Introduction
Numerous diseases, including cancer require
modulation in the immune system for disease management and care
(1). Under the circumstance of
weakened immune responsiveness, the host protection machinery has
to be activated to provide an alternative to conventional
chemotherapy (2). Potential
immunomodulatory agents (3,4) have
been identified based on the observed therapeutic effects of
phytochemicals isolated from various plants (5–7), and
segregates of microorganisms and mammalian proteins, including
immune mediators and certain synthetic chemicals (8).
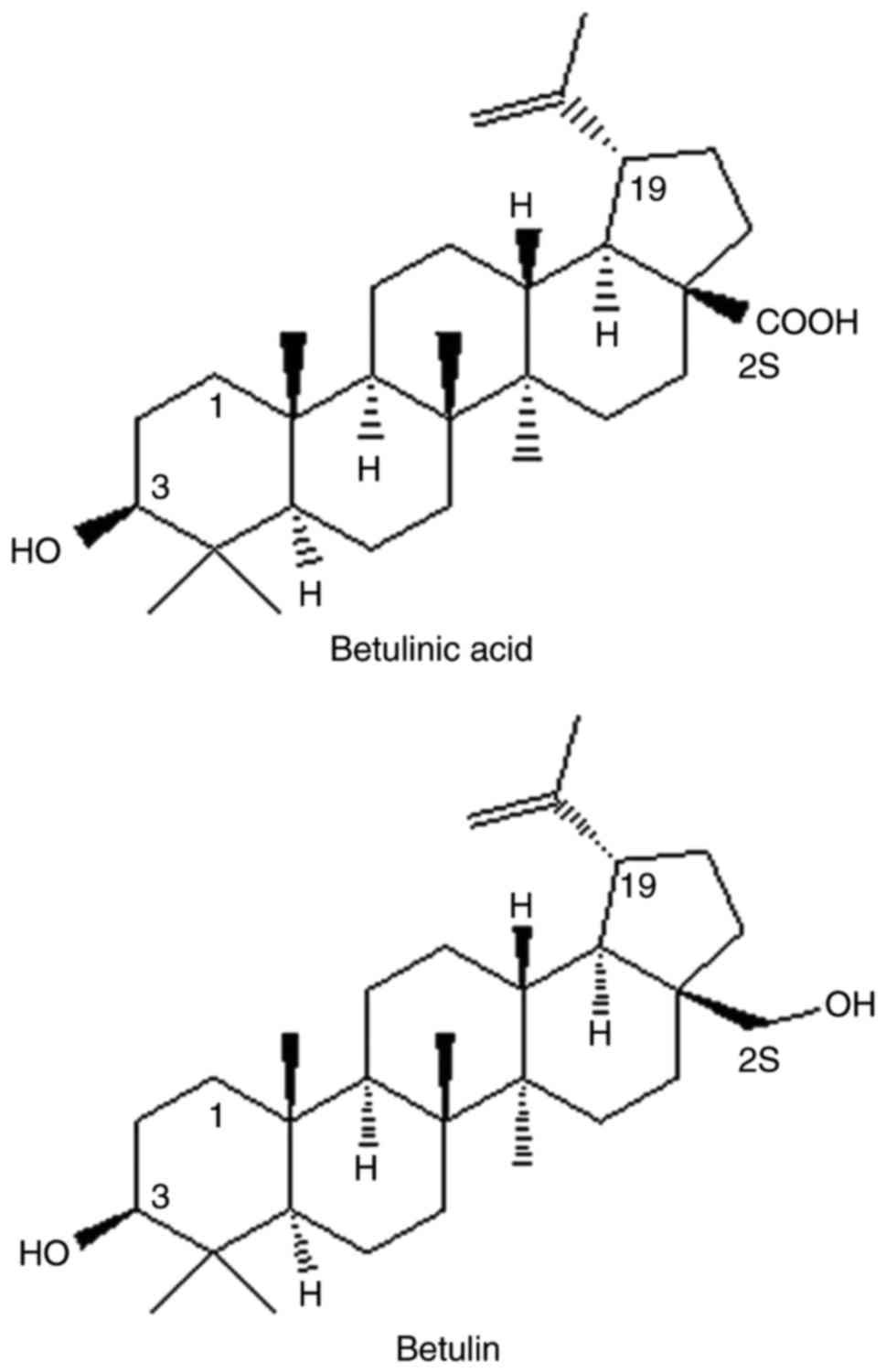
The triterpene alcohol, betulin and its equivalent
carboxylic acid, betulinic acid (BetA; Fig. 1) are isolated from betula birch
bark (9), which has been used for
various medicinal purposes in many countries (10). BetA is a pentacyclic lupane-type
triterpenoid of which a wide range of biological properties have
been shown against cancer, inflammation, malaria, and helmintic and
viral activities (11–14). Among these, the anticancer and
cytotoxic activities of BetA have received significant attention
(15,16). Selective cytotoxicity has been
demonstrated in multiple tumor cell lines (15–19)
without effect in normal cells, including dermal fibroblasts and
peripheral blood lymphocytes (20–22).
Furthermore, no systemic toxic effects have been observed in
rodents (23). However, BetA is
considered to be a weak antineoplastic agent, as it is required at
micromolar concentrations to inhibit cell proliferation in
vitro, and even higher doses are required (250 mg/kg body
weight) to control melanoma growth in athymic mice (24).
Despite a lack of toxicity, the poor potency of BetA
hinders its clinical development. Mullauer et al (23) cautioned that existing in
vivo data are insufficient to support that BetA does not cause
an effect on healthy cells. Furthermore, in contrast to previous
findings, a recent study by Heiss et al (24) reported that 10 µM BetA induces
changes in normal cellular metabolism. The study by Heiss et
al (24) raised concerns
regarding the clinical application of BetA and prompted us to
investigate whether BetA may cause any major effects on healthy
cells in the immune organs of mice.
