Introduction
Alzheimer's disease (AD) is caused by chronic and
progressive damage to the central nervous system. It has affected
60–65% of people worldwide (1).
One of the primary clinical manifestations reported in AD is a
decline in cognitive function. Patients with AD frequently succumb
to the development of a pulmonary embolism (2). However, the exact pathomechanism of
AD remains to be completely elucidated. The induction of apoptosis
in neurons has been proposed to be a potential theory behind AD
pathogenesis (3). Neurons exhibit
apoptotic features during the development of AD, including
apoptotic mitochondrial alterations (4). During mitochondrial apoptosis, the
accumulation of intracellular reactive oxygen species (ROS) and
calcium (Ca2+) overload is observed (5), which is responsible for functional
and structural damage to brain tissues. For research studies,
injection of D-galactose (D-gal) in combination with intragastric
treatment with AlCl3 successfully decreased memory
ability and has been used to establish an animal model of AD
(6).
Despite the considerable scientific manpower and
resources being devoted to developing novel AD therapies, there are
no adequate treatment options at present. Due to their various
biological responses, natural products have become a novel
repository for drug screening (7).
For example, Sparassis crispa polysaccharides exert
neuroprotective effects against L-glutamate (L-Glu)-induced cell
damage via the mitochondrial pathway of apoptosis (8). Hericium erinaceus has been
confirmed to have neuroprotective properties in L-Glu-induced
apoptotic differentiated (D)PC12 cells and mouse models of AD
(9).
Lycium barbarum (LB), a renowned functional
food and medicinal plant from Southeast Asia, exhibits
immunoregulatory and neuroprotective properties (10). It has been reported that
polysaccharides separated from LB can prevent the apoptosis of
6-hydroxydopamine-induced PC12 cells, in part through regulation of
the ROS-nitric oxide pathway (11). LB polysaccharides can also protect
retinal ganglion cells against acute ocular hypertension induced
ischemic injury (12).
Furthermore, LB polysaccharides exhibit neuroprotective effects in
differentiated PC12 cells against L-Glu induced toxicity through
the regulation of the mitochondrial pathway of apoptosis (8). The present study aimed to further
investigate the neuroprotective effects of LB and its underlying
mechanisms in DPC12 cells exposed to L-Glu and in an AD mouse model
established by AlCl3 and D-gal. The results suggested
that LB may possesses beneficial effects against L-Glu-induced
toxicity via the mitochondrial pathway of apoptosis. The
therapeutic effects of LB on AD were confirmed through the AD mouse
model, and provide evidence for LB as a potential functional food
that may be administered to patients with a neurodegenerative
disease.
Materials and methods
LB water extract preparation
LB (acquired from Beijing Tongren Tang Co., Ltd.,
Beijing, China) extract was obtained by soaking in double distilled
water at 90°C for 2 h twice. It was subsequently concentrated and
freeze-dried for further experiments. LB was analyzed via
3,5-dinitrosalicylic acid colorimetric estimation (13), phenol-sulfuric acid determination
(13) and the Kjeldahl method
(14). The constituents were as
follows: 9.2% total sugar; 1.9% reducing sugar; and 9.4% total
protein.
Cell culture
PC12 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), 5% horse serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 1% penicillin and 1%
streptomycin, in a humidified atmosphere containing 5%
CO2 at 37°C. Nerve growth factor (NGF; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 20 ng/ml dissolved in DMEM
(Invitrogen; Thermo Fisher Scientific, Inc.) with 1% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) was applied for 48 h
to differentiate cells.
MTT assay
DPC12 cells were seeded in 96-well plates at
1×104 cells/well. No LB, 200 or 400 µg/ml LB was applied
to pre-incubate DPC12 cells for 3 h prior to 24 h exposure to 20 mM
of L-Glu. An MTT assay (Sigma-Aldrich; Merck KGaA) was used to
determine cell viability. Following incubation with MTT solution
(0.5 mg/ml) for 3 h at 37°C in darkness, 100 µl dimethyl sulfoxide
was added to dissolve the purple formazan. A micro-plate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to detect
the absorbance at 490 nm. Viability values of treated cells were
expressed as a percentage of that of corresponding control
cells.
Assessment of caspase activity
DPC12 cells were seeded in a six-well plate at a
density of 3×105 cells/well. DPC12 cells were treated
with no LB, 200 or 400 µg/ml LB for 3 h, prior to co-incubation
with or without 20 mM L-Glu for a further 24 h. The activity of
caspase-3 in the cell lysate was measured with a caspase-3 activity
assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Assessment of apoptotic rate and the
cell cycle
DPC12 cells were seeded in a six-well plate at a
density of 3×105 cells/well. No LB, 200 or 400 µg/ml LB
was applied to DPC12 cells for 3 h, prior to 24 h exposure to 20 mM
L-Glu. The apoptotic rate and alterations in the cell cycle were
measured using a flow cytometer (FC500; Beckman Coulter, Inc.,
Brea, CA, USA) and analyzed using FlowJo 7.6 software (Tree Star,
Inc., Ashland, OR, USA). For the determination of apoptotic rate,
treated cells were stained with propidium iodide (PI) and Annexin V
(Annexin V-fluorescein isothiocyanate/PI double staining apoptosis
detection kit; G003; Nanjing Jiancheng Bioengineering Institute) at
room temperature in darkness for 20 min. In a separate experiment,
alterations in the cell cycle were determined in treated cells
stained with PI at room temperature in darkness for 10 min.
Mitochondrial membrane potential (MMP)
analysis
DPC12 cells were seeded in a six-well plate at a
density of 2×105 cells/well. DPC12 cells were pretreated
with no LB, 200 or 400 µg/ml LB for 3 h, and subsequently exposed
to 20 mM L-Glu for 12 h. Following staining with 2 µg/ml JC-1
(Sigma-Aldrich; Merck KGaA) in darkness for 15 min, alterations in
green (excitation, 490 nm and emission, 530 nm) and red,
(excitation, 540 nm and emission, 590 nm) fluorescence were
detected using a fluorescent microscope (magnification, ×20;
TE2000; Nikon Corporation, Tokyo, Japan).
