Introduction
Atherosclerosis is a chronic inflammatory disease
that causes vascular walls that can cause cardiovascular disease
such as stroke, myocardial infarction, and peripheral blood artery
disease (1,2). In developed countries,
atherosclerosis is a leading cause of mortality. It remains a huge
challenge to solve this global clinical problem. The pathogenesis
of atherosclerotic lesion formation is a multistage process.
Inflammation is a major component of atherosclerosis and considered
to play a role in all developmental stages of the diseases
(3,4). So far, few have known that the
complex upstream gene regulators involved in response to
atherosclerosis.
MicroRNAs are evolutionary conserved non-coding RNAs
of about 19–25 nucleotides, function by regulating one or more mRNA
to regulate gene expression for translation inhibition or cleavage
(5,6). With regard to miRNA function, they
play a key role in cell proliferation, cell death and organ
development (7,8). Recent studies have revealed that
miRNAs play a key role in the pathophysiological processes of
atherosclerosis. MicroRNAs control the senescence and dysfunction
of endothelial cells, proliferation and migration of vascular
smooth muscle cells, and macrophage-driven cytokine production and
polarization.
MicroRNA-34a (miR-34a) is located in the region of
chromosome 1p36.23, and usually, it is expressed aberrantly in
multiple types of diseases such as human tumors (9), atherosclerotic cardiovascular
diseases (10,11), and other types of diseases. MiR-34a
is suggested highly expressed in atherosclerosis patients and
played critical roles in the regulation of various cell biological
events including cell proliferation, differentiation, apoptosis,
etc. Endothelial cell (EC) apoptosis is a crucial process for the
development of atherosclerosis.
To the best of our knowledge, no precise studies
have been made about the role of miR-34a in cell apoptosis in
atherosclerosis progress. This study we will investigate the exact
role of miR-34a in cell apoptosis in atherosclerosis.
Materials and methods
Tissue samples
A total of 15 pairs of atherosclerotic lesion
tissues and normal veins were obtained during treatment from 15
patients at Dongyang People's Hospital. All tissues were
immediately stored in liquid nitrogen until use. All the protocols
were approved by the Ethics Committee of Dongyang People's
Hospital. Informed consent was obtained from each patient.
Cell culture
The human aortic endothelial cells (HAECs) were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin. All cells were incubated in a
humidified incubator at 37°C and 5% CO2.
Establishment of atherosclerotic cell
model
Ox-LDL was used to establish the atherosclerotic
cell model. Native LDL (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was oxidized by exposure to CuSO4 (5 µmol/l free Cu2+
concentration) in PBS at 37°C for 24 h. HAECs were treated with 50
µg/ml ox-LDL for 24 h.
Cell transfection
The miR-34a mimic (50 nM), miR-34a inhibitor (50
nM), the negative control (50 nM) (Shanghai GenePharma Co., Ltd.,
Shanghai, China), 1 µg Control-siRNA (empty vector), 1 µg
HDAC1-siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or 50
nM miR-34a inhibitor+1 µg HDAC1-siRNA, was transfected into HAECs
respectively using 30 µl Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's instructions. Transfected cells were incubated at
37°C in an atmosphere of 5% CO2 for 48 h.
RNA extraction and reverse
transcription-quantitative PCR
Total RNA of atherosclerotic tissues or HAECs was
extracted by using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), and cDNAs were synthesized using miScript
Reverse Transcription kit (Qiagen GmbH, Hilden, Germany) according
to the manufacturer's instructions. The primers for reverse
transcription and amplification of miR-34a, HDAC1 and U6 were
designed and synthesized by GenScript Co., Ltd., (Nanjing, China).
Quantitative real-time PCR was conducted to detect miR-34a and
HDAC1 mRNA using the SYBR Premix Ex Taq™ II (TliRNaseH Plus) kit
(Takara Biotechnology Co., Ltd., Dalian, China) using the Bio-Rad
Laboratories, Inc., (Hercules, CA, USA) machine, with the U6 small
nuclear RNA and GAPDH as internal normalized references,
respectively. The primer sequences for qPCR were as
follows:U6-Forward: 5′AAAGCAAATCATCGGACGACC3′; U6-Reverse:
5′GTACAACACATTGTTTCCTCGGA3′; GAPDH-Forward:
5′GAAGGTGAAGGTCGGAGTC3′; GAPDH-Reverse: 5′GAAGATGGTGATGGGATTTC3′;
miR-34a-Forward: 5′CAGCCTGGAGGAGGATCGA3′; miR34a-Reverse:
5′TCCCAAAGCCCCCAATCT3′; HDAC1-Forward: 5′CTACTACGACGGGGATGTTGG3′;
HDAC1-Reverse: 5′GAGTCATGCGGATTCGGTGAG3′. The 2−ΔΔCq
method was applied to quantify the relative gene expressions
(12).