Nitric oxide (NO) is a free radical and ubiquitous
signaling molecule (25,26) present in immune and endocrine
tissues, among others (27–29)
and a well-known mediator in numerous therapeutic and
immuomodulatory functions, indicating a regulatory function of NO
in primary and secondary immune organs. There are three isoforms of
NO synthases (NOSs), which are neuronal (nNOS), inducible (iNOS)
and endothelial (eNOS), and they are responsible for the synthesis
of NO from the amino acid, l-arginine (30). Previous studies have demonstrated
that all three isoforms express enzymatic activity of NADPH-d
(31,32), and indicated the expression pattern
of NADPH-d is an indirect indication of the presence of NOS and NO
(30,33). Therefore NADPH-d activity has been
used as a marker for NOS.
Therefore, the current study was performed to
determine whether 10 µM BetA modulates NO production or induces
changes in healthy spleen and thymus cells in mice, and to
investigate the possible functions of BetA in these organs. A
histochemical analysis of NADPH-d, a marker for NOS, was also
performed (34).
Materials and methods
Animals and reagents
Animal experiments were conducted in accordance with
the National University of Malaysia (UKM; Kuala Lumpur, Malaysia)
animal ethics guidelines and regulations laid out by the
Institutional Animal Ethics Committee (UKMAEC;
FF/2016/ALI/20-MAY/685-JUNE-2016). Six-week-old, female BALB/c mice
procured from the institutional animal holding facility (n=196),
were maintained (2–3 animals/cage) under pathogen-free conditions
and a 12-h light/dark cycle, with access to commercial pellets and
distilled water. BetA (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) served as a test drug, goniothalamin (GTN; provided by Dr
Ibrahim Jantan, Faculty of Pharmacy, UKM) served as a positive
control drug, and dimethyl sulfoxide (DMSO; Merck KGaA) served as a
vehicle.
Animal treatment and sample
collection
Animals were randomly divided into four main groups
as follows: Experimental group (10 µM BetA; n=48), a positive
control group (50 µM GTN; n=48), a negative control group (0.05%
DMSO; n=48) and a normal standard control group (n=48). Each main
group of animals was further divided into three equal subgroups
(n=6 per group) corresponding to the treatment period (4, 8 and 12
days). Chloral hydrate (10%; i.p.) was administered to anesthetize
the animals, subsequently animals were fixed using 4%
paraformaldehyde and a transcardiac perfusion method described by
Syed et al (27). The fixed
tissue blocks were maintained in the same fixative solution for a
further 6 h as post fixation, followed by rinsing in 0.1 M
phosphate buffer (PB) and cryoprotection in 30% sucrose. Finally,
10 µm frozen sections were sliced (Leica SM2010 Sliding microtome;
Leica Microsystems GmbH, Wetzlar, Germany). Three series of sliced
sections were collected on a glass slide and stained for
NADPH-d.
Histochemistry of NADPH-d and analysis
of tissue morphology
Frozen tissue sections were maintained at room
temperature for 30 min, washed twice in PB, followed by staining
for NADPH-d with the addition of substrate β-NADPH [Malaysia
Sigma-Aldrich (M) Sdn. Bhd, Kuala Lumpur, Malaysia] and nitroblue
tetrazolium, a salt that yields an insoluble blue formazan
precipitate visible under a light microscope (27). In the case of NADPH-d
histochemistry controls, sections were incubated at room
temperature for 45 min in a β-NADPH-free medium. The intensity of
the reaction in the thymus and spleen was determined by measurement
of optical density (OD; 0–260 nm), using Olympus cellSens (Olympus
Soft Imaging Solutions GmbH, Münster, Germany) software version
1.6, and was graphically represented. From each sample, three to
four slides were subjected to the staining procedure and multiple
areas were evaluated randomly using a light microscope. The
cross-sectional morphology of the tissues was observed using an
Olympus BX41TF microscope (Olympus Corp., Tokyo, Japan). Images of
the sections were captured using an Olympus UC30, with Olympus
cellSens software version 1.6. All measurements were performed in
duplicate or triplicate and repeated at least three times.
Statistical analysis
Statistical assessment of the intensity data was
performed using one-way ANOVA followed by Bonferroni multiple
comparison's tests (GraphPad Prism version 4.0; GraphPad Software,
In., La Jolla, CA, USA). All numerical data are expressed as the
mean ± standard error of the mean and P<0.05 was considered to
indicate a statistically significant difference.
Results
NADPH-d expression in the thymus
Although the control group (DMSO-treated vehicle)
received no drug treatment, weak or faint NADPH-d staining was
observed in the cortex and medulla region of the thymus (Fig. 2A-C), whereas in the
positive-control group (GTN), the capsule and cortex region
demonstrated moderate NADPH-d expression 4 days after the GTN
injection, which continuously increased at the eighth and twelfth
day of treatment (Fig. 2D-F). This
indicates that GTN induces time-dependent expression of NADPH-d in
the thymus. However, the NADPH-d distribution was initially similar
to that in the control group after day 4, and subsequently the
distribution spread to the whole lobule on the eighth and twelfth
day of treatment.
