Introduction
Neisseria meningitidis (N.
meningitidis) is a Gram-negative microorganism and a major
causative agent of severe sepsis and meningitis (1), both of which may lead to mortality in
children and young adults within h, although effective antibiotics
are available (2,3). N. meningitidis causes
meningococcal diseases in ~500,000 people annually worldwide,
mainly affecting children between the ages of 3 and 48 months,
followed by adolescents (4), and
although drug treatments such as penicillin are available, 10–15%
of children and adolescents succumb to infection. Of those who
survive, 11–19% exhibit problems in the nervous system, including
mental retardation, hearing loss and paralysis (5). N. meningitidis is also
responsible for the development of invasive meningococcal diseases,
such as septicemia, pneumonia and arthritis (6).
N. meningitidis is classified into 13
serogroups (A, B, C, D, H, I, K, L, X, Y, Z, 29E and W-135) based
on the molecular structure and antigenicity of bacterial capsular
polysaccharides (CPSs) (3); among
the serogroups A-C cause ≤90% of all meningitis cases. CPS-based
vaccines have been developed and used successfully to prevent the
invasive meningococcal disease that may be caused by serogroups A,
C, W135 and Y (7). However, the
CPS of N. meningitidis serogroup B (NMB) is an
α2-8-linked polysialic acid that resembles a molecule
that is present on the surface of human tissues, thus making a NMB
CPS-based vaccine poorly immunogenic, as well as presenting a
possible cause of autoimmunity (2,7,8).
Over the past 40 years there has been an increase in
the number of studies directed towards the identification of NMB
antigens as a basis of developing a new vaccine. Outer membrane
proteins of N. meningitidis have been implicated in
bacterial virulence and may induce immune responses; therefore,
they may present good antigen candidates for vaccine design
(9).
NMB outer membrane protein 0315 (NMB0315; NCBI Gene
ID: 902431) was confirmed as an outer membrane protein of NMB that
comprises 430 amino acids and has a molecular weight of 46 kDa, and
has been demonstrated to be a virulence factor for N.
meningitidis and a target for bactericidal antibodies (10). A previous study revealed that
certain proteins that were ≤98% identical to NMB0315 were not only
in the different serogroups of N. meningitidis, but also in
Neisseria gonorrhoeae (10), which suggested that NMB0315 may be
a potential candidate as a broad-spectrum vaccine against
meningococcal diseases.
An NMB0315 DNA vaccine was constructed. The
NMB0315 gene was amplified by polymerase chain reaction
(PCR) from the NMB MC58 standard strain genomic DNA (NCBI accession
no. NC_003112.2) and cloned into a pcDNA3.1(+) plasmid to
construct a recombinant plasmid, pcDNA3.1(+)/NMB0315
(designated pNMB0315). The constructed pNMB0315 was
transfected into eukaryotic COS-7 and RAW264.7 cells to express the
recombinant NMB0315 (rNMB0315) protein. To determine the
immunogenicity and protective efficacy of pNMB0315, female
BALB/c mouse were used as an in vivo model. The levels of
NMB0315-specific immunoglobulin G (IgG), IgG1 and IgG2a antibodies
that were induced by pcDNA3.1(+)/NMB0315 were
detected, and the protective immunogenicity was evaluated to
develop a novel DNA vaccine against NMB.
Materials and methods
Animals and housing
Pathogen free, female BALB/c inbred mice (125 mice,
3–4 weeks, average weight, 19.8 g) were purchased from The National
Resource Center for Rodent Laboratory Animal (Shanghai, China).
Animals were maintained in the animal facilities of The University
of South China (Hengyang, China), and raised on a normal diet (food
and water were available ad libitum) at 25°C and 50%
humidity on a 12-h light/dark cycle prior to euthanasia. All
experimental protocols involving mice were approved by the Ethics
Committee of The University of South China.
Plasmids, bacterial strains, cell
lines and reagents
The pcDNA3.1(+) plasmid (Addgene, Inc.,
Cambridge, MA, USA) and Escherichia coli strain JM109 (China
Center of Industrial Culture Collection, CICC®; Beijing,
China) were used in conventional recombinant experiments. NMB
strain MC58 was purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA). Neisseria mucosa was separated
from a normal population. COS-7 monkey kidney fibroblast (commonly
used for the production of recombinant proteins) and RAW264.7 mouse
leukemia cell lines (commonly used model of mouse macrophages for
the study of cellular responses to microbes and their products)
were purchased from Institute of Cell Biology of the Chinese
Academy Sciences (Shanghai, China). Restriction enzymes
BamHI and XhoI, T4 DNA ligase, Pfu DNA
polymerase, pre-stained protein molecular weight marker,
nuclease-free water and dNTP Mix were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). CpG (sequence TCC ATG ACG TTC
CTG ACG TT) was synthesized by Beijing Genomics Institute
(Shenzhen, China). Horseradish peroxidase (HRP)-conjugated goat
anti-mouse IgG (cat. no. ab97023), IgG1 (cat. no. ab97240), IgG2a
(cat. no. ab97245) antibodies were all purchased from Abcam
(Cambridge, UK). ELISA kits (cat. nos. IFN-r/88-7314-22 and
IL-4/88-7044-22) were purchased from eBioscience (Thermo Fisher
Scientific, Inc.). N. meningitidis Serogroup B Diagnostic
Antiserum from BD Biosciences (Franklin Lakes, NJ, USA) (3).
Construction and identification of
pcDNA3.1(+)/NMB0315
BamHI and XhoI were selected as the
restriction sites for the upstream and downstream primers,
respectively. The full length NMB0315 gene (GI: 902431,
location 326,142-327,434, length 1293 bp) was amplified by PCR
(pre-denaturation at 94°C, for 2 min, denaturation at 94°C, for 30
sec; annealing at 60°C for, 30 sec and extension at 72°C, for 2
min, 34 cycles; a final extension/72°C for 10 min. DNA polymerase,
nuclease-free water and dNTP Mix were purchased from Thermo Fisher
Scientific, Inc. Successful amplification was confirmed using 1%
agarose gel, stained with Gold View (cat. no. 200601; BLKW
Biotechnology Co., Ltd., Beijing, China) using the NMB strain MC58
genomic DNA (extracted by Bacteria Genomic DNA kit; CoWin
Bioscience Co., Ltd., Beijing, China; cell density 1×106
cells/ml) as a template and the upstream primer (BamHI),
5′-CGCGGATCCATGGCTGTCTTCCCACTTTC-3′ and downstream primer
(XhoI), 5′-CCGCTCGAGTCAATCCGATTGCGACAC-3′ were used. The
amplified PCR product was digested with restriction enzymes
BamHI and XhoI (1 µg PCR product, 0.4 µM
BamHI, 0.4 µM XhoI/25 µl) and cloned into
pcDNA3.1(+) to generate a recombinant plasmid
pcDNA3.1(+)/NMB0315 (pNMB0315), which was used
as a DNA vaccine. The constructed recombinant pNMB0315 was
confirmed by restriction digestion and sequencing, and subsequently
transformed (200 µl competent JM109/OD600 = 0.6 plus 10
ng pcDNA3.1(+)/NMB0315 plasmid were transformed at
room temp for 30 min then 42°C for 90 sec, 4°C for 3 min. Then 800
µl LB broth was added and incubated at 37°C for 1 h followed by
centrifugation at 4°C, 4,000 × g for 60 sec. The positive
transformants were selected on LB agar containing ampicillin into
E. coli JM109 for overexpression. The clones containing the
insert NMB0315 were selected by resistance to ampicillin and
stored for further use.
