Introduction
Due to the aging population, obesity is common and
is frequently associated with non-alcoholic fatty liver disease
(NAFLD), which includes non-alcoholic fatty liver and non-alcoholic
steatohepatitis (NASH) (1,2). Globally, NAFLD is a widespread
disorder which is now considered to be among the most common types
of liver disease. Although initially benign, the disease may
progress from non-alcoholic steatosis (NAS) to NASH and
subsequently to hepatic fibrosis, liver cirrhosis and hepatoma
(3). The prevalence of NAFLD in
the general population of the western world has been reported to be
20–30% (3). In the general
population of Asia, the prevalence of NAFLD has been reported to be
15–30%, and >50% in patients with diabetes and metabolic
syndromes (4). In mainland China,
ultrasound surveys assessing fatty liver due to any cause have been
published since the mid-1990s (5,6).
From these surveys, the median prevalence of ultrasonographic
steatosis in the Chinese population has been observed to be 10%,
with a range of between 1 and 30% (5,7).
A recent study reported that the adiponectin-derived
active peptide ADP355 exerts anti-inflammatory and anti-fibrotic
activity in thioacetamide-induced liver injury (8). There has been interest in the
isolation and characterization of mesenchymal stem cells (MSCs) and
in the potential application of these cells to the treatment of
liver disease. MSCs are a heterogeneous subset of stromal stem
cells, which may be isolated from various adult tissues (9). They are able to differentiate into
cells of a mesodermal lineage, including adipocytes, osteocytes and
chondrocytes, in addition to cells of other embryonic lineages. The
multipotency of mesenchymal stem cells makes them an attractive
choice for clinical applications (10–12).
Immune modulation is an additional important issue in MSC
transplantation. It has been demonstrated that MSCs exhibited
potent anti-inflammatory and immunomodulatory activity in
vitro and in vivo (13). In liver disease, MSC
transplantation has been observed to exert therapeutic effects in
acute and chronic liver injury (14–18).
In a recent study, it was demonstrated that bone-derived MSC
transplantation was effective in treating experimental liver
fibrosis induced by consecutive intraperitoneal injections of
CCl4, and molecules secreted by the cells ameliorated
fulminant hepatic failure induced by thioacetamide (19). MSCs have been demonstrated to exert
a positive effect on the immune micro-environment in animal models
of fulminant hepatic failure (FHF) and chronic liver fibrosis
(19).
In the present study, the potential beneficial
effects of MSCs were investigated in a high fat diet (HFD)-induced
NAFLD model, including further examination of whether MSCs induced
immunosuppression. A mouse model of NAFLD was established through
treatment with a TROPHIC (T)/HFD (20). Isolation and culture of murine MSCs
from compact bone were acquired using modified previously-described
procedures (19,21). The results of the present study
demonstrated that NASH induced by a HFD was ameliorated by
treatment with MSCs, as indicated by a decrease in obesity, the
expansion of subcutaneous adipose tissue, hepatic lipid
accumulation, liver inflammation and fibrosis. MSC-mediated
immunomodulation resulted from a decrease in cluster of
differentiation (CD)4+ T lymphocytes in the spleen.
Materials and methods
Animals and diet
A total of 18 of male C57BL/6 mice, aged 6–8 weeks,
weighing 16–18 g, were purchased from the Academy of Military
Medical Science (Beijing, China) and were housed in a pathogen-free
room, with a 12 h light/dark cycle at 20–25°C. They were maintained
on a normal diet or HFD obtained from TROPHIC Animal Feed High-Tech
Co., Ltd. (Nantong, Jiangsu, China). The food compositions of the
two dietary groups are presented in Table I. The total energy and cholesterol
content in the two dietary groups are presented in Table II. The total protein, carbohydrate
and total fat content within the total energy in the two dietary
groups are presented in Table
III. The mice were randomized into two groups: i) Normal mice;
and ii) T/HFD mice. A total of 21 weeks subsequently, the T/HFD
mice were randomized into two groups: i) T/HFD mice; and ii)
T/HFD+MSC mice, which were intravenously injected twice with
1×106 MSCs/mouse, at 21 and 23 weeks. A total of 21
weeks subsequently, the diets of the T/HFD and T/HFD+MSC mice were
replaced with a normal diet. A total of 28 weeks subsequently, all
of the animals were sacrificed and tissues were harvested. Mice
were weighed weekly. The present study was approved by the Animal
Ethics Committee of Tianjin Medical University (Tianjin,
China).
 | Table I.Food composition in the two dietary
groups. |
Table I.
Food composition in the two dietary
groups.
|
| Mass, g |
|---|
|
|
|
|---|
| Component | Normal diet | High-fat diet |
|---|
| Casein | 193.000 | 262.000 |
| Corn starch | 296.500 | 0.000 |
| Maltodextrin | 33.000 | 161.000 |
| Sucrose | 332.000 | 89.000 |
| Soybean oil | 24.000 | 32.000 |
| Lard | 19.000 | 317.000 |
| Cellulose | 47.000 | 65.000 |
| Mineral mix | 43.000 | 58.000 |
| Vitamin mix | 9.500 | 13.000 |
| L-cysteine | 3.000 | 4.000 |
| Choline
bitartrate | 3.000 | 3.000 |
| TBHQ | 0.008 | 0.069 |
| Total | 1,000.000 | 1,000.000 |
 | Table II.Total energy and cholesterol content
in the two dietary groups. |
Table II.
Total energy and cholesterol content
in the two dietary groups.
|
| Diet |
|---|
|
|
|
|---|
| Component | Normal diet | High-fat diet |
|---|
| Total energy,
kcal/g |
3.8 |
5.2 |
| Total cholesterol,
mg/kg | 40.8 | 228.0 |
 | Table III.Total Protein, carbohydrate and fat
content within the total energy in the two dietary groups. |
Table III.