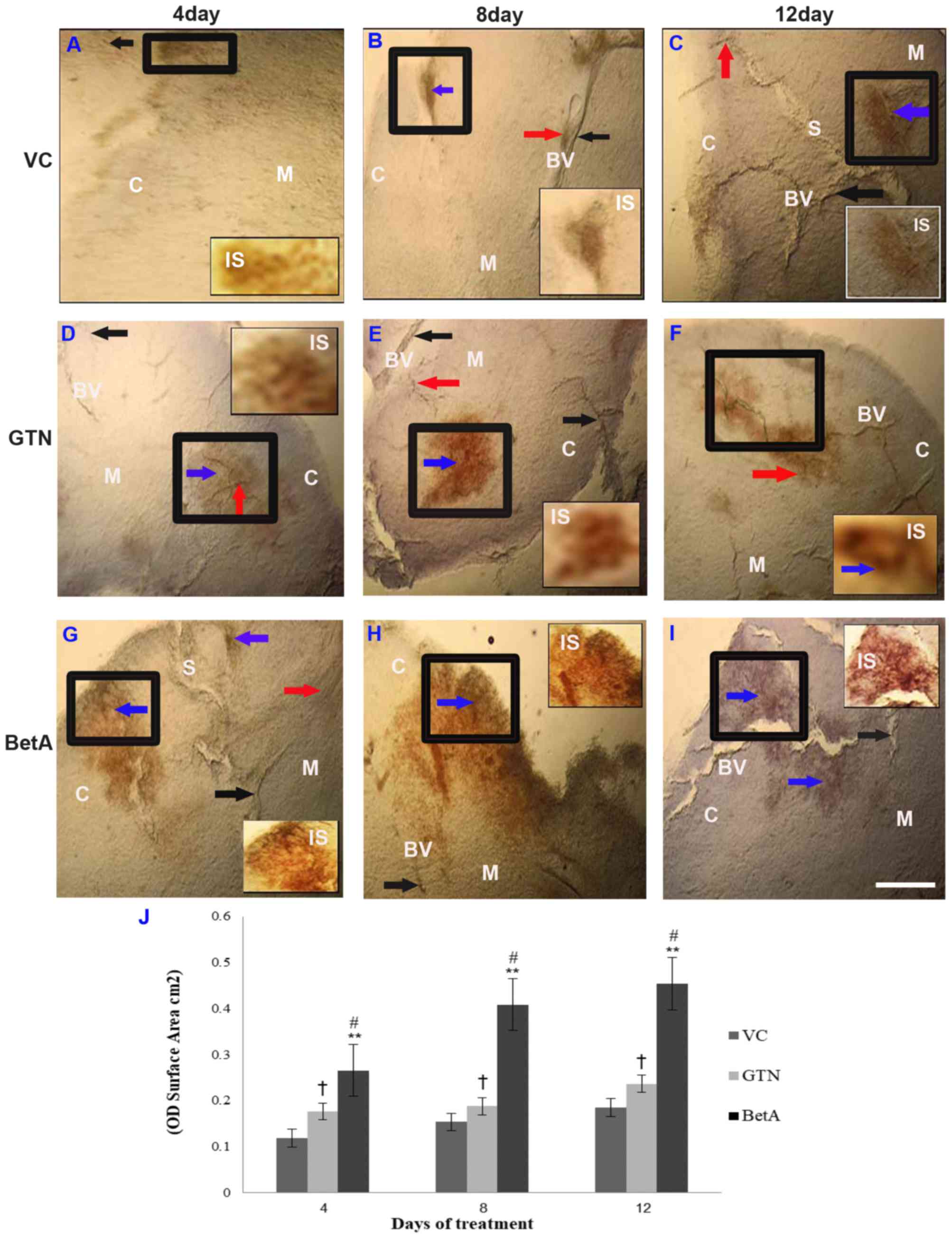 | Figure 2.Representative photomicrographs
demonstrating thymus sections of the (A-C) VC (dimethyl sulfoxide),
(D-F) positive (GTN) and (G-I) BetA-treated groups on days 4, 8 and
12 after treatment. Inset (IS) is representative of the area in the
figure outlined in black. Black arrows identify the NADPH-d-stained
blood vessels (BV), the red arrow points the perivascular nerve
fibres and blue arrow demonstrates the NADPH-d positive cells in
the cortex (denoted by the letter C) and medulla (denoted by the
letter M). The letter ‘S’ indicates the interlobular septum. Scale
bar, 100 µm. (J) The intensity (OD) of the NADPH-d staining was
quantified using Olympus Soft Imaging cellSens software version
1.6. Data are expressed as means ± standard deviation of mice (n=6
per group) **P<0.001 vs. the VC group, #P<0.001
vs. the GTN group. †P<0.01 vs. the VC group. GTN, goniothalamin;
BetA, betulinic acid; NADPH-d, nicotinamide adenine dinucleotide
phosphate diaphorase; VC, vehicle control; OD, optical density. |
Similarly, a gradual increase of NADPH-d
distribution (P<0.001) was observed in the BetA treatment group
and its activity increased along with longer BetA exposure
(Fig. 2G-I). However, the thymus
displayed only slight to moderate NADPH-d staining after 4 days
(Fig. 2G), 8 days (Fig. 2H) and 12 days of BetA treatment
(Fig. 2I), in the cortex and the
medullary region. The interlobular septum extends from the capsule
into the thymus, subdividing the thymus into interconnecting
lobules, which are of varying size and orientation. The expression
pattern of NADPH-d spread to a wider area at the medullary region
across the septum 8 days after BetA treatment, demonstrating higher
NADPH-d activity. As the septum is the site where blood
vasculatures in the thymus are oriented, staining in this region
(Fig. 2C and G) supports that NO
has a role in vascular physiology. The graph (Fig. 2J) demonstrates the intensity (OD)
per staining area (cm2) of the thymus tissue sections
after 4, 8 and 12 days of treatment. Increased NADPH-d expression
indicates that the effect of BetA on the thymus depends on exposure
time.
