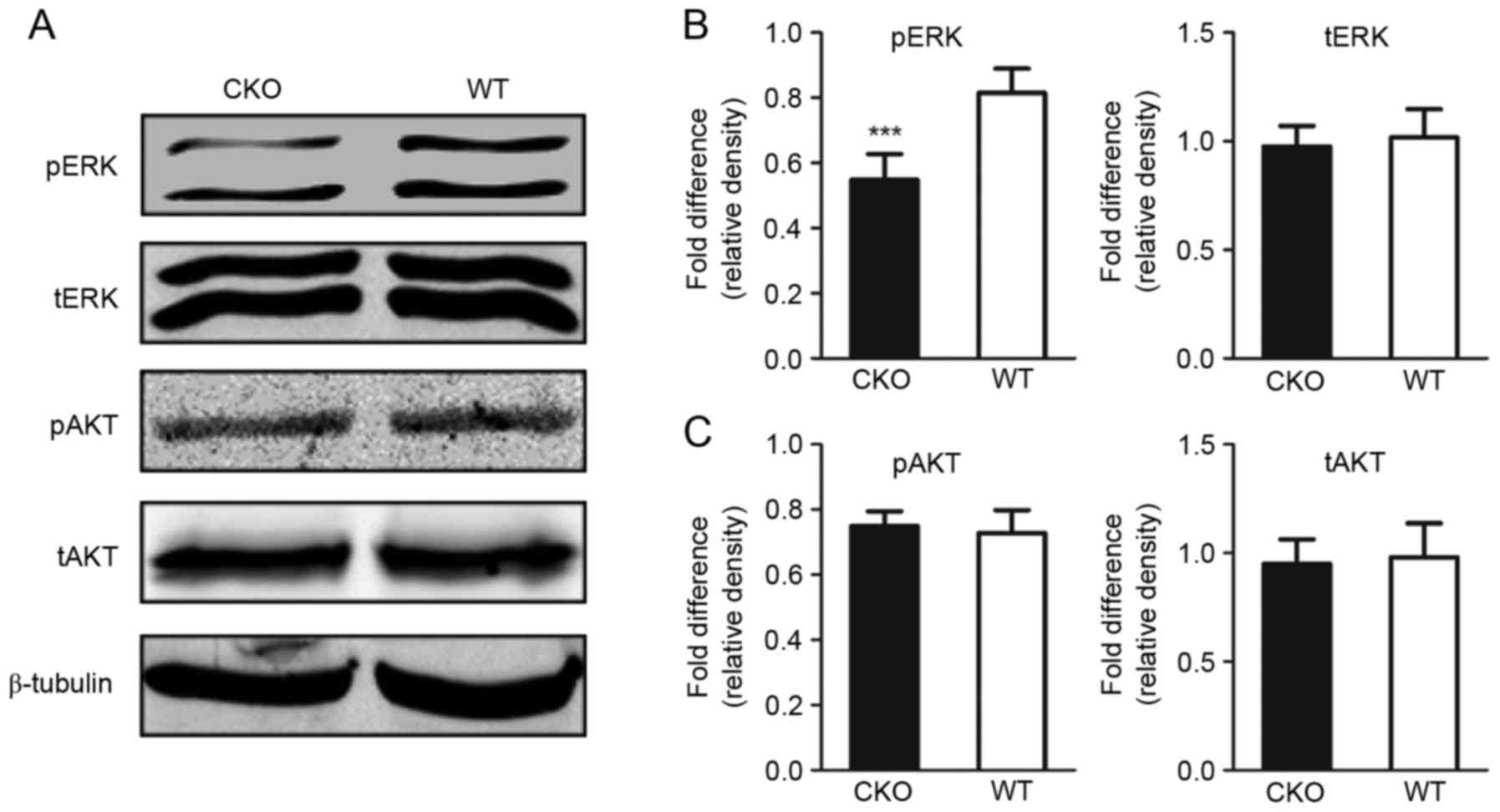
tERK, pERK, tAKT and pAKT expression levels revealed a decrease in
pERK levels within the cerebral cortex of Shp2 CKO mice compared
with in Shp2 WT mice; however, significant alterations in tERK,
tAKT and pAKT levels were not observed (Fig. 3A-C). These results suggested that
the ERK signaling pathway within the cerebral cortex is affected by
Shp2 KO in radial glia.