Intracellular Ca2+and ROS
concentration analysis
DPC12 cells were seeded in a six-well plate
(2×105 cells/well) and were pretreated with no LB, 200
or 400 µg/ml of LB for 3 h, prior to co-incubation with or without
20 mM L-Glu for 12 h. The treated cells were stained with 5 µM
Fluo-4-AM (Molecular Probes; Thermo Fisher Scientific, Inc.) to
detect Ca2+ levels, or 10 µM of 2,7′-dichlorofluorescein
diacetate (Sigma-Aldrich; Merck KGaA) to detect ROS levels. After
15-min staining in darkness at 37°C, cells were washed with HBSS
for three times. Alterations in green fluorescence intensity were
subsequently analyzed by flow cytometry (FC500; Beckman Coulter,
Inc.). FlowJo v7.6 software (Tree Star, Inc.) was used to analyze
fluorescence intensity.
Western blot analysis
DPC12 cells were seeded in a six-well plate at a
density of 3×105 cells/well. Cells were pretreated with
200 or 400 µg/ml LB for 3 h, and subsequently co-incubated with or
without 20 mM L-Glu for a further 24 h. Treated cells were lysed
with radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck
KGaA) containing 1% protease inhibitor cocktail (Sigma-Aldrich;
Merck KGaA). A Bicinchoninic Acid protein assay kit (Merck KGaA)
was used to determine the concentration of the lysed cell protein
according to the manufacturer's protocols. Proteins (40 µg) were
separated on a 10–12% SDS-PAGE gel. Following electrophoretic
transfer, the nitrocellulose membranes (0.45 µm; Bio Basic, Inc.,
Markham, ON, Canada) were exposed to primary antibodies including
cleaved caspase-3 (9662), caspase-8 (9746) and caspase-9 (9502),
apoptosis regulator Bcl-2 (Bcl-2; 2872), apoptosis regulator BAX
(Bax; 2772) and GAPDH (5174; all obtained from Cell Signaling
Technology, Inc., Danvers, MA, USA) at a dilution of 1:2,000 for 12
h at 4°C. The membranes were subsequently incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (sc-3836; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) accordingly at a dilution of 1:500, for 2 h at room
temperature. An enhanced chemiluminescence detection kit (Merck
KGaA) was used to detect the chemiluminescence of blots and ImageJ
v1.38 software (National Institutes of Health, Bethesda, MD, USA)
was used to quantify the intensity.
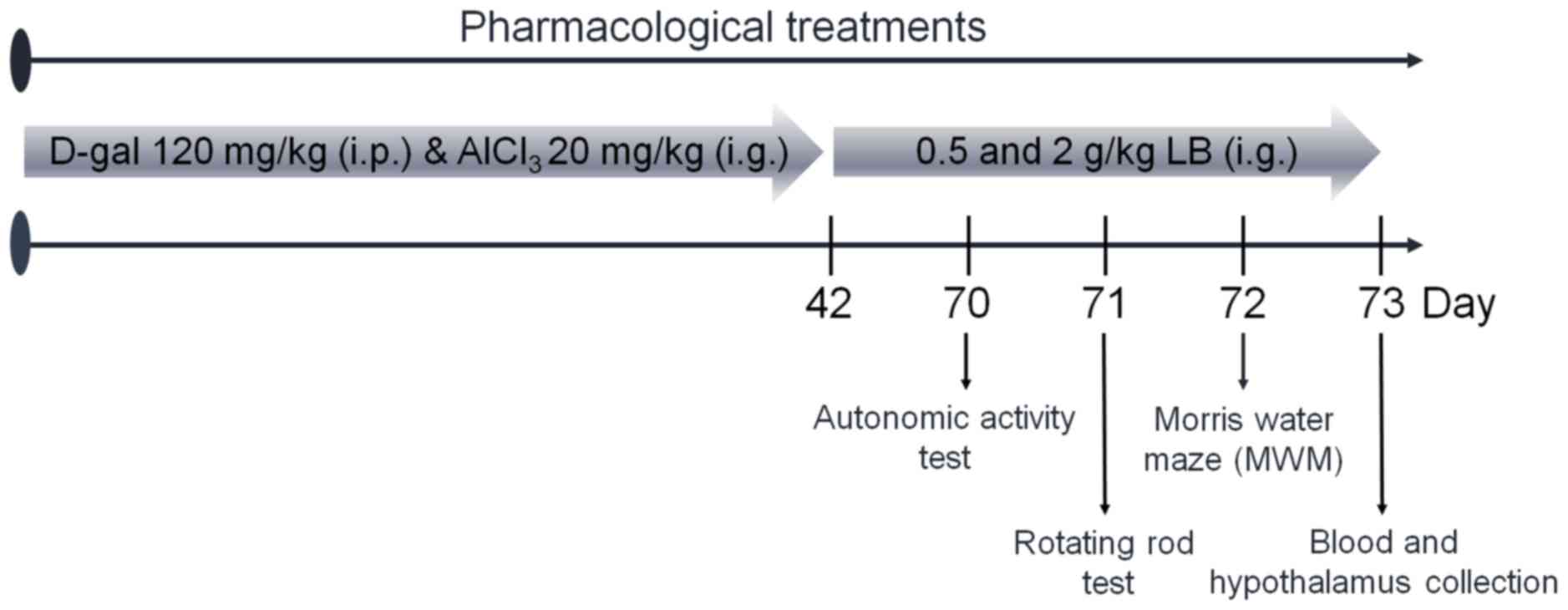
Animal care and drug treatment
process
The experimental protocol was approved by the
Institutional Animal Ethics Committee of Jilin University
(Changchun, China). Male BALB/c mice (20–22 g; 8 weeks old; n=40)
were housed in groups of 6 in transparent cages and maintained on a
12-h light/dark cycle at 23±1°C with water and food available ad
libitum. Mice were treated intragastrically with 20 mg/kg
AlCl3 and subcutaneously injected with 120 mg/kg D-gal
once daily for 6 weeks to establish the AD model, which was
determined via a Morris water maze test as described below. AD mice
were treated orally with normal saline, 0.5 or 2.0 g/kg LB for 4
weeks (n=10). Non-induced BALB/c mice (n=10) were treated with
normal saline as a control. Following the final treatment, mice
underwent behavioral testing. The protocol is presented in Fig. 1.
