Introduction
Glioma is the most common malignant tumor of the
central nervous system in adults. According to the WHO
classification of tumors of the nervous system, glioma may be
further divided into four grades (grades I–IV) with increasing
malignancy (1). Grade IV,
additionally termed glioblastoma multiforme (GBM), is the most
malignant type of brain tumor. Despite the improved survival
associated with modern surgical, chemotherapy and radiotherapy
treatments, the prognosis of patients with glioma remains poor due
its rapid and invasive growth, its genetic heterogeneity, and a
lack of understanding of its underlying molecular mechanisms
(2,3).
Vasculogenic mimicry (VM) refers to non-endothelial
tumor cell-lined microvascular channels in aggressive, malignant
and genetically dysregulated tumors (4,5). A
previous report indicated that VM had been implicated in invasion,
metastasis and cancer progression (6). However, there is limited data
regarding the correlation with VM and abnormally-expressed genes in
human gliomas. Aquaporins (AQPs) are a family of water-selective
transmembrane transport channels that allow rapid movement of
H2O across normally hydrophobic cell membranes (7). In previous studies, AQP1 and AQP4
have received the most attention due to their contributions to
brain edema (8,9). In brain tumors, studies have
demonstrated that AQP1 expression is increased with the grade of
malignancy, and was associated with tumor blood vessels (10,11).
However, the role of AQP1 remains speculative in glioma.
In the present study, the AQP1 expression was
measured in clinical glioma tissue samples and GBM cell lines.
Subsequently, short hairpin (shRNA)-mediated AQP1 silencing was
used to assess the potential effects of AQP1 on migration, invasion
and VM formation using two types of GBM cell lines in vitro
and in vivo. The present study aimed to assess the
expression and functional role of aquaporin-1 (AQP1) in human GBM
migration, invasion and VM formation.
Materials and methods
Clinical samples and cell lines
All the clinical glioma tissues samples were
obtained from Guangzhou Overseas Chinese Hospital (Guangzhou,
China) and, according to criteria from the World Health
Organization (WHO), classified into 4 grades with increasing
malignancy: Grade I pilocytic astrocytoma; grade II astrocytoma;
grade III anaplastic astrocytoma; and grade IV GBM, the most
malignant brain tumor. For each grade, 10 cases were used in the
present study. All samples were freshly frozen in liquid nitrogen
and stored at −80°C until RNA extraction. The present study was
approved by the Institutional Review Boards of the Guangzhou
Overseas Chinese Hospital, and all participants provided written
informed consent. The GBM cell lines A172 and U251, and the normal
glial cell line HEB were obtained from the Shanghai Cell Collection
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences) at 37°C in a 5% CO2 humidified
atmosphere.
Cell transfection
The shRNA-AQP1 was synthesized and cloned into the
pSUPER-retro-puromycin plasmid (Shangahi GenePharma Co., Ltd.,
Shanghai, China). The shRNA-AQP1 sequence was:
5′-GATCACACACAACTTCAGCAACTCGAGTTGCTGAAGTTGTGTGTGATC-3, and the
negative control sequence was:
5′-CACCGTTCTCCGAACGTGTCACGTCGAAACGTGACACGTTCGGAGAA-3′. The plasmids
above were combined with PIK vector, and lentiviral vectors were
constructed. Following 10 h transfection (multiplicity of
infection=20) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc. Waltham, MA, USA), according to the manufacturer's
protocol, the transfected A172 and U251 cells were selected with
puromycin, and the stably transfected cell lines were prepared by
monoclonal screening. Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blot analyses were used to
assess the transfection efficiency as detailed below.
RT-qPCR assay
Total RNA was extracted from the tissues and cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The cDNA synthesis kit (Takara Biotechnology Co., Ltd., Dalian,
China) was used, according to the manufacturer's protocol. qPCR was
performed to detect the expression levels of AQP1 mRNA using the
LightCycler 480 detection system (Roche Diagnostics, Indianapolis,
IN, USA). The thermocycling conditions were as follows: Initial
denaturation at 95°C for 3 min, followed by 45 cycles of 95°C (15
sec) and 60°C (30 sec). The primer sequences of AQP1 were: Forward,
5′-TCATCTACGACTTCATCCTGGC-3′ and reverse,
5′-GGAAGCTCCTGGAGTTGATGT-3′. β-actin mRNA levels were used for
normalization: Forward 5′-GTCCACACCCGCCACCAGTTC-3′ and reverse
5′-TCCCACCATCACACCCTGGTG-3′. The qPCR results were analyzed and
expressed as relative mRNA levels of the Cq value, which was then
converted to a fold change (12).
Western blot analysis
The tissue samples and cells were lysed in a
radio-immunoprecipitation assay buffer [50 mM Tris-HCl (pH 7.4),
150 mM NaCl, 1% NP-40] containing a protease inhibitor cocktail
(Roche Diagnostics), and the protein concentration was measured
using a micro bicinchoninic acid protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.). A total of 50 µg per lane of the total
cell lysates was resolved on 10% SDS-PAGE gels and transferred to
polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific,
Inc.). The PVDF membranes were blocked with 5% non-fat dry milk for
1 h at room temperature, and followed by immunoblot detection and
visualization with enhanced chemiluminescence western blot
detection reagents (Pierce; Thermo Fisher Scientific, Inc.).