Cell proliferation assay
Cell Count Kit-8 assay (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used as a qualitative
marker for cell viability. After 48 h transfection with miR-34a
mimic, miR-34a inhibitor, the negative control or miR-34a
inhibitor+ HDAC1-siRNA, HAECs were seeded into 96 well plates in
triplicate at 5×103 cells per well. At 0, 24, 48, and 72
h, 10 µl of CCK-8 solution mixed with 90 µl of RPMI-1640 was added
to each well. And after 2 h of incubation, the absorbance was
measured at 570 nm.
Apoptosis assay
HAECs were transfected with miR-34a mimic, miR-34a
inhibitor, the negative control or miR-34a inhibitor+ HDAC1-siRNA,
and 48 h after transfection, the cells then were subjected to
apoptosis assay. Then 106 treated cells were stained with Annexin V
and PI using an apoptosis detection kit (BD Biosciences, Franklin
Lakes, NJ, USA). According to the manufacturer's instructions,
after incubation for 15 min in the dark, cell apoptosis were then
detected by flow cytometry.
Luciferase reporter assay
For confirmation of direct target binding, the wild
type and mutant 3′UTR of HDAC1 identified by TargetScan was cloned
into a pmiR-RB-ReportTM dual luciferase reporter gene plasmid
vector (Guangzhou RiboBio Co., Ltd., Guangzhou, China). The UTR
region of candidate target gene was inserted downstream of the
sequence of renilla luciferase, which is designed for reporter
fluorescence. For luciferase reporter analysis, HAECs were
co-transfected with luciferase reporter vectors and control mimic
or miR-34a mimic using Lipofectamine 2000. After 48 h, luciferase
activity was analyzed by the dual-luciferase assay system (Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocols.
Western blot analysis
HAECs were transfected with miR-34a mimic, miR-34a
inhibitor, the negative control or miR-34a inhibitor+ HDAC1-siRNA
for 48 h, then cells were collected and total proteins were
extracted in 40 mM Tris-HCl (pH 7.4) containing 150 mM NaCl and 1%
(v/v) Triton X-100, supplemented with protease inhibitors. Protein
concentration was determined using the bicinchoninic acid protein
assay (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of
protein were resolved on 10% SDS-PAGE gels, and then transferred to
a PVDF membrane (EMD Millipore, Billerica, MA, USA). After blocking
with 5% skimmed milk in TBST, then probed with antibodies against
Bcl-2 (cat no. 4223; dilution: 1:1,000), procaspase-3 (cat no.
9664; dilution: 1:1,000), procaspase-9 (cat no. 9501; dilution:
1:1,000), c-Myc (cat no. 13,987; dilution: 1:1,000); p21 (cat no.
2947; dilution: 1:1,000); β-actin (cat no. 4970; dilution: 1:1,000)
(all from Cell Signaling Technology, Inc., Danvers, MA, USA),
membranes were subsequently incubated with horseradish peroxidase
(HRP) conjugated secondary antibody (Anti-rabbit IgG, HRP-linked
Antibody; cat no. 7074; dilution: 1:5,000). Immunoreactive bands
were visualized using the enhanced chemiluminescence detection
system. The protein levels of the stripes were normalized based on
the gray value of β-actin.
Statistical analysis
SPSS v17.0 software was used to analyze the data.
Values are expressed as mean ± SD of experiments performed in
triplicate. Data were analyzed by one-way ANOVA or Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
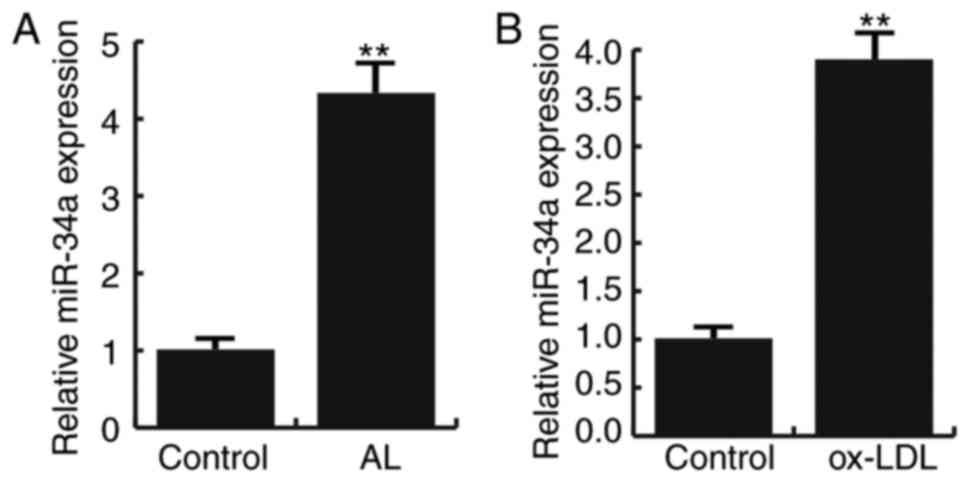
MiR-34a is highly expressed in
atherosclerosis
Fifteen pairs of atherosclerotic lesion tissues and
normal veins were recruited in this study. Q-RT PCR result showed
that compared to the normal veins, the expression of miR-34a were
significantly increased in atherosclerotic lesion tissues (Fig. 1A). In addition, Ox-LDL induction
significantly increased the expression of miR-34a in HAECs
(Fig. 1B). These data suggest that
miR-34a is highly expressed in atherosclerosis.