Behavioral tests
Autonomic activity test
As described previously (9), the horizontal and vertical locomotor
activities of the mice were recorded for 5 min following placement
into squares.
Fatigue rotarod test
Following a previous study protocol (9), training was repeated three times and
mice were placed on the turning device (Chengdu Techman Software
Co., Ltd., Chengdu, China) at a speed of 20 rpm and their time
until exhaustion was recorded.
Morris water maze
Following a training period of 5 days, mice were
placed in an open swimming arena with a depth of 10 cm and a
temperature of 25±2°C. Following a previous study protocol
(9), the time spent within the
target quadrant over a 120 sec probe test period was recorded.
Measurement of acetylcholine (ACh) and
choline acetyltransferase (ChAT) levels
Following behavioral testing, blood from caudal
veins of mice and the hypothalamus were collected. The hypothalamus
was homogenized in saline (1–5 w/v). ELISA kits were used according
to manufacturer's protocols to analyze the levels of Ach and ChAT
(A105-1 and A079-1, respectively, Nanjing Jiancheng Bioengineering
Institute) in the serum and hypothalamus.
Statistical analysis
The data are expressed as the mean ± standard
deviation. A one-way analysis of variance was used to detect
statistical significance followed by post hoc Dunn's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
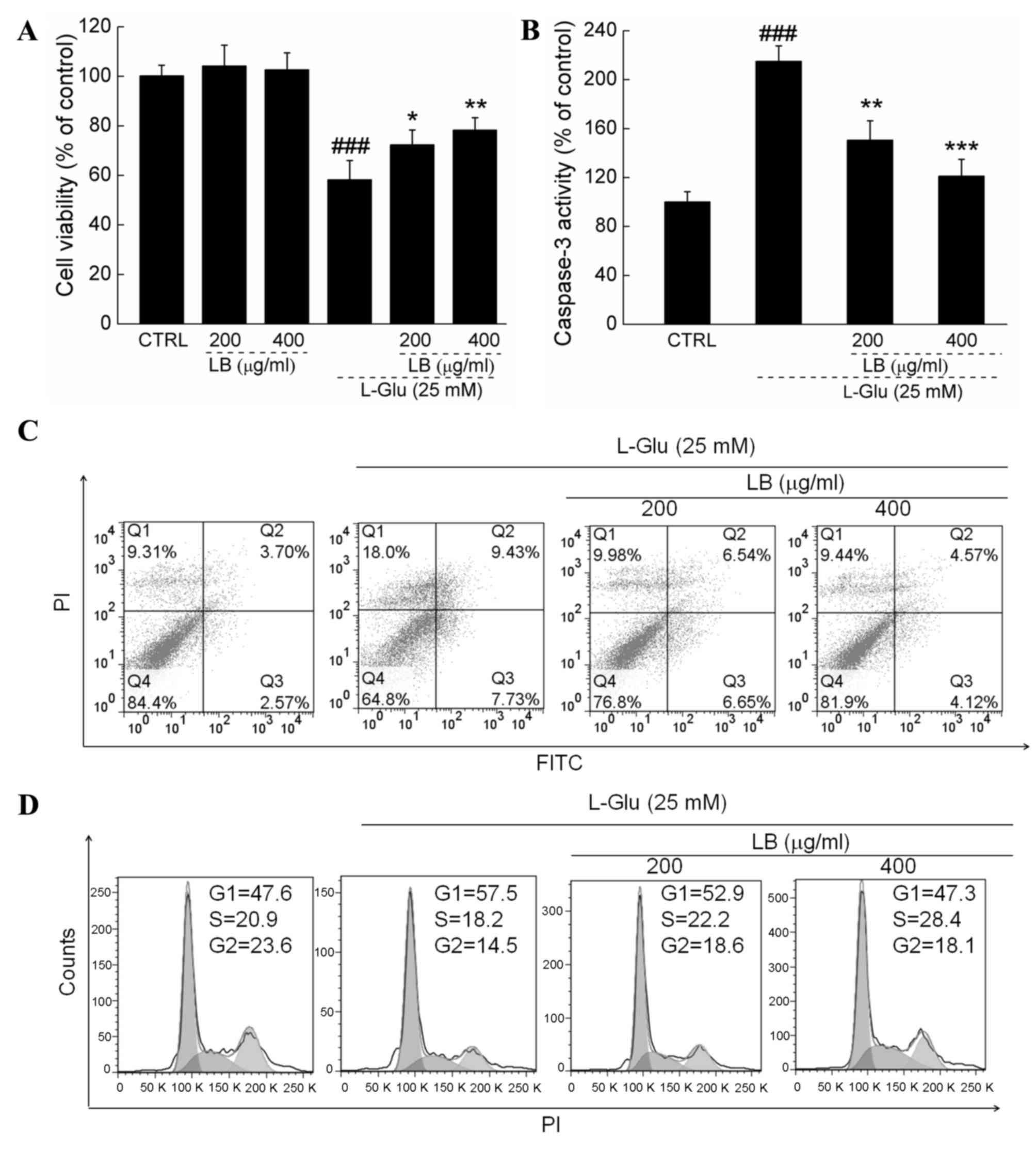
LB improves cell viability, inhibited
apoptotic rate and normalized cell cycle
LB alone failed to influence the levels of cell
proliferation (Fig. 2A). In L-Glu
treated cells, 3 h pretreatment with 200 and 400 µg/ml LB improved
cell viability by >20% (P<0.05; Fig. 2A). Treatment with LB suppressed
caspase-3 activity by >30% in L-Glu treated cells (P<0.05;
Fig. 2B). L-Glu caused a cellular
apoptotic rate of 17.2% of in DPC12 cells, whereas LB reduced the
rate of apoptosis by ~50% (Fig.
