Introduction
Ischemic heart disease is considered to be a leading
cause of morbidity and mortality in humans, particularly those
suffering from acute myocardial infarction (AMI). These patients
are considered to be more susceptible to complications, including
cardiogenic shock, or the development of chronic heart failure
(CHF), although emergency revascularization has been widely used
(1,2). It is likely that the loss of blood
flow to the myocardium, primarily due to the occlusion of coronary
arteries, may lead to myocardiocyte death and myocardial
remodeling. As a result, improving myocardial recovery following
AMI may exert positive effects in reducing the incidence of
associated complications and leading to improved clinical
outcomes.
MicroRNAs (miRNAs/miRs) are non-protein coding RNAs
with a length of 22–24 nucleotides, which are able to interact with
the 3′-untranslated regions of target mRNAs and inhibit
transcription or translation (3).
It has been previously reported that a number of miRNAs, including
miRNA-34a, miRNA-208a and miRNA-495, may be important gene
regulators for remodeling or inducing angiogenesis in response to
AMI, and these beneficial effects have made non-coding RNAs
potentially important therapeutic tools for preventing the
appearance of CHF following AMI (4–6).
miRNA-210 serves an important role in response to
hypoxic conditions and may be upregulated by hypoxia-inducing
factors, and resultant alterations of cellular processes, including
apoptosis, angiogenesis and metastasis, have been observed
(7). Recently, Zeng et al
(8) established ischemic brain
models in mice and demonstrated that overexpression of miRNA-210
was able to enhance the microvessel density (MVD) in brain samples
using immunohistochemistry analysis. Similar results were
additionally reported by Yang et al (9), who demonstrated that miRNA-210
promoted hepatocellular carcinoma angiogenesis. In addition, Wang
et al (10,11) reported different results pertaining
to the effects of miRNA-210 in AMI, although the same AMI rat
models were used. It may be noted that the earlier formation of
collateral circulation may reduce the risk of cardiomyocyte death
and myocardial remodeling following AMI and facilitate improved
clinical outcomes. However, these controversial data raised
questions regarding the role of miRNA-210 in AMI. Therefore, the
present study was designed to investigate the efficacy of miRNA-210
in AMI, and sought to elucidate the potential associated
mechanisms.
Materials and methods
Animal experiments
Animals
A total of 48 Sprague-Dawley rats (4-week old; male)
weighing 220–240 g were obtained from the Animal Center of Nanjing
Medical University (Nanjing, China). All the rats were housed in
the laboratory animal room maintained at a temperature of 20±2°C
with a relative humidity of 50–70% and on a regular 12 h light-dark
cycle, with access to standard chow and water for 1 week prior to
any operative procedures. The protocol of the animal experiments
was approved by the Institutional Animal Care and Use Committee of
Nanjing Medical University.
Rat model of AMI
The rats were anesthetized by intraperitoneal
injection of 10% chloral hydrate (3 ml/kg) and a tracheotomy was
subsequently performed, supported by a small animal ventilator
(ALC-V8S; Shanghai Alcott Biotech Co., Ltd., Shanghai, China). The
establishment of the AMI models was achieved by ligating the
proximal left anterior descending (LAD) coronary artery with a 6-0
silk suture. A successful procedure was confirmed if the followed
criteria were fulfilled: i) Observation of rapid discoloration over
the anterior surface; and ii) electrocardiogram exhibiting
persistent ST segment elevation ≥0.2 mV in two or more contiguous
limb leads three times (reexamined every 10 min after the
ligation). At the end of the surgery, all rats received an
intramuscular injection of penicillin sodium (400,000 units) to
prevent potential infection.
Animal groups and myocardial
transfection with lentiviral vectors in vivo
The animals were randomly divided into 4 groups: i)
Sham-operated (Sham; 12 rats) rats received all surgical procedures
except for ligation of the LAD coronary artery; ii) AMI and
treatment with negative control vector (AMI + NV; 7 rats); iii) AMI
(AMI; 8 rats); and iv) AMI and treatment with lentivirus-mediated
miRNA-210 agonist (AMI + LV-miR-210 agonist; 9 rats). The
myocardial transfection in vivo was performed via
intravenous injection with LV-miR-210 agonist
(precursor-hsa-miR-210; Shanghai Genechem Co., Ltd., Shanghai,
China) or negative control vector
(hu6-MCS-Ubiquitin-EGFP-IRES-puromycin; Shanghai Genechem Co.,
Ltd.). The forward primer sequence for hsa-miR-210 was
5′-GGAAAGGACGAAACACCGGGGACAAGAGAGGAGTGGCTCTG-3′, and the reverse
primer sequence was
5′-TGTCTCGAGGTCGAGAATTAAAAAACTAGTGGCCCACTACCCTGTC-3′, which were
amplified into the 301-bp product as described previously (4). The negative control vector and
LV-miRNA210 agonist were added to PBS to a final volume of 0.5 ml,
which was applied to each rat, while untreated rats received PBS
(0.5 ml) only.
Cardiac function assessment
A total of 4 weeks post-surgery, echocardiography
using the Vevo2100 system (Fujifilm VisualSonics, Inc., Toronto,
ON, Canada) with a high-frequency (30 MHz) MS-400 transducer was
performed to evaluate the cardiac functions of the rats
noninvasively. Prior to echocardiography, 3% isoflurane was used to
induce anesthesia and maintenance with 1.5% isoflurane was applied
during the procedure. The thickness of interventricular septum in
systole and diastole, left ventricle internal dimension in systole
(LVIDs) and diastole (LVIDd), thickness of LV posterior wall in
systole (LVPWs) and diastole (LVPWd), left atrium (LA) internal
dimension, LV volume in systole (LV VOLs) and diastole (LV VOLd),
LV mass and LV mass corrected were measured. In addition, the LV
fractional shortening percentage (FS) and LV ejection fraction
percentage (EF) were calculated as followed: FS
(%)=[(LVIDd−LVIDs)/LVIDd] ×100; EF (%)=[(LV VOLd-LV VOLs)/LV VOLd]
×100.