Deletion of Shp2 in radial glia leads
to glial defects in cerebellum
Radial glia possess the ability to generate glial
and neuronal cells within the cerebral cortex. A previous study
demonstrated that the majority of radial glia bypass neurogenesis
and retain the glial cell phenotype within the cerebellum (22), similar to the gross appearance of
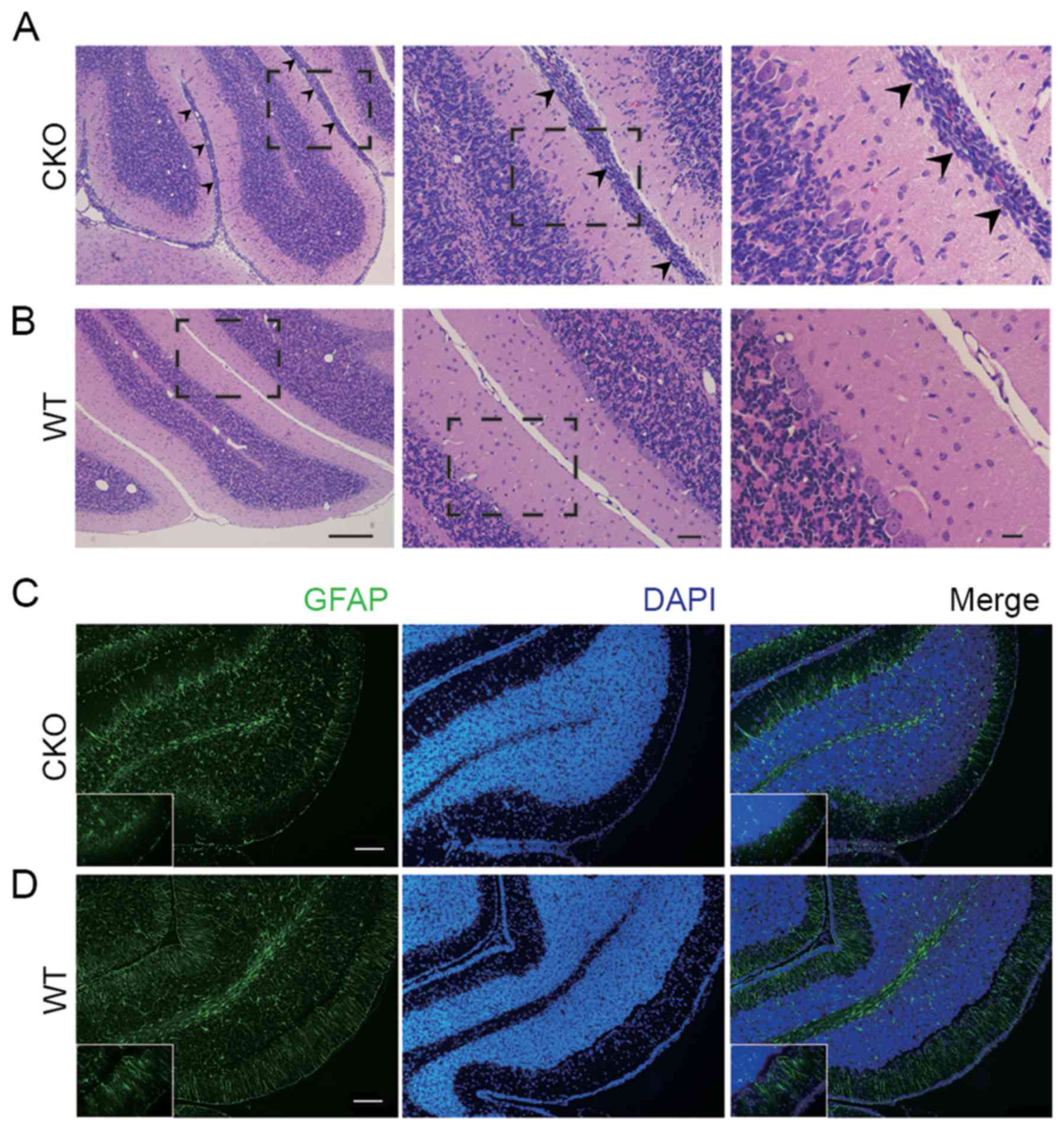
cerebellum tissue of the Shp2 CKO mice (Fig. 1E). However, histological analysis
by H&E staining of cerebellar sections indicated that at P14 in
the Shp2 CKO cerebellum there were abnormal cell clusters at the
pial surface, which is the site of the neonatal transient external
granular layer (EGL), while in WT this outer region was not present
(Fig. 4A and B). Furthermore,
immunohistochemical analysis of cerebellar sections fromP14 mice
showed aberrant alignment of the glial cells in the EGL of Shp2 CKO
mice (Fig. 4C and D). As radial
glia-derived glial cells in the EGL have critical functions in
development of cerebellum by mediating granular cell migration from
EGL to the internal granular layer (IGL). These results suggest
that Shp2 deficiency in radial glia resulted in defective glial
cells in the EGL, which led to failed migration of granular cells
and abnormal retention of cell clusters. Together, these data
suggest that expression of Shp2 in the radial glia has a pivotal
role in regulating cerebellar development.
Deletion of Shp2 in radial glia is
associated with impaired sensory-motor development
The emergence of coordinated sensory-motor behaviors
are dependent upon the establishment of distinct connections
between the discrete populations of functioning neurons within the
cortex and cerebellum (23,24).