NADPH-d expression in the spleen
Compared with that in the control group (Fig. 3A-C), the spleen of the GTN
(Fig. 3D-F) and BetA groups
(Fig. 3G-I) demonstrated a
particularly strong NADPH-d reaction, with increased expression
levels in the cortex and medullary regions (Fig. 3D-I), as well as in the vasculature
(Fig. 3C, F and I), which
increased with longer exposure times. The spleen is enclosed by a
fibro elastic outer connective tissue, the capsule, and composed of
white and red pulp, forming two functionally and morphologically
different units. The NADPH-d staining (brown precipitate) was more
widely present in the red pulp than in the white pulp area. The red
pulp region is comprised of various cells, including sinusoids
(35). The graph (Fig. 3J) demonstrates the intensity (OD)
per staining area (cm2) of the spleen tissue sections
after 4, 8 and 12 days of treatment
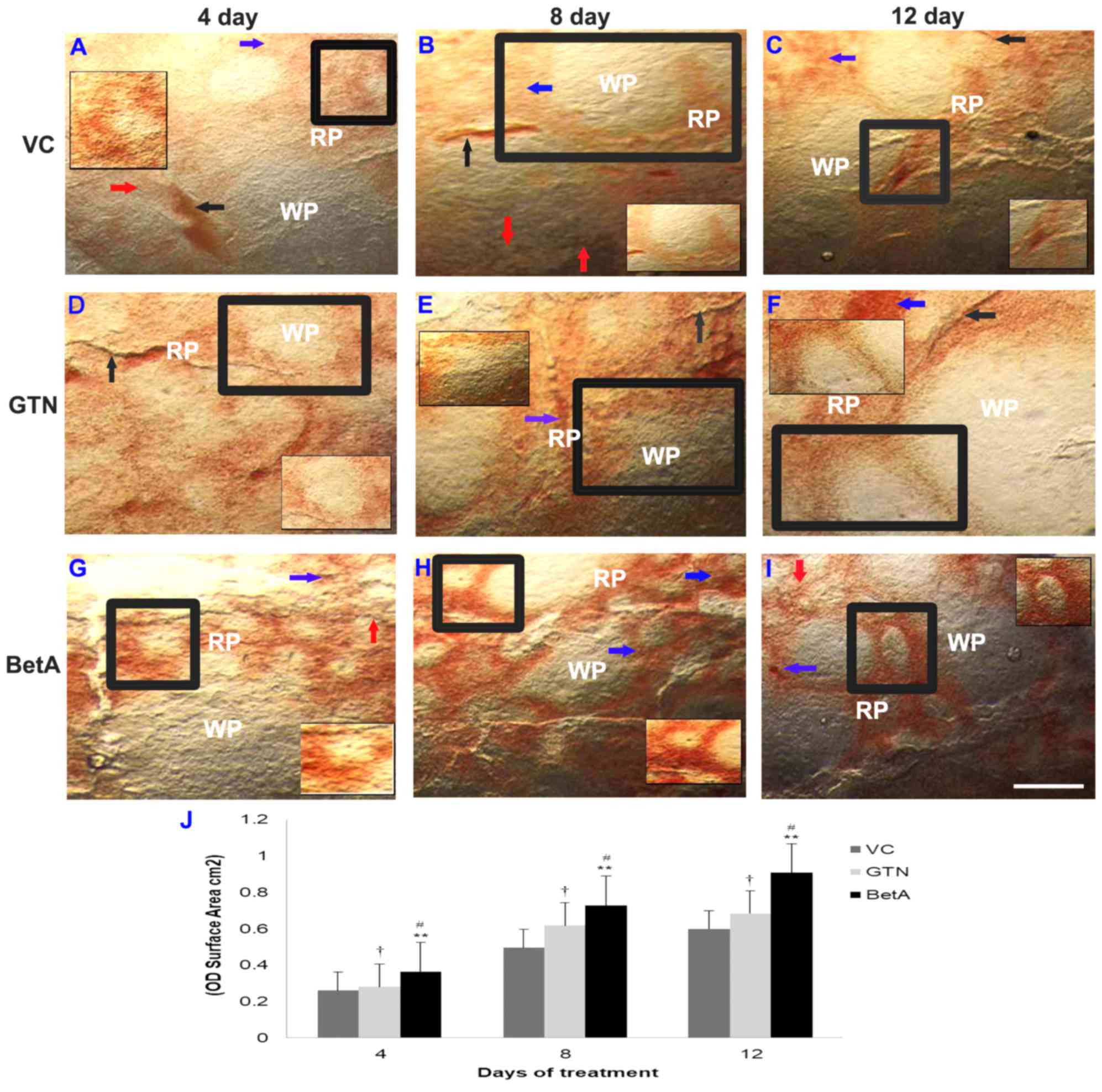 | Figure 3.Representative photomicrographs
demonstrating spleen sections the (A-C) VC (dimethyl sulfoxide),
(D-F) positive (GTN) and (G-I) BetA-treated groups on days 4, 8 and
12 after treatment. Inset (IS) is representative of the area in the
figure outlined in black. Black arrows identify the NADPH-d-stained
blood vessels (BV), the red arrow points the perivascular nerve
fibres and blue arrow demonstrates the NADPH-d positive cells in
the red pulp (RP) region. ‘WP’ indicates the white pulp region.
