Introduction
Cholangiocarcinoma (CCA) is a malignant tumor that
arises from the epithelial cells in intrahepatic or extrahepatic
bile ducts, and is difficult to diagnose and has a poor prognosis
(1–3). Although surgical resection and liver
transplantation are potentially curative treatments, the majority
of patients present with unresectable tumors and succumb to disease
within a year of diagnosis (4).
CCA is frequently resistant to chemotherapy as results of
monotherapy and combination chemotherapy are often disappointing
(5–7). Therefore, finding novel chemical
agents for the treatment of CCA is required.
The diabetes therapeutic biguanide compound,
phenformin, has been demonstrated to inhibit complex I of the
mitochondria (8). In previous
studies, phenformin was identified to inhibit tumor growth in
xenograft, transgenic and carcinogen-induced mouse cancer models
(9,10). As phenformin increases
intracellular adenosine monophosphate (AMP) and adenosine
diphosphate and subsequently induces the activation of liver kinase
B1 (LKB1)/5′ AMP-activated protein kinase(AMPK) signaling. The
LKB1/AMPK axis may inhibit cancer cell proliferation and growth and
functions as a tumor suppressive signaling pathway (8). Phenformin has the potential to be a
novel agent that inhibits CCA cell proliferation and tumor
growth.
In the present study, CCA cell lines were treated
with phenformin and the biological effects were observed.
Phenformin inhibited CCA cell proliferation and tumor growth and
induced cell apoptosis and autophagy.
Materials and methods
Cell culture
BE and Huh28 human CCA cell lines were purchased
from American Type Culture Collection (Manassas, VA, USA) and were
maintained under standard culture conditions (37°C and 5%
CO2) in RPMI 1640 supplement with 10% (v/v) FBS (both
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
RNA isolation and quantitative
real-time polymerase chain reaction (RT-qPCR)
Total RNA was purified from CCA cells or CCA
adjacent tissues using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 1 µg RNA was reverse transcribed using SuperScript Reverse
Transcriptase II (Invitrogen; Thermo Fisher Scientific, Inc.) in
37°C for 1 h. RT-qPCR was performed using SYBR® Green
Supermix (4473369; Applied Biosystems; Thermo Fisher Scientific,
Inc.) on an Applied Biosystems 7500 PCR system with the following
program: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 31 sec, and finally 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. The relative expression of each gene was
analyzed by the comparative cycle threshold method
(2−ΔΔCq method) (11)
which was normalized to GAPDH. Primers used in the present study
are described in Table I.
 | Table I.Sequences of primers and siRNAs. |
Table I.
Sequences of primers and siRNAs.
| Name | Sequence (5′-3′) |
|---|
| BECN1 | F,
TGGACACGAGTTTCAAGATCC |
|
| R,
CTCCTGGGTCTCTCCTGGTT |
| ATG5 | F,
AAAGATGTGCTTCGAGATGTGT |
|
| R,
CACTTTGTCAGTTACCAACGTCA |
| ATG7 | F,
ATGATCCCTGTAACTTAGCCCA |
|
| R,
CACGGAAGCAAACAACTTCAAC |
| GAPDH | F,
AGCCTCAAGATCATCAGCAATGCC |
|
| R,
TGTGGTCATGAGTCCTTCCACGAT |
| siRNA-NC |
ACGUGACACGUUCGGAGAATT |
| siRNA-LKB1 |
CTGGTTGATACCCACTCAAAAAG |
Western blotting
Cells were lysed in IP lysis buffer (catalog no.
P0013, Beyotime Institute of Biotechnology, Haimen, China),
according to the manufacturer's protocol. Subsequently, the protein
concentration was measured by BCA assay kit (Thermo Fisher
Scientific, Inc.) and cell lysates were boiled in 5X SDS-PAGE
loading buffer for 10 min and resolved by 8% SDS-PAGE prior to
transfer onto a nitrocellulose membrane. The membrane was blocked
by 5% (m/v) non-fat milk (BD Biosciences, Franklin Lakes, NJ, USA).