Patients with mutations in the PTPN11 gene associated with Noonan
syndrome exhibit numerous sensory and motor disorders, including
hearing loss, muscle hypotonia, motor development delay and
learning disabilities (25). Shp2
CKO mice exhibited marked ataxia at P14. As cerebral cortical and
cerebellar development was observed to be severely affected by Shp2
loss, the effect of Shp2 CKO for sensory-motor function development
of newborn mice was investigated. The nest finding test was applied
to evaluate the comprehensive ability of newborn mice to identify
and return to the nest using motor skills and motivation. The
results of the present study demonstrated a decrease in nest
finding scores in the Shp2 CKO group compared with in the WT and
heterozygous groups from P7 in both sexes; however, all mice
achieved maximal scores at postnatal day 14 (Fig. 5A and B). The capability of newborn
mice to react to auditory stimulus was investigated ~2 days after
the nest finding test; development of this ability within Shp2 CKO
mice of both sexes were delayed compared with in the Shp2 WT and
Shp2 heterozygous mice groups from P9; the marked variation
remained evident at P14 (Fig. 5C and
D). Muscle strength of the newborn mice was assessed via the
wire hanging and righting reflex tests. In both sexes, Shp2 CKO
mice demonstrated a delayed acquisition of righting ability from P9
(Fig. 5E and F) and a significant
reduction in wire hanging duration from P11 (Fig. 5G and H) compared with in the WT and
heterozygous groups. The results of the present study suggested
that Shp2 expression in radial glial is involved in the development
of somatosensory function and motor function in newborn mice.