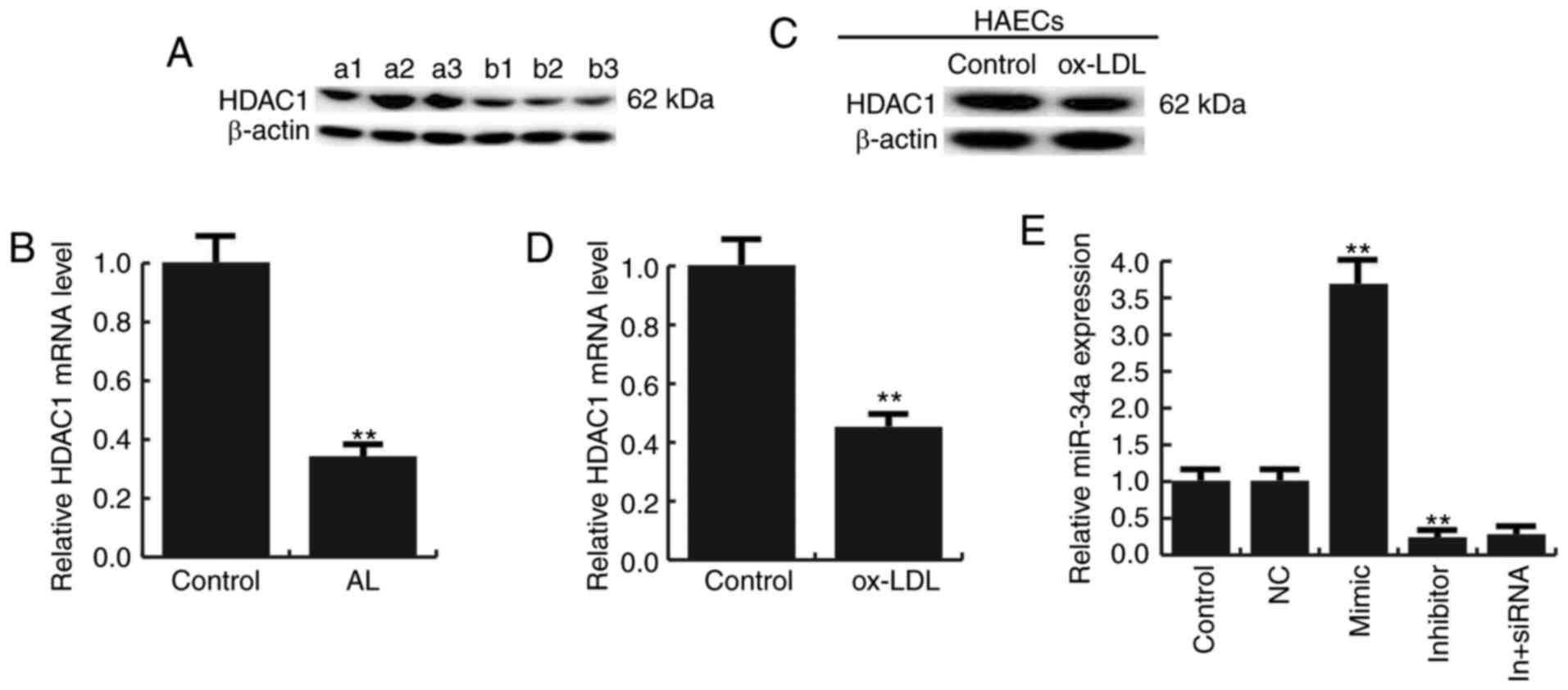
HDAC1 is low expressed in
atherosclerosis
We next studied the expression of HDAC1 in
atherosclerosis, and we found that the expression of HDAC1 was
reduced both in atherosclerotic lesions and Ox-LDL treated HAECs
(Fig. 2A-D).