Expression and identification of
rNMB0315
Eukaryotic COS-7 and RAW264.7 cell lines were
cultured in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% heat-inactivated fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
5% CO2. The cell lines (2×105/ml) were
transfected with pNMB0315 (8 µg/ml) in a 24-well plate at
room temperature for 48 h using the X-treme GENE HP DNA
Transfection Reagent (Roche Diagnostics, Shanghai, China),
according to the manufacturer's instructions. COS-7 or RAW264.7
cells were transfected with empty vector pcDNA3.1(+) or PBS
as controls. The transfected cells were harvested 48 h
post-transfection to evaluate the expression of rNMB0315 by
immunocytochemical method and western blot analysis. For
immunocytochemical method and western blot analysis, rabbit immune
serum-containing antibodies of recombinant rNMB0315 (prepared by
the authors' group, prokaryotic expression product) were used as
primary antibody (1:1,000) and HRP-conjugated goat anti-rabbit IgG
as secondary antibody (1:5,000, cat. no. SC-2357; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA).
Immunocytochemistry
COS-7 or RAW264.7 cells (106/ml) were
cultured in Dulbecco's modified Eagle's medium containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), at 37°C in a
humidified atmosphere containing 5% CO2. Cells were
washed three times with pre-cooled PBS and seeded onto glass
coverslips (Thermo Fisher Scientific, Inc). The cells were fixed
with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 20 min at room temperature and then washed three times
with PBS prior to the addition of 3% H2O2 for
10 min (inhibitor of endogenous peroxidase activity). Blocking was
performed with 5% bovine serum albumin (BSA; Gibco; Thermo Fisher
Scientific, Inc.) for 30 min at 37°C, incubated the primary
antibody [1:1,000; preparation of primary antibody: New Zealand
rabbits (6 rabbits, 6 months, female, 2–2.5 kg, maintained under
specific pathogen-free conditions in isolated cages, with a 12-h
light-dark cycle at 25°C, 50% humidity and ventilation facility,
food and water were available ad libitum)] were immunized
with rNMB0315 protein (200 µg/200 µl each time, prepared by the
authors' group) in subcutaneous injection above the gluteals, with
Freund's adjuvant (Sigma-Aldrich; Merck KGaA) at 0 week (complete
Freund's adjuvant; cat. no. F5881), 2, 4 and 6 weeks (incomplete
Freund's adjuvant; cat. no. F5506); two weeks following the last
immunization, blood was collected via ear vein and centrifuged (800
× g) at room temperature for 20 min, the serum was stored at −70°C]
at 4°C overnight, washed three times with PBS. HRP-conjugated goat
anti-rabbit IgG as secondary antibody (1:5,000; Abcam, Cambridge,
UK) was added for 60 min at room temperature, then washed three
times. Stained with DAB for 1 min, at room temperature and then
with hematoxylin (both from Sigma-Aldrich; Merck KGaA) for 8 min,
at room temperature was performed. Images were captured using an
inverted microscope (XD202; Nanjing Jiangnan Novel Optical Co.,
Ltd., Nanjing, China). A total of 50 fields were examined
(magnification, ×200).
Western blotting
COS-7 or RAW264.7 cells (106/ml) were
harvested 48 h following transfection and resuspended in lysis
buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 5 mM EDTA and 1% NP-40]
supplemented with Complete Mini (Roche Diagnostics) for protein
extraction. Protein concentrations were determined using the
bicinchoninic acid Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Protein extracts (10 µg/10
µl/lane) were separated by 10% SDS-PAGE, transferred to
polyvinylidene fluoride membrane (Beyotime Institute of
Biotechnology) blocked by 1% BSA for 3 h at room temperature. The
membranes were incubated with the primary antibody (1:1,000)
anti-NMB0315 (prepared by the authors' group) at 4°C overnight then
washed three times with PBS-T. β-actin was used for internal
control (β-actin rabbit polyclonal antibody, cat. no. 20536-1-AP,
1:3,000; Proteintech, Group, Inc., Chicago, IL, USA).
HRP-conjugated goat anti-rabbit IgG was added as a secondary
antibody (1:5,000; Abcam) for 45 min at room temperature, washed
three times. Signals detection was performed with an enhanced
chemiluminescence kit (GE Healthcare, Chicago, IL, USA).
Animal immunization and specimen
collection
A total of 125 4-week-old female BALB/c mice were
randomly and equally divided into five groups (25 mice/group), and
were subsequently immunized intramuscularly as follows (total
volume of 100 µl each group): i) pNMB0315 (50 µg) +
CpG (10 µg; as an adjuvant); ii) pNMB0315-alone (50 µg);
iii) pcDNA3.1(+) (50 µg); iv) CpG (10 µg); or v) PBS. The
inoculation was performed three times with an interval of 2 weeks
four times (0, 2, 4 and 6 weeks), and blood samples were collected
from the immunized mice by tail bleeding prior to each
immunization. The collected serum was stored at −70°C until
use.
Specific antibody assay
NMB0315-specific IgG, IgG1 and IgG2a antibody levels
were determined by indirect ELISA as previously described (11). Briefly, ELISA plates were coated
with 1 µg/well of purified rNMB0315 (prokaryotic expression
product), sealed and incubated overnight at 37°C. Following 3
washes with PBS + 0.05% Tween-20 (PBST), the coated plates were
blocked with 150 µl blocking buffer (0.5% skim milk in PBST) at
37°C for 1 h. The plate was washed 3 times with PBST and incubated
with 100 µl NMB0315-immune mouse serum (1:10,000 in blocking buffer
was used as a working dilution following testing of serial
dilutions) each well in a 96 well plate for 1 h at room
temperature. A blank control without serum was set up concurrently.
Following 3 washes, 100 µl HRP-conjugated goat anti-mouse IgG, IgG1
or IgG2a secondary antibody (1:5,000 in PBST) was added to the
respective ELISA plate wells, and the plates were incubated at room
temperature in the dark for 30 min. The plates were washed 5 times,
100 µl 3,3,5,5-tetramethylbenzidine Microwell Peroxidase Substrate
System (Tiangen Biotech Co., Ltd., Beijing, China) was added and
the plates were incubated 37°C for 20 min. The reactions were
stopped with the addition of 100 µl H2SO4 (2
mol/l) to each well, and signal detected using a Bio-Rad microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at an
absorbance of 450 nm. All assays were performed in triplicate. The
cutoff value was set as previously described (12).