Immunoblotting was performed with AQP1 antibodies (Abcam,
Cambridge, UK; cat. no. ab15080; 1:1,000) at 37°C for 2 h, followed
by incubation with the horseradish-peroxidase-conjugated
immunoglobulin G secondary antibodies (Abcam; cat. no. ab97023;
1:5,000) for 1 h at room temperature. GAPDH (Abcam; cat. no.
ab8245; 1:1,000) levels were used for the control and
normalization. The protein bands were scanned and quantified using
ChemiDoc MP imaging analysis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) as the relative grey value.
Transwell migration and invasion
assay
The transfected A172 and U251 cells were trypsinized
and resuspended to a density of 5×105 cells/ml in
serum-free medium. A total of 200 µl cell suspension was added to
the upper chamber of each well in 24-well polycarbonate Transwell
membrane inserts (BD, 353097, USA) coated with 40 µl extracellular
matrix (ECM) Matrigel (invasion assay; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) or without Matrigel (migration assay), and 600
µl Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum was added in the lower chamber. Following incubation
for 24–48 h at 37°C, cells on the upper membrane surface were
removed by careful wiping with a cotton swab, and the Transwells
were fixed in 4% paraformaldehyde solution for 30 min at room
temperature and stained with 0.2% crystal violet solution for 30
min at room temperature. Migrated and invaded cells adhering to the
underside of the Transwell were counted using an inverted
microscope (magnification, ×400).
VM assay
A total of 50 µl ECM Matrigel (Sigma-Aldrich; Merck
KGaA) was dropped onto 18-mm glass coverslips in 6-well plates and
incubated at 37°C for 1 h. Subsequently, the transfected A172 and
U251 cells (2×105 cells/well) were seeded onto the
coated coverslips. Following incubation for 24–48 h, the cells were
fixed in 4% paraformaldehyde-PBS for 10 min at room temperature,
oxidized in a 0.5% periodic acid solution for 5 min and rinsed with
PBS. The coverslips were dried at room temperature and the VM
images were captured at ×400 magnification (CX71, Olympus
Corporation, Tokyo, Japan).
In vivo xenograft experiments
A total of 6 female BALB/C nude mice (weight, 20–22
g) at the age of 4 weeks were obtained from the Laboratory Animal
Centre of Jinan University (Guangzhou, China). The animals were
maintained on a 12 h light/dark cycle under room temperature
(24±1°C) and humidity of 50±10%, and free fed with standard forage
and clean water. They were randomly divided into two groups (three
mice per group). The silenced A172 cell and negative control cell
suspensions (5×106 cells/ml) in 200 µl serum-free medium
were subcutaneously injected into the flanks of nude mice. Tumor
growth was measured twice per week for 4–5 weeks. Following 5
weeks, tumor samples were carefully isolated, weighed and analyzed
by hematoxylin-eosin (HE) staining. The experimental protocol was
approved by the Laboratory Animal Ethics Committee of Jinan
University (Guangzhou, China).
Invasion-associated protein
measurement and HE staining
The isolated tumor tissues were divided into two
parts: One for the measurement of the invasion-associated proteins
αvβ3 integrin (Abcam, Cambridge, UK; cat. no. ab78289; dilution,
1:500), 72 kDa type IV collagenase (MMP-2; Abcam; cat. no. ab37150;
dilution, 1:500) and matrix metalloproteinase-9 (MMP-9; Abcam; cat.
no. ab38898; dilution, 1;1,000) using western blot analysis as
aforementioned; and the other for HE staining.
The tissues were fixed in a 4% paraformaldehyde-PBS
solution for 30 min at room temperature, and sliced into 3–5 µm
sections following paraffin-embedding. Slides were stained as
follows: 70% ethyl alcohol for 10 sec; diethylpyrocarbonate-treated
water for 5 sec; hematoxylin with RNAase inhibitor for 20 sec; 70%
ethyl alcohol for 30 sec; eosin Y in 100% ethyl alcohol for 20 sec
followed by dehydration with a series of alcohols for 30 sec each;
and xylene for 2 min.
Statistical analyses
The data were analyzed by one-way analysis of
variance and data from the multiple groups were also analyzed using
analysis of variance with repeated measures. The Student's t-test
to determine statistical significance using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). In addition, the least
significant difference post-hoc test was employed where equal
variances were assumed, while Dunnett's T3 test used when equal
variances were not assumed. The results are expressed as the mean ±
standard deviation with at least three times. Two-tailed P<0.05
was considered to indicate a statistically significant
difference.