Total Protein, carbohydrate and fat
content within the total energy in the two dietary groups.
|
| Diet |
|---|
|
|
|
|---|
| Component | Normal diet | High fat diet |
|---|
| Total protein,
% | 18 | 18 |
| Total carbohydrate,
% | 72 | 22 |
| Total fat, % | 10 | 60 |
Isolation and culture of bone-derived
MSCs
MSCs obtained from murine compact bone were isolated
and culture-expanded as described previously (19,21).
Femurs and tibiae were collected from a total of 3 2–3-week-old
female C57BL/6 mice (weighing 6–10 g) and were purchased from the
Academy of Military Medical Science, Beijing, China. They were
housed in a pathogen-free room, with a 12 h light/dark cycle at
20–25°C. Bone marrow was flushed with PBS or α-modified minimal
essential medium (α-MEM) (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) using a syringe. The compact bones were excised
into chips of ~1 mm into plastic culture dishes, and washed with
PBS or α-MEM until the released cells were removed. The cells were
incubated in α-MEM containing 10% select fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C, in an atmosphere
containing 5% CO2. The medium was replaced every 4–5
days. Adherent cells (passage 0) were confluent following 1–2 weeks
of incubation and were harvested using a cell scraper. The cells
were passaged by digestion with 0.25% trypsin-EDTA (Gibco; Thermo
Fisher Scientific, Inc.). Subsequent to 5–8 passages, the cells
were used for further experiments.
Examination of immunophenotypic
features of MSCs
The prepared MSCs, as described above, were
harvested by digestion with trypsin, and stained for 30 min at 4°C
with fluorescein isothiocyanate-conjugated anti-mouse CD11b, CD45,
CD105 and ataxin-1 (Sca-1) antibodies, or phycoerythrin-conjugated
anti-mouse CD29, CD44 and CD135 antibodies (all eBioscience, Inc.;
Thermo Fisher Scientific, Inc.). Cells were analyzed using a
FACSCalibur instrument using a laser at a wavelength of 488 nm (BD
Biosciences, Franklin Lakes, NJ, USA). Flow cytometric data were
analyzed using Flow Jo software (version 7.6; Tree Star, Inc.,
Ashland, OR, USA).
Histological analysis of livers
Livers were perfused with PBS, removed, weighed and
sliced into 0.5×0.5 cm sections. The sections were embedded in
paraffin subsequent to being fixed in 4% (w/v) paraformaldehyde and
were cut into 6-µm-thick sections. The paraffin sections were
stained with 0.5 % w/v hematoxylin for 5 min and 1% w/v eosin for
10 sec (HE) at room temperature to assess inflammation and
steatosis, and picrosirius red to assess fibrosis. Evaluation of
the extent of resultant NASH (3)
was performed using the following scaling scores.
Steatosis
Hepatocytes containing fat vacuoles were
subjectively visualized and graded according to the following
scale: 0, Normal, no hepatocytes affected; 1, minor, <5% of
hepatocytes affected; 2, mild, 5–33% of hepatocytes affected; 3,
moderate, 34–66% of hepatocytes affected; and 4, severe, >66% of
hepatocytes affected.
Lobular inflammation
Grading of lobular inflammation was performed as
follows: 0, None; 1, 1–2 foci/x20 field; 2, 2–4 foci/x20 field; and
3, >4 foci/x20 field.
Stages of NASH
Staging of NASH was performed as follows: 0, None;
1, extensive zone 3 perisinusoidal fibrosis; 2, zone 3
perisinusoidal, and portal or periportal fibrosis; 3, bridging
fibrosis; and 4, cirrhosis.
Analysis of spleen leukocytes
Single-cell suspensions derived from spleens were
prepared by mechanical disruption and filtered through a 40-µm cell
strainer (BD Biosciences). The cells were placed in H2O
30–50s, soon later added with 1/10 volume 10× PBS, and red blood
cells were removed with cytolysis. The cells were stimulated with
50 ng/ml phorbol 12-myristate 13-acetate, 1 µg/ml ionomycin (Enzo
Life Sciences, Inc., Farmingdale, NY, USA) and 3 µg/ml brefeldin A
(eBioscience, Inc.; Thermo Fisher Scientific, Inc.) for 5 h. The
cells were subsequently stained for surface markers using rat
anti-mouse CD4-allophycocyanin (catalog no. 17-0042-81,
eBioscience, Inc., San Diego, CA, USA) for 30 min at 4°C. The cells
were analyzed using the 488 nm laser of a FACSCalibur instrument
and the data generated were analyzed using FlowJo software version
7.6.1 (Tree Star Inc., Ashland, OR, USA).