Cytokine assays
A total of 5 immunized mice from each group were
euthanized 2 weeks following the third immunization, and their
spleens were isolated for preparation of splenic suspension. The
spleen from the immunized mice were obtained using aseptic
techniques, ground and filtered by 200-mesh sieve (70-µM pore size)
and prepared for single-cell suspension. The suspension was used to
seed 96-well plates (1×106 cells/well), and the cells
were incubated with rNMB0315 (10 µg), as a specific stimulator, at
37°C in 5% CO2 for 48 h. The culture supernatant was
collected and the levels of T helper 1 (Th1)- and Th2-type
cytokines, interleukin (IL)-4 and interferon (IFN)-γ, respectively,
were detected by indirect ELISA, method according to the
manufacturer's instructions.
Serum bactericidal assay (SBA)
Two weeks following the third immunization (20 mice
in each group), immune serum was collected for SBA as described
previously (3). Briefly, a
suspension of MNB strain MC58 (40,000 CFU/ml) was mixed with
newborn rabbit complement (Pel-Freez Biologicals, Rogers, AR, USA)
at a 1:1 ratio; the mixture was subsequently combined with the
immune serum, serially diluted in 2-fold from 1:2 to 1:256, and
cultured for 1 h at 37°C prior to being inoculated on chocolate
agar plates and incubated at 37°C overnight. MNB diagnostic
antiserum was used as a positive control; negative controls
included MC58 suspension + complement, UV-inactivated MC58 +
complement, and immune serum + MC58 + heat-inactivated complement.
When the serum with a bactericidal rate (determined by counting of
the cultured bacteria colonies, compared with the negative and
positive controls) was >50%, the highest serum dilution was
determined using serum bactericidal antibody titers (3).
Immunoprotection of pNMB0315
Cultured MC58 cells were diluted to an optical
density with A600 = 0.005 (a concentration equivalent to
4,000 colony-forming units/ml, which is a lethal dose of MC58; mice
were infected with 500, 1,000, 2,000, 3,000, 4,000 colony-forming
units/ml MC58 respectively, a lethal dose resulted in mice
succumbing in 72 h was confirmed) to form the strain suspension.
The MC58 suspension (40,000 CFU/ml) was immediately injected into
the abdominal cavity of immunized mice (20 mice in each group), 2
weeks following the third immunization. Signs of MC58 infection and
survival rate of the mice were recorded daily for 14 days,
following which all mice were humanely euthanized with 100%
CO2.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA) was
used for one-way analysis of variance for data sets containing
multiple comparisons and a Dunnett's test as a post-hoc test.
Kaplan-Meier with log-rank test was used for comparison of the
survival rate of mice. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction of a recombinant plasmid
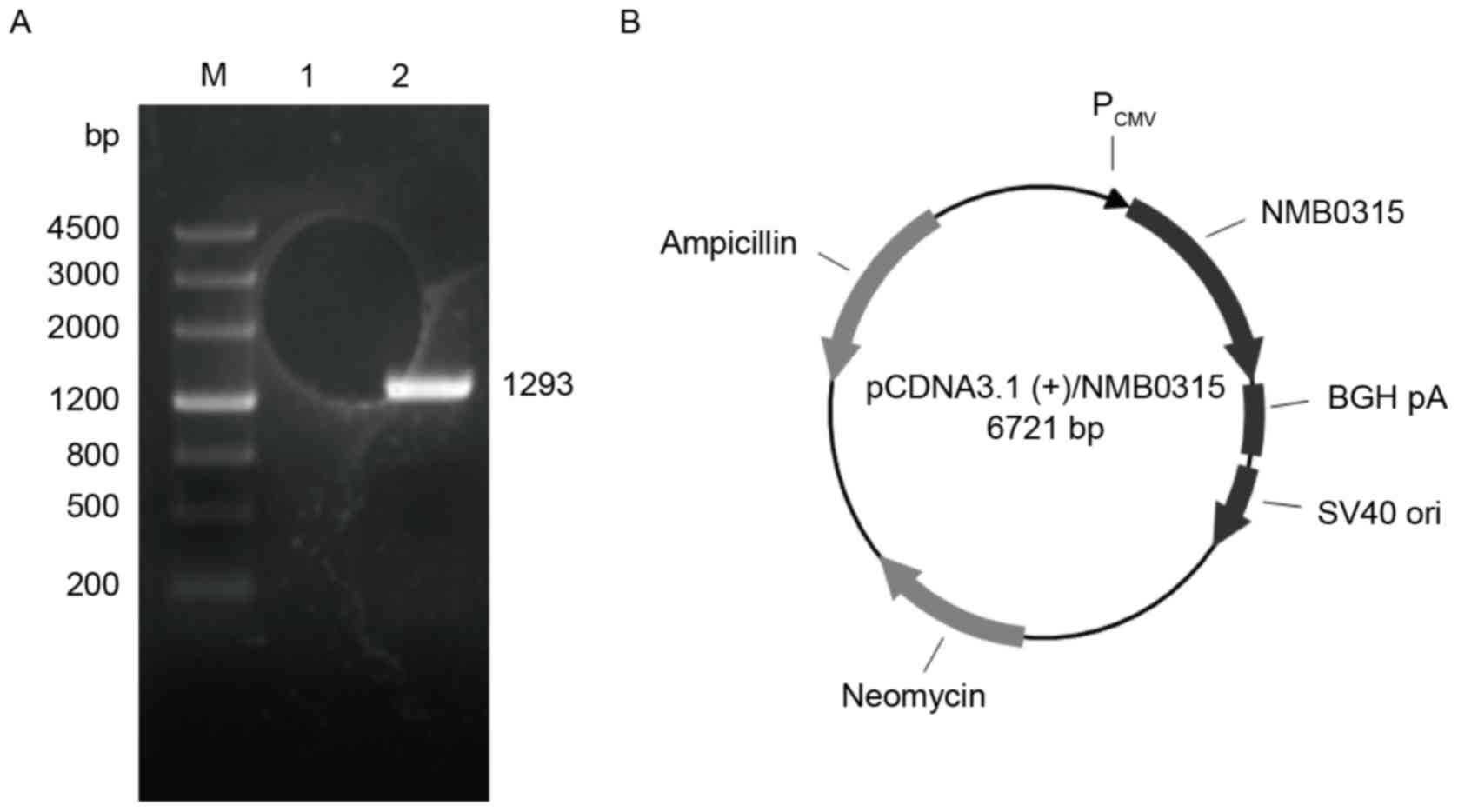
pNMB0315
The full-length NMB0315 gene sequence from
the genomic DNA of NMB standard strain MC58 was amplified by PCR,
and a NMB0315 gene (1,293 bp) was obtained (Fig. 1A). The NMB0315 gene was
cloned into the mammalian expression vector pcDNA3.1(+) to
generate the recombinant plasmid pNMB0315 (Fig. 1B).