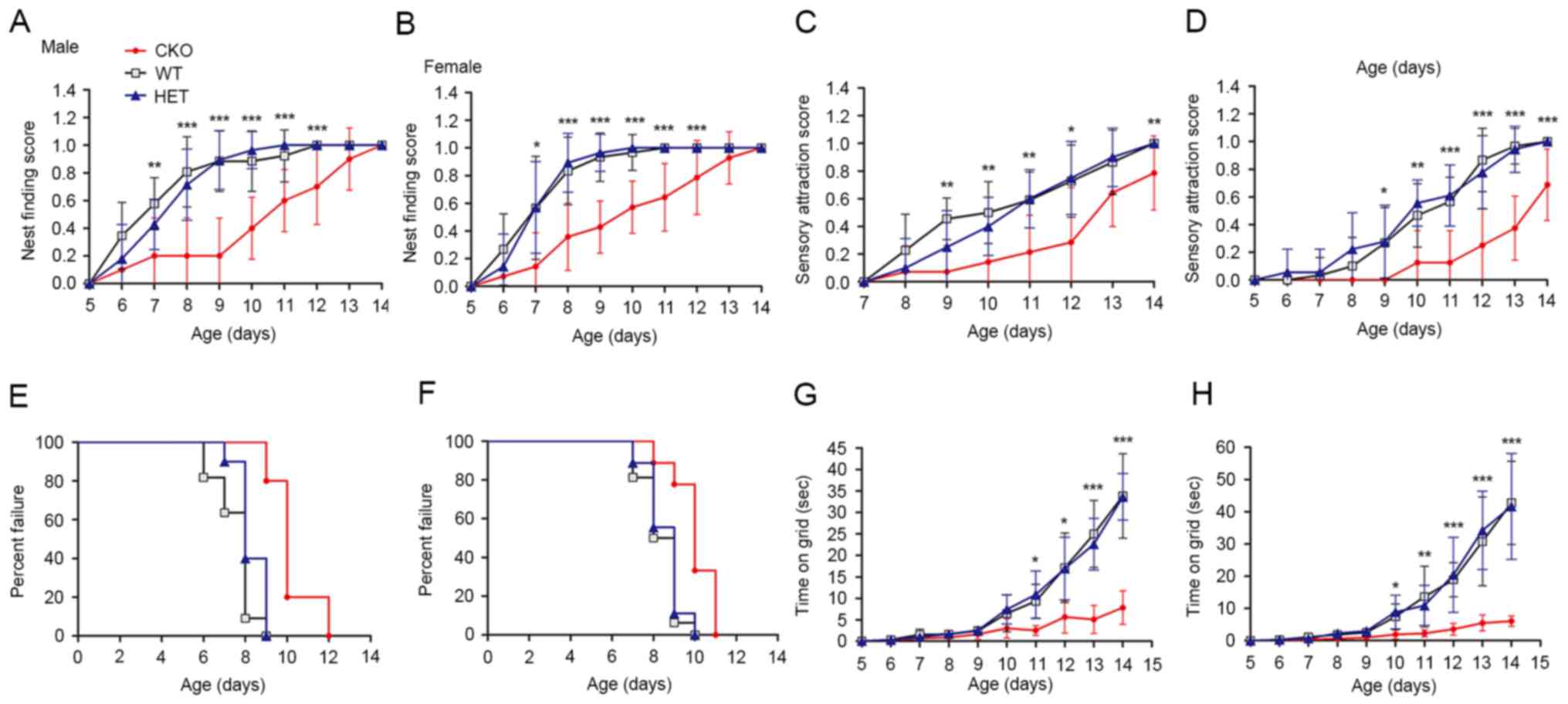 | Figure 5.Deletion of Shp2 in radial glia
impairs sensory-motor development. Nest finding score of (A) male
and (B) female Shp2 CKO, HET and WT newborn mice. Scores are
represented on a scale from 0, no response, to 1, success arrival
to the nest in three trials. Sensory attraction score of (C) male
and (D) female newborn mice. Scores are represented on a scale from
0, no response, to 1, moved towards the stimulus in three trials.
Righting reflex of (E) male and (F) newborn mice. ‘Success’ was
defined as the day on which newborns achieved the criteria of
righting in <3 sec. Wire hanging ability of (G) male and (H)
female newborn mice. Duration was measured until the newborn
release their hold of a vertical wire. Data are expressed as the
mean ± standard deviation. CKO and HET groups were compared.