Statistical analysis
GraphPad PRISM software (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA) was used to perform a Student's
t test Results are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
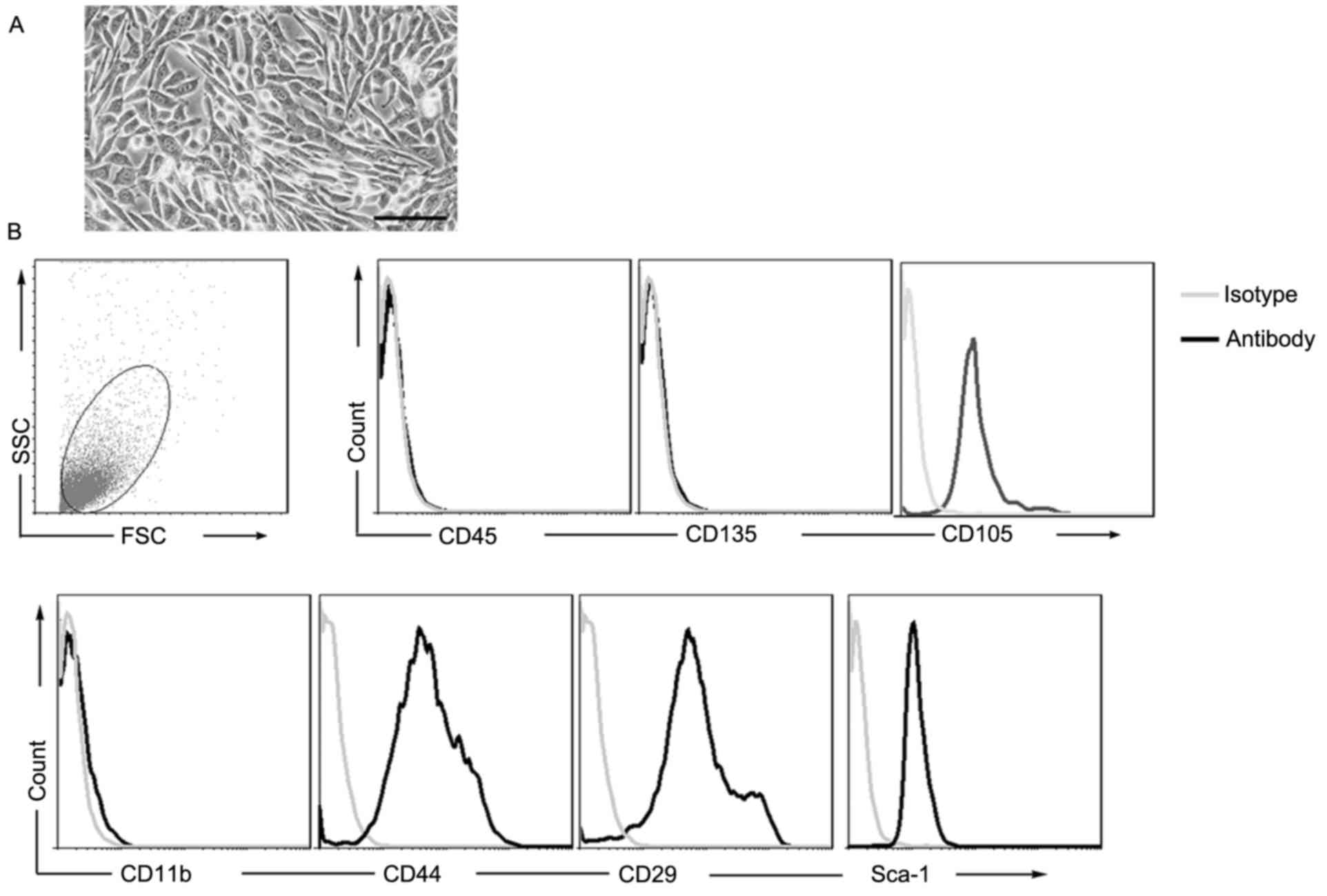
Cells isolated from compact bone were
characterized as MSCs
According to modified previously-described
procedures (19), mMSCs were
isolated from compact bone (from 2–3-week-old female C57BL/6 mice)
and cultured. The MSCs appeared to be vortex-shaped and
fibroblast-like, although not polygon-shaped (osteocytes) (Fig. 1A). In order to further identify the
adherent cells, immunophenotypic features were analyzed. As
presented in Fig. 1B, negative
surface markers of MSCs, including CD11b, CD45 and CD135, were not
expressed; however, the cells expressed surface markers
characteristic of MSCs, including CD29, CD44, CD105 and Sca-1. The
results of the present study indicated that these cells were
MSCs.
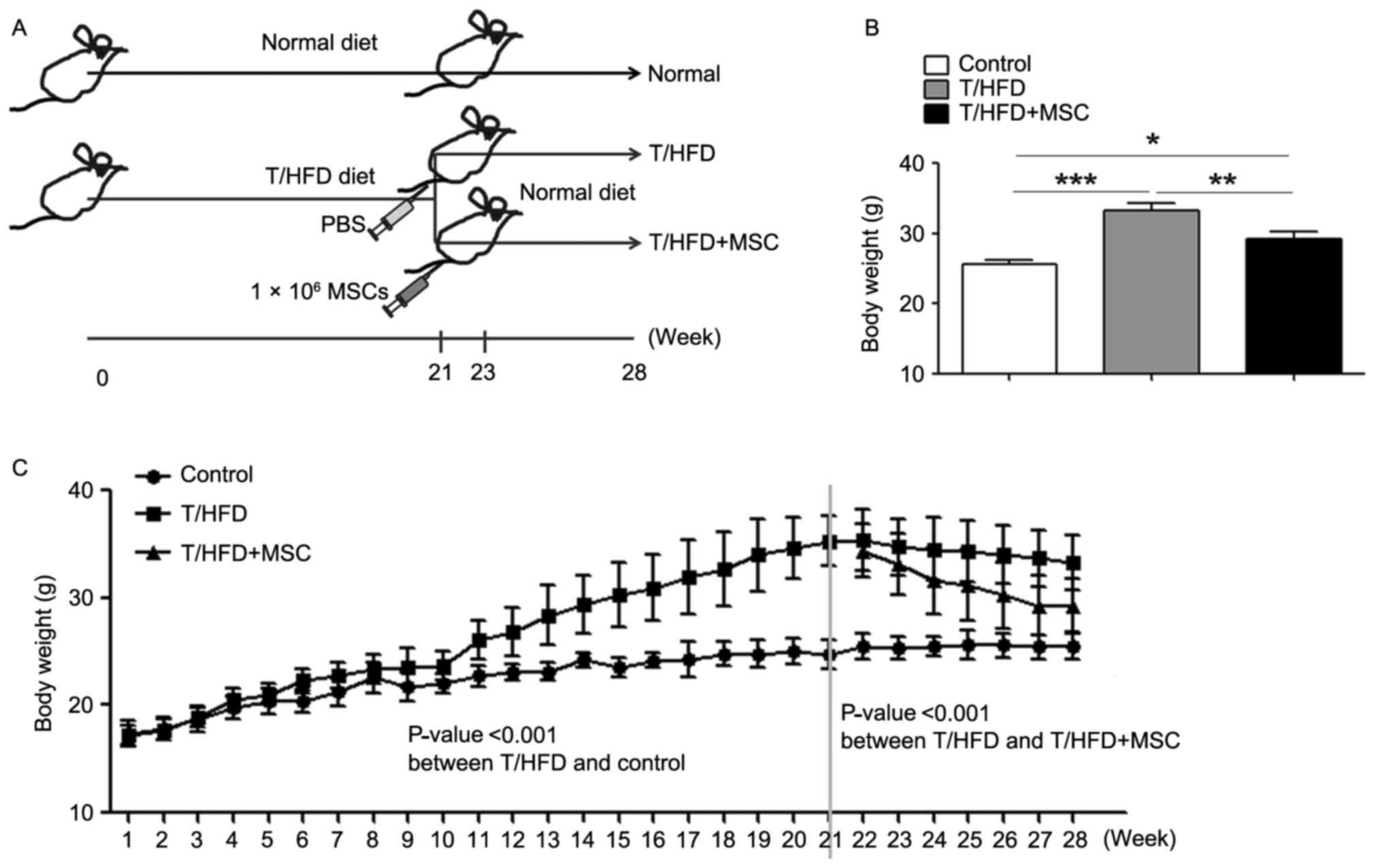