Scale bar, 100 µm. (J) The intensity (OD) of the NADPH-d staining
was quantified using Olympus Soft Imaging cellSens software version
1.6. Data are expressed as means ± standard deviation of mice (n=6
per group) **P<0.001 vs. the VC group, #P<0.001
vs. the GTN group, †P<0.01 vs. the VC group. GTN,
goniothalamin; BetA, betulinic acid; NADPH-d, nicotinamide adenine
dinucleotide phosphate diaphorase; VC, vehicle control; OD, optical
density. |
Morphological changes
No phenotypical appearance or significant
morphological changes were observed in either the GTN- or the
BetA-treated thymus and spleen.
Discussion
The present study demonstrated positive NADPH-d
staining in the thymus and in the spleen of non-immunized GTN and
BetA-treated mice. However, its expression and distribution pattern
varied with these agents throughout the treatment period. Although
no morphological or phenotypical changes were evident in these
organs, the NADPH-d activity was more prominent in the spleen than
in the thymus. The red pulp region of the spleen strongly expressed
NADPH-d, whereas only a moderate reactivity was observed in the
white pulp region. Although NADPH-d expression was detected after 4
days, a significant increase in expression was observed after 8
days of BetA treatment, indicating the contribution of NADPH-d in
spleen NO generation during the entire treatment period. Similarly,
a steady increase in NADPH-d activity in the thymus beginning from
day 4 may specify an interactive outcome of NO on the thymus
vascular and medulla regions, which may be involved in immune
modulation (33,36–37).
Although the function of NO in the thymus and spleen has not been
demonstrated, the findings of the current study indicate that there
is an association between the immunological effects induced by
BetA. The major effect of BetA-induced NO appears to be involved in
maintenance of the immune system (38–40)
and the current results support other reports in which the presence
of NO was demonstrated in the immune system (33,41).
It has been demonstrated that the expression of NOS
and production of NO are characteristic of cells involved in immune
responses (33,36,42).
Although NO is less active in cells from normal mice (43), a spleen cell subpopulation produces
enough NO during an in vitro immune response to completely
prevent multiplication of T cells (44,45).
Similarly, previous studies have demonstrated that a wide range of
potential immunomodulatory functions may be expected for NO in the
thymus, including the following: Induction of tolerance,
restriction of major histocompatibility complex, lymphocyte
trafficking and regulation of thymic endocrine output (33,36,37,42).
Furthermore, if there are effects or changes in the cells due to
BetA treatment, it is assumed that NO may be produced to perform
its regulatory roles in the cells of these immune organs.
Betulin and BetA trigger and modify cytokine
production in human whole blood cell cultures (46). To support this hypothesis, previous
studies have reported that BetA augments mouse immune function,
including cellular and humoral immunity, and activity of phagocytic
macrophages (39,47). BetA was identified as a modulatory
agent of cytokine production by T helper cells (Th1/Th2) and other
immune cells in animals. Consistently, the results of numerous
studies indicated that BetA may be useful for modulation of the
immune system (40,48–51).
Furthermore, various bioactive materials derived from other plants
exhibit immunomodulatory abilities (52–55).
In addition, NO is recognized to act as a
vasodilator following its release from endothelial cells (56–58),
including BetA-treated endothelial cells (59–62).
This function is mediated by NO inhibitory action on vascular
smooth muscles. However, Moncada et al (25) and other studies (63,64)
have demonstrated that NO-mediated vasodilation occurs without
endothelial cells present, where the NO-positive nitrergic nerves
may act directly on smooth muscle cells, leading to vasodilation.
Such control by neuronal NO has also been demonstrated in the
peripheral nervous system (65,66).
The innervation of NO-positive perivascular nerves
has also been demonstrated in many types of vascular tissue
(67–69). The distribution of supply of nerves
in the thymus are not entirely known; however, previous studies
focused on the neural structures contained within the thymus
(70,71). The activity of NADPH-d was
demonstrated in many parts of the nervous system in mammals
(72–74). The present results are consistent
with previous findings that NADPH-d-labelled cells are present in
the rat thymus (28,30,75).
Although the current study observed NADPH-d-stained nerve fibers in
the perivascular area, no NADPH-d neuronal cell body-like
structures were observed in either the thymus or the spleen. The
moderate distribution of NO-containing nerves travelling along
blood vessels may reflect a particularly significant role that
neuronal NO may perform in controlling blood flow through the
thymus and spleen. The fact that NO-positive nerves and the
vascular endothelium may produce NO to influence blood flow has
been described in the nervous system (74), pancreas (76), thyroid (27,29)
and various other tissues. This observation indicates that NO
participates in neurotransmission in the thymus and spleen. It is
important to note that in addition to blood vessels, NO may
regulate the secretory activity of immune cells by its generation
in these cells (77). Thus, BetA
may present a promising biological response modifier and may
reinforce the immune response of a host.
In conclusion, the current study demonstrates that
BetA treatment induces the expression of NADPH-d activity in the
thymus and spleen without causing any significant changes to the
morphology of the organs. These findings are of direct clinical
relevance and may contribute to the progression of drug discovery.
To the best of our knowledge, this is the most significant study
describing BetA-induced, NADPH-d-mediated NO signaling, which may
be the mechanism underlying BetA-elicited immunomodulation in these
organs.
Acknowledgements
The present study was partly supported by an
internal research grant (grant no. 1437-0195) obtained from the
Deanship of Scientific Research University of Tabuk (Tabuk, Saudi
Arabia).