Results
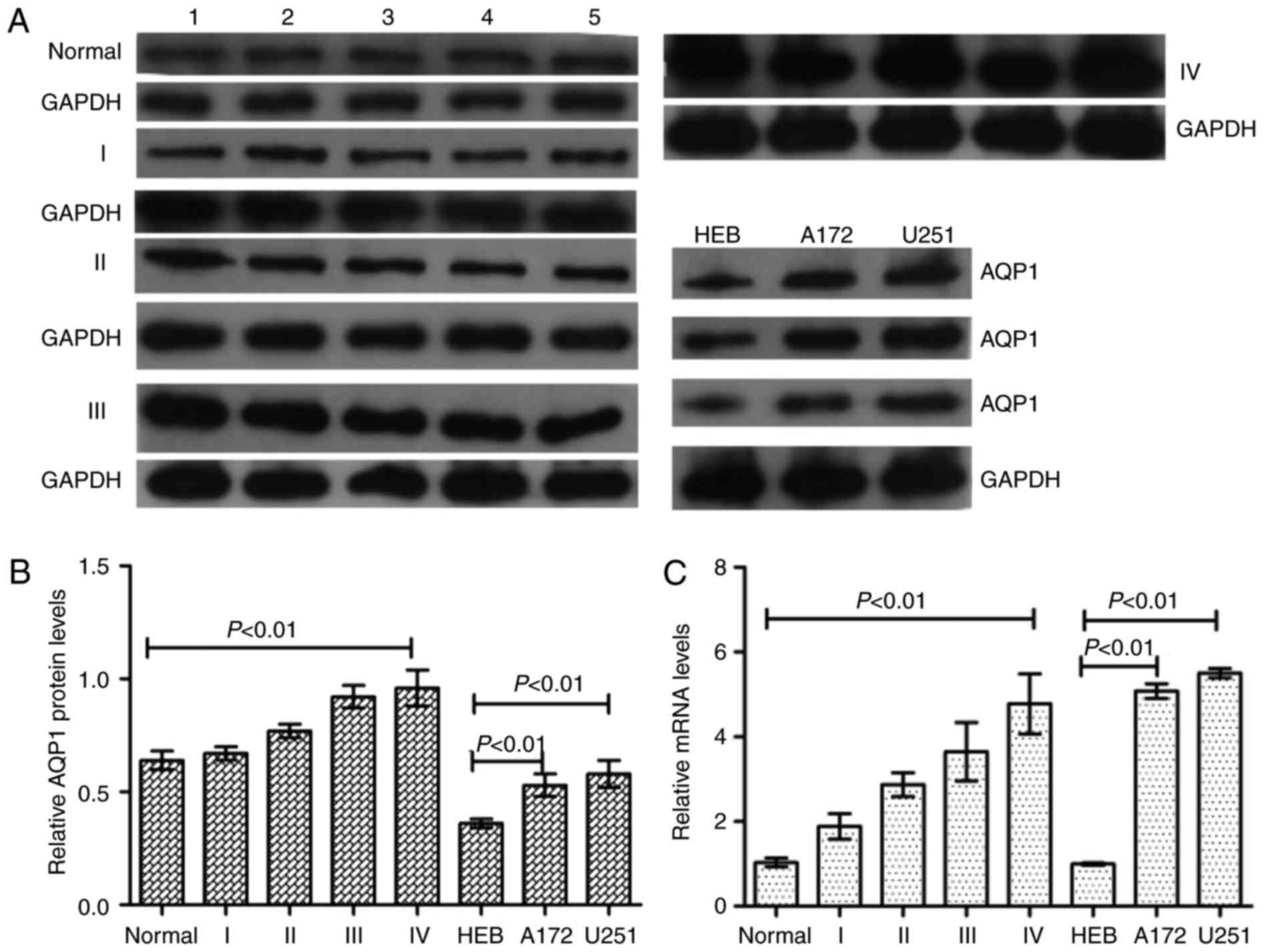
AQP1 is upregulated in glioma tissues
and cell lines, and is associated with malignancy grade
The expression of AQP1 in normal brain tissues,
glioma tumor tissues and glioma cell lines was analyzed by western
blotting and RT-qPCR analysis. Western blotting and RT-qPCR
analysis demonstrated that AQP1 was expressed at higher levels in
glioma tumor tissues and cell lines compared with normal brain
tissues and HEB cells, and was positively associated with glioma
malignancy (glioma grades; Fig.
1), meaning that a higher malignant grade of glioma was
associated with a higher AQP1 expression level.
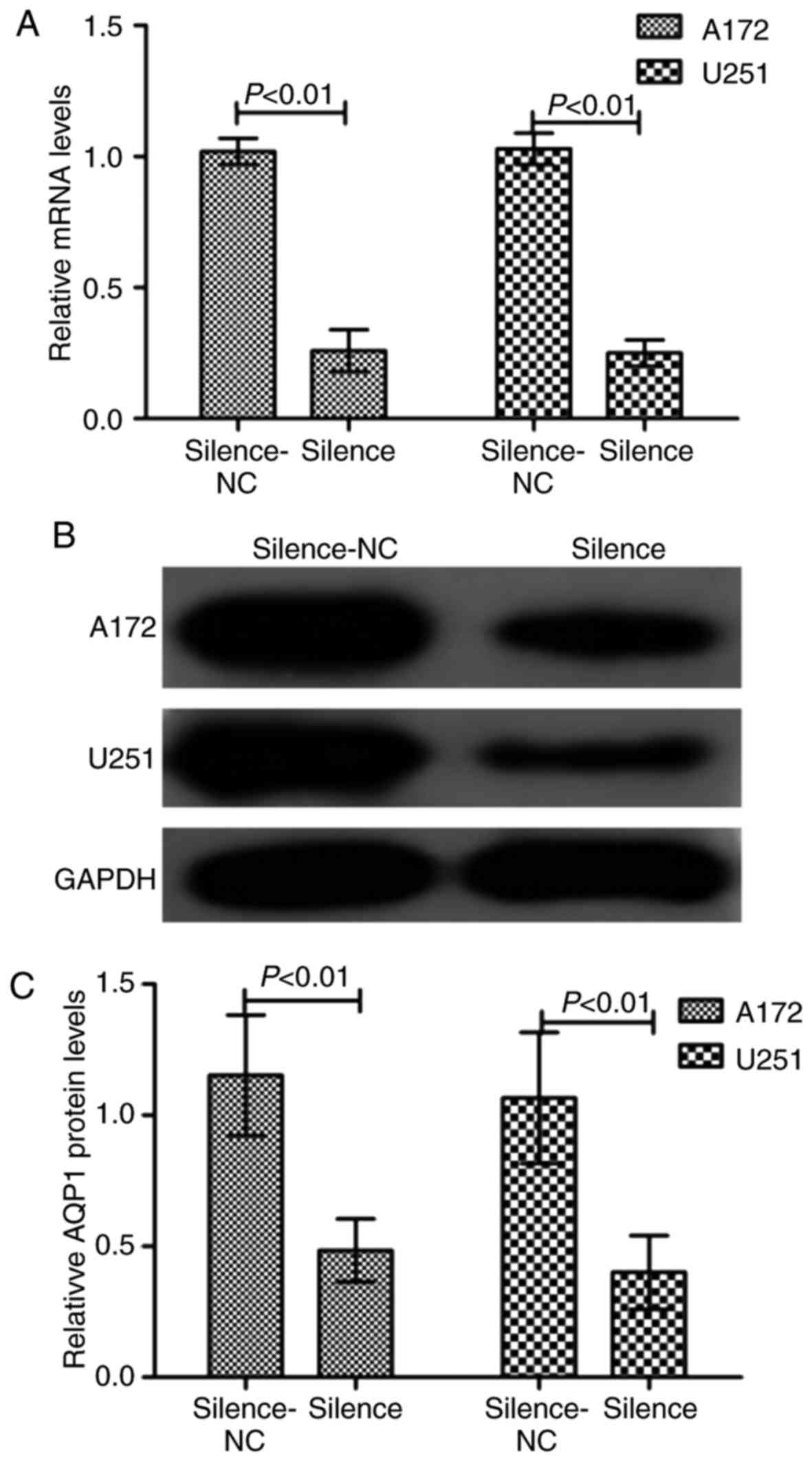
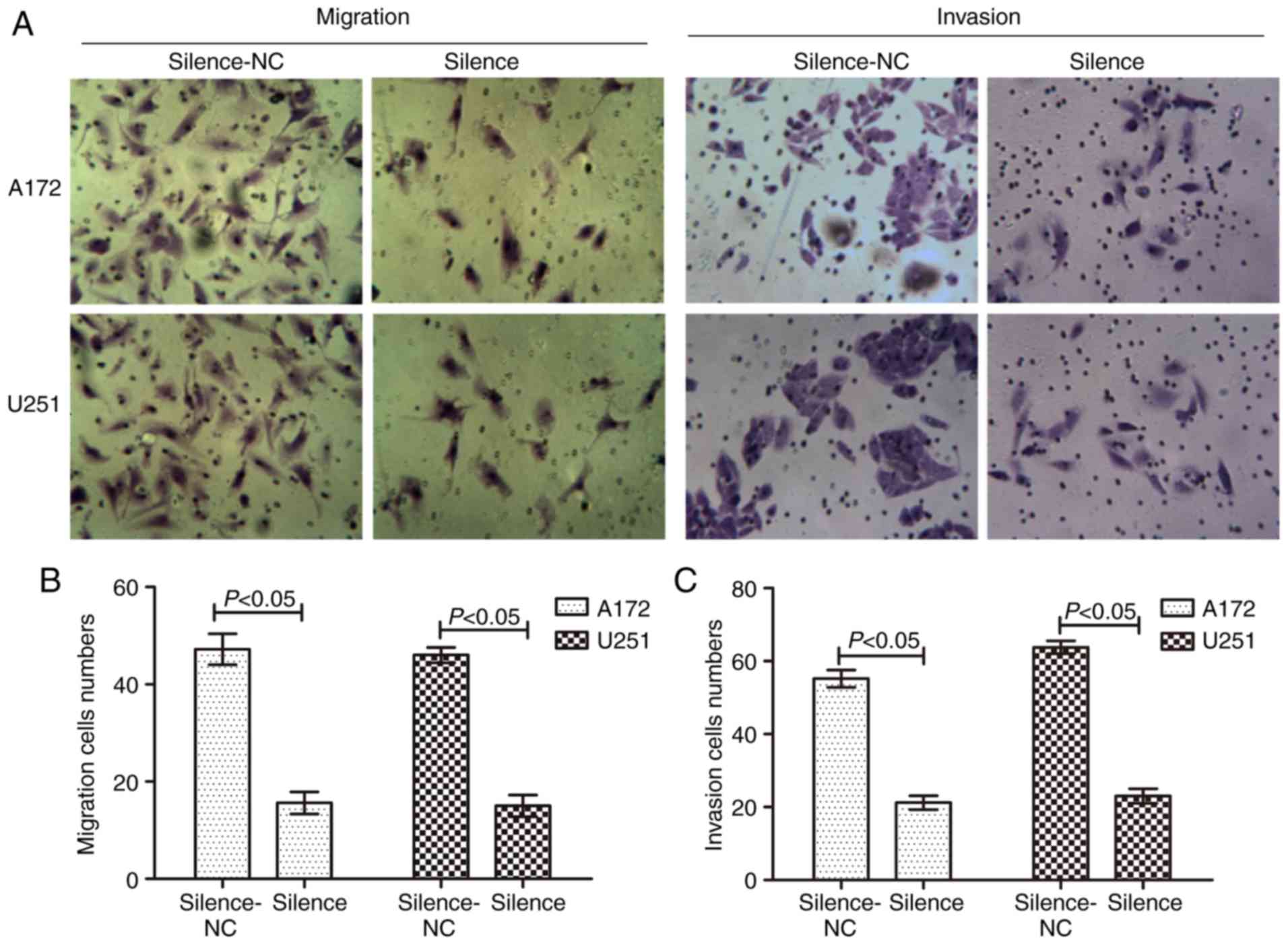
Silencing of AQP1 inhibits GBM
migration and invasion
To examine the role of AQP1 in glioma cells, AQP1
was stably silenced using shRNA-AQP1 in A172 and U251 cells. As
presented in Fig. 2, RT-qPCR
analysis and western blotting demonstrated that the transfection
was successful in reducing the expression levels of AQP1. Following
transfection, a Transwell assay demonstrated that the migratory and
invasive capacity were markedly reduced in the silenced group,
demonstrating that the number of migrated and invaded cells
decreased (Fig. 3). There was a
statistical difference between the NC and transfected groups
(P<0.05). These results suggested that silencing of AQP1 reduced
the abilities of migration and invasion in GBM cells.