Treatment with MSCs reverses
HFD-induced weight gain and expansion of subcutaneous adipose
tissue
In order to investigate the beneficial effects of
MSCs on NAFLD, a mouse model of NAFLD was established using HFD,
and the mice were intravenously-injected twice with
1×106 MSCs/mouse at weeks 21 and 23 (Fig. 2A). A marked difference was observed
between T/HFD and T/HFD+MSC mice at 28 weeks post-treatment
(Fig. 2B). Compared with normal
control mice, the T/HFD-fed mice exhibited an accelerated elevation
of body weight between 1 and 21 weeks (Fig. 2C). Following the two intravenous
injections of MSCs, the weight of the T/HFD-fed mice decreased
markedly at 21–28 weeks (Fig.
2C).
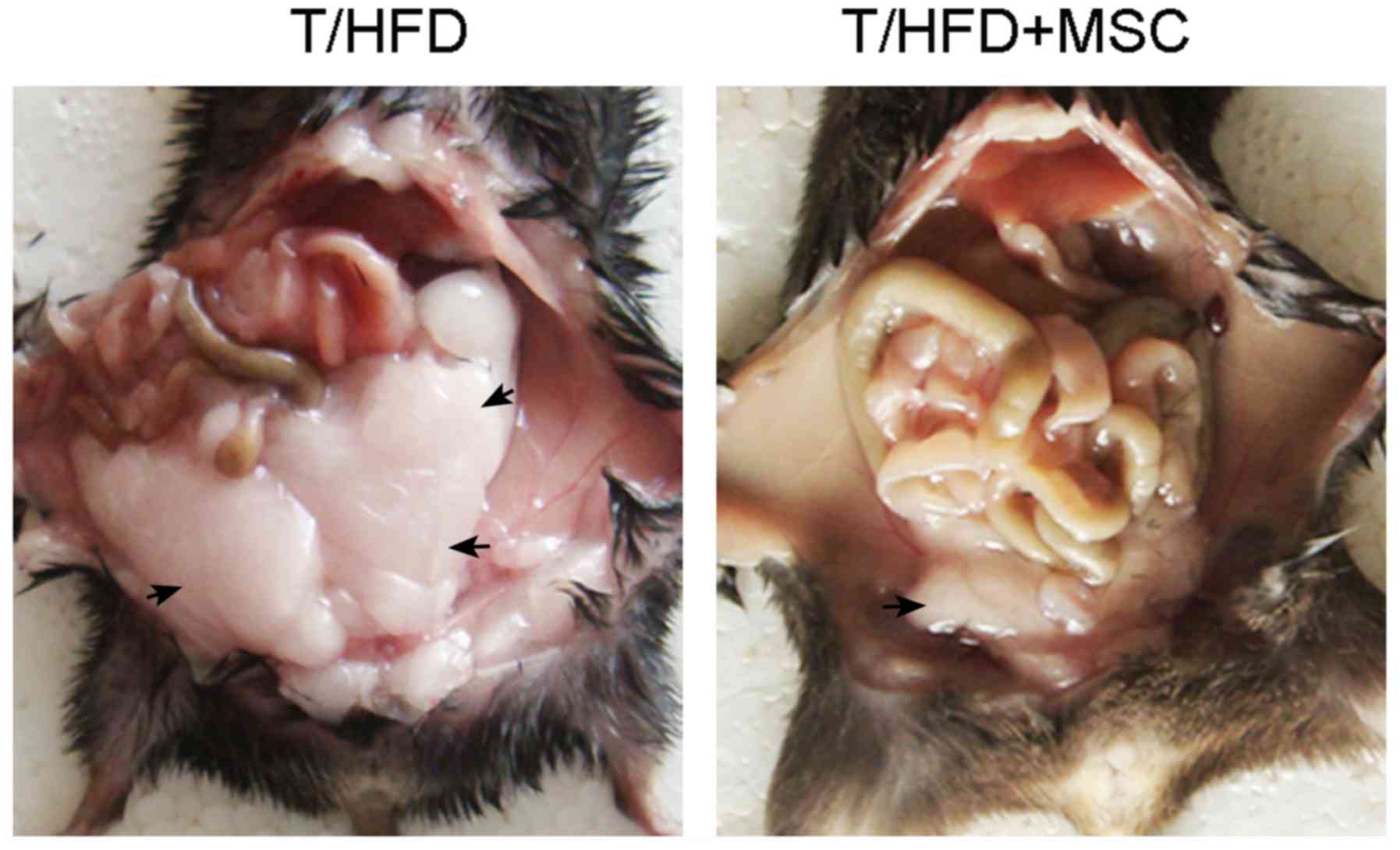
Weight gain is often accompanied by the expansion of
subcutaneous adipose tissue. The results of the present study
demonstrated that the mass of subcutaneous abdominal adipose tissue
increased in the T/HFD-fed mice, an effect which was reversed by
the MSC treatment (Fig. 3).