References
|
1
|
Geetha S, Singh V, Ram MS, Ilavazhagan G,
Banerjee PK and Sawhney RC: Immunomodulatory effects of
seabuckthorn (Hippophae rhamnoides L.) against chromium (VI)
induced immunosuppression. Mol Cell Biochem. 278:101–109. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aravindaram K and Yang NS:
Anti-inflammatory plant natural products for cancer therapy. Planta
Med. 76:1103–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winkler C, Wirleitner B, Schroecksnadel K,
Schennach H, Mur E and Fuchs D: In vitro effects of two extracts
and two pure alkaloid preparations of Uncaria tomentosa on
peripheral blood mononuclear cells. Planta Med. 70:205–210. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patwardhan B and Gautam M: Botanical
immunodrugs: Scope and opportunities. Drug Discov Today.
10:495–502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sreesha T: In-vitro study of cytotoxicity
activity of flavonoid fraction from the petals of Cassia senna. Int
J Ethnomed Pharmacol Res. 1:52–55. 2013.
|
|
6
|
Nagarathna PKM, Reena K, Sriram Reddy and
Johnson W: Review on immunomodulation and immunomodulatory activity
of some herbal plants. Int J Pharm Sci Rev Res. 22:223–230.
2013.
|
|
7
|
Ibrahim J, Waqas A and Syed Nasir AB:
Plant-derived immunomodulators: An insight on their preclinical
evaluation and clinical trials. Front Plant Sci.
6:6552015.PubMed/NCBI
|
|
8
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eiznhamer DA and Xu ZQ: Betulinic acid: A
promising anticancer candidate. IDrugs. 7:359–373. 2004.PubMed/NCBI
|
|
10
|
Higa M, Noha N, Yokaryo H, Ogihara K and
Yogi S: Three new naphthoquinone derivatives from Diospyros
maritima Blume. Chem Pharm Bull (Tokyo). 50:590–593. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanson JR: Pentacyclic triterpenes as
promising agents in cancer. Salvador JAR: Nova Science Publishers,
Inc; New York, NY: pp. 1912010
|
|
12
|
Santos RC, Salvador JA, Marín S, Cascante
M, Moreira JN and Dinis TC: Synthesis and structure-activity
relationship study of novel cytotoxic carbamate and
N-acylheterocyclic bearing derivatives of betulin and betulinic
acid. Bioorg Med Chem. 18:4385–4396. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang DM, Xu HG, Wang L, Li YJ, Sun PH, Wu
XM, Wang GJ, Chen WM and Ye WC: Betulinic acid and its derivatives
as potential antitumor agents. Med Res Rev. 35:1127–1155. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ali-Seyed M, Jantan I, Vijayaraghavan K
and Bukhari SN: Betulinic acid: Recent advances in chemical
modifications, effective delivery, and molecular mechanisms of a
promising anticancer therapy. Chem Biol Drug Des. 87:517–536. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alakurtti S, Mäkelä T, Koskimies S and
Yli-Kauhaluoma J: Pharmacological properties of the ubiquitous
natural product betulin. Eur J Pharm Sci. 29:1–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Csuk R: Betulinic acid and its
derivatives: A patent review (2008–2013). Expert Opin Ther Pat.
24:913–923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuco V, Supino R, Righetti SC, Cleris L,
Marchesi E, Gambacorti-Passerini C and Formelli F: Selective
cytotoxicity of betulinic acid on tumor cell lines, but not on
normal cells. Cancer Lett. 175:17–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thurnher D, Turhani D, Pelzmann M,
Wannemacher B, Knerer B, Formanek M, Wacheck V and Selzer E:
Betulinic acid: A new cytotoxic compound against malignant head and
neck cancer cells. Head Neck. 25:732–740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mukherjee R, Kumar V, Srivastava SK,
Agarwal SK and Burman AC: Betulinic acid derivatives as anticancer
agents: Structure activity relationship. Anticancer Agents Med
Chem. 6:271–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galgon T, Wohlrab W and Dräger B:
Betulinic acid induces apoptosis in skin cancer cells and
differentiation in normal human keratinocytes. Exp Dermatol.
14:736–743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selzer E, Pimentel E, Wacheck V, Schlegel
W, Pehamberger H, Jansen B and Kodym R: Effects of betulinic acid
alone and in combination with irradiation in human melanoma cells.
J Invest Dermatol. 114:935–940. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Periasamy G, Teketelew G, Gebrelibanos M,
Sintayehu B, Gebrehiwot M, Karim A and Geremedhin G: Betulinic acid
and its derivatives as anti-cancer agent: A review. Arch Appl Sci
Res. 6:47–58. 2014.
|
|
23
|
Mullauer FB, Kessler JH and Medema JP:
Betulinic acid, a natural compound with potent anticancer effects.
Anticancer Drugs. 21:215–227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heiss EH, Kramer MP, Atanasov AG, Beres H,
Schachner D and Dirsch VM: Glycolytic switch in response to
betulinic acid in non-cancer cells. PLoS One. 9:e1156832014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moncada S, Palmer RM and Higgs A: Nitric
oxide: Physiology, pathophysiology and pharmacology. Pharmacol Rev.