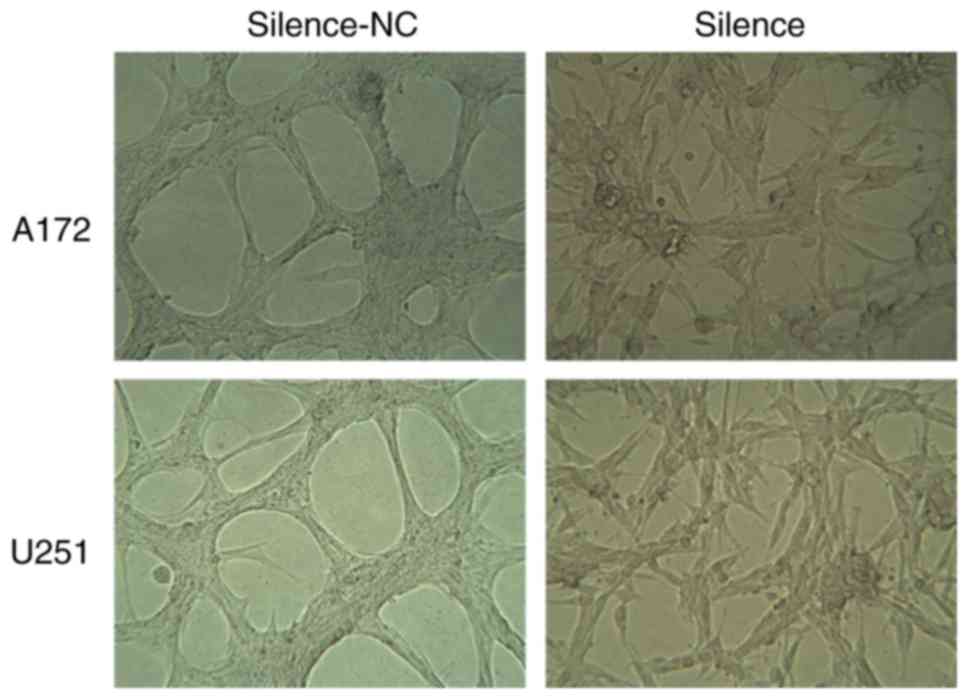
Silencing of AQP1 inhibits the
development of VM in vitro
VM is associated with tumor blood supply and
metastasis. The number of vessels (nodes) and the remodeling of the
microcirculation are used as histological markers of tumor
progression. In in vitro experiments, the vessel numbers of
the network channels, which reflect VM development, were calculated
and analyzed. It was observed that A172 and U251 cells in the
silence-NC group, which express high levels of AQP1, formed
classical VM networks on Matrigel. Following silencing of AQP1 via
transfection, it was observed that the classical VM networks became
less obvious, and the number of vessels decreased significantly in
the silence group, compared with the silence-NC group (Fig. 4). These results suggested that AQP1
may regulate the development of VM in GBM cells in
vitro.
Silencing of AQP1 inhibits
tumorigenesis and induces invasion-associated protein
expression
In order to study the effect of AQP1 on
tumorigenesis and tumor infiltration in vivo, the
transfected A172 and U251 cells were planted into the nude mouse
xenograft model. Across the 35 days, there was a marked decrease in
tumor weight in the silence group compared with the silence-NC
group (Fig. 5A and B). In
addition, western blot analysis of invasion-associated proteins
demonstrated that in the case of decreased AQP1 expression, the
expression of αvβ3 integrin, MMP-2 and MMP-9 in the silence group
decreased (Fig. 5C and D). There
were statistical differences between the silence-NC group and the
silence group (P<0.05). These results suggested that silencing
of AQP1 reduced the abilities of tumorigenesis and tumor
infiltration in vivo.