The following primary antibodies were used: LKB1 (3047;1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), phosphorylated
(p)-AMPK (2532; 1:1,000; Cell Signaling Technology, Inc.), AMPK
(2532; 1:1,000; Cell Signaling Technology, Inc.) and GAPDH
(60004-1-Ig; 1:5,000; ProteinTech Group, Inc., Chicago, IL, USA).
All these antibodies were incubated at 4°C overnight. Then the
membranes were incubated with HRP-linked anti-rabbit IgG (7074;
1:2,000; Cell Signaling Technology, Inc.) or HRP-linked anti-mouse
IgG (7076, 1:2,000; Cell Signaling Technology, Inc.) for 1 h at
room temperature. Proteins were then visualized with an Enhanced
Chemiluminescence kit (Thermo Fisher Scientific, Inc.).
Cell Counting Kit-8 (CCK-8) cell
viability assays
Cells were seeded into a 96-well plate at
3×103 cells per well with 100 µl culture medium
containing 0 or 2 mM phenformin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and cultured at 37°C and 5% CO2 for
12, 24 or 48 h. The cell viability was quantified by addition 10 µl
of CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). After a 1.5 h incubation, the optical density (OD) was
measured using a Power Wave XS microplate reader (Omega Bio-Tek,
Inc., Norcross, GA, USA) at a wavelength of 450 nm.
Colony formation assays
A total of 500 cells/well were seeded in 6-well
plates and incubated for 10 days at 37°C and 5% CO2.
Colonies were fixed and stained with 0.5% crystal violet at room
temperature and the number of colonies was counted.
Flow cytometry cell apoptosis
assays
A total of 2×105 cells/well were seeded
into 6-well plates and treated with 0 or 2 mM phenformin in an
incubator at 37°C. After 24 h, cells were washed twice with 1X cold
PBS, and stained with Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI; BD Biosciences, Franklin Lakes, NJ,
USA), according to the manufacture's protocol. BD FACSCalibur (BD
Biosciences) was used for the detection and CellQuest Pro software
(BD Biosciences) for the analysis.
In vivo tumor formation assay
A total of 10 male BALB/c (nu/nu) mice (Shanghai
SLAC Laboratory Animal Co., Ltd.; 5 weeks old, mean weight 15 g)
were kept at 25°C and 45% humidity with 12-h light/dark cycle with
free access to sterile food and water. Then, 4×106 RBE
cells were subcutaneously injected into the right flank of the
mice. After 2 weeks, the mice were intratumorally injected with 0
(physiological saline) or 2 mM phenformin (5 mice/group) every 4
days for a month. Tumor sizes were measured once a week and mice
were sacrificed for the analysis of tumor burden after 4 weeks. The
procedures in the present study were approved by the Ethics
Committee of the Second Military Medical University (Shanghai,
China).
siRNA treatment
The sequences for LKB1 siRNAs are described in
Table I. A total of
3×105 RBE and Huh28 cells were transfected with 100 nM
LKB1 siRNA or with 100 nM negative control siRNA (Table I) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 24 h these
transfected cell lines were used to perform the subsequent
assays.