*P<0.05, **P<0.01, ***P<0.001 CKO vs. HET groups. Shp2,
tyrosine-protein phosphatase non-receptor type 11; CKO, conditional
knockout; HET, heterozygous; WT, wild-type. |
Discussion
The size and complexity of cerebral cortex in
mammals has been demonstrated to be developmentally associated with
the generation of neural progenitor cells (26,27);
structural development of the cerebral cortex has been reported to
be regulated by a variety of signaling molecules and transcription
factors (28,29). In the present study, newborn mice
deficient of Shp2 in radial glia, the key organizer and progenitor
cells in cerebral cortex, exhibited growth retardation and
postnatal lethality accompanied with significant defects in the
cerebral cortex. Smaller and thinner cerebral cortices were
associated with decreased cortical cell density, reduced
proliferative ability of VZ neural progenitors and increased
cortical cell apoptosis. As radial glia are considered to be the
primary progenitor cell type that comprise the majority of
mitotically active cells within the VZ (30), the results of the present study may
indicate that Shp2 is indispensable for the self-renewal and
proliferation of cortical radial glia. In addition, appropriate
functioning of the cerebral cortex depends on accurate regulation
of the generation of various types of neural cells, in particular
neurons and glia (31,32). Almost all neurogenesis occurs
prenatally, except in the subventricular and subgranular zones;
however, the generation of glia predominantly perinatally or in the
early postnatal period. The sequential processes of neuro- and
gliogenesis are required for normal cytoarchitecture of the
cerebral cortex (33,34). The present study reported that Shp2
KO within radial glia resulted in the generation of fewer cortical
neurons, but an increase in the number of glial cells. Therefore,
Shp2 may affect the differentiation process of radial glia of the
cerebral cortex.
A previous study demonstrated that the conditional
deletion of Erk2 within radial glia using the hGFAP-Cre recombinase
system was associated with cerebral deficits, analogous to the
results of the present study, including reduced brain size and
ventricular proliferative ability, as well as increased gliogenesis
and decreased neurogenesis (19).
ERK1/2 has been demonstrated to be a critical downstream factor of
Shp2; ERK2 is 13 times more abundant than ERK1 in the superficial
cortex of nude mice (35).
Analysis of the ERK signaling pathway revealed a marked reduction
in cortical pERK levels within Shp2 CKO mice compared with WT. The
results of the present study supported the pivotal role of the ERK
pathway in the regulation of corticogenesis. In addition, Shp2 may
be involved in the regulation of radial glia proliferation and
differentiation via modulation of ERK activity.
Unlike its role in the cerebral cortex, radial glia
do not generate granular cells inn mammalian cerebellum (36); however, radial fibers develop from
the radial glia and facilitate inward migration of granular cells
from the EGL to the IGL post-birth (22). In the present study, abnormal
retention of granular cells within the EGL was observed,
accompanied with defective glial fibers between the EGL and the
IGL. However, histological analysis did not reveal significant
alterations in cell density of granular cells within the IGL of
Shp2 CKO. The gross appearance of Shp2 CKO cerebellum was similar
to that of Shp2 WT mice. These findings indicated that Shp2
expression in radial glia of the cerebellum is involved in the
migration process of granular cells during cerebellar development;
however, Shp2 function may be limited to the regulation of
scaffolding within radial glia, with less influence on granular
cell proliferation and maturation. However, the limited duration of
observation (~3 weeks) may affect the observed effects of Shp2 CKO
in further cerebellar lamination and foliation.
In the present study, developmental delay of
sensory-motor function within Shp2 CKO mice was demonstrated in
numerous aspects, including somatosensory function via the sound
attraction test, muscle strength via the wire holding test and
comprehensive sensory-motor function via the nest finding test.
These results indicate a key role for radial glial Shp2 in the
acquisition of complex neurological functions and behaviors.
Additionally, these findings further suggested that radial glia may
be an important neural cell type involved in human Noonan syndrome,
associated with disease-associated sensory-motor dysfunctions and
developmental deficits.