Preparation of tissue samples
Heart tissues were quickly removed from the
sacrificed rats following the assessment of cardiac function. Each
of the tissue samples was divided into two parts which were
prepared for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis (temporarily stored at −80°C) and
immunohistochemical analysis (fixed in 4% paraformaldehyde at 20°C,
for 2 h) separately.
RT-qPCR analysis
In order to measure the expression levels of
miRNA-210 and associated genes, heart samples were analyzed via
RT-qPCR. Total RNA was extracted using TRIzol reagent (Zoonbio
Biotechnology Co., Ltd., Nanjing, China) and transcribed into cDNA
using the TransScript miRNA RT Enzyme Mix (TransGen Biotech Co.,
Ltd., Beijing, China; www.transbionovo.com/). The amplification of cDNA was
subsequently performed following the protocol (95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec, 60°C for 20 sec) of the
SYBR Premix Ex Taq II kit (Takara Biotechnology Co., Ltd., Dalian,
China) using an ABI7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Human GAPDH was used as an
internal control. The expression levels of miRNA-210, vascular
endothelial growth factor (VEGF) and hepatocyte growth factor (HGF)
were measured. The primer sequences of relative genes were as
follows: GAPDH forward, 5′-GCAAGTTCAACGGCACAG-3′ and reverse,
5′-GCCAGTAGACTCCACGACAT-3′; HGF forward, 5′-GGGGCTACACTGGATTGA-3′
and reverse, 5′-GCCTTGATGGTGCTGACT-3′; miRNA-210 forward,
5′-AGCCACTGCCCACAGCACACTG-3′ and reverse,
5′-CAGTGCAGGGTCCGAGGTATT-3′; and VEGF forward,
5′-CTCACCAAAGCCAGCACAT-3′ and reverse, 5′-TTCTCCGCTCTGAACAAGG-3′.
The comparative Cq method (2−ΔΔCq) was used to analyze
the data (12).
Immunohistochemistry
The paraffin-embedded heart samples were sliced into
4–5 µm thicknesses following dehydration, and were stained with
hematoxylin-eosin (H&E; hematoxylin solution and 1% eosin
solution, at room temperature for 10 min each) and prepared for
Masson's trichrome staining (Bouin's solution, at 56°C for 1 h at
room temperature, Weigert hematoxylin working fluid for 20 min,
then dying with ponceau red solution for 5 min, rinsing with
distilled water for 30 sec, slides were subsequently soaked in 1%
phosphotungstic solution and dying with aniline blue solution, for
8 min each, at room temperature). MVD counting was performed on the
basis of platelet endothelial cell adhesion molecule (CD31)
immunohistochemistry. After deparaffinization [twice in xylene, 5
min each, followed by three sequential (90, 75 and 50%) solutions
of ethanol, 5 min each], heart tissues were soaked in citrate
buffer (0.01 mol/l, pH 6.0) to recover antigenicity. Subsequently,
slides were incubated with 0.5% hydrogen peroxide-methanol solution
for 10 min at room temperature. After rinsing with distilled water,
rabbit anti-CD31 polyclonal antibody (1:100; cat. no. BA2966; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) was applied to
sections as the primary antibody for 2 h at room temperature and
the secondary antibody solution (1:500; cat. no. K5007; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) was added to
induce horseradish peroxidase binding, maintained at room
temperature for 30 min, following the methods described by Mineo
et al (13). For each
sample, at least 2 fields were selected for analysis. All the
images were obtained using a light microscope (magnification, ×200;
Nikon Corporation, Tokyo, Japan), among which three areas with
highest MVD in each section were selected for counting and the
average of these vascular counts (mean intensity=integral optical
density sum/area) was recorded as the MVD level in each case,
quantified using image analysis software (Image Pro Plus 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
Cell experiments
Cell culture and regent exposure
Human umbilical vein endothelial cells (HUVECs)
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) were obtained from the American Type
Culture Collection (Manassas, VA, USA). Cells cultured under normal
oxygen levels were maintained at 37°C and supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified chamber with 5% CO2 and 95% air (labeled as
the normoxia condition). The fresh culture medium was changed every
day. Simultaneously, parallel cultured cells under hypoxic
conditions were cultured as previously described (14).
Small interfering RNA (siRNA)
transfection
The cells cultured under hypoxic condition were
divided into two groups when they reached 50% confluence and were
transfected with specific siRNA (The sequences were as follows:
Upstream primer, GCUACAAGAAACCGCCUAUTT; downstream primer,
AUAGGCGGUUUCUUGUAGCTT) for silencing HGF expression (100
nM/1.5×105 cells; labeled siHGF) or scrambled probe, as
the negative control (both Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Lipofectamine RNAiMax (Invitrogen; Thermo Fisher
Scientific, Inc.) was used, according to the manufacturer's
protocol. Following 48 h of incubation, further western blot
analyses were performed to evaluate the transfection efficiency and
detect alterations in CD31 protein expression levels in these
cells.
Western blot analysis
Each of heart samples was homogenized in lysis
buffer with protease inhibitors (p0013c; Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged at 16,200 × g for 15
min at a temperature of 4°C. To determine the protein
concentration, the Pierce Bicinchoninic Acid protein assay (Thermo
Fisher Scientific, Inc.) was used. Extracted protein samples were
separated using 8–15% SDS-PAGE and subsequently transferred to
polyvinylidene difluoride membranes (40 µg protein/lane). The
membranes were blocked with 5% skimmed milk in TBS for 1 h at 37°C.
Rabbit polyclonal antibodies against HGF (1:400; cat. no. BA0911;
Wuhan Boster Biological Technology, Ltd.) and β-myosin heavy chain
(MHC) (1:500; cat. no. ab50967; Abcam, Cambridge, UK) were diluted
with blocking buffer, in which the membranes were maintained
overnight at 4°C and washed with TBS-Tween-20 four times for 5 min
each the following day. The membranes were subsequently incubated
in blocking buffer with added secondary antibodies (1:5,000; cat.
no. ABP103; Zoonbio Biotechnology Co., Ltd.) for 2 h at room
temperature. Rabbit anti-rat β-actin (1:2000; cat. no. 8457; Cell
Signaling Technology, Inc., USA) was applied as an internal
control.