2C). Exposure to LB for 24 h markedly reversed G1 arrest in
DPC12 cells induced by L-Glu (Fig.
2D).
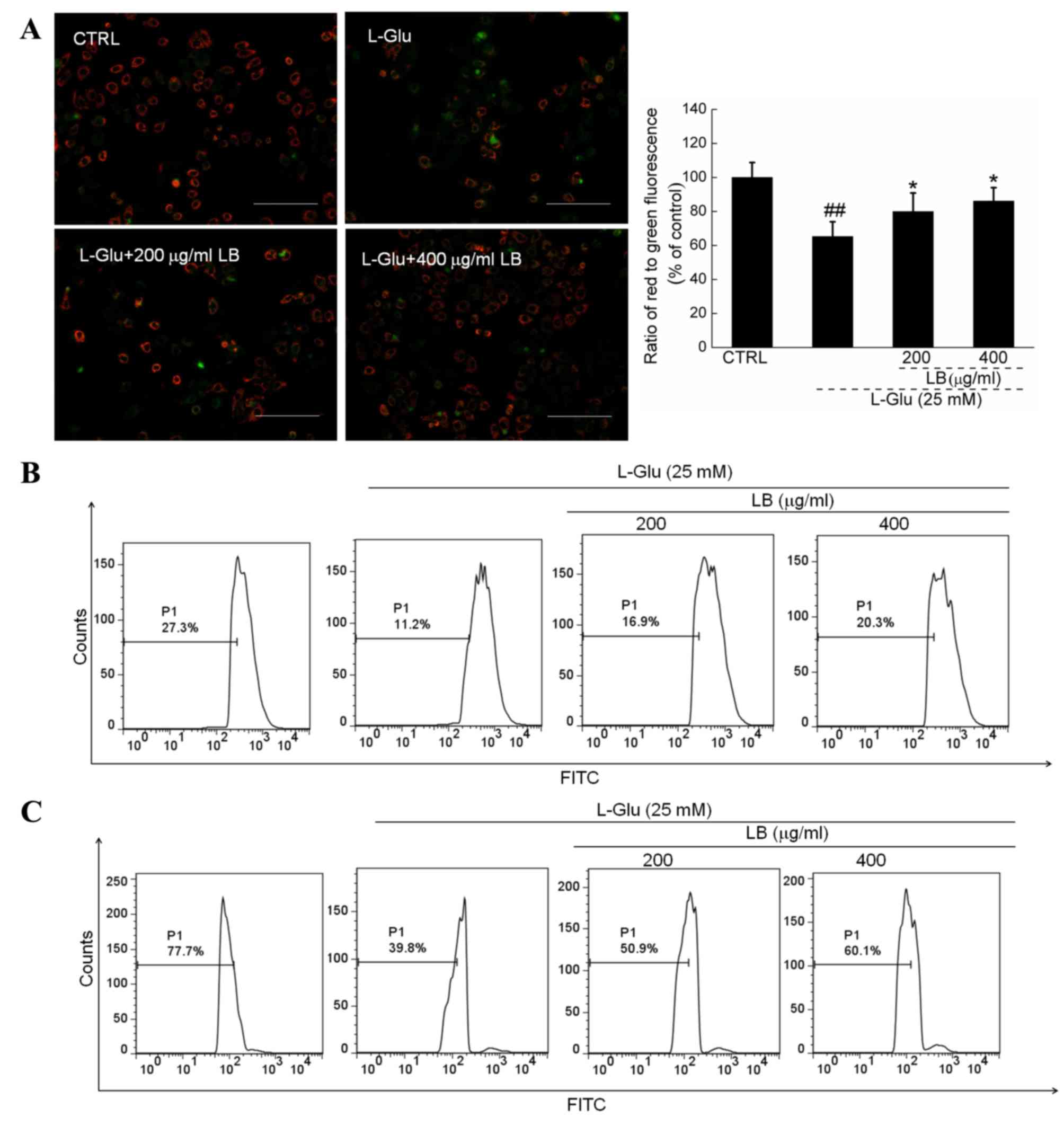
LB reverses mitochondrial
apoptosis
Intense green fluorescence was observed in 12-h
L-Glu-incubated cells, indicating MMP depolarization.
Comparatively, LB pre-incubation strongly enhanced the ratio of red
to green fluorescence, suggesting a beneficial effect on
mitochondrial function (P<0.05; Fig. 3A). Furthermore, by comparison with
L-Glu incubated cells, LB markedly suppressed the intracellular
levels of ROS (Fig. 3B) and
Ca2+ (Fig. 3C)
following 3 h pretreatment in combination with 12 h co-incubation
with L-Glu.