In conclusion, loss of Shp2 within radial glia
resulted in cortical dysplasia with decreased ERK signaling. Shp2
CKO was associated with glial defects and impaired sensory-motor
development of newborn mice. The results of the present study
provide a critical insight into the function of radial glia Shp2
regulation of neural and behavioral development.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371371, 2014).
Glossary
Abbreviations
Abbreviations:
|
CKO
|
conditional knockout
|
|
EGL
|
external granular layer
|
|
hGFAP
|
human glial fibrillary acidic
protein
|
|
IGL
|
internal granular layer
|
|
VZ
|
ventricular zone
|
|
WT
|
wild-type
|
References
|
1
|
Grossmann KS, Rosario M, Birchmeier C and
Birchmeier W: The tyrosine phosphatase Shp2 in development and
cancer. Adv Cancer Res. 106:53–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tajan M, de Rocca Serra A, Valet P,
Edouard T and Yart A: SHP2 sails from physiology to pathology. Eur
J Med Genet. 58:509–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saxton TM and Pawson T: Morphogenetic
movements at gastrulation require the SH2 tyrosine phosphatase
Shp2. Proc Natl Acad Sci USA. 96:pp. 3790–3795. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kontaridis MI, Swanson KD, David FS,
Barford D and Neel BG: PTPN11 (Shp2) mutations in LEOPARD syndrome
have dominant negative, not activating, effects. J Biol Chem.
281:6785–6792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lajiness JD, Snider P, Wang J, Feng GS,
Krenz M and Conway SJ: SHP-2 deletion in postmigratory neural crest
cells results in impaired cardiac sympathetic innervation. Proc
Natl Acad Sci USA. 111:pp. E1374–E1382. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kusakari S, Saitow F, Ago Y, Shibasaki K,
Sato-Hashimoto M, Matsuzaki Y, Kotani T, Murata Y, Hirai H, Matsuda
T, et al: Shp2 in forebrain neurons regulates synaptic plasticity,
locomotion, and memory formation in mice. Mol Cell Biol.
35:1557–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li K, Leung AW, Guo Q, Yang W and Li JY:
Shp2-dependent ERK signaling is essential for induction of Bergmann
glia and foliation of the cerebellum. J Neurosci. 34:922–931. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Y, Park J, Hu X, Zheng K, Li H, Cao Q,
Feng GS and Qiu M: Control of oligodendrocyte generation and
proliferation by Shp2 protein tyrosine phosphatase. Glia.
58:1407–1414. 2010.PubMed/NCBI
|
|
9
|
Rakic P: Elusive radial glial cells:
Historical and evolutionary perspective. Glia. 43:19–32. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyata T, Kawaguchi D, Kawaguchi A and
Gotoh Y: Mechanisms that regulate the number of neurons during
mouse neocortical development. Curr Opin Neurobiol. 20:22–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barry DS, Pakan JM and McDermott KW:
Radial glial cells: Key organisers in CNS development. Int J
Biochem Cell Biol. 46:76–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Z, Wang X, Xiao J, Wang Y, Lu H, Teng
J and Wang W: Early postnatal GFAP-expressing cells produce
multilineage progeny in cerebrum and astrocytes in cerebellum of
adult mice. Brain Res. 1532:14–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan Q, Lee A, Suzuki R, Yamagami T, Stokes
A, Nguyen BC, Pleasure D, Wang J, Chen HW and Zhou CJ: Pax6
mediates β-catenin signaling for self-renewal and neurogenesis by
neocortical radial glial stem cells. Stem Cells. 32:45–58. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Way SW, McKenna J III, Mietzsch U, Reith
RM, Wu HC and Gambello MJ: Loss of Tsc2 in radial glia models the
brain pathology of tuberous sclerosis complex in the mouse. Hum Mol
Genet. 18:1252–1265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuo L, Theis M, Alvarez-Maya I, Brenner
M, Willecke K and Messing A: hGFAP-cre transgenic mice for
manipulation of glial and neuronal function in vivo. Genesis.