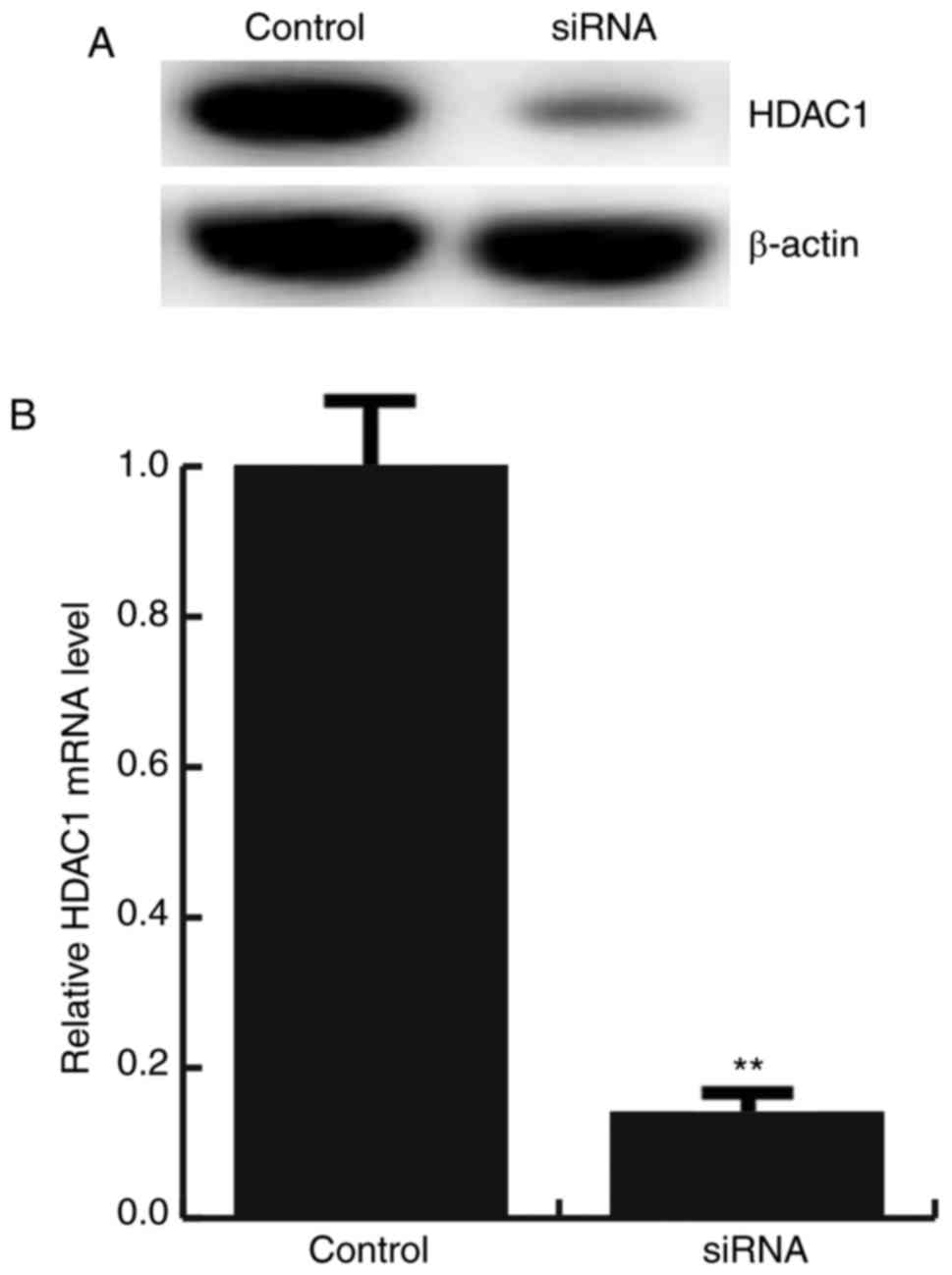
For further study the role of miR-34a in
atherosclerosis, miR-34a mimic, miR-34a inhibitor, the negative
control (NC), Control-siRNA (empty vector), HDAC1-siRNA, or miR-34a
inhibitor+HDAC1-siRNA was transfected into HAECs respectively, the
transfection efficiency was detected by qRT-PCR and western
blotting (Figs. 2E and 3).
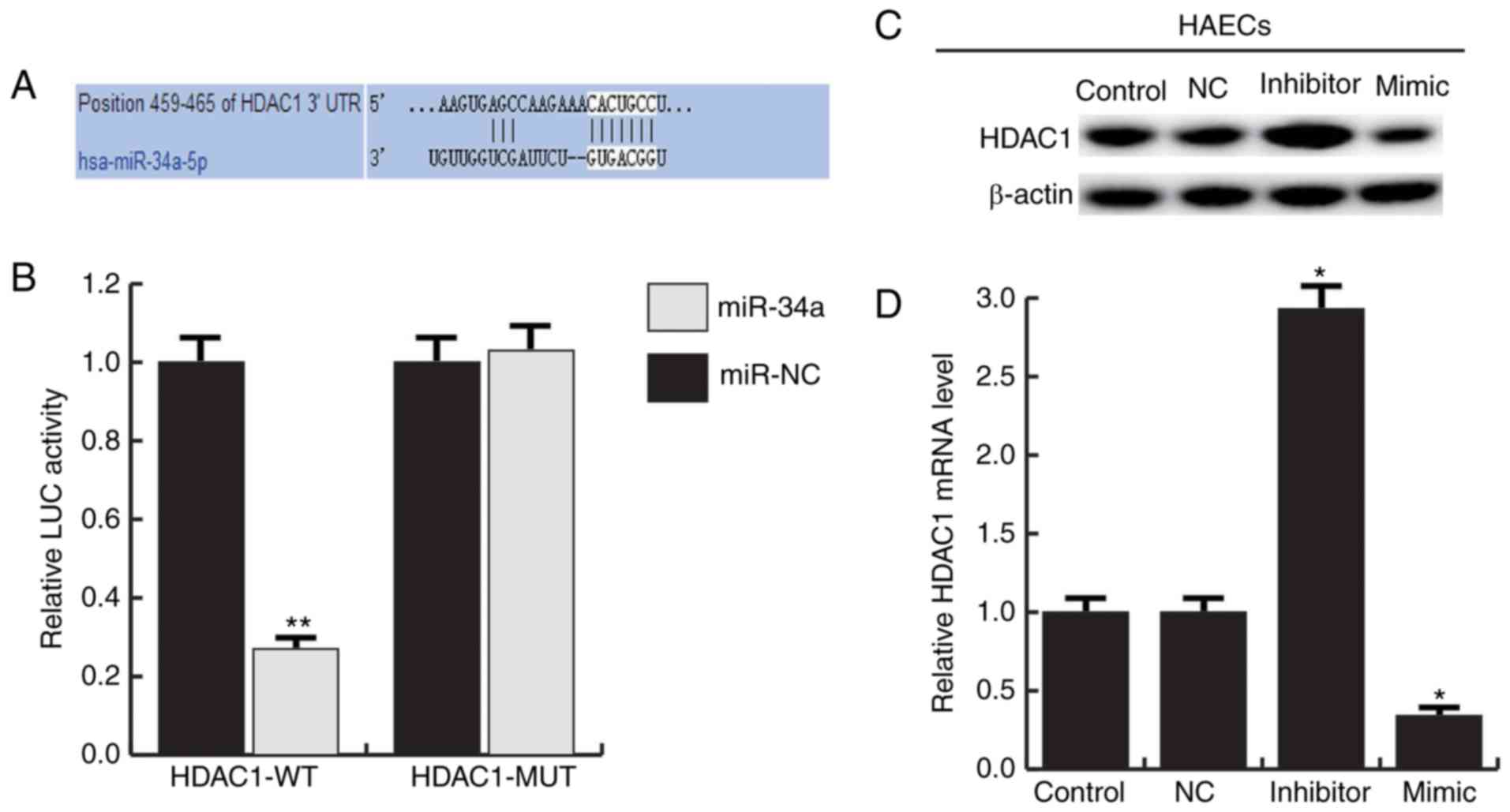
HDAC1 is the target gene of
miR-34a
We used TargetScan to predict the potential targets
of miR-34a, HDAC1 was identified as a potential miR-34a target gene
(Fig. 4A). And the luciferase
reporter assay showed that the luciferase activity was
significantly reduced in the HAECs co-transfected of miR-34a with
HDAC1-UTR-WT reporter plasmids, but co-transfection of miR-34a with
HDAC1-UTR-MUT reporter plasmids did not (Fig. 4B). To further confirm that miR-34a
regulates HDAC1 expression in HAECs, miR-34a mimic, miR-34a