Identification of pNMB0315
The constructed recombinant plasmid pNMB0315
(total length, 6,721 bp) was identified by double digestion of the
recombinant plasmid with restriction enzymes BamHI and
XhoI, followed by sequencing (data not shown). The digested
products were 1,293 bp, which corresponded to the NMB0315
gene insert, and 5,428 bp, which corresponded to the
pcDNA3.1(+) plasmid vector (Fig. 2A). The recombinant plasmid
pNMB0315 was transfected into eukaryotic COS-7 and RAW264.7
cells, and the expressed NMB0315 protein was identified by
immunocytochemical method and western blot analysis (Fig. 2B and C, respectively). Detection
was similar for RAW264.7 cells (data not shown). For
immunocytochemical method and for western blotting, rabbit immune
serum containing NMB0315-specific antibodies was used as the
primary antibody and HRP-conjugated goat anti-rabbit IgG as the
secondary antibody. Cells transfected with pNMB0315 were
stained brown, indicating the detection of rNMB0315 expression
(Fig. 2B-a), whereas no rNMB0315
protein was detected in the control cells (Fig. 2B-b-d). The recombinant plasmid
pNMB0315 was transfected into eukaryotic COS-7 and RAW264.7
cells and the expressed NMB0315 protein was identified by western
blot analysis (Fig. 2C). The
results indicated that a eukaryotic recombinant plasmid
pNMB0315 was successfully constructed and could effectively
expressed rNMB0315 protein in mammalian cells.
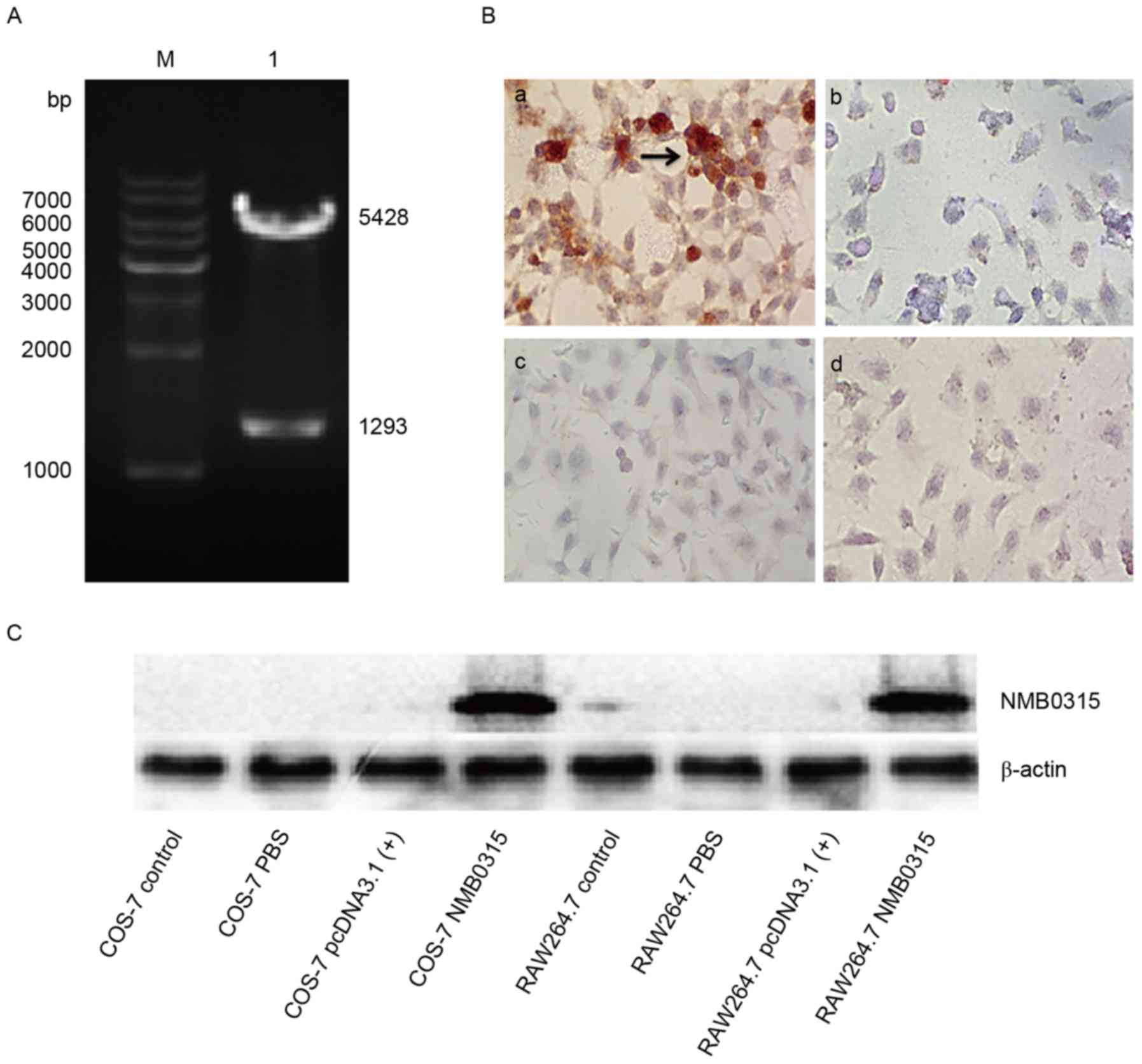 | Figure 2.Enzyme digestion, immunocytochemical
staining and western blot analysis of pNMB0315. (A)
Recombinant plasmid pNMB0315 was digested with BamHI
and XhoI to confirm successful cloning of the NMB0315
gene into the plasmid vector. M, DNA marker; lane 1, digested
fragments of pNMB0315, in which the 5,428 bp fragment is the
pcDNA3.1(+) vector, and the 1,293 bp fragment is
NMB0315. (B) Expression of NMB0315 protein in eukaryotic
COS-7 cells by immunocytochemical assay; magnification, ×200. (B-a)