Following 48 h of incubation, transfected cells were
lysed with radioimmunoprecipitation buffer (p0013c; Beyotime
Institute of Biotechnology) containing protease and phosphatase
inhibitors, and centrifuged at 13,800 × g for 20 min at 4°C. The
remainder of the protocol was as described above. However, the
protein expression levels of HGF and CD31 (1:1,000; cat. no. 77699;
Cell Signaling Technology, Inc., Danvers, MA, USA) were detected in
the cells and GAPDH (1:1,000; cat. no. 5174; Cell Signaling
Technology, Inc.) was used as the internal control. The Syngene Bio
Imaging Device (Syngene Europe, Cambridge, UK) was used to
visualize immunoreactive protein bands.
Statistical analysis
Statistical analysis was performed using the SPSS
22.0 software package (IBM Corp., Armonk, NY, USA) and all data are
expressed as the mean ± standard deviation. One-way analysis of
variance followed by Bonferroni corrections was used for
comparisons between multiple groups. P<0.05 (two-tailed) was
considered to indicate a statistically significant difference.
Results
AMI increases myocardial miRNA-210
expression
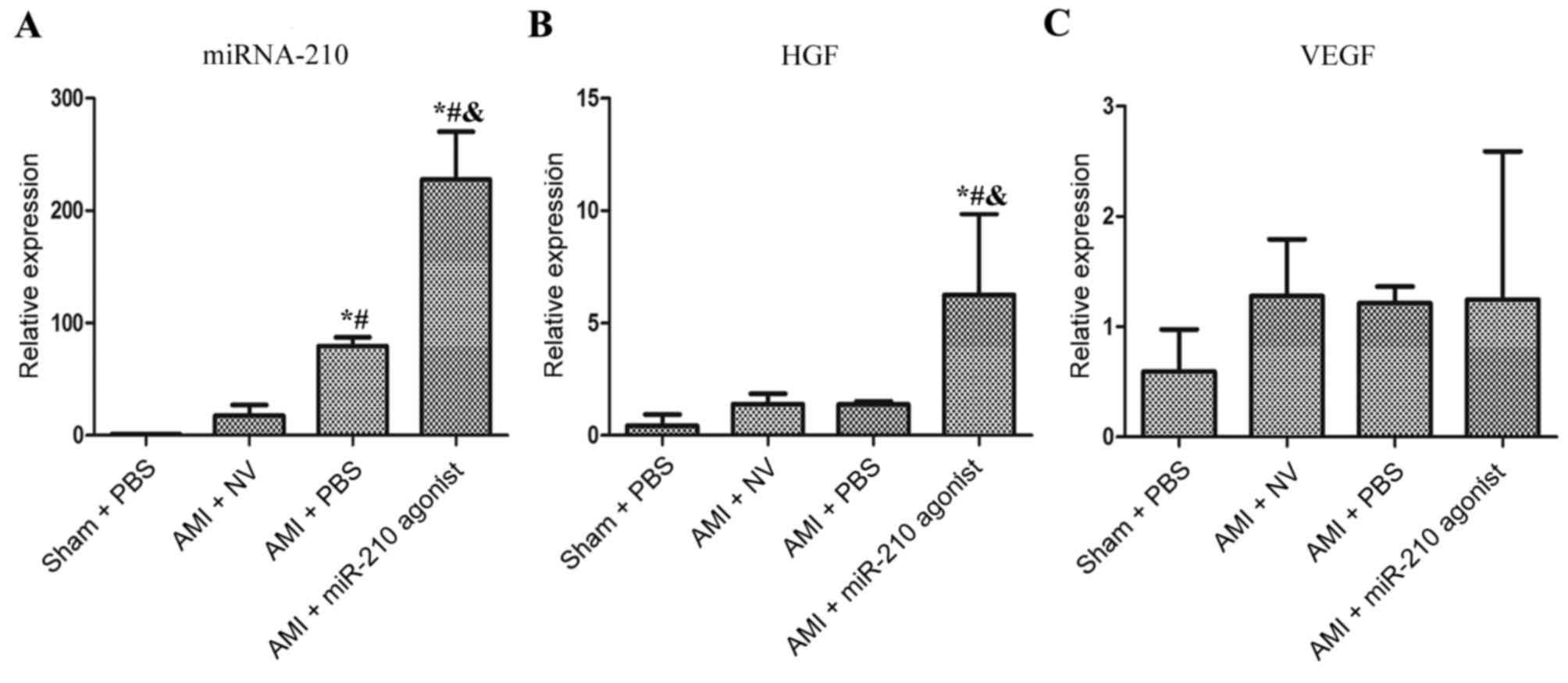
As depicted in Fig.
1A, myocardial miRNA-210 expression was increased significantly
in the AMI + PBS subgroup compared with the sham group (P=0.011) at
4 weeks post-surgery. The expression level of miRNA-210 in the AMI
+ miR-210 agonist group was additionally significantly increased,
compared with the other three subgroups (P<0.001 vs. Sham + PBS,
AMI + NV and AMI + PBS, respectively). Notably, the comparison
between the AMI + PBS group and AMI + NV group demonstrated a
significant decrease in miRNA-210 expression in the latter group
(P=0.038) and no statistical difference between the Sham + PBS
group and the AMI + NV group was observed (P=0.799).
Overexpression of miRNA-210
up-regulates HGF expression
An apparent upregulation of HGF was observed in the
AMI + miR-210 agonist subgroup compared with the other three
subsets (P=0.02 vs. Sham + PBS; P=0.049 vs. AMI + NV; P=0.048 vs.