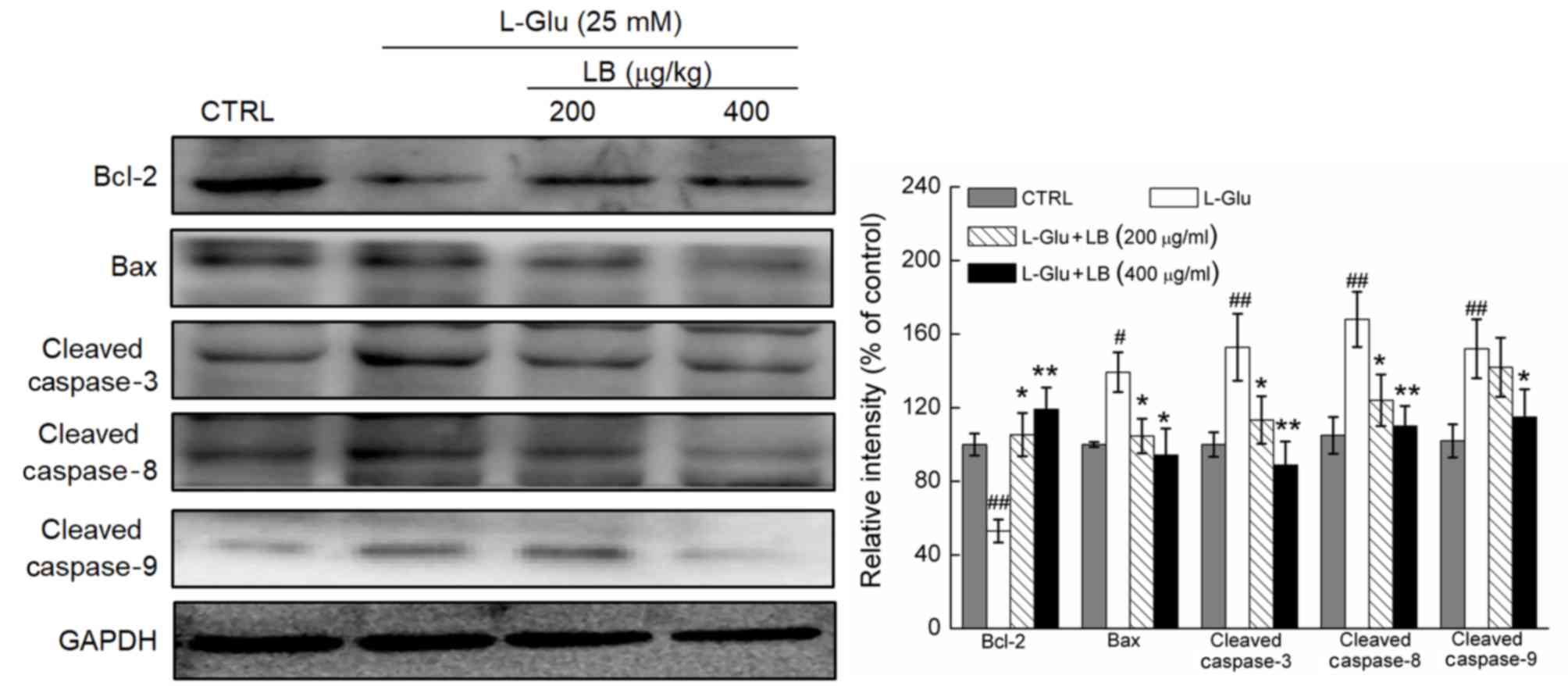
LB reverses alterations in pro- and
anti-apoptotic protein expression
A significant decrease in Bcl-2 expression levels,
and a significant increase in the expression levels of Bax, and
cleaved caspase-3, −8 and −9 was observed in L-Glu-treated DPC12
cells (P<0.05; Fig. 4). DPC12
cells pre-treated with LB at doses of 200 and 400 µg/ml exhibited a
significant increase in the levels of Bcl-2 expression, and the
expression levels of Bax, and cleaved caspase-3, −8 and −9 were
significantly decreased compared with cells treated with L-Glu only
(P<0.05; Fig. 4).
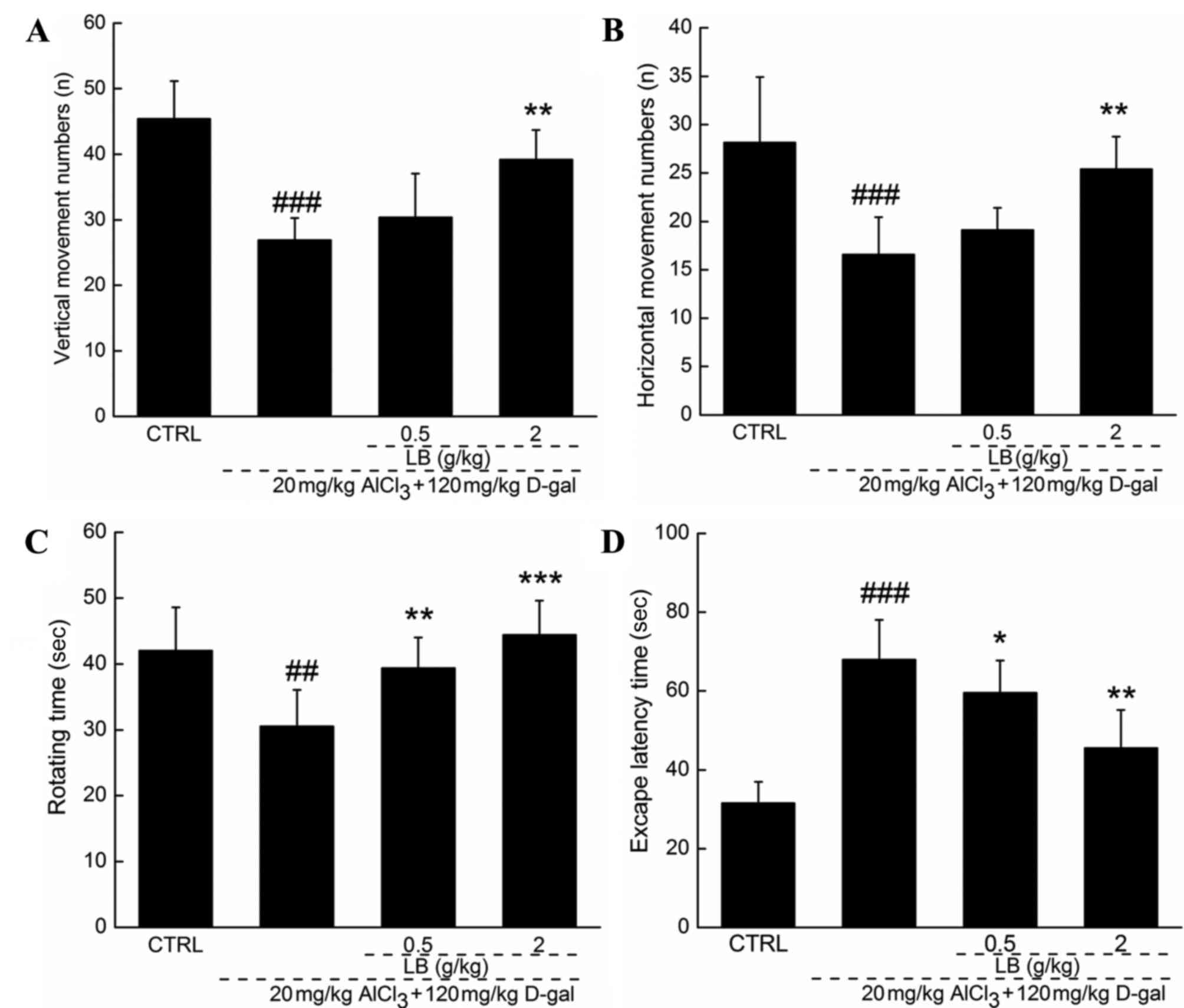
Effects of LB on AD mouse
behavior
Following 4 weeks of treatment with LB, the quantity
of horizontal and vertical movements was increased by >25%
(P<0.01; Fig. 5A and B) by
comparison with AD mice. Endurance time was increased in the
rotarod test by 30% following 4 weeks of treatment with LB, by
comparison with AD mice (P<0.01; Fig. 5C). The Morris water maze test is
commonly applied to evaluate the effects of a drug on the learning
and memory of an animal. Escape latency time increased by over
two-fold in AD mice compared with the wild-type control group