Statistical analysis
Data are expressed as the mean ± standard deviation;
two-way analysis of variance followed by the LSD post-hoc test was
performed for comparisons between groups. GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA) was used for analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
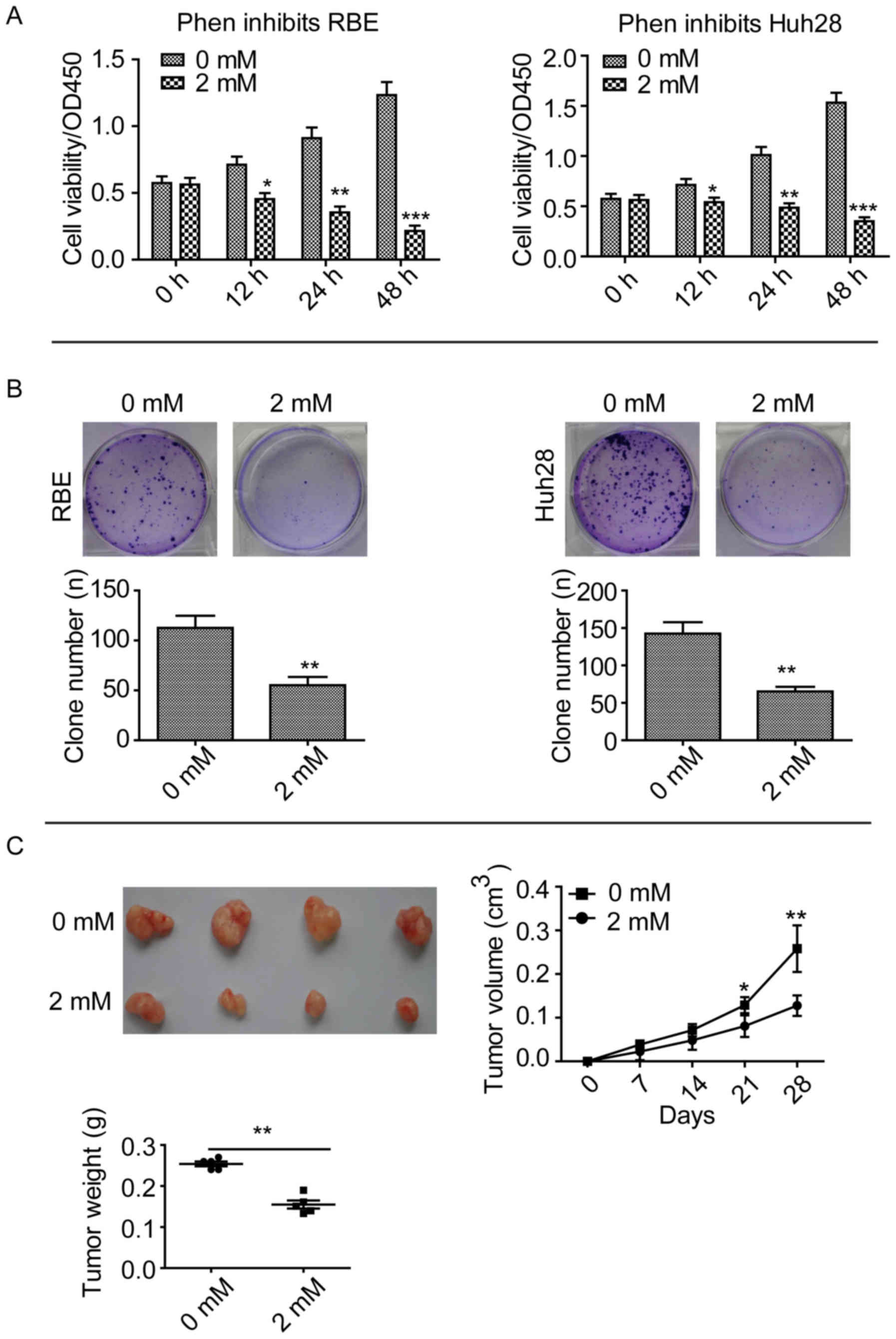
Phenformin inhibits CCA cell line
proliferation and growth in vitro and in vivo
An inhibitor of mitochondrial complex 1, phenformin,
has been demonstrated to inhibit tumor growth in lung cancer and
delay tumor progression in a lymphoma mouse model (8). In the present study, CCK-8 assays
were performed after treating two CCA cell lines, RBE and Huh28,
with 2 mM phenformin. Phenformin significantly inhibited the
proliferation of these cell lines compared with cells treated with
0 mM, after 12, 24 and 48 h (Fig.
1A). Clone formation assays revealed that phenformin
significantly inhibited the ability of the cell lines to form
clones compared with cells treated with 0 mM phenformin (P<0.01;
Fig. 1B). Subcutaneous tumor
formation assays revealed that mice injected with 2 mM phenformin
exhibited a significantly smaller RBE tumor volume compared with
the 0 mM control group after 28 days (P<0.01; Fig. 1C). These results suggested that
phenformin inhibits CCA cell proliferation and growth in
vitro and in vivo.