AMI + PBS; Fig. 1B), which was
principally driven by the overexpression of miRNA-210. However, the
AMI-induced overexpression of miRNA-210 did not promote myocardial
HGF expression (P=0.921 vs. Sham group) and the comparisons between
multiple groups demonstrated no significant differences in the
expression of VEGF (Fig. 1C). In
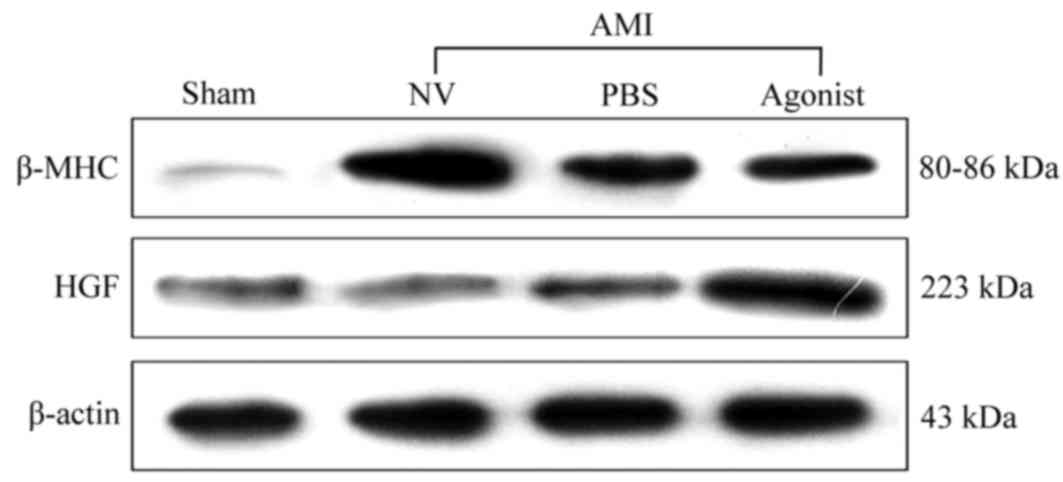
addition, similar results in terms of protein expression levels
exhibited an upregulation of HGF associated with miRNA-210
overexpression (Fig. 2). The
attenuating increase of myocardial β-MHC protein expression induced
by AMI was observed following treatment with miR-210 agonists,
particularly compared with the AMI + NV group. These findings
indicated that HGF may be a target gene of miRNA-210 in AMI.
Role of HGF in HUVEC
proliferation
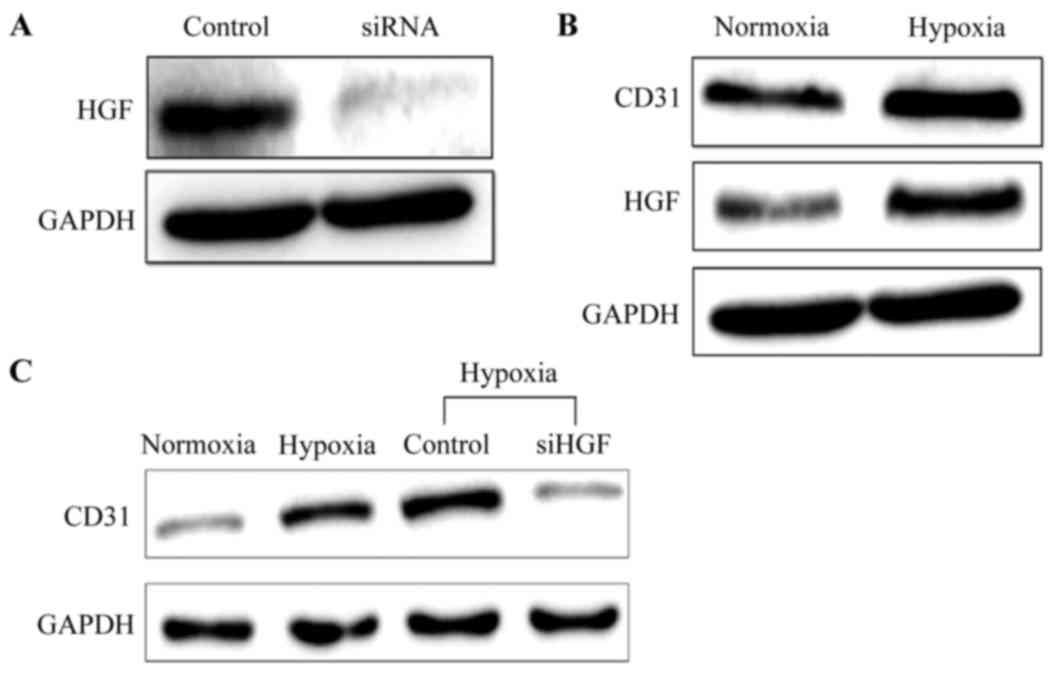
As presented in Fig.
3A, the protein expression level of HGF in HUVECs was decreased
markedly following transfection with siRNA, demonstrating the
suppressive effect of siRNA on HGF expression. Additionally,
simultaneously increased CD31 and HGF protein expression was
observed in the cells under hypoxic condition (Fig. 3B). When the HGF expression was
silenced through transfection with siRNA, the level of CD31 protein
expression in cells under hypoxic conditions was decreased, as
depicted in Fig. 3C, indicating
that HGF may be an important stimulator of HUVEC growth and
proliferation.