43:109–142. 1991.PubMed/NCBI
|
|
26
|
Knowles RG and Moncada S: Nitric oxide
synthase in mammals. Biochemical J. 298:249–258. 1994. View Article : Google Scholar
|
|
27
|
Syed MA, Leong SK and Chan AS:
Localization of NADPH-diaphorase reactivity in the chick and mouse
thyroid gland. Thyroid. 4:475–478. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gulati P, Leong SK and Chan AS: Ontogeny
of NADPH-d expression in the thymic microenvironment of the chick
embryo. Cell Tissue Res. 294:335–343. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akbari Z, Rohani MH and Behzadi G:
NADPH-d/NOS reactivity in the lumbar dorsal horn of congenitally
hypothyroid pups before and after formalin pain induction. Int J
Dev Neurosci. 27:779–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837,
837a-837d. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andronowska A and Chruściel M: Expression
and cellular distribution of NADPH-diaphorase and nitric oxide
synthases in the porcine uterus during early pregnancy. Folia
Histochem Cytobiol. 45:375–380. 2007.PubMed/NCBI
|
|
32
|
Ali SM, Chan AS and Leong SK: Localization
of nitrergic neuronal and non-neuronal cells in the ultimobranchial
glands of the chicken. Anat Embryol (Berl). 193:161–168. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dorko F, Špakovská T, Lovasová K, Patlevič
P and Kluchová D: NADPH-d activity in rat thymus after the
application of retinoid acid. Eur J Histochem. 56:e72012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hope BT, Michael GJ, Knigge KM and Vincent
SR: Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl
Acad Sci USA. 88:pp. 2811–2814. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Steiniger BS: Human spleen microanatomy:
Why mice do not suffice. Immunology. 145:334–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danko J, Ondrasovic M, Svický E, Jenca A,
Pospieszny N and Ondrasovicová O: Histochemical study of
innervation and NADPH-D activity of the thymus. Anat Histol
Embryol. 32:233–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Svický E, Ondraovic M, Danko J,
Ondrasovicová O, Jenca A, Pospieszny N and Toropila M: Localisation
of NADPH-diaphorase-positive structures in the thymus of the rat,
mouse and rabbit. Folia Morphol (Warsz). 62:167–170.
2003.PubMed/NCBI
|
|
38
|
Jacobs AT and Ignarro LJ:
Lipopolysaccharide-induced expression of interferon-beta mediates
the timing of inducible nitric-oxide synthase induction in RAW
264.7 macrophages. J Biol Chem. 276:47950–47957. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi JE, Obminska-Mrukowicz B, Yuan LY and
Yuan H: Immunomodulatory effects of betulinic acid from the bark of
white birch on mice. J Vet Sci. 11:305–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jine Y, Lis M, Szczypka M and
Obmińska-Mrukowicz B: Influence of betulinic acid on lymphocyte
subsets and humoral immune response in mice. Pol J Vet Sci.
15:305–313. 2012.PubMed/NCBI
|
|
41
|
Hibbs JB Jr: Infection and nitric oxide. J
Infect Dis. 185 Suppl 1:S9–S17. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ibiza S and Serrador JM: The role of
nitric oxide in the regulation of adaptive immune responses.
Immunología. 27:103–117. 2008.
|
|
43
|
Filep JG, Földes-Filep E, Rousseau A,
Sirois P and Fournier A: Vascular responses to endothelin-1
following inhibition of nitric oxide synthesis in the conscious
rat. Br J Pharmacol. 110:1213–1221. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Umeshappa CS, Singh KP, Nanjundappa RH,
Channappanavar R, Maan S and Maan NS: Bluetongue virus-23
stimulates inducible nitric oxide synthase expression and nitric
oxide production in mononuclear cells of blood and/or regional
lymphoid organs. Vet Res Commun. 36:245–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Darwiche SS, Pfeifer R, Menzel C, Ruan X,
Hoffman M, Cai C, Chanthaphavong RS, Loughran P, Pitt BR, Hoffman
R, et al: Inducible nitric oxide synthase contributes to immune
dysfunction following trauma. Shock. 38:499–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zdzisińska B, Rzeski W, Paduch R,
Szuster-Ciesielska A, Kaczor J, Wejksza K and Kandefer-Szerszeń M:
Differential effect of betulin and betulinic acid on cytokine
production in human whole blood cell cultures. Pol J Pharmacol.
55:235–238. 2003.PubMed/NCBI
|
|
47
|
Chen S, Bai Y, Li Z, Jia K, Jin Y, He B,
Qiu WW, Du C, Siwko S, Chen H, et al: A betulinic acid derivative
SH479 inhibits collagen-induced arthritis by modulating T cell
differentiation and cytokine balance. Biochem Pharmacol. 126:69–78.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sultana N and Saify ZS: Naturally
occurring and synthetic agents as potential anti-inflammatory and
immunomodulants. Antiinflamm Antiallergy Agents Med Chem. 11:3–19.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang P, Li Q, Li K, Zhang X, Han Z, Wang
J, Gao D and Li J: Betulinic acid exerts immunoregulation and
anti-tumor effect on cervical carcinoma (U14) tumor-bearing mice.
Pharmazie. 67:733–739. 2012.PubMed/NCBI
|
|
50
|
Dash SK, Chattopadhyay S, Tripathy S, Dash
SS, Das B, Mandal D, Mahapatra SK, Bag BG and Roy S: Self-assembled
betulinic acid augments immunomodulatory activity associates with
IgG response. Biomed Pharmacother. 75:205–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yi J, Zhu R, Wu J, Wu J, Xia W, Zhu L,
Jiang W, Xiang S and Tan Z: In vivo protective effect of betulinic
acid on dexamethasone induced thymocyte apoptosis by reducing
oxidative stress. Pharmacol Rep. 68:95–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kovalenko LP, Balakshin VV, Presnova GA,
Chistyakov AN, Shipaeva EV, Alekseeva SV and Durnev AD:
Immunotoxicity and allergenic properties of betulin-containing
birch bark dry extract. Pharm Chem J. 41:17–19. 2007. View Article : Google Scholar
|
|
53
|
Naithani R, Huma LC, Moriarty RM,
McCormick DL and Mehta RG: Comprehensive review of cancer
chemopreventive agents evaluated in experimental carcinogenesis
models and clinical trials. Curr Med Chem. 15:1044–1071. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Paszkiewicz M, Budzyńska A, Różalska B and
Sadowska B: The immunomodulatory role of plant polyphenols. Postepy
Hig Med Dosw (Online). 66:637–636. 2012.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mathew NS and Negi PS: Traditional uses,
phytochemistry and pharmacology of wild Banana (Musa acuminata
Colla): A review. J Ethnopharmacol. 196:124–140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Schmidt A, Bilgasem S, Lorkowski S,
Vischer P, Völker W, Breithardt G, Siegel G and Buddecke E:
Exogenous nitric oxide regulates activity and synthesis of vascular
endothelial nitric oxide synthase. Eur J Clin Invest. 38:476–485.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Son Y, Lee JH, Cheong YK, Jung HC, Jeong
SO, Park SH and Pae HO: Piceatannol, a natural hydroxylated analog
of resveratrol, promotes nitric oxide release through
phosphorylation of endothelial nitric oxide synthase in human
endothelial cells. Eur Rev Med Pharmacol Sci. 19:3125–3132.