(P<0.001; Fig. 5D). Treatment
with LB (0.5 and 2 g/kg) returned the escape latency time into the
normal range (P<0.05; Fig.
5D).
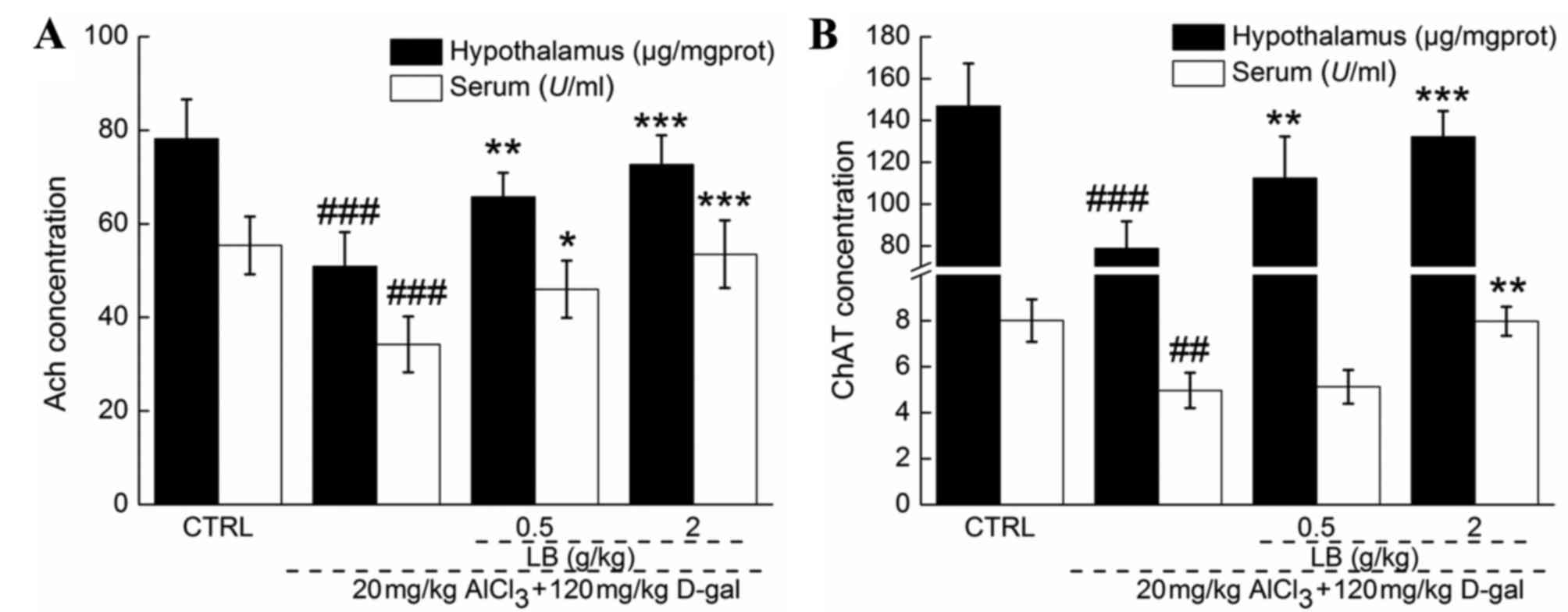
LB upregulates ACh and ChAT levels in
the serum and hypothalamus
Significantly reduced serum and hypothalamic levels
of ACh and ChAT were observed in AD mice compared with the control
group (P<0.001; Fig. 6),
suggesting that central cholinergic function was disturbed by
AlCl3 and D-gal. Comparatively, the levels of ACh and
ChAT in the serum and hypothalamus were significantly increased
(P<0.05; Fig. 6) following 4
weeks of treatment with LB, demonstrating the ability of LB to
improve central cholinergic system function in AD mice.
Discussion
LB is different from other potential therapies
currently being investigated for AD. The water extract of this
functional food contains natural active ingredients that have been
consumed safely in Southeast Asia for centuries. The present study
confirmed the neuroprotective effects of LB in in vitro and
in vivo models. LB was demonstrated to increase cell
viability, inhibit cellular apoptosis, ameliorate mitochondrial
apoptotic alterations and normalize behaviors in AD mice.