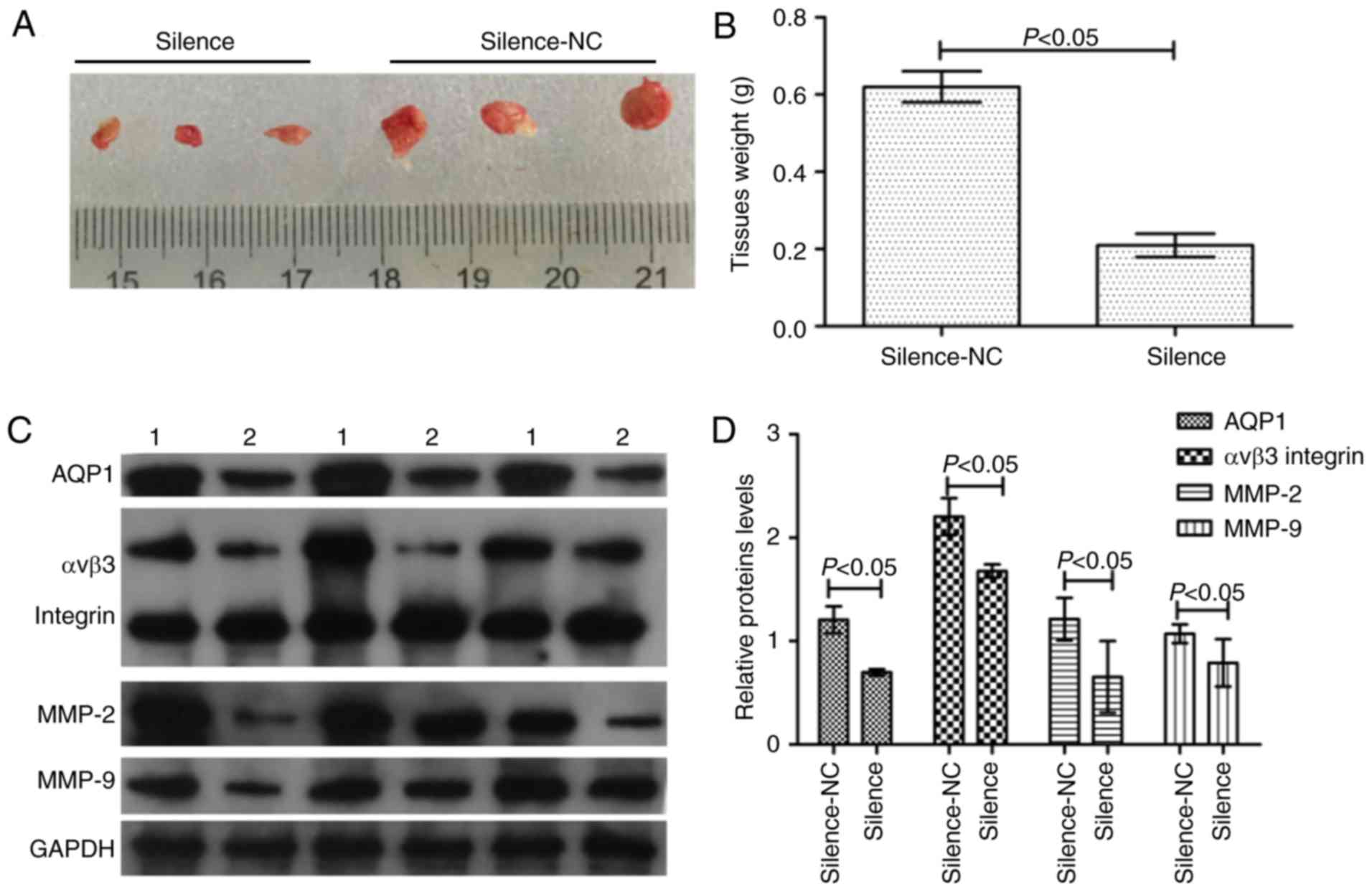 | Figure 5.AQP1 regulates tumorigenesis in
vivo. (A) Macroscopic appearance of xenotransplanted tumors.
(B) Quantitative analysis of tumor weights. (C) The western
blotting images of AQP1, αvβ3 integrin, MMP-2, MMP-9 protein. 1,
Silence-NC group; 2, silence group. (D) Gray scale analysis of
relative AQP1, αvβ3 integrin, MMP-2, MMP-9 expression levels in the
western blotting. Values are presented as the mean ± standard
deviation. n=3. AQP1, aquaporin-1; MMP-2, 72 kDa type IV
collagenase; MMP-9, matrix metalloproteinase-9; NC, negative
control. |
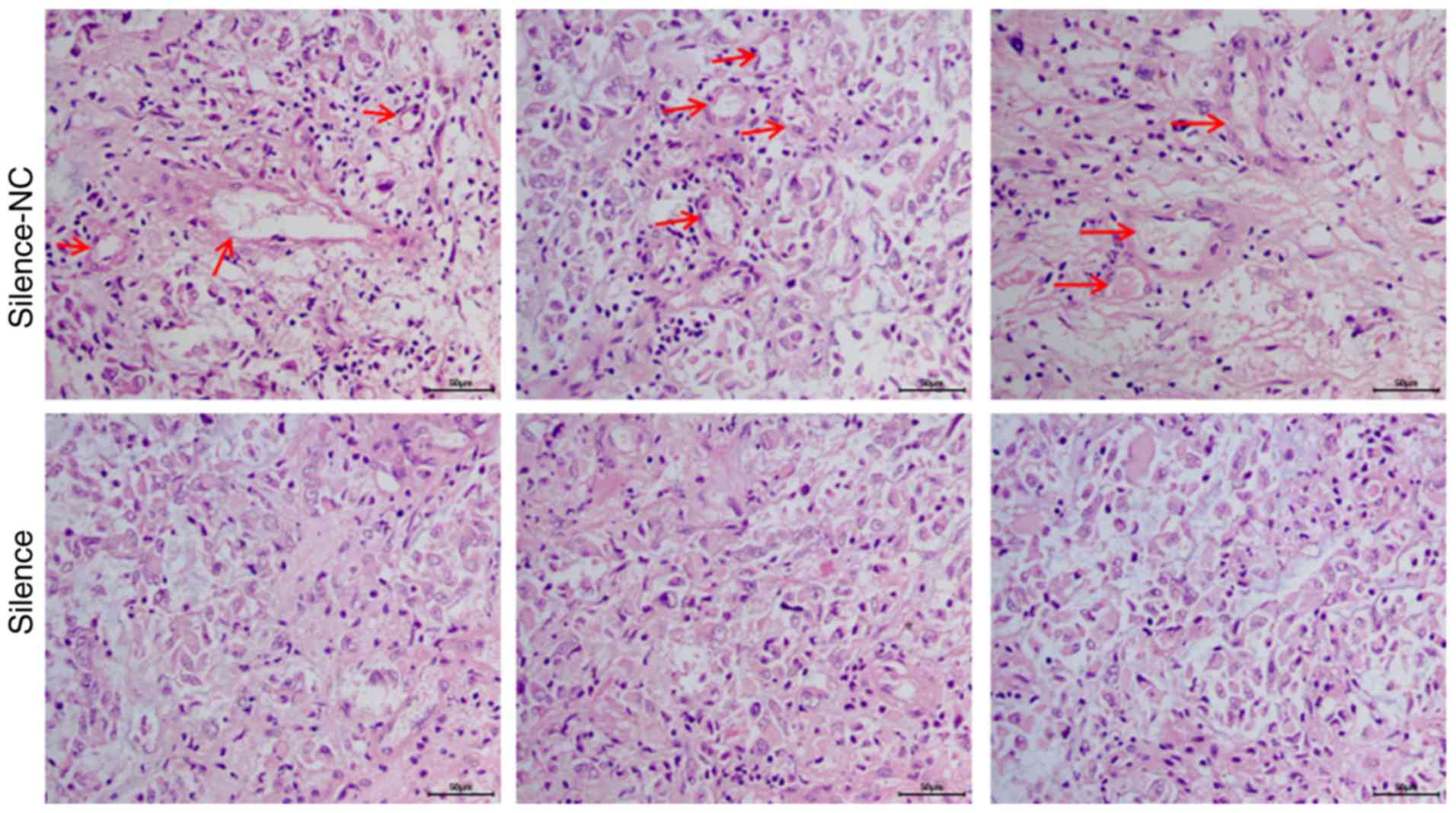
Silencing of AQP1 inhibited the
development of VM in vivo
In vivo, cancer tissues require an adequate
blood supply for growth, and VM serves as an alternative pathway
for maintaining this supply. In the in vivo experiment, VM
was analyzed using the in vivo xenograft tumors via HE
staining. HE staining demonstrated that A172 cells in the
silence-NC group formed a large number of typical vascular
structures (red arrows; Fig. 6).
Following silencing of AQP1 via transfection, the number of
vascular structures decreased significantly in the silence group,
compared with the silence-NC group. These results suggested that
AQP1 may regulate the development of VM in vivo.
Discussion
Using western blotting and RT-qPCR, the present
study demonstrated that AQP1 expression levels were upregulated and
positively associated with glioma grade, and that the highest
expression levels were observed in GBM cells. Based on the
characteristics of malignant glioma and this observation, it was
hypothesized that AQP1 may be associated with GBM migration and