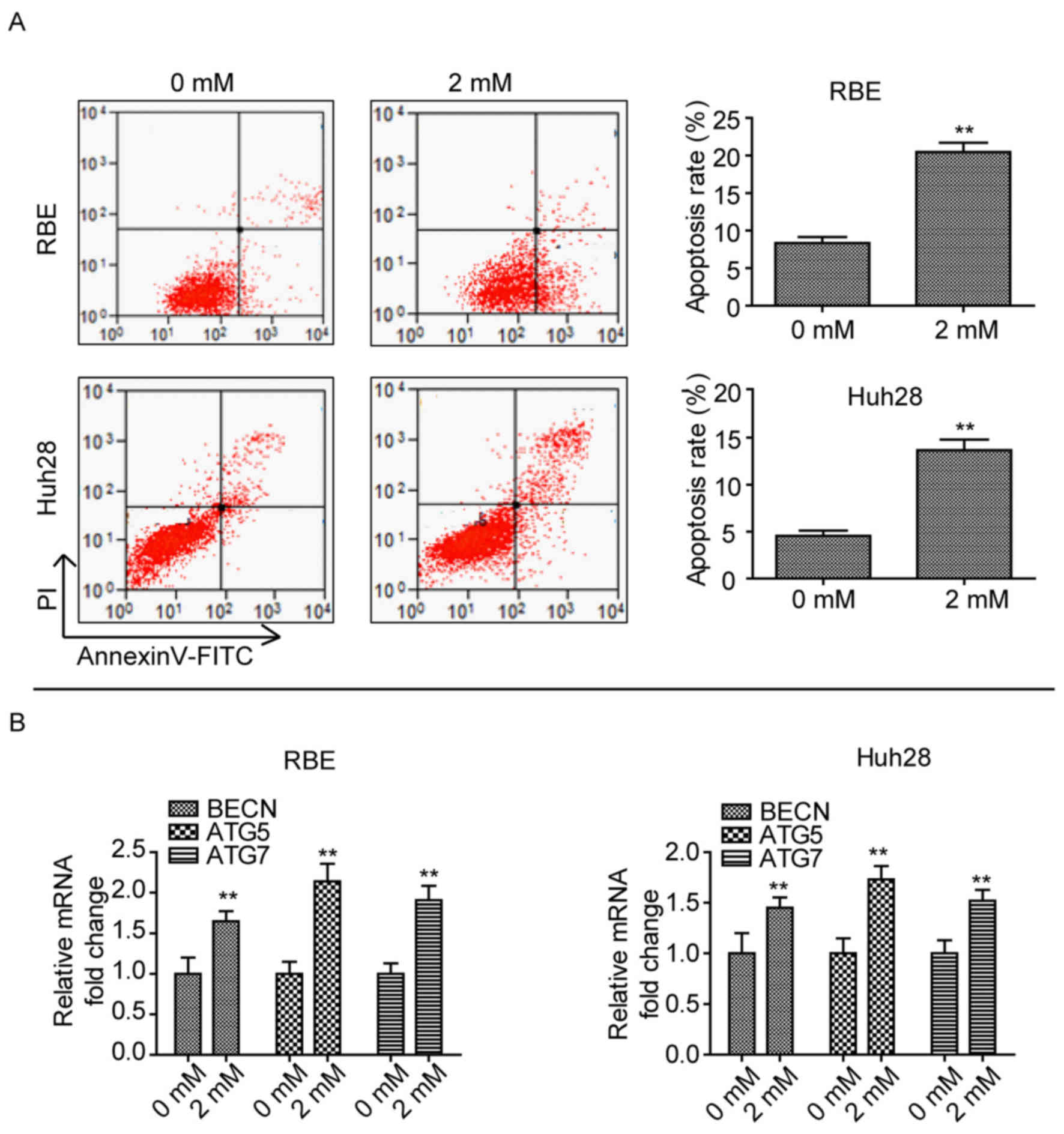
Phenformin induces CCA cell apoptosis
and autophagy
As phenformin inhibited cell proliferation, the role
of phenformin in CCA cell apoptosis and autophagy was determined.