COS-7 cells transfected with pNMB0315; (b) COS-7 cells
transfected with pcDNA3.1(+); (c) COS-7 cells transfected
with PBS; and (d) untransfected COS-7 cells. (C) Eukaryotic
expression of recombinant NMB0315 protein was detected by western
blot analysis. COS-7 control, untransfected COS-7 cells; COS-7 PBS,
cells transfected with PBS; COS-7 pcDNA3.1(+), cells
transfected with pcDNA3.1(+); COS-7 NMB0315, cells
transfected with pNMB0315; RAW264.7 control, untransfected
RAW264.7 cells; RAW264.7 PBS, cells transfected with PBS; RAW264.7
pcDNA3.1(+), cells transfected with pcDNA3.1(+);
RAW264.7 NMB0315, cells transfected with pNMB0315. NMB,
Neisseria meningitidis serogroup B. |
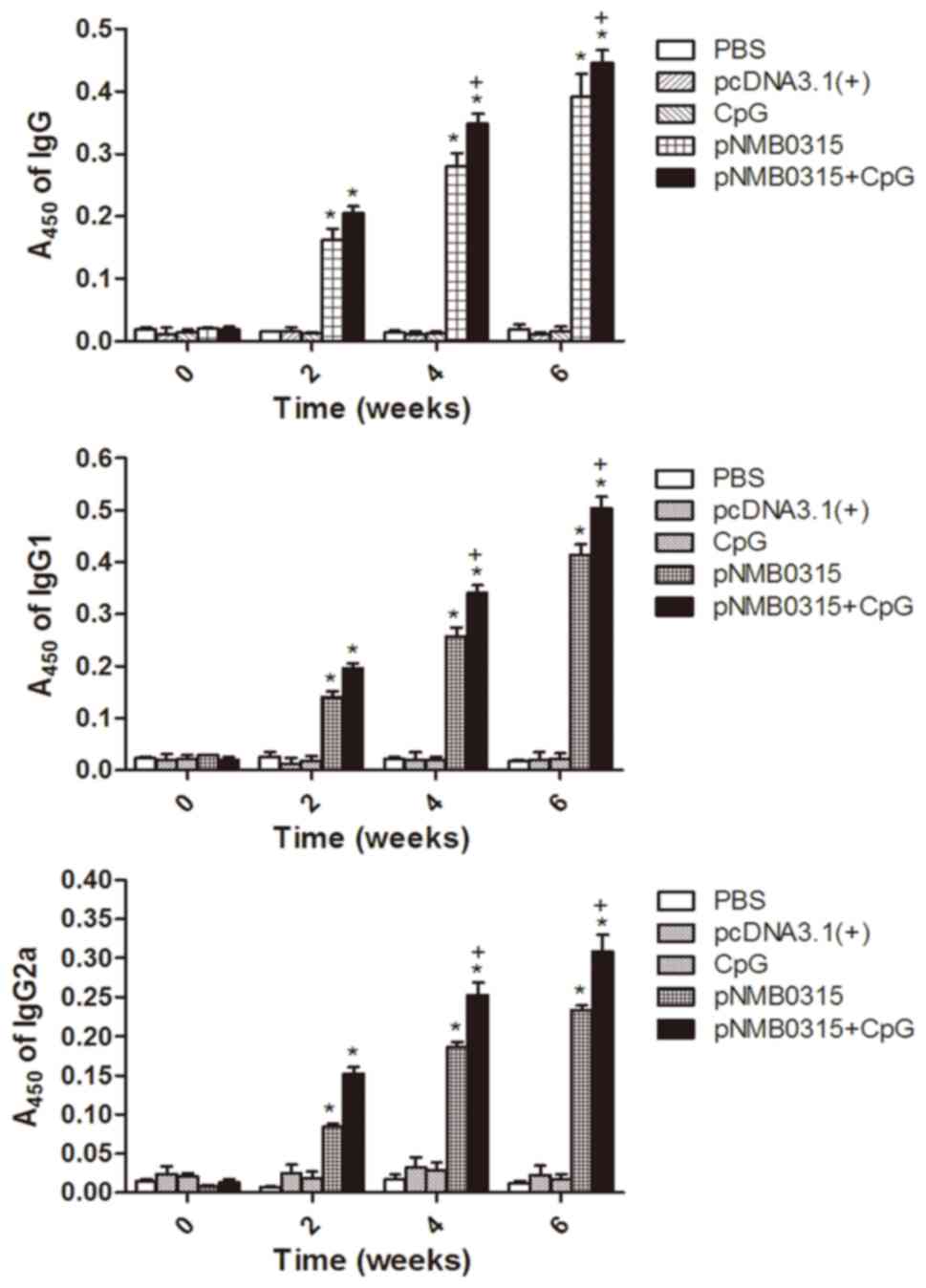
Humoral immune response is induced by
pNMB0315
The levels of NMB0315-specific IgG, IgG1 and IgG2a
antibodies in the pNMB0315 + CpG group and the
pNMB0315-only group notably increased between week 2 and
week 6 following the initial vaccination, and were significantly
higher compared with the respective antibody levels in the control
groups, pcDNA3.1(+), PBS and CpG-only at weeks 2, 4 and 6
(P<0.01; Fig. 3). The specific
IgG, IgG1 and IgG2a antibody levels in the pNMB0315 + CpG
group were significantly higher compared with the levels in the
pNMB0315-only group at weeks 4 and 6 (P<0.05; Fig. 3). It has been reported previously
that IgG2a predominantly indicates cellular immunity and IgG1
predominantly indicates humoral immunity (3); therefore, the serum IgG2a/IgG1 ratios
were calculated in the pNMB0315-only group at weeks 2, 4 and
6 post-immunization were 0.775 (0.152/0.196), 0.744 (0.253/0.340)
and 0.614 (0.309/0.503), respectively; in the pNMB0315 + CpG
group at weeks 2, 4 and 6 post-immunization 0.595 (0.084/0.141),
0.723 (0.186/0.257) and 0.565 (0.234/0.414), respectively. All
ratios were <1, which suggested that the DNA vaccine
pNMB0315 predominantly induced humoral immunity
responses.