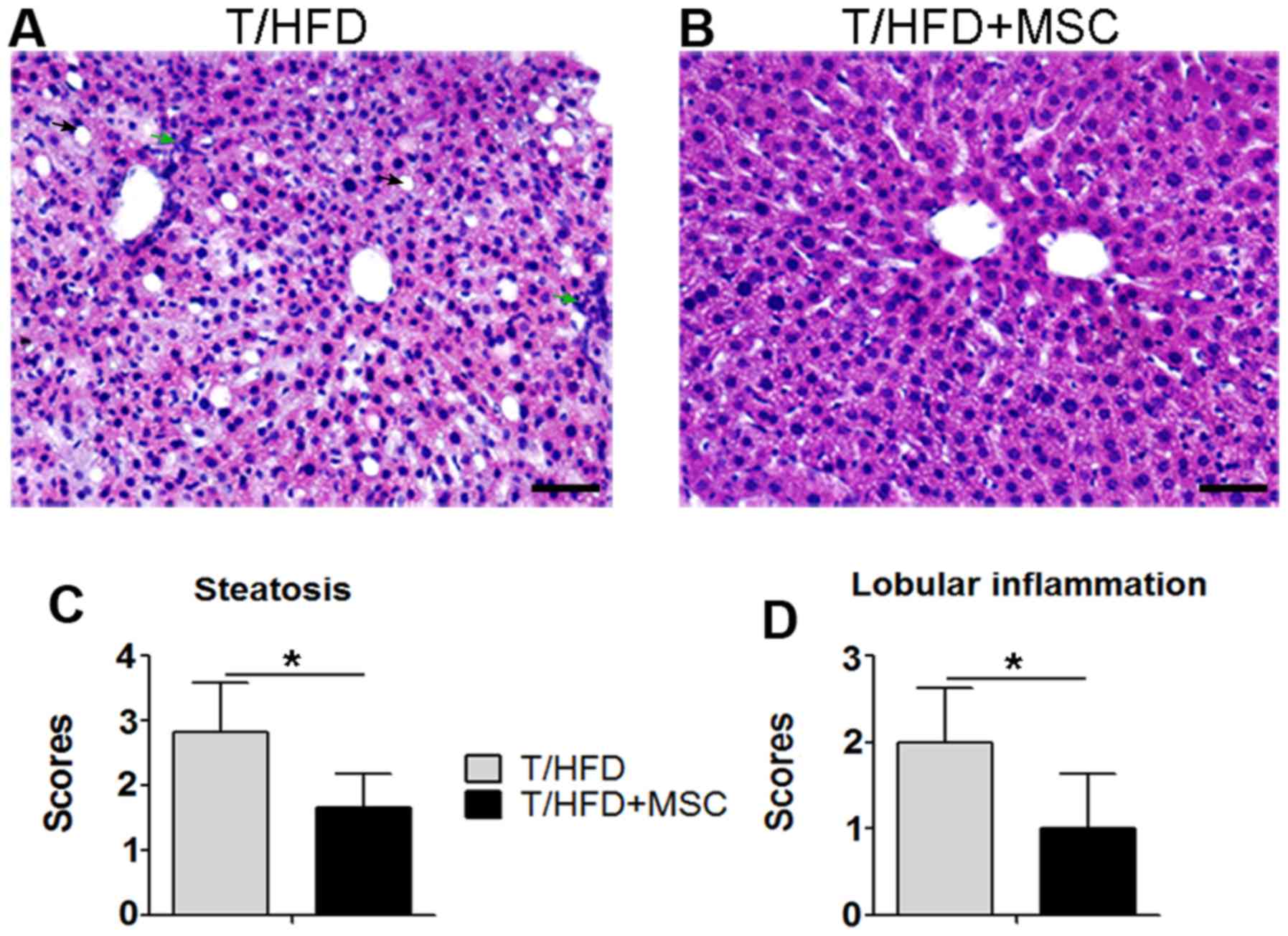
Treatment with MSCs decreases
HFD-induced steatosis and lobular inflammation
NFLD may progress via hepatic lipid accumulation to
NAS, and via lobular inflammation to NASH (3). Hepatocytes containing fat vacuoles
may be visualized as clear bubbles following liver section HE
staining. As presented in Fig. 4,
hepatic lipid accumulation indicated by clear bubbles occurred in
T/HFD-fed mice (Fig. 4A), while it
was decreased in T/HFD+MSC mice (Fig.