Alterations induced by overexpression of
miRNA-210 in histology and cardiac function
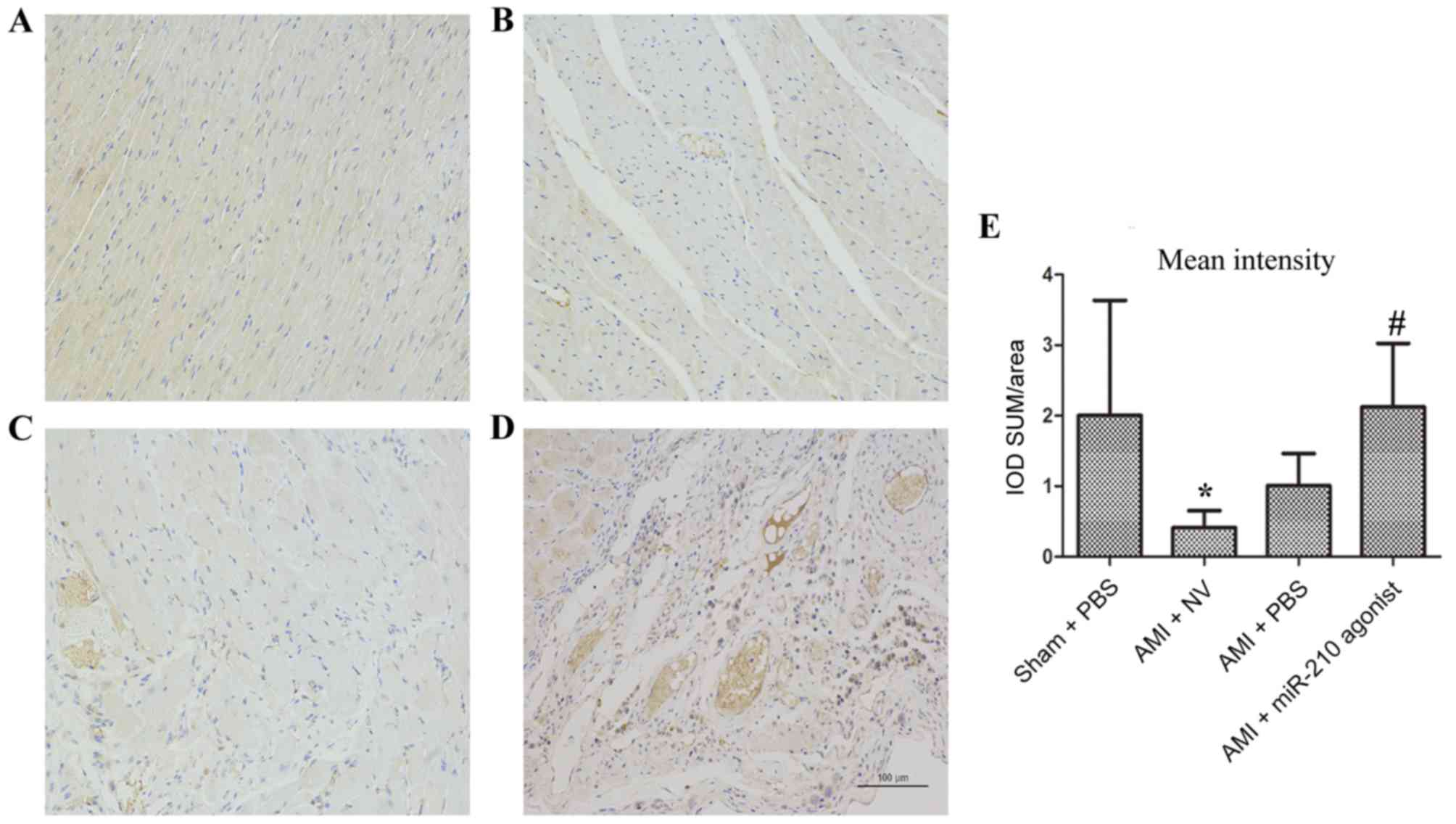
MVD in heart samples
MVD was measured in myocardial samples (Fig. 4). Compared with the sham group
(Fig. 4A), a markedly decreased
optical intensity of CD31 cells was noted in the myocardium in the
AMI + NV subgroup (P=0.045; Fig. 4B
and E). By contrast, the optical intensity of CD31 cells in the
infarcted area of the heart treated with the miR-210 agonist was
significantly increased compared with the AMI + NV group (P=0.029;
Fig. 4D and E), whereas no
statistical significance was observed when compared with the Sham
group (P=0.996). The comparison between the AMI + NV group and AMI
+ PBS group indicated no significant difference (P=0.716; Fig. 4C and E). Therefore, better
promoting angiogenesis effects with respect to over expression of
miRNA-210 were observed.
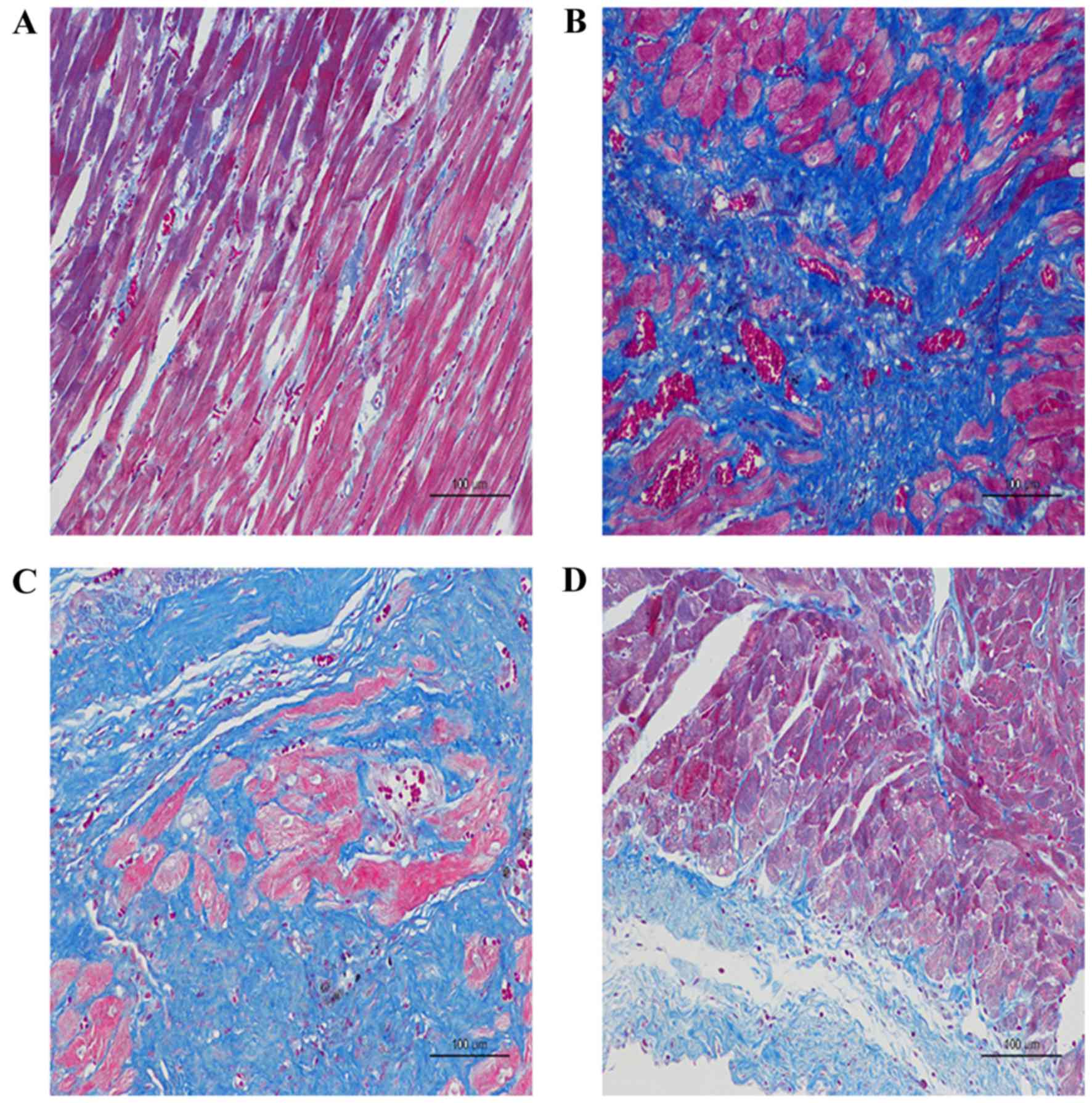
Histopathological observation of rat
heart tissue
As presented in the images of H-E and Masson's
trichrome staining, the diffuse, edematous myocardial fibers were
severely broken, taking the form of deranged cellular structures
among which a large number of collagen fibers had been accumulated
in the AMI + NV group and the AMI + PBS group, compared with the
Sham group (Fig. 5A-C). Improved
results were observed with miRNA-210 agonists, indicating reduced
accumulation of collagen fibers and decreased destruction of
myocardial fibers (Fig. 5D).