PC12 cells are able to differentiate into
neuron-like cells that form clear synapses and produce
nerve-associated proteins (15).
Glutamate is reported to be an excitatory neurotransmitter in the
central nervous system; however, excessive levels are responsible
for excitoxicity (16). Glutamate
receptors are excessively activated in patients with
neuropathological conditions, which induces neuronal death
processes (17). In the present
in vitro study, 25 mM L-Glu was used to establish an
apoptotic DPC12 cell model, in order to investigate the
neuroprotective effects of LB. The data revealed that LB
significantly suppressed L-Glu-induced Ca2+ overload and
ROS accumulation. Energy metabolizing mitochondria are recognized
to be Ca2+ hubs (18),
and Ca2+ overload is responsible for mitochondrial
depolarization, which in turn leads to further release of free
radicals, particularly ROS (19).
Intracellular free radicals are an essential factor during
apoptosis, and thus they have become a target for the prevention of
apoptosis (20). High ROS levels
stimulate the opening of the mitochondrial permeability transition
pore, which contributes to the activation of the mitochondrial
pathway of apoptosis (21).
Notably, a feedback loop between intracellular ROS levels and
mitochondrial function has been demonstrated, with ROS accumulation
inducing MMP dissipation, which further contributes to excessive
ROS production (22). The results
of the present study demonstrated that LB enhanced Bcl-2 expression
levels and reduced the expression levels of Bax, and cleaved
caspase-3, −8 and −9 in L-Glu-exposed DPC12 cells. Bcl-2 family
members, located in the outer mitochondrial membrane, serve as
measures of mitochondrial function (23). The activation of caspase family
members has a central role in neurodegeneration, particularly
caspase-3 (24). MMP disruption
activates the enzymatic apoptotic machinery of caspases, which are
responsible for cellular fragmentation into apoptotic bodies
(25). In response to
extracellular stimuli, caspase-8 directly activates caspase-3
through the mitochondrial apoptotic pathway (26,27).
Mitochondria subsequently release cytochrome c into the cytoplasm,
which is associated with the activation of caspase-9 (28). Caspase-3 is subsequently activated,
which has a critical role in the mitochondrial apoptotic cascade,
in part through the amplification of initiator caspase-8 and −9
signals (29). Results indicate
that LB-mediated neuroprotection against L-Glu induced DPC12 cell
apoptosis may be associated with mitochondrial apoptotic
signaling.
Due to the complexity of AD pathology, establishing
a representative animal model for basic research is difficult
(30). D-gal induces the swelling
and dysfunction of brain cells (31), and aluminum promotes amyloid β
production in astrocytes (32),
the two of which result in cognitive and memory dysfunction in
animals (32). The combination of
AlCl3 and D-gal establishes a mouse model displaying
AD-like behaviors which are more stabilized than that of
AlCl3 or D-gal alone (6). HB has been confirmed to improve the
cognition of mice in an AlCl3 and D-gal-induced AD model
(9). Similarly, LB significantly
alleviated the loss of memory and learning ability in AD mice.
Furthermore, LB significantly increased the serum and hypothalamic
expression levels of ACh and ChAT. Low levels of ChAT and ACh are
consistently observed in brain tissues of patients with AD, which
is thought to be responsible for the decline in learning and memory
abilities (33). H.
erinaceus has been demonstrated to improve learning and memory
abilities in AD mice via modulation of ACh and ChAT expression
levels (9). Additionally,
Flammulina velutipes polysaccharides increase the expression
levels of ACh and ChAT in scopolamine-induced neuron damaged rats
(34). The modulating effect of LB
on ACh and ChAT expression levels suggests that its neuroprotective
effects in AD mice may be mediated in part through the improvement
of cholinergic function.
In conclusion, the neuroprotective effects of LB
were successfully verified through a L-Glu-induced DPC12 apoptosis
cell model and an AlCl3 and D-gal-induced AD mouse
model. The present study revealed that this effect may be
associated with modulation of the mitochondrial pathway of
apoptosis and the cholinergic system. Thus, LB may be a potential
candidate for the treatment or prevention of neurodegenerative
disease.
References
|
1
|
Chang CH, Chen Y, Yew XX, Chen HX, Kim JX,
Chang CC, Peng CC and Peng RY: Improvement of erinacine A
productivity in Hericium erinaceus mycelia and its neuroprotective
bioactivity against the glutamate-insulted apoptosis. LWT Food Sci
Technol. 65:1100–1108. 2016. View Article : Google Scholar
|
|
2
|
Bermejo-Pareja F, Llamas Velasco S and
Villarejo-Galende A: Alzheimer's disease prevention: A way forward.
Revista Clínica Española (English Edition). 216:495–503. 2016.
View Article : Google Scholar
|
|
3
|
Rosello A, Warnes G and Meier UC: Cell
death pathways and autophagy in the central nervous system and its
involvement in neurodegeneration, immunity and central nervous
system infection: To die or not to die-that is the question. Clin
Exp Immunol. 168:52–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karbowski M and Neutzner A:
Neurodegeneration as a consequence of failed mitochondrial
maintenance. Acta Neuropathol. 123:157–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Niu F, Sun Z, Cao W, Zhang X, Guan
D, Lv Z, Zhang B and Xu Y: Altered expression of A beta
metabolism-associated molecules from D-galactose/AlCl(3) induced
mouse brain. Mech Ageing Dev. 130:248–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SP, Yang FQ and Tsim KW: Quality
control of Cordyceps sinensis, a valued traditional Chinese
medicine. J Pharm Biomed Anal. 41:1571–1584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu S, Wang D, Zhang J, Du M, Cheng Y, Liu
Y, Zhang N, Wang D and Wu Y: Mitochondria related pathway is
essential for polysaccharides purified from sparassis crispa
mediated neuro-protection against glutamate-induced toxicity in
differentiated PC12 cells. Int J Mol Sci. 17:pii: E1332016.