4B). It was additionally observed that HFD-induced lobular
inflammation was present within the THFD-fed mouse livers (Fig. 4A), which was suppressed in the
T/HFD+MSC mice (Fig. 4B). As
indicated by scores obtained from the methods described above,
increases in steatosis (Fig. 4C)
and lobular inflammation (Fig. 4D)
induced by HFD were significantly decreased in response to MSC
treatment.
Treatment with MSCs suppresses
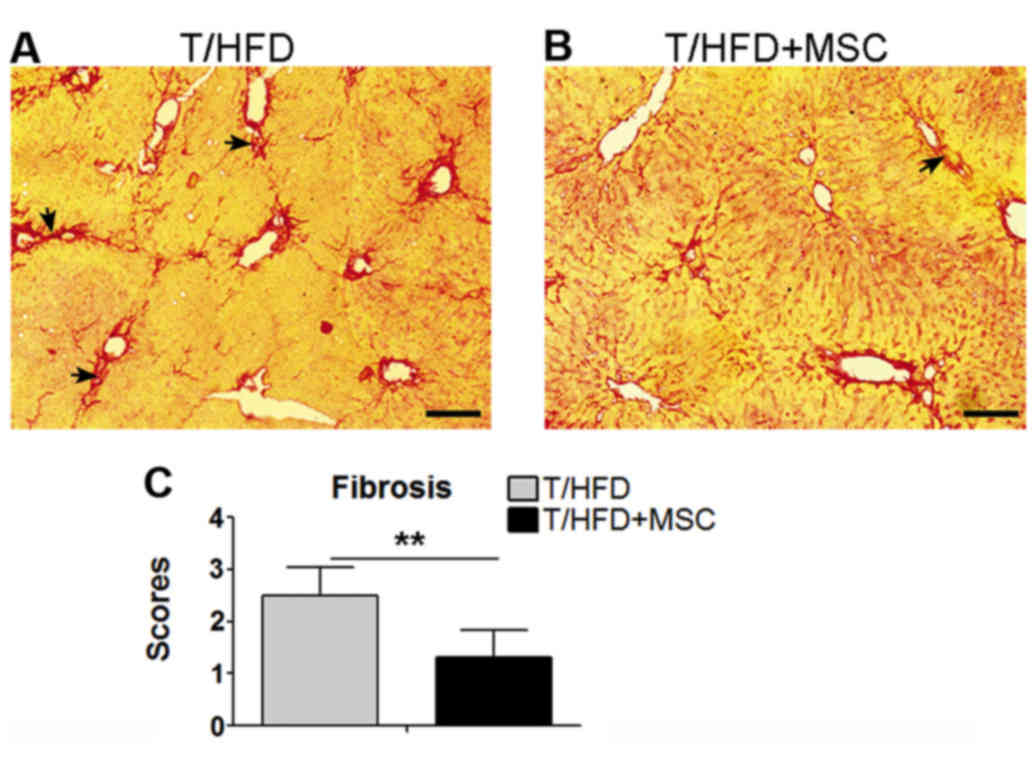
HFD-induced liver fibrogenesis
NAFLD frequently progresses to fibrosis (3). Therefore, the stages of fibrosis in
the liver samples were assessed by staining with picrosirius red.
Fibrogenesis was observed within the T/HFD-fed mouse livers
(Fig. 5A), and a reduction of
hepatic fibrogenesis occurred in the T/HFD+MSC mice (Fig. 5B). Significant differences in
fibrosis stage scores were observed between the T/HFD and T/HFD+MSC
mice (Fig. 5C).
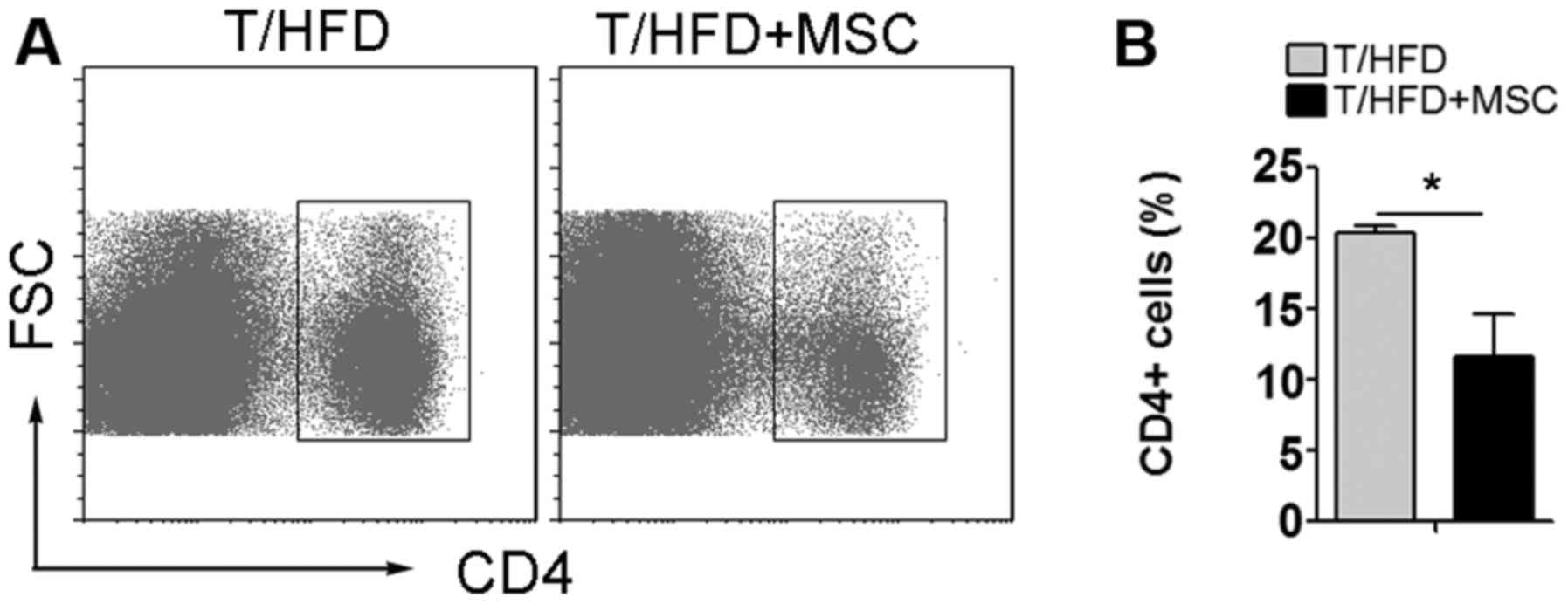
The number of CD4+ T
lymphocytes in the spleen is decreased by treatment with MSCs
MSCs are able to interact with innate and adaptive
immune system cells, leading to the modulation of numerous effector
functions (9). Analysis of splenic
leukocytes was performed in order to explore the effect of MSCs on
immunological responses. It was observed that, compared with
T/HFD-fed mice, the number of CD4+ T lymphocytes in the
spleen was decreased by treatment with MSCs in the T/HFD+MSC mice
(Fig. 6).