inhibitor and negative control (NC) were transfected into HAECs.
The results showed that miR-34a mimic significantly inhibited the
expression of HDAC1, and miR-34a inhibitor significantly enhanced
the expression of HDAC1 (Fig. 4C and
D). Together, these data indicate that miR-34a directly target
HDAC1.
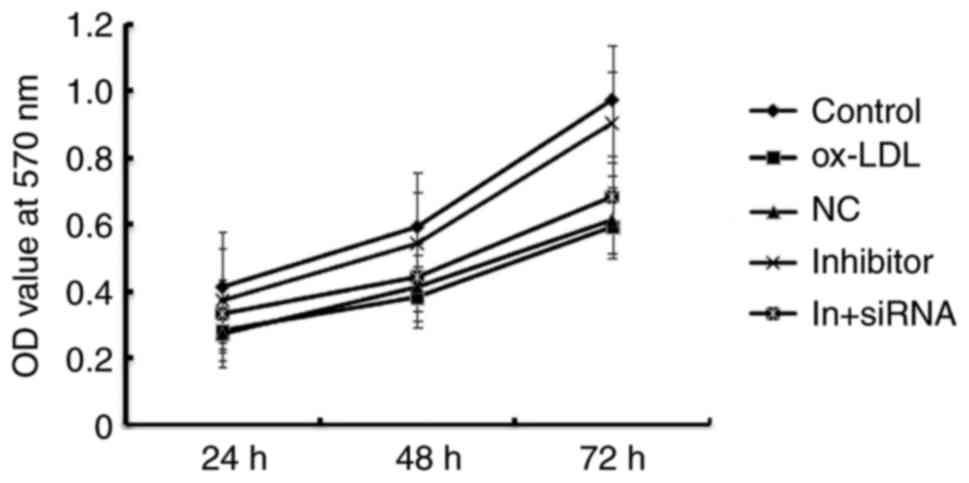
MiR-34a inhibitor enhances the cell
viability of HAECs
Given our limited understanding of the role played
by miR-34a in HAECs, we examined the effects of miR-34a gain and
loss-of-function on atherosclerosis. The CCK-8 results showed that
Ox-LDL induction significantly reduced the viability of HAECs, and
when compared with ox-LDL treatment alone, miR-34a inhibitor
significantly enhanced the cell viability of HAECs, whereas
HDAC1-siRNA eliminated the increased viability of HAECs induced by
miR-34a inhibitor (Fig. 5). These
results indicated that miR-34a inhibitor enhances the cell
viability of HAECs through regulating HDAC1.