When cells were treated with 2 mM phenformin for 24 h, a
significant amount of cell death was observed compared with cells
treated with 0 mM phenformin (P<0.01; Fig. 2A). In addition, the mRNA expression
levels of autophagy-associated genes, Beclin-1 (BECN1),
autophagy (ATG)5 and ATG7 (12) were upregulated after treatment with
2 mM phenformin in the two cell lines compared with cells treated
with 0 mM phenformin (Fig. 2B).
These findings suggested that phenformin induces CCA cell apoptosis
and autophagy.
Phenformin exerts its effects via the
LKB1/AMPK axis in CCA
A previous study revealed that phenformin induces
apoptosis in LKB1-deficient non-small cell lung cancer cell lines
(13). This finding suggests that
phenformin may activate the LKB1/AMPK signaling pathway to inhibit
CCA cell proliferation and induce apoptosis and autophagy. In the
present study, the protein expression levels of LKB1 was reduced in
RBE and Huh28 cell lines following knockdown of LKB1 using a
specific siRNA, compared with cells treated with negative control
siRNA (Fig. 3A). The expression
levels of the target of LKB1, p-AMPK, following knockdown of LKB1
in the cell lines was measured after treatment with phenformin.
Knockdown of LKB1 reduced the expression levels of p-AMPK when
treated with phenformin for 12 h (Fig.
3B). In addition, the cell viability was reduced in LKB1
knockdown cells with phenformin treatment, as determined by a CCK-8
cell viability assay (Fig. 3C).
The mRNA expression levels of autophagy-associated genes, BECN1,
ATG5 and ATG7, were reduced in LKB1 knockdown cell lines
treated with phenformin, compared with cells transfected with
negative control siRNA (P<0.01; Fig. 3D). These results suggested that