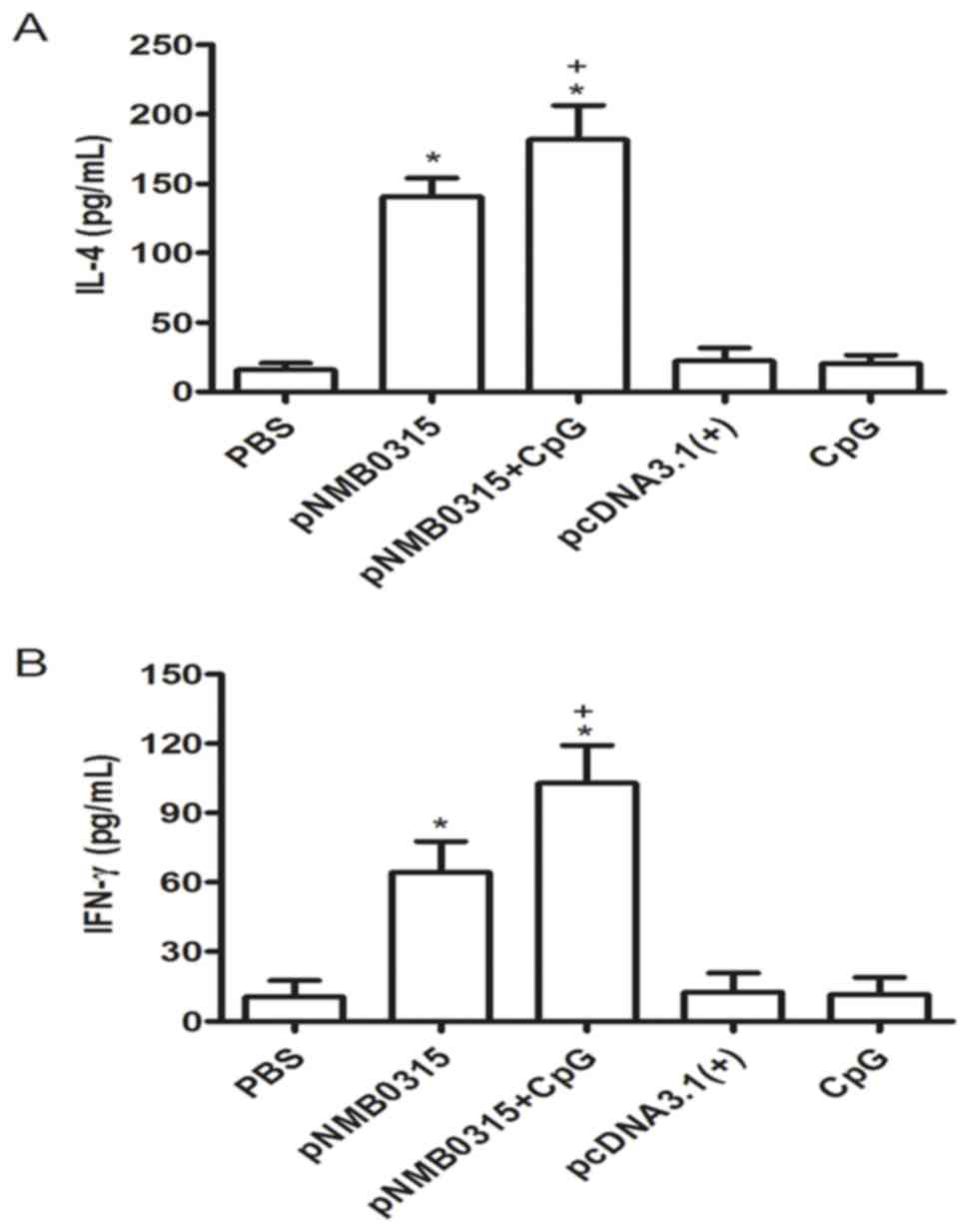
Th1-type and Th2-type cytokines are
induced by pNMB0315
Splenic cells from the immunized mice were harvested
2 weeks following the third immunization, and were subsequently
stimulated in vitro with 10 µg/ml of rNMB0315 protein for 48
h. The levels of IL-4 and IFN-γ in the splenic lymphocytes culture
supernatant were detected by indirect ELISA. The concentrations of
IL-4 and IFN-γ in the pNMB0315 + CpG group and in the
pNMB0315-only group were significantly higher compared with
the respective expression levels in the pcDNA3.1(+), PBS and
CpG control groups (P<0.01; Fig. 4A
and B). In addition, the concentrations of IL-4 and IFN-γ in
the pNMB0315 + CpG group were significantly higher compared
with the pNMB0315-only group (P<0.05; Fig. 4). These results suggested that
pNMB0315 may elicit a potent Th1-type cytokine (IFN-γ) and
Th2-type cytokine (IL-4) response in the immunized mice.
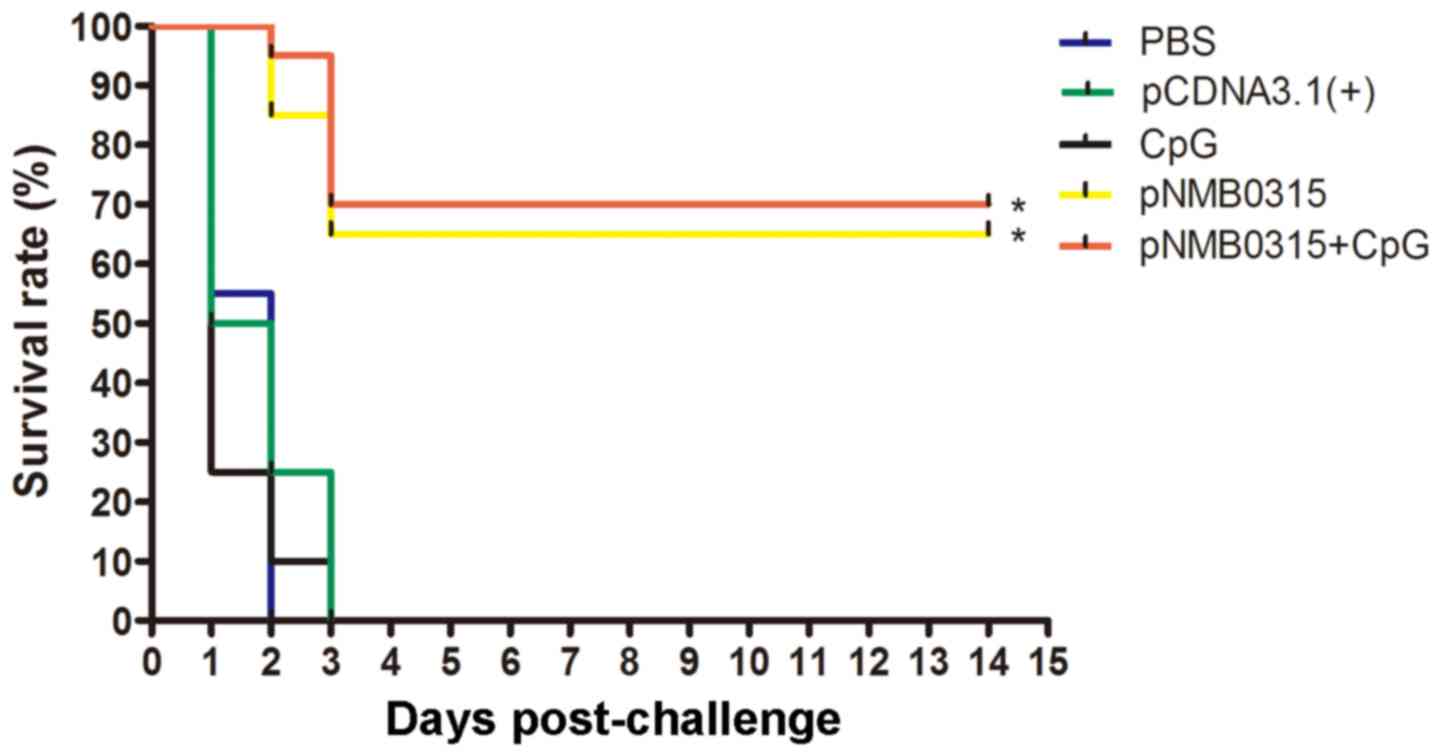
Immunoprotection efficacy against
challenge with NMB MC58
Two weeks following the third vaccination, immunized
mice were challenged with a lethal dose of NMB MC58. All
mice in the pcDNA3.1(+), PBS and CpG control groups
displayed signs of severe clinical symptoms, including rough hair,
shivering, decreased mobility and severe ataxia, and succumbed to
the infection within 3 days (Fig.