invasion. In addition, a previous report revealed that AQP1
regulated cell volume via the rapid transmembrane transport of
water, thereby promoting cell migration (13). In a study into the metastasis of
lung cancer, the researchers reported that AQP1 led to
extravasation and spread (14).
In order to verify the above hypothesis,
shRNA-mediated AQP1 silencing was used to assess the potential
effects of AQP1 on migration and invasion using two types of GBM
cell line. The Transwell assay demonstrated that silencing of AQP1
was able to suppress GBM cell migration and invasion in
vitro. In angiogenesis, tumor growth and metastasis, MMPs
(including MMP-2 and MMP-9) and integrins (including αvβ3) degrade
the ECM and release and/or activate growth factors, finally
resulting in cancer cell migration and invasion (15,16).
Previous reports demonstrated that elevated αvβ3 integrin, MMP-2
and MMP-9 expression levels in tumor cells markedly increased the
adhesion and migration of the tumor cells (17–20).
In the present study, western blot analysis using in vivo
xenograft tumor tissues demonstrated that invasion-associated
protein (αvβ3 integrin, MMP-2 and MMP-9) expression decreased in
the AQP1 silence group, indicating that silencing of AQP1 was able
to suppress GBM migration and invasion in vivo.
Additionally, AQP1 knockdown or inhibition may effectively inhibit
cell proliferation, invasion and tumorigenesis in osteosarcoma and
hepatocellular carcinoma (21,22).
From the above data, it was inferred that AQP1 was indeed
associated with GBM migration and invasion, and that silencing of
AQP1 was able to suppress GBM migration and invasion, in
vitro and in vivo.