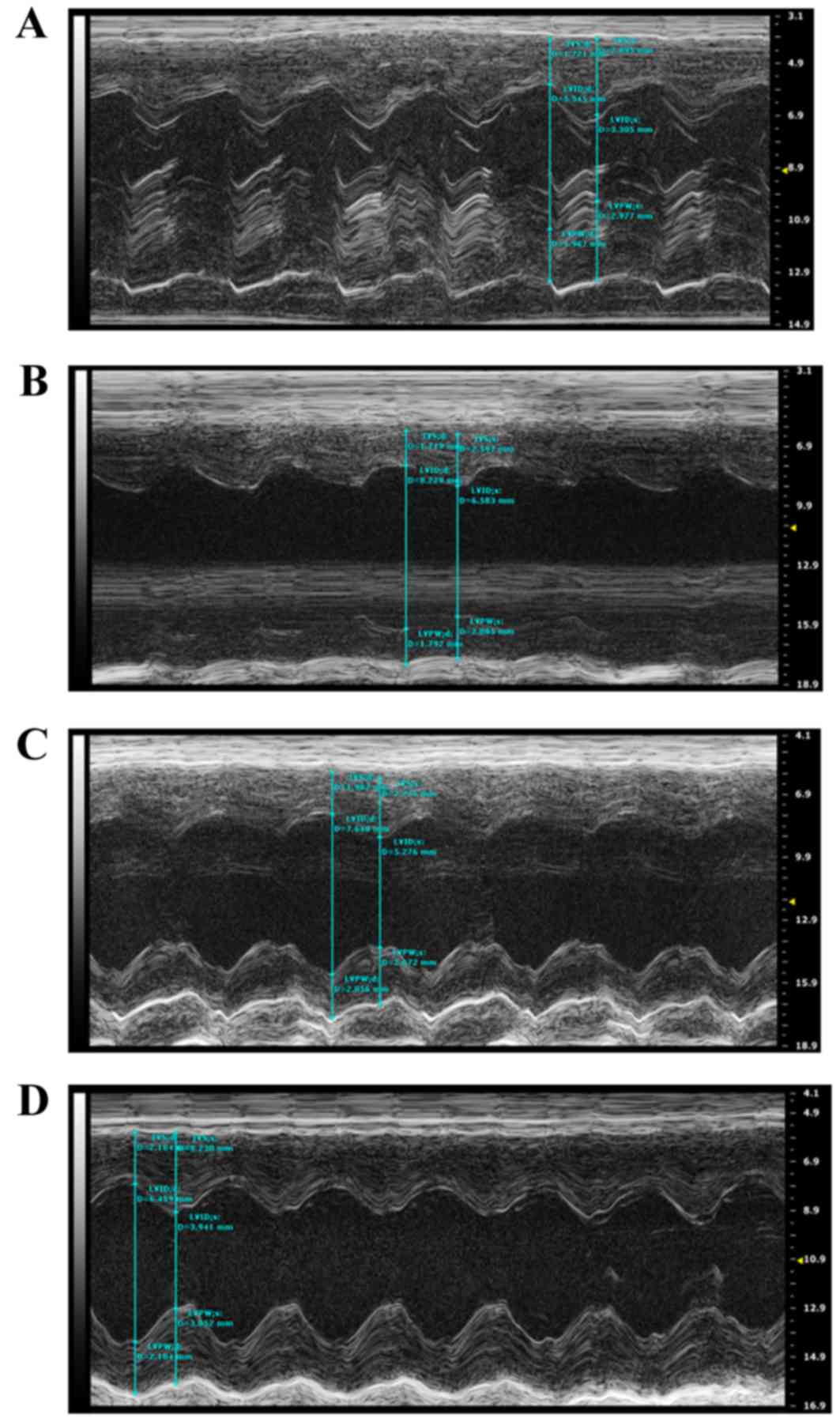
Cardiac function changes
Echocardiography was performed on the rats to
evaluate alterations in cardiac structure and function at 4 weeks
post-surgery. Compared with the Sham group, the cardiac
contractility was markedly decreased in the AMI + NV group, which
was primarily derived from the enlarged LVD and LV VOL in systole,
resulting in a decrease in LVEF and LVFS. By contrast, the hearts
receiving the miRNA-210 agonist exhibited improved cardiac
contractility compared with the AMI + NV groups at the same points,
and improved results in LVEF and LVFS were acquired, while no
significant difference compared with the Sham group was observed.
The characteristics of alterations in cardiac structure and
function among the rats are summarized in Fig. 6 and Table I.
 | Table I.Alterations in cardiac structure and
function at 4 weeks post-surgery. |
Table I.