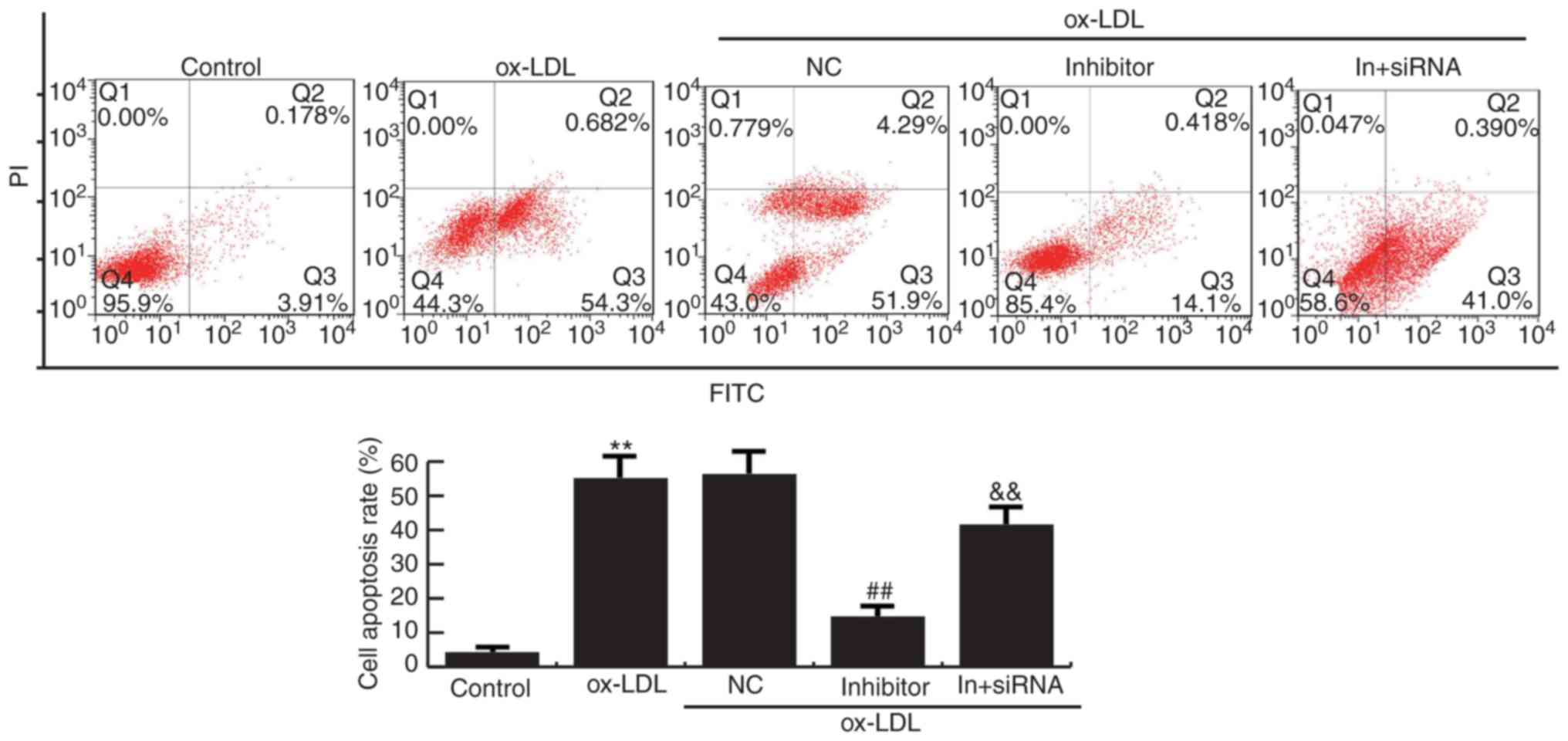
MiR-34a inhibitor suppresses apoptosis
in HAECs
Flow cytometry analysis demonstrated that cell
apoptosis were increased in Ox-LDL induction HAECs compared to the
control group. Compared with ox-LDL treatment alone, miR-34a
inhibitor significantly reduced the apoptosis of HAECs, whereas
HDAC1-siRNA eliminated the decrease of apoptosis of HAECs induced
by miR-34a inhibitor (Fig. 6).
Together, these data indicated that miR-34a inhibits cell grow and
promotes apoptosis of HAECs via regulating HDAC1.