phenformin exerts its effects via activation of the LKB1/AMPK
signaling pathway.
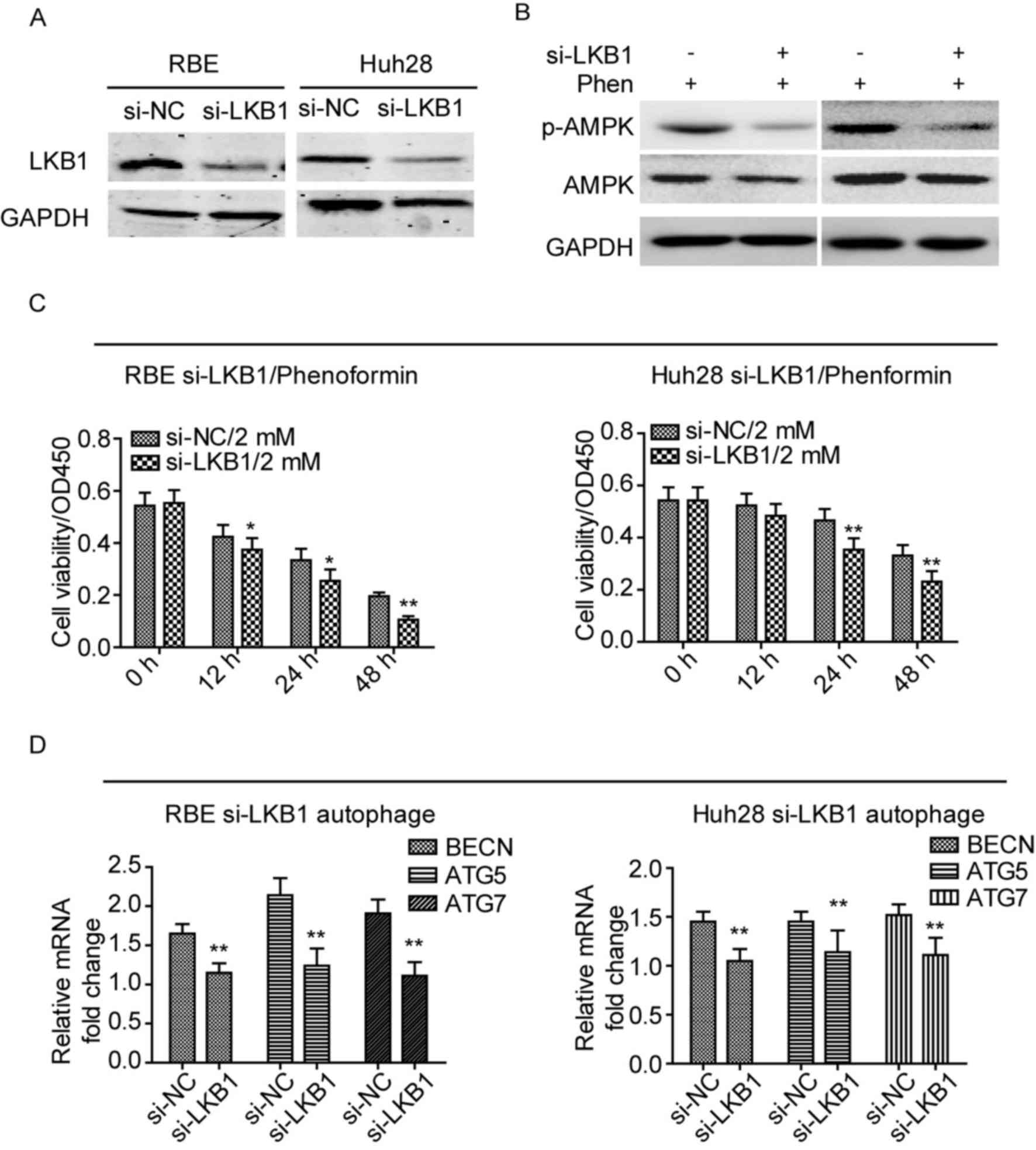 | Figure 3.Phenformin exerts its effects via the
LKB1/AMPK axis in cholangiocarcinoma cell lines. (A) Protein
expression levels of LKB1 were reduced following transfection of
cells with an LKB1-specific siRNA. (B) Knockdown of LKB1 inhibited
the activation and phosphorylation of AMPK in the two cell lines
following treatment with 2 mM phenformin. (C) Knockdown LKB1
significantly inhibited the cell viability of the two cell lines.
(D) mRNA expression levels of autophagy-associated genes, BECN1,
ATG5 and ATG7, were reduced following knockdown of LKB1
in cell lines treated with phenformin for 24 h. Assays were
performed in triplicate. *P<0.05 and **P<0.01 vs. siRNA-NC.
NC, negative control; BECN1, Beclin1 gene; ATG5,
autophagy 5 gene; ATG7, autophagy 7 gene; LKB1, liver kinase
B1; AMPK, 5′ AMP-activated protein kinase; Phen, phenformin; siRNA;
short interfering RNA. |
Discussion
The present study investigated the biological
effects of phenformin in CCA cell lines and demonstrated that
phenformin inhibited CCA cell proliferation and growth and induced
cell apoptosis and autophagy.
Phenformin is a biguanide compound and paralog to
metformin, which is widely used in as a therapy for type 2 diabetes
worldwide (14). Phenformin has
been previously demonstrated to exhibit potential anti-cancer
properties. Shackelford et al (13) revealed that phenformin inhibits
LKB1-deficient non-small cell lung cancer progression. Liu et
al (15) reported that
phenformin induces cell cycle alterations and apoptosis in breast
cancer. Cancer cells exhibit alterations in cellular metabolism to
enable their continued growth and proliferation (16,17).