Inhibiting angiogenesis is an important therapeutic
approach in cancer (23,24). The proteins that regulate abnormal
angiogenesis have attracted intense interest. AQP1, as a water
channel membrane protein, was able to promote tumor angiogenesis by
allowing faster endothelial cell migration in a mouse model of
melanoma (25,26). In human glioma, it was demonstrated
that elevated AQP1 in GBM cells led to a typical angiogenesis
structure, and that AQP1 knockdown reduced VM structure formation
in vitro. In vivo, VM channels are patterned networks
with red blood cells readily detectable inside such channels, and
are arranged in arcs, loops and networks (27). In the present in vivo
xenograft experiment, HE staining exhibited typical VM channels in
GBM cells and, following AQP1 silencing, the number of typical VM
channels decreased. These results demonstrated that VM formation
may be inhibited by regulating AQP1 expression in human GBM.
In conclusion, AQP1 was positively associated with
glioma grade and promoted glioma cell migration, invasion and VM
formation in vitro and in vivo. In the treatment of
malignant glioma, the present study may provide a strategy and
facilitate the development of AQP1-directed diagnostics and
therapeutics against glioma. Further studies ought to be aimed at
performing a systematic evaluation of AQP1 in gliomas of different
grades, and correlating such findings with clinical survival
parameters.
Acknowledgements
The present study was supported by the Scientific
Research and Cultivation of Special Fund of the First Clinical
Medical College of Jinan University (grant no. 2015109).
References
|
1
|
Kleihues P, Louis DN, Scheithauer BW,
Rorke LB, Reifenberger G, Burger PC and Cavenee WK: The WHO
classification of tumors of the nervous system. J Neuropathol Exp
Neurol. 61:215–225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, van den Bent MJ and Hegi ME:
Optimal role of temozolomide in the treatment of malignant gliomas.
Curr Neurol Neurosci Rep. 5:198–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson HJ and Galileo DS: Small-molecule
inhibitors of FGFR, integrins and FAK selectively decrease
L1CAM-stimulated glioblastoma cell motility and proliferation. Cell
Oncol (Dordr). 39:229–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folberg R, Hendrix MJ and Maniotis AJ:
Vasculogenic mimicry and tumor angiogenesis. Am J Pathol.
156:361–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guzman G, Cotler SJ, Lin AY, Maniotis AJ
and Folberg R: A pilot study of vasculogenic mimicry
immunohistochemical expression in hepatocellular carcinoma. Arch
Pathol Lab Med. 131:1776–1781. 2007.PubMed/NCBI
|
|
7
|
King LS, Yasui M and Agre P: Aquaporins in
health and disease. Mol Med Today. 6:60–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mohammadi MT and Dehghani GA: Nitric oxide
as a regulatory factor for aquaporin-1 and 4 gene expression
following brain ischemia/reperfusion injury in rat. Pathol Res
Pract. 211:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao X, Derugin N, Manley GT and Verkman
AS: Reduced brain edema and infarct volume in aquaporin-4 deficient
mice after transient focal cerebral ischemia. Neurosci Lett.
584:368–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshio K, Binder DK, Liang Y, Bollen A,
Feuerstein B, Berger MS and Manley GT: Expression of the
aquaporin-1 water channel in human glial tumors. Neurosurgery.
56:375–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rouzaire-Dubois B, Ouanounou G and Dubois
JM: Relationship between extracellular osmolarity, NaCl
concentration and cell volume in rat glioma cells. Gen Physiol
Biophys. 30:162–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Y, Wen X, Jiang Z, Fu HQ, Han H and
Dai L: Aquaporin 1 and aquaporin 4 are involved in invasion of lung
cancer cells. Clin Lab. 58:75–80. 2012.PubMed/NCBI
|
|
15
|
Klein G, Vellenga E, Fraaije MW, Kamps WA
and de Bont ES: The possible role of matrix metalloproteinase
(MMP)-2 and MMP-9 in cancer, eg acute leukemia. Crit Rev Oncol
Hematol. 50:87–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiao Y, Feng X, Zhan Y, Wang R, Zheng S,
Liu W and Zeng X: Matrix metalloproteinase-2 promotes αvβ3
integrin-mediated adhesion and migration of human melanoma cells by
cleaving fibronectin. PLoS One. 7:e415912012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brooks PC, Clark RA and Cheresh DA:
Requirement of vascular integrin alpha v beta 3 for angiogenesis.
Science. 264:569–571. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuphal S, Bauer R and Bosserhoff AK:
Integrin signaling in malignant melanoma. Cancer Metastasis Rev.
24:195–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.PubMed/NCBI
|
|
21
|
Wu Z, Li S, Liu J, Shi Y, Wang J, Chen D,
Luo L, Qian Y, Huang X and Wang H: RNAi-mediated silencing of AQP1
expression inhibited the proliferation, invasion and tumorigenesis
of osteosarcoma cells. Cancer Biol Ther. 16:1332–1340. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelagalli A, Nardelli A, Fontanella R and
Zannetti A: Inhibition of AQP1 hampers osteosarcoma and
hepatocellular carcinoma progression mediated by bone
marrow-derived mesenchymal stem cells. Int J Mol Sci. 17:E11022016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K,
Pang JC, Ng HK and Chen ZP: Clinical significance of vasculogenic
mimicry in human gliomas. J Neurooncol. 105:173–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nicchia GP, Stigliano C, Sparaneo A, Rossi
A, Frigeri A and Svelto M: Inhibition of aquaporin-1 dependent
angiogenesis impairs tumour growth in a mouse model of melanoma. J
Mol Med(Berl). 91:613–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yue WY and Chen ZP: Does vasculogenic
mimicry exist in astrocytoma? J Histochem Cytochem. 53:997–1002.
2005. View Article : Google Scholar : PubMed/NCBI
|