Alterations in cardiac structure and
function at 4 weeks post-surgery.
|
| Group |
|---|
|
|
|
|---|
| Parameter | Sham + PBS
(n=12) | AMI + NV (n=7) | AMI + PBS (n=8) | AMI + LV miR-210
agonist (n=9) |
|---|
| IVSs, mm |
2.56±0.35 |
1.97±0.41 |
2.91±0.15 |
3.08±0.52b |
| IVSd, mm |
1.61±0.13 |
1.47±0.38 |
1.80±0.17 |
1.84±0.27 |
| LVIDs, mm |
3.76±0.77 |
6.64±0.46a |
5.24±0.65 |
4.39±0.90b |
| LVIDd, mm |
6.40±1.18 |
8.76±1.19 |
8.02±0.62 |
7.44±0.81 |
| LA, mm |
3.72±0.80 |
4.98±0.80 |
4.14±0.15 |
4.20±0.65 |
| LVPWs, mm |
2.93±0.18 |
2.68±0.33 |
2.77±0.20 |
2.98±0.37 |
| LVPWd, mm |
1.90±0.18 |
1.93±0.16 |
1.97±0.09 |
2.01±0.15 |
| LV Mass, mg | 751.03±175.18 | 1202.90±332.57 | 1180.24±84.26 | 1073.77±75.05 |
| LV Mass Cor,
mg | 600.82±140.14 |
962.32±266.05 |
944.20±67.41 |
859.02±60.05 |
| LV VOLs, µl |
69.92±31.48 |
227.71±35.30a |
133.61±37.85 |
91.07±45.20b |
| LV VOLd, µl | 215.07±90.12 | 428.11±131.76 | 348.11±61.50 | 295.68±70.30 |
| EF, % |
71.15±3.46 |
45.22±8.43a |
61.90±6.52b |
67.26±5.84b |
| FS, % |
41.37±2.87 |
23.73±5.51a |
34.76±4.90 |
38.45±4.16b |
Discussion
The resulting myocardiocyte death and myocardial
remodeling post-AMI, derived from the occlusion of associated
coronary arteries which may completely block blood flow to the
myocardium, has been hypothesized to be the most important risk
factor for the development of heart failure among these patients
(2). Therefore, reperfusion
therapy strategies have been widely applied in the clinic and
demonstrated to be beneficial, regardless of emergency
revascularization or thrombolytic therapy (15). The concept of using gene therapy to
stimulate the regenerative abilities of the body for damaged
tissues had been proposed as a potential therapeutic strategy for
AMI. A number of miRNAs have been reported to act as potential
therapeutic tools, primarily as regulators of target gene
expression inducing therapeutic angiogenesis or attenuating
remodeling in response to ischemic states (4–6,14,16).
miRNA-210 serves a role in response to hypoxic conditions, and has
been observed to be an important regulator in angiogenesis. Wang
et al (10) reported that
the knockdown of miRNA-210 expression led to improved cardiac
function in AMI, while the opposite results were described by Wang
et al (11), in which
protective effects of the upregulation of miRNA-210 and VEGF were
observed in AMI, which were hypothesized to be derived from the
promotion of myocardial angiogenesis. These controversial data, in
addition to the unclear mechanisms, called the role of miRNA-210 in
AMI into question. As a result, the present study was performed and
the principal finding was that overexpression of miRNA-210 induced
by agonists exerted positive effects on promoting angiogenesis in
AMI through the potential mechanism of upregulating HGF expression,
which may serve a role in reducing remodeling following AMI and
lead to better clinical outcomes in terms of improved cardiac
contractility.
HGF, similar to VEGF, has been demonstrated to be an
important stimulator of angiogenesis, exerting promotive effects on
endothelial cell (EC) growth, proliferation, migration and
differentiation (17–19). Gorin et al (17) demonstrated that HGF was exhibited a
high angiogenic potential due to the direct targeting of ECs, and
the results of the present study were consistent with these
findings. Among the rats receiving miRNA-210 agonists in the
present study, the upregulation of HGF induced by miRNA-210
overexpression exhibited promotive effects on angiogenesis in in
vivo and in vitro experiments, as confirmed by CD31
immunohistochemistry. In addition, the increased proliferation and
migration of ECs associated with HGF expression was indicated by
Kaga et al (18). The
underlying mechanism was reported to be associated with enhanced
intracellular signaling molecules, including focal adhesion kinase
and RAC-α serine/threonine-protein kinase, with respect to the
regulation of cytoskeletal remodeling, cellular migration and
morphogenesis (20). The results
of the present study indicated that angiogenesis in AMI was induced
by miRNA-210 overexpression via direct targeting of HGF, instead of
VEGF, which was different from the observations of certain previous
studies (10,11). The balance between proliferation
and migration in ECs and vascular smooth muscle cells (VSMCs) may
be considered to serve an important role in angiogenesis. Basic
fibroblast growth factor (bFGF) has been reported to upregulate
aquaporin 1 mRNA expression in HUVECs, thereby influencing vascular
permeability (21). However, bFGF
promoted the production of inflammatory factors, including C-C
motif chemokine 2 and interleukin-8, as the activation of nuclear
factor-κB and the number of VSMCs was additionally increased,
whereas HGF and VEGF did not (18). HGF has been suggested to be an
anti-inflammatory gene, due to its antioxidant effect in VSMCs
(22,23). To the best of our knowledge, the
acute phase of AMI is frequently complicated by a severe
inflammatory response, leading to negative effects on the
proliferation and migration of ECs; this may explain the results
indicated in the present study, as the anti-inflammatory effect of
HGF appeared to facilitate the balance between proliferation and
migration in ECs and VSMCs, and subsequently to promote
angiogenesis in AMI.