View Article : Google Scholar
|
|
9
|
Zhang J, An S, Hu W, Teng M, Wang X, Qu Y,
Liu Y, Yuan Y and Wang D: The Neuroprotective properties of
Hericium erinaceus in glutamate-damaged differentiated PC12 cells
and an Alzheimer's disease mouse model. Int J Mol Sci. 17:pii:
E18102016. View Article : Google Scholar
|
|
10
|
Zareisedehizadeh S, Tan CH and Koh HL: A
review of botanical characteristics, traditional usage, chemical
components, pharmacological activities and safety of pereskia bleo
(Kunth) DC. Evid Based Complement Alternat Med. 2014:3261072014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao K, Liu M, Cao J, Yao M, Lu Y, Li J,
Zhu X, Yang Z and Wen A: Protective effects of Lycium barbarum
polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through
the ROS-NO pathway. Molecules. 20:293–308. 2015. View Article : Google Scholar
|
|
12
|
Mi XS, Feng Q, Lo AC, Chang RC, Lin B,
Chung SK and So KF: Protection of retinal ganglion cells and
retinal vasculature by Lycium barbarum polysaccharides in a mouse
model of acute ocular hypertension. PLoS One. 7:e454692012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Li Q, Wang J and Teng L:
Screening of Irpex lacteus mutant strains and optimizing
fermentation conditions. J Food Agric Environ. 12:1213–1219.
2014.
|
|
14
|
Wang H, Pampati N, McCormick WM and
Bhattacharyya L: Protein nitrogen determination by kjeldahl
digestion and ion chromatography. J Pharm Sci. 105:1851–1857. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su WT and Shih YA: Nanofiber containing
carbon nanotubes enhanced PC12 cell proliferation and
neuritogenesis by electrical stimulation. Biomed Mater Eng. 26
Suppl 1:S189–S195. 2015.PubMed/NCBI
|
|
16
|
Shimmyo Y, Kihara T, Akaike A, Niidome T
and Sugimoto H: Three distinct neuroprotective functions of
myricetin against glutamate-induced neuronal cell death:
Involvement of direct inhibition of caspase-3. J Neurosci Res.
86:1836–1845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheriyan J, Balsara RD, Hansen KB and
Castellino FJ: Pharmacology of triheteromeric N-Methyl-d-Aspartate
receptors. Neurosci Lett. 617:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feissner RF, Skalska J, Gaum WE and Sheu
SS: Crosstalk signaling between mitochondrial Ca2+ and ROS. Front
Biosci (Landmark Ed). 14:1197–1218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernardi P and Rasola A: Calcium and cell
death: The mitochondrial connection. Subcell Biochem. 45:481–506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thatte U, Bagadey S and Dahanukar S:
Modulation of programmed cell death by medicinal plants. Cell Mol
Biol (Noisy-le-grand). 46:199–214. 2000.PubMed/NCBI
|
|
21
|
Christophe M and Nicolas S: Mitochondria:
A target for neuroprotective interventions in cerebral
ischemia-reperfusion. Curr Pharm Des. 12:739–757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL,
Cui Y, Guo RX and Yu HM: Protection of oxidative preconditioning
against apoptosis induced by H2O2 in PC12 cells: Mechanisms via
MMP, ROS and Bcl-2. Brain Res. 1057:57–64. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo M, Lu Z, Sun H, Yuan K, Zhang Q, Meng
S, Wang F, Guo H, Ju X, Liu Y, et al: Nuclear entry of active
caspase-3 is facilitated by its p3-recognition-based specific
cleavage activity. Cell Res. 20:211–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hippe D, Gais A, Gross U and Luder CG:
Modulation of caspase activation by Toxoplasma gondii. Methods Mol
Biol. 470:275–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SY, Cherla RP, Caliskan I and Tesh VL:
Shiga toxin 1 induces apoptosis in the human myelogenous leukemia
cell line THP-1 by a caspase-8-dependent, tumor necrosis factor
receptor-independent mechanism. Infect Immun. 73:5115–5126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang D, Yaguchi T, Nakano T and Nishizaki
T: Adenosine-induced caspase-3 activation by tuning
Bcl-XL/DIABLO/IAP expression in HuH-7 human hepatoma cells. Cell
Biol Toxicol. 26:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boucher D, Blais V, Drag M and Denault JB:
Molecular determinants involved in activation of caspase 7. Biosci
Rep. 31:283–294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Espín R, Roca FJ, Candel S, Sepulcre MP,
González-Rosa JM, Alcaraz-Pérez F, Meseguer J, Cayuela ML, Mercader
N and Mulero V: TNF receptors regulate vascular homeostasis in
zebrafish through a caspase-8, caspase-2 and P53 apoptotic program
that bypasses caspase-3. Dis Model Mech. 6:383–396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hall AM and Roberson ED: Mouse models of
Alzheimer's disease. Brain Res Bull. 88:3–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Salminen A, Haapasalo A, Kauppinen A,
Kaarniranta K, Soininen H and Hiltunen M: Impaired mitochondrial
energy metabolism in Alzheimer's disease: Impact on pathogenesis
via disturbed epigenetic regulation of chromatin landscape. Prog
Neurobiol. 131:1–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Wei X, Yang J, Suo J, Chen J, Liu
X and Zhao X: Chronic exposure to aluminum and risk of Alzheimer's
disease: A meta-analysis. Neurosci Lett. 610:200–2 06. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Farkas E and Luiten PGM: Cerebral
microvascular pathology in aging and Alzheimer's disease. Prog
Neurobiol. 64:575–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang W, Yu J, Zhao L, Ma N, Fang Y, Pei F,
Mariga AM and Hu Q: Polysaccharides from Flammulina velutipes
improve scopolamine-induced impairment of learning and memory of
rats. J Funct Foods. 18:411–422. 2015. View Article : Google Scholar
|