Discussion
MSCs were originally isolated from bone marrow by
Friedenstein et al (22) in
1976, and have subsequently been observed to exist in other organs
and tissues (21). However, the
bone marrow-derived method based on plastic adherence has proved
unsuccessful for mMSCs, due to the low frequency of mMSCs and the
contamination of hematopoietic cells in culture (23). In the present study, the compact
bone-derived method established originally by Zhu et al
(21) was used. MSCs that appeared
vortex-shaped and fibroblast-like were successfully isolated, and
were demonstrated to express putative surface markers of MSCs,
including CD29, CD44, CD105 and Sca-1, which was consistent with a
previous report (21).
MSCs exhibit potential clinical value in the
treatment of liver disease. In a previous study, it was
demonstrated that compact bone-derived MSCs improved gross and
microscopic liver histopathology and prolonged the survival of mice
with thioacetamide-induced FHF, in addition to suppressing
CCl4-induced chronic liver fibrosis (19). In addition, it was demonstrated
that treatment with MSCs partially ameliorated FHF, and markedly
improved chronic liver fibrosis (19). In the present study, a model of
NAFLD was established using T/HFD (20), in order to explore whether MSCs
exhibit potential clinical value in NAFLD. It was observed that
HFD-fed mice with MSC intervention exhibited a decrease in weight
gain. Obesity is often accompanied by the expansion of subcutaneous
adipose tissue (4), which was
demonstrated to be reversed by treatment with MSCs in the present
study. In addition, the steatosis and liver fibrosis in the present
model of NAFLD were ameliorated by treatment with MSCs. The results
of the present study suggested that MSCs may exhibit clinical value
in NAFLD.
The immunomodulatory and immunosuppressive functions
of MSCs are potentially involved in the beneficial effect of MSC
transplantation, in chronic and acute liver disease (24). Recently, it was demonstrated that
MSC therapy suppressed liver fibrosis by downregulating immune cell
infiltration (19). In the present
study, MSC intervention suppressed lobular inflammatory cell
infiltration, indicated by HE staining, in the livers of T/HFD
mice. The results of the present study demonstrated that the
immunomodulatory and immunosuppressive functions of MSCs may serve
a role in the beneficial effect of MSC transplantation observed in
the present model of NAFLD.
The immunosuppressive effect of treatment with MSCs,
inducing an anti-inflammatory state, has been demonstrated to be
associated with an altered distribution of CD4+ T
lymphocytes (19). Autologous and
allogeneic bone marrow-derived MSCs have been demonstrated to
dose-dependently and contact-independently reduce CD4+ T
cell proliferation, induced by cellular or nonspecific mitogenic
stimuli (25). The suppressive
capacities of MSCs were further confirmed in preclinical studies,
demonstrating that treatment with MSC is able to modulate
pathogenic T cell responses (26–28).
In the present study, it was demonstrated that transplantation of
compact bone-derived MSCs led to a suppression of CD4+ T
cell proliferation in the spleens of T/HFD mice. It is hypothesized
that the suppression of CD4+ T cells is one mechanism by
which MSCs exert immunomodulatory and immunosuppressive
functions.
In summary, compact bone-derived MSCs exhibit
potential clinical value in a mouse model of NAFLD. MSC
transplantation decreased weight gain and the expansion of
subcutaneous adipose tissue, decreased HFD-induced steatosis and
lobular inflammation, and suppressed liver fibrogenesis. MSCs exert
beneficial effects in the mouse model of NAFLD via immunomodulation
and immunosuppression, including the suppression of CD4+
T cells.
Acknowledgements
The present study was supported by the One College
One Policy Project, Modern College of Arts and Science, Shanxi
Normal University and the Scientific Research Foundation for
Doctors, Shanxi Normal University (grant no. 0505/02070293).