Phenformin is a biguanide compound that inhibits complex 1 of
mitochondria, resulting in increased intracellular AMP and ADP that
induces the LKB1/AMPK signaling loop (8). AMPK is a conserved sensor of
intracellular energy levels (18)
and phenformin treatment and the activation of the LKB1/AMPK
signaling pathway may significantly inhibit anabolic pathways and
cell proliferation (19). In the
present study, phenformin significantly inhibited CCA cell
proliferation and induced cell apoptosis and autophagy. These
effects may be induced by the LKB1/AMPK signaling pathway in CCA
cell lines.
Wang et al (20) reported that LKB1 is frequently
downregulated in CCA tissues. The findings of the present study
suggested that silencing of LKB1 in combination with phenformin led
to these cells being more susceptible to death. The current
findings suggest that Phenformin has the potential to be a
therapeutic agent for CCA treatment.
Acknowledgements
The present study was funded by The National Natural
Science Fund (grant no. 81472280).
References
|
1
|
Bismuth H, Nakache R and Diamond T:
Management strategies in resection for hilar cholangiocarcinoma.
Ann Surg. 215:31–38. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim J and Park C: Pathology of
cholangiocarcinoma. Abdom Imaging. 29:540–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan SA, Toledano MB and Taylor-Robinson
SD: Epidemiology, risk factors, and pathogenesis of
cholangiocarcinoma. HPB (Oxford). 10:77–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan SA, Davidson BR, Goldin R, Pereira
SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC,
Thursz MR and Wasan H; British Society of Gastroenterology, :
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
Consensus document. Gut. 51 Suppl 6:VI1–VI9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson CD, Pinson CW, Berlin J and Chari
RS: Diagnosis and treatment of cholangiocarcinoma. Oncologist.
9:43–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al: Guidelines for the diagnosis and
treatment of cholangiocarcinoma: An update. Gut. 61:1657–1669.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knol JA and Pelletier SJ: Primary
Carcinomas of the Liver. Surgical treatment: Resection and
transplantation. Cambridge University Press; Cambridge: pp.
177–194. 2009
|
|
8
|
Hawley SA, Ross FA, Chevtzoff C, Green KA,
Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM and
Hardie DG: Use of cells expressing γ subunit variants to identify
diverse mechanisms of AMPK activation. Cell Metab. 11:554–565.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Algire C, Amrein L, Bazile M, David S,
Zakikhani M and Pollak M: Diet and tumor LKB1 expression interact
to determine sensitivity to anti-neoplastic effects of metformin in
vivo. Oncogene. 30:1174–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Memmott RM, Mercado JR, Maier CR, Kawabata
S, Fox SD and Dennis PA: Metformin prevents tobacco
carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila).
3:1066–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pyo JO, Yoo SM and Jung YK: The interplay
between autophagy and aging. Diabates Metab J. 37:333–339. 2013.
View Article : Google Scholar
|
|
13
|
Shackelford DB, Abt E, Gerken L, Vasquez
DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS
and Shaw RJ: LKB1 inactivation dictates therapeutic response of
non-small cell lung cancer to the metabolism drug phenformin.
Cancer Cell. 23:143–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidson MB and Peters AL: An overview of
metformin in the treatment of type 2 diabetes mellitus. Am J Med.
102:99–110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Ren L, Liu C, Xia T, Zha X and Wang
S: Phenformin induces cell cycle change, apoptosis, and
mesenchymal-epithelial transition and regulates the
AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells. PloS
One. 10:e01312072015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muñoz-Pinedo C, El Mjiyad N and Ricci JE:
Cancer metabolism: Current perspectives and future directions. Cell
Death Dis. 3:e2482012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaw RJ: Glucose metabolism and cancer.
Curr Opin Cell Biol. 18:598–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shackelford DB and Shaw RJ: The LKB1-AMPK
pathway: Metabolism and growth control in tumour suppression. Nat
Rev Cancer. 9:563–575. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Zhang K, Wang J, Wu X, Liu X, Li
B, Zhu Y, Yu Y, Cheng Q, Hu Z, et al: Underexpression of LKB1 tumor
suppressor is associated with enhanced Wnt signaling and malignant
characteristics of human intrahepatic cholangiocarcinoma.
Oncotarget. 6:18905–18920. 2015.PubMed/NCBI
|