31:85–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen J, Yang HB, Zhou B, Lou HF and Duan S:
β-catenin is critical for cerebellar foliation and lamination. PLoS
One. 8:e644512013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Casper KB and McCarthy KD: GFAP-positive
progenitor cells produce neurons and oligodendrocytes throughout
the CNS. Mol Cell Neurosci. 31:676–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li FF, Shen J, Shen HJ, Zhang X, Cao R,
Zhang Y, Qui Q, Lin XX, Xie YC, Zhang LH, et al: Shp2 plays an
important role in acute cigarette smoke-mediated lung inflammation.
J Immunol. 189:3159–3167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samuels IS, Karlo JC, Faruzzi AN,
Pickering K, Herrup K, Sweatt JD, Saitta SC and Landreth GE:
Deletion of ERK2 mitogen-activated protein kinase identifies its
key roles in cortical neurogenesis and cognitive function. J
Neurosci. 28:6983–6995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haziza S, Magnani R, Lan D, Keinan O,
Saada A, Hershkovitz E, Yanay N, Cohen Y, Nevo Y, Houtz RL, et al:
Calmodulin methyltransferase is required for growth, muscle
strength, somatosensory development and brain function. PLoS Genet.
11:e10053882015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neel BG, Gu H and Pao L: The ‘Shp’ing
news: SH2 domain-containing tyrosine phosphatases in cell
signaling. Trends Biochem Sci. 28:284–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Yang Y, Tang X, Zhao M, Liang F, Xu
P, Hou B, Xing Y, Bao X and Fan X: Bergmann glia function in
granule cell migration during cerebellum development. Mol
Neurobiol. 47:833–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loeb GE and Tsianos GA: Major remaining
gaps in models of sensorimotor systems. Front Comput Neurosci.
9:702015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dasen JS: Transcriptional networks in the
early development of sensory-motor circuits. Curr Top Dev Biol.
87:119–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts AE, Allanson JE, Tartaglia M and
Gelb BD: Noonan syndrome. Lancet. 381:333–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kriegstein A, Noctor S and
Martínez-Cerdeño V: Patterns of neural stem and progenitor cell
division may underlie evolutionary cortical expansion. Nat Rev
Neurosci. 7:883–890. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Namba T and Huttner WB: Neural progenitor
cells and their role in the development and evolutionary expansion
of the neocortex. Wiley Interdiscip Rev Dev Biol. 6:2016.PubMed/NCBI
|
|
28
|
Molyneaux BJ, Arlotta P, Menezes JR and
Macklis JD: Neuronal subtype specification in the cerebral cortex.
Nat Rev Neurosci. 8:427–437. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sur M and Rubenstein JL: Patterning and
plasticity of the cerebral cortex. Science. 310:805–810. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Malatesta P and Götz M: Radial glia - from
boring cables to stem cell stars. Development. 140:483–486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian X, Shen Q, Goderie SK, He W, Capela
A, Davis AA and Temple S: Timing of CNS cell generation: A
programmed sequence of neuron and glial cell production from
isolated murine cortical stem cells. Neuron. 28:69–80. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sauvageot CM and Stiles CD: Molecular
mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol.
12:244–249. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dwyer ND, Chen B, Chou SJ, Hippenmeyer S,
Nguyen L and Ghashghaei HT: Neural stem cells to cerebral cortex:
Emerging mechanisms regulating progenitor behavior and
productivity. J Neurosci. 36:11394–11401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guerout N, Li X and Barnabé-Heider F: Cell
fate control in the developing central nervous system. Exp Cell
Res. 321:77–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lefloch R, Pouysségur J and Lenormand P:
Single and combined silencing of ERK1 and ERK2 reveals their
positive contribution to growth signaling depending on their
expression levels. Mol Cell Biol. 28:511–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang VY and Zoghbi HY: Genetic regulation
of cerebellar development. Nat Rev Neurosci. 2:484–491. 2001.
View Article : Google Scholar : PubMed/NCBI
|