5). Survival rates in the pNMB0315-only group and in the
pNMB0315 + CpG group were 65% (13 out of 20) and 70% (14 out
of 20), respectively, at 14 days post-challenge. The survival rates
of the pNMB0315 and pNMB0315 + CpG groups were
significantly higher compared with the PBS, pcDNA3.1(+) and
CpG control groups (P<0.01; Fig.
5); no significant difference was identified in survival rate
was identified between the pNMB0315 and the pNMB0315
+ CpG (P>0.05)). In addition, SBA demonstrated that the serum
bactericidal titers of the pNMB0315 group and the
pNMB0315 + CpG group reached 1:64 and 1:128, respectively
(SBA titers >8 or a 4 fold increase are considered as protective
in many meningococcal species, including group B) (13), following the three immunizations;
however, the titers of the control groups were all <1:2 (results
not shown). These results indicated that pNMB0315-induced
immune serum may have a potent complement-dependent bactericidal
activity in vitro and may be highly protective in mice
against NMB strain MC58 infection in vivo.
Discussion
N. meningitides, also known as meningococcus,
is a human-specific pathogenic organism that is the cause of
encephalomyelitis epidemics (14,15).
The most common presentation of invasive meningococcal infection
(or meningococcal disease) is meningitis, which results from the
spread of the bacterium through the bloodstream. In ~50% of cases,
N. meningitides crosses the blood-brain barrier and enters
the cerebrospinal fluid, causing purulent meningitis. The mortality
rate of meningococcemia is up to 40% as meningococcemia is more
severe than general meningococcal diseases. (2,6).
CPS-based vaccines are available and are used for the prevention of
infection by N. meningitides serogroups A, C, W135 and Y
infections, and the development of an effective vaccine against NMB
is important for the prevention and control of the disease
(8).
DNA vaccines are a relatively new vaccination
strategy. Compared with the traditional vaccines, such as live
attenuated or inactivated viral vaccines, or protein subunit
vaccines, DNA vaccines offer a number of advantages (16). For example, DNA vaccines are able
to replicate and express the protein of interest in the host cells,
which is similar to a live attenuated vaccine, but is inherently
safer than live attenuated or inactivated viral vaccines. In
addition, the expressed protein from DNA vaccines maintains its
natural conformation, which potentially produces increased native
immunogenicity. Furthermore, DNA plasmids are simple and
inexpensive to design and create, and the plasmids themselves are
not immunogenic (17). The factors
associated with the immune effects of a DNA vaccine mainly include
regulatory elements, immunization routes and adjuvants. The
promoter region is an important regulatory element that directly
influences the expression levels of an exogenous gene in
vivo. The plasmid used in this study, pcDNA3.1(+), is a
eukaryotic expression vector with a strong promoter from
cytomegalovirus, which promotes high-efficiency expression of an
exogenous gene in mammalian cells (18). In the present study, a recombinant
pNMB0315 was constructed and transfected into eukaryotic
cell lines, which effectively expressed rNMB0315 protein,
indicating the successful construction of a DNA vaccine. CpG was
used as an adjuvant to enhance immune effects of the DNA vaccine
(19). CpGs are recognized by
toll-like receptor 9 (TLR9), a receptor found on antigen presenting
cells (APCs), which results in the activation of TLR9 and the
enhancement of antigen presenting capacity of APCs (20,21).
As N. meningitides is an extracellular
pathogen, anti-N. meningitides infections are mainly
dependent on humoral immunity (22). The DNA vaccine pNMB0315 used
in the present study induced high-level NMB0315-specific antibodies
IgG, IgG1 and IgG2a in female BALB/c mice. The results indicated
that pNMB0315 provided exceptional immunogenicity and that
the CpG adjuvant aided pNMB0315 in eliciting the production
of antibodies in mice. It has been reported previously that the
subclasses of IgG present in the serum may reflect the type of
immune response, and that IgG2a was predominant in cellular
immunity and IgG1 was predominant in humoral immunity (23). The Th1-type cytokine IFN-γ
positively correlates with cell-mediated immune responses, which
promote the production of IgG2a, whereas the Th2-type cytokine IL-4
is correlated with humoral immune response and promotes the
production of IgG1 (24). Serum
IgG2a/IgG1 ratios in the pNMB0315 + CpG and the
pNMB0315 groups were <1 at weeks 2, 4 and 6
post-inoculation, which suggested that the pNMB0315 DNA
vaccine predominantly induced humoral immunity responses.
SBA is used to quantify the levels of antibodies
that are specific for bacterial surface determinants and results in
complement-mediated lysis of bacteria (25). SBA in vitro is considered as
the gold standard test for the evaluation of functional
anti-meningococcal antibodies and as an accepted surrogate for
protection (26). The serum
bactericidal antibody titer of the pNMB0315 + CpG and the
pNMB0315 groups in vitro reached 1:128 and 1:64,
respectively, following three immunizations, which may be
correlated with the high levels of IgG antibodies in the immunized
serum. In addition, the pNMB0315 vaccine exhibited an high
immunoprotective efficacy. The vaccine pNMB0315 + CpG group
and pNMB0315 group offered 70 and 65% protection against NMB
MC58, respectively, two weeks following innoculation. The survival
rate (70%) of pNMB0315 immunization is lower than that of
rNspA immunization (85% survival rate) reported by the authors,
previously (3), which might be
associated with lower transfection efficiency of eukaryotic plasmid
pNMB0315. Therefore, a prokaryotic expression vector will be
constructed to express recombinant protein NMB0315 and research its
immunocompetence and immunoprotection.
In conclusion, the present study successfully
constructed and effectively transfected eukaryotic cells with a
pNMB0315 DNA vaccine. pNMB0315 induced high levels of
NMB0315-specific IgG, IgG1 and IgG2a antibodies and offered
effective immunoprotection against NMB in inoculated mice. In
addition, the immune serum containing NMB0315-specific antibodies
exhibited strong bactericidal activity, which provided a
preliminary proof that the outer membrane protein NMB0315 may be a
potential vaccine candidate antigen, and that the pNMB0315
may serve as a promising DNA vaccine against NMB. However, there
are a variety of surface-exposed proteins on the MNB that may be
associated with bacterial virulence and complicated pathogenesis.