References
|
1
|
Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q,
Ren C, Ponomarenko A and DeCarli LM: Model of nonalcoholic
steatohepatitis. Am J Clin Nutr. 79:502–509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Flegal KM, Carroll MD, Kit BK and Ogden
CL: Prevalence of obesity and trends in the distribution of body
mass index among US adults, 1999–2010. Jama. 307:491–497. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmed M: Non-alcoholic fatty liver disease
in 2015. World J Hepatol. 7:1450–1459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong VW: Nonalcoholic fatty liver disease
in Asia: A story of growth. J Gastroenterol Hepatol. 28:18–23.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan J: Steatohepatitis studies in China.
World Chi J Digestology. 9:6–10. 2001.
|
|
6
|
Fan JG and Farrell GC: Epidemiology of
non-alcoholic fatty liver disease in China. J Hepatol. 50:204–210.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HJ, Zhuang H and Liu XE: Advances in
the epidemiological study of fatty liver. Zhonghua Liu Xing Bing
Xue Za Zhi. 25:630–632. 2004.(In Chinese). PubMed/NCBI
|
|
8
|
Wang H, Zhang H, Zhang Z, Huang B, Cheng
X, Wang D, La Gahu Z, Xue Z, Da Y, Li D, et al: Adiponectin-derived
active peptide ADP355 exerts anti-inflammatory and anti-fibrotic
activities in thioacetamide-induced liver injury. Sci Rep.
6:194452016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Uccelli A, Moretta L and Pistoia V:
Mesenchymal stem cells in health and disease. Nat Rev Immunol.
8:726–736. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding DC, Shyu WC and Lin SZ: Mesenchymal
stem cells. Cell Transplant. 20:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang B, Li G and Jiang XH: Fate
determination in mesenchymal stem cells: A perspective from
histone-modifying enzymes. Stem Cell Res Ther. 6:352015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke C, Biao H, Qianqian L, Yunwei S and
Xiaohua J: Mesenchymal stem cell therapy for inflammatory bowel
diseases: Promise and challenge. Curr Stem Cell Res Ther.
10:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rankin S: Mesenchymal stem cells. Thorax.
67:565–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y, Araki H, Kato J, Nakamura K,
Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M,
et al: Human mesenchymal stem cells xenografted directly to rat
liver are differentiated into human hepatocytes without fusion.
Blood. 106:756–763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang B, Shi M, Liao L, Yang S, Liu Y and
Zhao RC: Systemic infusion of FLK1(+) mesenchymal stem cells
ameliorate carbon tetrachloride-induced liver fibrosis in mice.
Transplantation. 78:83–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aurich I, Mueller LP, Aurich H,
Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J,
Hengstler JG, Fleig WE and Christ B: Functional integration of
hepatocytes derived from human mesenchymal stem cells into mouse
livers. Gut. 56:405–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parekkadan B, Van Poll D, Suganuma K,
Carter EA, Berthiaume F, Tilles AW and Yarmush ML: Mesenchymal stem
cell-derived molecules reverse fulminant hepatic failure. PLoS One.
2:e9412007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuo TK, Hung SP, Chuang CH, Chen CT, Shih
YR, Fang SC, Yang VW and Lee OK: Stem cell therapy for liver
disease: Parameters governing the success of using bone marrow
mesenchymal stem cells. Gastroenterology. 134:2111–2121, e1-e3.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang B, Cheng X, Wang H, Huang W, La Ga
Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y, et al: Mesenchymal
stem cells and their secreted molecules predominantly ameliorate
fulminant hepatic failure and chronic liver fibrosis in mice
respectively. J Transl Med. 14:452016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XJ, Wang BW, Zhang C, Xia MZ, Chen YH,
Hu CQ, Wang H, Chen X and Xu DX: Vitamin d deficiency attenuates
high-fat diet-induced hyperinsulinemia and hepatic lipid
accumulation in male mice. Endocrinology. 156:2103–2113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu H, Guo ZK, Jiang XX, Li H, Wang XY,
Yao HY, Zhang Y and Mao N: A protocol for isolation and culture of
mesenchymal stem cells from mouse compact bone. Nat Protoc.
5:550–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Friedenstein AJ, Gorskaja J and Kulagina
NN: Fibroblast precursors in normal and irradiated mouse
hematopoietic organs. Exp Hematol. 4:267–274. 1976.PubMed/NCBI
|
|
23
|
Phinney DG, Kopen G, Isaacson RL and
Prockop DJ: Plastic adherent stromal cells from the bone marrow of
commonly used strains of inbred mice: Variations in yield, growth,
and differentiation. J Cell Biochem. 72:570–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meier RP, Müller YD, Morel P,
Gonelle-Gispert C and Bühler LH: Transplantation of mesenchymal
stem cells for the treatment of liver diseases, is there enough
evidence? Stem Cell Res. 11:1348–1364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Di Nicola M, Carlo-Stella C, Magni M,
Milanesi M, Longoni PD, Matteucci P, Grisanti S and Gianni AM:
Human bone marrow stromal cells suppress T-lymphocyte proliferation
induced by cellular or nonspecific mitogenic stimuli. Blood.
99:3838–3843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zappia E, Casazza S, Pedemonte E,
Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti
F, Frassoni F, et al: Mesenchymal stem cells ameliorate
experimental autoimmune encephalomyelitis inducing T-cell anergy.
Blood. 106:1755–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Augello A, Tasso R, Negrini SM, Cancedda R
and Pennesi G: Cell therapy using allogeneic bone marrow
mesenchymal stem cells prevents tissue damage in collagen-induced
arthritis. Arthritis Rheum. 56:1175–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urbán VS, Kiss J, Kovács J, Gócza E, Vas
V, Monostori E and Uher F: Mesenchymal stem cells cooperate with
bone marrow cells in therapy of diabetes. Stem Cells. 26:244–253.
2008. View Article : Google Scholar : PubMed/NCBI
|