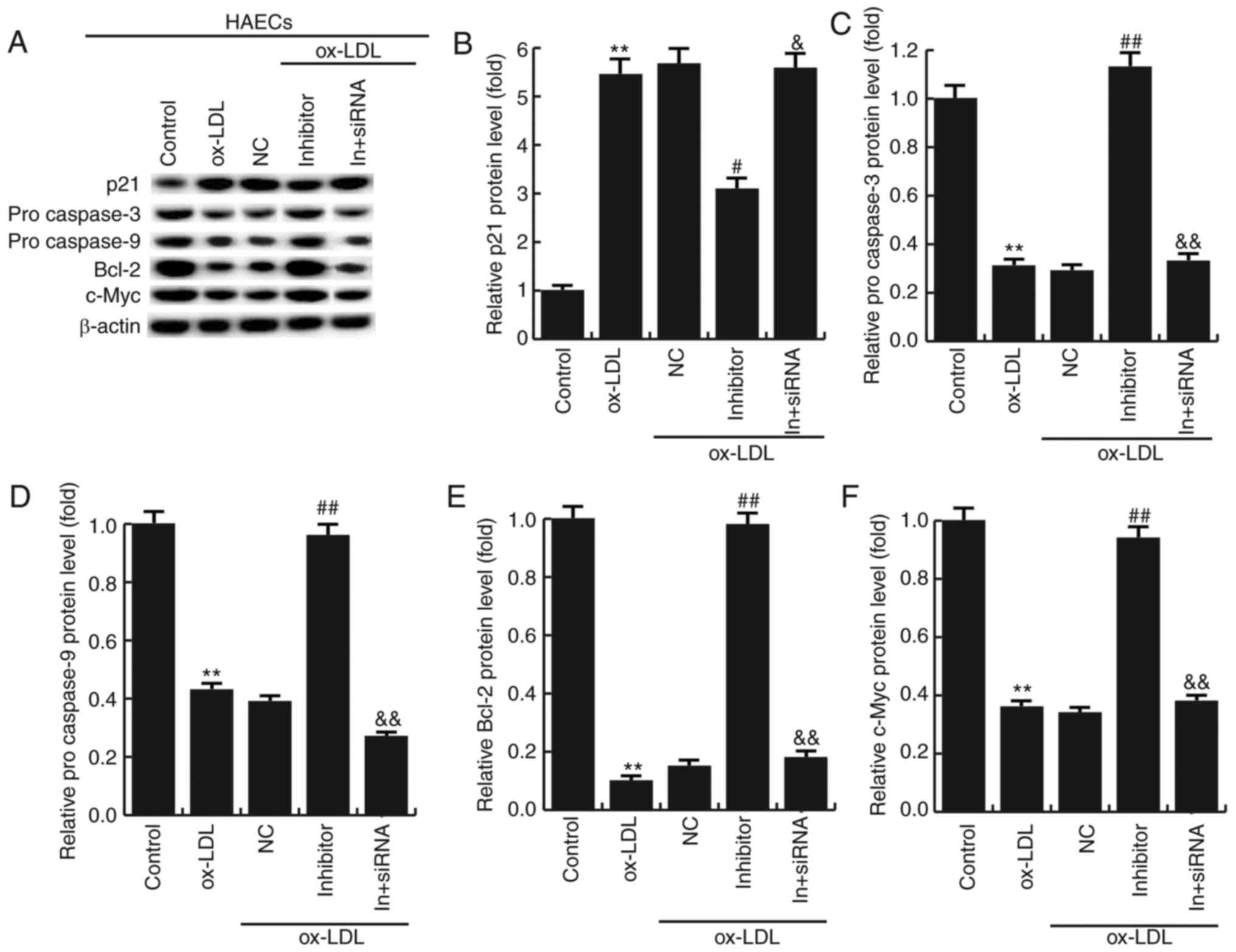
Effects of miR-34a inhibitor on the
expression of apoptosis-related genes
To further investigate the molecular mechanism of
the miR-34a effects on HAECs, the expression of apoptosis-related
genes were detected by western blotting. The results showed that
miR-34a inhibitor significantly increased the expression of Bcl-2,
procaspase-3, procaspase-9 and c-Myc, and the expression of p21 was
significantly decreased compared with ox-LDL treatment alone. In
addition, HDAC1-siRNA eliminated the effects induced by miR-34a
inhibitor (Fig. 7). It is further
demonstrated that miR-34a regulates the expression of
apoptosis-related proteins by targeting HDAC1.
Discussion
Functional miRNA of miR-34a have been highly
concerned by researchers in recent years. Welch et al
(13), reported that ectopic
miR-34a induces apoptosis resulting in the activation of a
caspase-mediated apoptotic pathway when reintroduced into the
neuroblastoma cell lines, which show a decrease in expression of
miR-34a. Then, a number of data showed that miR-34a regulates a
plethora of target proteins to induce cell apoptosis, thus acting
as a tumor suppressor (14–17).
The present study found that miR-34a inhibitor could repress cell
apoptosis in atherosclerosis.
In the present study, our preliminary data showed
that miR-34a was significantly increased in atherosclerotic lesion
tissues compared with the normal tissues, and consistent results
was found in ox-LDL induced HAECs. Also, we found that the
expression of HDAC1 was negative correlation with miR-34a. So we
hypothesis HDAC1 gene was associated with miR-34a. In order to
confirm this hypothesis, firstly we use TargetScan to predict the
potential targets of miR-34a. HDAC1 was identified as a potential
miR-34a target gene. In addition, luciferase reporter assay
verified miR-34a directly binding to 3′-UTR of HDAC1. Moreover, the
expression of HDAC1 was significantly increased in miR-34a
inhibitor transfected HAECs. These results demonstrated that
miR-34a directly targets HDAC1.