It is improbable that the selection of a single virulence factor as
a protective antigen may provide complete protection. Therefore,
future studies should focus on the development of a multicomponent
or multivalent vaccine against NMB.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant no. 81172890), The Hunan
Province Cooperative Innovation Center for Molecular Target New
Drug Study (grant no. 2015-351) and The Construct Program of the
Key Discipline in Hunan Province and Hunan Provincial Key
Laboratory for Special Pathogens Prevention and Control (grant no.
2014-5-2012-312). The authors thank Mrs. Chunxue Lu and Mr. Yukuai
Zhang (University of South China, Hengyang, China) for their
excellent technical assistance and advice.
References
|
1
|
Shahbaaz M, Bisetty K, Ahmad F and Hassan
MI: Towards new drug targets? function prediction of putative
proteins of Neisseria meningitidis MC58 and their virulence
characterization. OMICS. 19:416–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silva GP, Cruz SC, Cruz AC and Milagres
LG: Short-term and long-term antibody response by mice after
immunization against Neisseria meningitidis B or diphtheria toxoid.
Braz J Med Biol Res. 46:148–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ying S, He J, Yu M, Zhang Y, Deng S, Zhang
L, Xie M and Hu S: Recombinant Neisseria surface protein A is a
potential vaccine candidate against Neisseria meningitides
serogroup B. Mol Med Rep. 10:1619–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelton SI: The global evolution of
meningococcal epidemiology following the introduction of
meningococcal vaccines. J Adolesc Health. 59 Suppl 2:S3–S11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Braunstein M, Rajkumar P, Claus CL,
Vaccarelli G, Moore AJ, Wang D and Anderson MK: HEBAlt enhances the
T-cell potential of fetal myeloid-biased precursors. Int Immunol.
22:963–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brehony C, Rodrigues CM, Borrow R, Smith
A, Cunney R, Moxon ER and Maiden MCJ: Distribution of
bexsero® antigen sequence types (BASTs) in invasive
meningococcal disease isolates: Implications for immunisation.
Vaccine. 34:4690–4697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung JW, Hsieh SY, Lin CL, Leng CH, Liu
SJ, Chou AH, Lai LW, Lin LH, Kwok Y, Yang CY and Chong P:
Biochemical characterizations of Escherichia coli-expressed
protective antigen Ag473 of Neisseria meningitides group B.
Vaccine. 28:8175–8182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khatami A and Pollard AJ: The epidemiology
of meningococcal disease and the impact of vaccines. Expert Rev
Vaccines. 9:285–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin D, Brodeur BR, Hamel J, Couture F,
de Alwis U, Lian Z, Martin S, Andrews D and Ellis RW: Candidate
Neisseria meningitidis NspA vaccine. J Biotechnol. 83:27–31. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Yang X, Yang C, Wu Z, Xu H and
Shen Y: Crystal structure of outer membrane protein NMB0315 from
Neisseria meningitidis. PLoS One. 6:e268452011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Kang HE and Woo HJ: Protective
immunity conferred by the C-terminal fragment of recombinant
Pasteurella multocida toxin. Clin Vaccine Immunol. 19:1526–1531.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang Y, Lin H, Ma Z and Fan H:
Construction and immunogenicity of recombinant swinepox virus
expressing outer membrane protein L of salmonella. J Microbiol
Biotechnol. 26:1173–1181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borrow R, Carlone GM, Rosenstein N, Blake
M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff
DM, et al: Neisseria meningitidis group B correlates of protection
and assay standardization-International Meeting Report Emory
University, Atlanta, Georgia, United States, 16–17 March 2005.
Vaccine. 24:5093–5107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johansson L, Rytkonen A, Bergman P,
Albiger B, Källström H, Hökfelt T, Agerberth B, Cattaneo R and
Jonsson AB: CD46 in meningococcal disease. Science. 301:373–375.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sjölinder H and Jonsson AB: Olfactory
nerve-a novel invasion route of Neisseria meningitidis to reach the
meninges. PLoS One. 5:e140342010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaudhari A, Pathakota GB and Annam PK:
Design and construction of shrimp antiviral DNA vaccines expressing
long and short hairpins for protection by RNA interference. Methods
Mol Biol. 1404:225–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Abdulhaqq SA and Weiner DB: DNA vaccines:
Developing new strategies to enhance immune responses. Immunol Res.
42:219–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu QQ, Zhang QH and Ma L: Construction of
recombinant eukaryotic expression plasmid pcDNA3.1(+)-mtDNA of
human colorectal carcinoma cells. Di Yi Jun Yi Da Xue Xue Bao.
25:1016–1019. 2005.(In Chinese). PubMed/NCBI
|
|
19
|
Cheng WK, Plumb AW, Lai JC, Abraham N and
Dutz JP: Topical CpG oligodeoxynucleotide adjuvant enhances the
adaptive immune response against influenza A infections. Front
Immunol. 7:2842016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hemmi H, Takeuchi O, Kawai T, Kaisho T,
Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K and
Akira S: A Toll-like receptor recognizes bacterial DNA. Nature.
408:740–745. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu HM, Wang J, Zhang B, Fang L, Xu K and
Liu RY: CpG-ODN promotes phagocytosis and autophagy through JNK/P38
signal pathway in Staphylococcus aureus-stimulated macrophage. Life
Sci. 161:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buchanan RM, Briles DE, Arulanandam BP,
Westerink MA, Raeder RH and Metzger DW: IL-12-mediated increases in
protection elicited by pneumococcal and meningococcal conjugate
vaccines. Vaccine. 19:2020–2028. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong W, Qi Y, Xiong X, Jiao J, Duan C and
Wen B: Rickettsia rickettsii outer membrane protein YbgF induces
protective immunity in C3H/HeN mice. Hum Vaccin Immunother.
11:642–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trotter CL, Yaro S, Njanpop-Lafourcade BM,
Drabo A, Kroman SS, Idohou RS, Sanou O, Bowen L, Findlow H,
Diagbouga S, et al: Seroprevalence of bactericidal, specific IgG
antibodies and incidence of meningitis due to group A Neisseria
meningitidis by age in Burkina Faso 2008. PLoS One. 8:e554862013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bash MC, Lynn F, Mocca B, Borrow R,
Findlow H, Hassan-King M, Preziosi MP, Idoko O, Sow S, Kulkarni P
and Laforce FM: Development and use of a serum bactericidal assay
using pooled human complement to assess responses to a
meningococcal group A conjugate vaccine in African toddlers. Clin
Vaccine Immunol. 21:755–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Findlow J, Holland A, Andrews N, Weynants
V, Sotolongo F, Balmer P, Poolman J and Borrow R: Comparison of
phenotypically indistinguishable but geographically distinct
Neisseria meningitidis group B isolates in a serum bactericidal
antibody assay. Clin Vaccine Immunol. 14:1451–1477. 2007.
View Article : Google Scholar : PubMed/NCBI
|