2015.PubMed/NCBI
|
|
58
|
Tillery LC, Epperson TA, Eguchi S and
Motley ED: Differential regulation of endothelial nitric oxide
synthase phosphorylation by protease-activated receptors in adult
human endothelial cells. Exp Biol Med (Maywood). 241:569–580. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Steinkamp-Fenske K, Bollinger L, Xu H, Yao
Y, Horke S, Förstermann U and Li H: Reciprocal regulation of
endothelial nitric-oxide synthase and NADPH oxidase by betulinic
acid in human endothelial cells. J Pharmacol Exp Ther. 322:836–842.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian LB, Fu JY, Cai X and Xia ML:
Betulinic acid inhibits superoxide anion-mediated impairment of
endothelium-dependent relaxation in rat aortas. Ind J Pharmacol.
44:588–592. 2012. View Article : Google Scholar
|
|
61
|
Hohmann N, Xia N, Steinkamp-Fenske K,
Förstermann U and Li H: Estrogen receptor signaling and the
PI3K/Akt pathway are involved in betulinic acid-induced eNOS
activation. Molecules. 21:E9732016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jin SW, Choi CY, Hwang YP, Kim HG, Kim SJ,
Chung YC, Lee KJ, Jeong TC and Jeong HG: Betulinic acid increases
eNOS phosphorylation and no synthesis via the calcium-signaling
pathway. J Agric Food Chem. 64:785–791. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen MF, Huang YC, Long C, Yang HI, Lee
HC, Chen PY, Hoffer BJ and Lee TJ: Bimodal effects of fluoxetine on
cerebral nitrergic neurogenic vasodilation in porcine large
cerebral arteries. Neuropharmacology. 62:1651–1658. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lies B, Beck K, Keppler J, Saur D,
Groneberg D and Friebe A: Nitrergic signalling via interstitial
cells of Cajal regulates motor activity in murine colon. J Physiol.
593:4589–4601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wenisch S and Arnhold S: NADPH-diaphorase
activity and NO synthase expression in the olfactory epithelium of
the bovine. Anat Histol Embryol. 39:201–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Şelaru M, Rusu MC and Jianu AM: Expression
of nNOS in the human larynx. Anat Sci Int. 90:327–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Knipping S, Holzhausen HJ, Berghaus A,
Bloching M and Riederer A: Ultrastructural detection of nitric
oxide in human nasal mucosa. Otolaryngol Head Neck Surg.
132:620–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Koyama T, Hatanaka Y, Jin X, Yokomizo A,
Fujiwara H, Goda M, Hobara N, Zamami Y, Kitamura Y and Kawasaki H:
Altered function of nitrergic nerves inhibiting sympathetic
neurotransmission in mesenteric vascular beds of renovascular
hypertensive rats. Hypertens Res. 33:485–491. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shimada S, Todoki K, Omori Y, Toyama T,
Matsuo M, Wada-Takahashi S, Takahashi SS and Lee MC: Contribution
of nitrergic nerve in canine gingival reactive hyperemia. J Clin
Biochem Nutr. 56:98–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mignini F, Sabbatini M, D'Andrea V and
Cavallotti C: Intrinsic innervation and dopaminergic markers after
experimental denervation in rat thymus. Eur J Histochem.
54:e172010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dorko F, Danko J, Flešárová S, Boroš E and
Sobeková A: Effect of pesticide bendiocar-bamate on distribution of
acetylcholine- and butyrylcholine-positive nerves in rabbit's
thymus. Eur J Histochem. 55:e372011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu C, Yang Y, Hu X, Li JM, Zhang XM, Cai
Y, Li Z and Yan XX: Ontogenesis of NADPH-diaphorase positive
neurons in guinea pig neocortex. Front Neuroanat. 9:112015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jung J, Na C and Huh Y: Alterations in
nitric oxide synthase in the aged CNS. Oxid Med Cell Longev.
2012:7189762012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cossenza M, Socodato R, Portugal CC,
Domith IC, Gladulich LF, Encarnação TG, Calaza KC, Mendonça HR,
Campello-Costa P and Paes-de-Carvalho R: Nitric oxide in the
nervous system: Biochemical, developmental, and neurobiological
aspects. Vitam Horm. 96:79–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Downing JE: Multiple nitric oxide synthase
systems in adult rat thymus revealed using NADPH diaphorase
histochemistry. Immunology. 82:659–664. 1994.PubMed/NCBI
|
|
76
|
Villanueva C and Giulivi C: Subcellular
and cellular locations of nitric-oxide synthase isoforms as
determinants of health and disease. Free Radic Biol Med.
49:307–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bogdan C: Nitric oxide synthase in innate
and adaptive immunity: An update. Trends Immunol. 36:161–178. 2015.
View Article : Google Scholar : PubMed/NCBI
|