Histone acetylation function as reducing histone-DNA
interactions or creating an open chromatin configuration (18). Histone deacetylases
(HDAC)-dependent regulation of protein acetylation levels leads to
cell- and gene-specific transcriptional repression or activation
(18). HDAC1 is considered as a
positive regulator of cell proliferation, HDAC1 depletion in mice
results in growth deficiencies, and the p21 cyclin-dependent
inhibitor were correlated increased (19,20).
Our research have revealed that miR-34a directly targets HDAC1, and
miR-34a inhibitor could promote cell viability and prevent cell
apoptosis. We further explored underlying mechanism of the effects
on HAECs caused by miR-34a, and the relationship between HDAC1 and
apoptosis was explored. The expression levels of the anti-apoptotic
protein Bcl-2, procaspase-3, procaspase-9 and c-Myc were detected
after transfection with miR-34a inhibitor or miR-34a inhibitor
+HDAC1-siRNA. Results showed that miR-34a inhibitor significantly
improved the expression of anti-apoptotic protein, and HDAC1-siRNA
eliminated the effects of miR-34a inhibitor on HAECs.
Moreover, p21 was also determined in the present
study, and the findings suggested that miR-34a inhibitor
significantly inhibited p21 expression, and this inhibition was
reversed by HDAC1-siRNA. It has been demonstrated that the most
significant down-regulation by miR-34a is represented by ribosomal
proteins (17). Of interest, some
ribosomal proteins have the ability to up-regulate miR-34 and
down-regulate p21. Established anticancer treatments were recently
introduced into atherosclerosis therapeutic strategies to prevent
restenosis after angioplasty and endarterectomy. miR-34a increases
the sensitivity of cells to 5-FU treatment (21) and recently it has been demonstrated
that some ribosomal proteins are essential to mediate cell
apoptotis caused by 5-FU through molecular mechanisms involving
crucial pro-inflammatory factors as NFkB (22). In the light of these findings, one
possibility is that miR-34a inhibitor down-regulated p21 expression
through regulating ribosomal proteins thus preventing cell
apoptosis.
In conclusion, this study demonstrated that miR-34a
was up-regulated in atherosclerosis. MiR-34a inhibition could
promote the proliferation and induce apoptosis of endothelial cells
through regulating the expression of apoptotic-associated proteins
by targeting HDAC1. Thus, our research provides evidence that
miR-34a might serve as a new potential therapeutic target for
atherosclerosis.
References
|
1
|
Libby P and Theroux P: Pathophysiology of
coronary artery disease. Circulation. 111:3481–3488. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams KJ and Tabas I: Atherosclerosis
and inflammation. Science. 297:521–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK and Libby P: The immune
response in atherosclerosis: A double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hammond SM: An overview of microRNAs. Adv
Drug Deliv Rev. 87:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito T, Yagi S and Yamakuchi M:
MicroRNA-34a regulation of endothelial senescence. Biochem Biophys
Res Commun. 398:735–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao T, Li J and Chen AF: MicroRNA-34a
induces endothelial progenitor cell senescence and impedes its
angiogenesis via suppressing silent information regulator 1. Am J
Physiol Endocrinol Metab. 299:E110–E116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuroblastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cole KA, Attiyeh EF, Mosse YP, Laquaglia
MJ, Diskin SJ, Brodeur GM and Maris JM: A functional screen
identifies miR-34a as a candidate neuroblastoma tumor suppressor
gene. Mol Cancer Res. 6:735–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita Y, Kojima K, Hamada N, Ohhashi R,
Akao Y, Nozawa Y, Deguchi T and Ito M: Effects of miR-34a on cell
growth and chemoresistance in prostate cancer PC3 cells. Biochem
Biophys Res Commun. 377:114–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji Q, Hao X, Meng Y, Zhang M, Desano J,
Fan D and Xu L: Restoration of tumor suppressor miR-34 inhibits
human p53-mutant gastric cancer tumorspheres. BMC Cancer.
8:2662008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen QR, Yu LR, Tsang P, Wei JS, Song YK,
Cheuk A, Chung JY, Hewitt SM, Veenstra TD and Khan J: Systematic
proteome analysis identifies transcription factor YY1 as a direct
target of miR-34a. J Proteome Res. 10:479–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reichert N, Choukrallah MA and Matthias P:
Multiple roles of class I HDACs in proliferation, differentiation
and development. Cell Mol Life Sci. 69:2173–2187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jurkin J, Zupkovitz G, Lagger S,
Grausenburger R, Hagelkruys A, Kenner L and Seiser C: Distinct and
redundant functions of histone deacetylases HDAC1 and HDAC2 in
proliferation and tumorigenesis. Cell Cycle. 10:406–412. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haberland M, Montgomery RL and Olson EN:
The many roles of histone deacetylases in development and
physiology: Implications for disease and therapy. Nat Rev Genet.
10:32–42. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Zhao H, Zhou X and Song L:
Inhibition of lactate dehydrogenase A by microRNA-34a resensitizes
colon cancer cells to 5-fluorouracil. Mol Med Rep. 11:577–582.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|