In order to examine the potential mechanisms of
miRNA-208a with respect to myocardial remodeling post-AMI, Shyu
et al (4) performed
experiments in vivo and in vitro, and demonstrated
that miRNA-208a served an important role in the development of
myocardial fibrosis following AMI, primarily through its regulatory
effects on endoglin expression, in addition to potent targeting
effects on β-MHC. In the present study, improved cardiac functions
were observed on the basis of improved cardiac contractility among
the rats receiving miRNA-210 agonists, as demonstrated by
echocardiography and western blot analysis of β-MHC. Left
ventricular remodeling post-AMI may lead to a temporary
compensatory enhancement of left ventricular systolic function,
indicating a marked increase in β-MHC protein expression, which may
increase myocardial oxygen demand and result in worse clinical
outcomes. The results of the present study illustrated the
attenuating increase of β-MHC protein expression, and were
consistent with the results described by Wang et al
(24); therefore, this may be an
effective interpretation of the antifibrotic effect associated with
microvascular formation induced by miRNA-210 overexpression, which
was primarily due to the improved blood flow following maturation
of new blood vessels, forming complete collateral circulation to
increase the supply of oxygenated blood to myocardial cells.
In conclusion, the present study provided evidence
to support the protective effects of overexpression of miRNA-210 in
AMI, which were considered to be due to the promotion of
angiogenesis in the infarcted myocardium by targeting HGF
expression and inducing improved left ventricular remodeling
post-AMI. Therefore, the overexpression of miRNA-210 may be
suggested to be an advantageous therapeutic tool for treating AMI
in order to obtain improved clinical outcomes.
Acknowledgements
The present study was supported by the Jiangsu
Provincial Special Program of Medical Science (grant no.
BL2013001).
References
|
1
|
Goldberg RJ, Spencer FA, Gore JM, Lessard
D and Yarzebski J: Thirty-year trends (1975 to 2005) in the
magnitude of, management of, and hospital death rates associated
with cardiogenic shock in patients with acut: A population-based
perspective. Circulation. 119:1211–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McManus DD, Chinali M, Saczynski JS, Gore
JM, Yarzebski J, Spencer FA, Lessard D and Goldberg RJ: 30-year
trends in heart failure in patients hospitalized with acute
myocardial infarction. Am J Cardiol. 107:353–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shyu KG, Wang BW, Cheng WP and Lo HM:
MicroRNA-208a increases myocardial endoglin expression and
myocardial fibrosis in acute myocardial infarction. Can J Cardiol.
31:679–690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Cheng HW, Qiu Y, Dupee D, Noonan
M, Lin YD, Fisch S, Unno K, Sereti KI and Liao R: MicroRNA-34a
plays a key role in cardiac repair and regeneration following
myocardial infarction. Circ Res. 117:450–459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang J, Huang W, Cai W, Wang L, Guo L,
Paul C, Yu XY and Wang Y: Inhibition of microRNA-495 enhances
therapeutic angiogenesis of human induced pluripotent stem cells.
Stem Cells. 35:337–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dang K and Myers KA: The role of
hypoxia-induced miR-210 in cancer progression. Int J Mol Sci.
16:6353–6372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng LL, He XS, Liu JR, Zheng CB, Wang YT
and Yang GY: Lentivirus-mediated overexpression of MicroRNA-210
improves long-term outcomes after focal cerebral ischemia in mice.
CNS Neurosci Ther. 22:961–969. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Zhang J, Xia T, Li G, Tian T, Wang
M, Wang R, Zhao L, Yang Y, Lan K and Zhou W: MicroRNA-210 promotes
cancer angiogenesis by targeting fibroblast growth factor
receptor-like 1 in hepatocellular carcinoma. Oncol Rep.
36:2553–2562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Pan X, Fan Y, Hu X, Liu X, Xiang M
and Wang J: Dysregulated expression of microRNAs and mRNAs in
myocardial infarction. Am J Transl Res. 7:2291–2304.
2015.PubMed/NCBI
|
|
11
|
Wang J, Zhang Y, Liu YM, Guo LL, Wu P,
Dong Y and Wu GJ: Huoxue Anxin Recipe (HAR) promotes myocardium
angiogenesis of acute myocardial infarction rats by up-regulating
miR-210 and vascular endothelial growth factor. Chin J Integr Med.
22:685–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mineo TC, Ambrogi V, Baldi A, Rabitti C,
Bollero P, Vincenzi B and Tonini G: Prognostic impact of VEGF,
CD31, CD34, and CD105 expression and tumour vessel invasion after
radical surgery for IB-IIA non-small cell lung cancer. J Clin
Pathol. 57:591–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye P, Liu J, He F, Xu W and Yao K:
Hypoxia-induced deregulation of miR-126 and its regulative effect
on VEGF and MMP-9 expression. Int J Med Sci. 11:17–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2015 ACC/AHA/SCAI focused update on primary
percutaneous coronary intervention for patients with ST-elevation
myocardial infarction: An update of the 2011 ACCF/AHA/SCAI
guideline for percutaneous coronary intervention and the 2013
ACCF/AHA guideline for the management of ST-elevatio myocardial
infarction. J Am Coll Cardiol. 67:1235–1250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Yu P, Zeng Q, Luo B, Cai S, Hui K,
Yu G, Zhu C, Chen X, Duan M and Sun X: Neuroprotective effect of
hydrogen-rich saline in global cerebral ischemia/reperfusion rats:
Up-regulated tregs and down-regulated miR-21, miR-210 and NF-κB
expression. Neurochem Res. 41:2655–2665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gorin C, Rochefort GY, Bascetin R, Ying H,
Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, et
al: Priming dental pulp stem cells with fibroblast growth factor-2
increases angiogenesis of implanted tissue-engineered constructs
through hepatocyte growth factor and vascular endothelial growth
factor secretion. Stem Cells Transl Med. 5:392–404. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaga T, Kawano H, Sakaguchi M, Nakazawa T,
Taniyama Y and Morishita R: Hepatocyte growth factor stimulated
angiogenesis without inflammation: Differential actions between
hepatocyte growth factor, vascular endothelial growth factor and
basic fibroblast growth factor. Vascul Pharmacol. 57:3–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Awada HK, Johnson NR and Wang Y: Dual
delivery of vascular endothelial growth factor and hepatocyte
growth factor coacervate displays strong angiogenic effects.
Macromol Biosci. 14:679–686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sulpice E, Ding S, Muscatelli-Groux B,
Bergé M, Han ZC, Plouet J, Tobelem G and Merkulova-Rainon T:
Cross-talk between the VEGF-A and HGF signalling pathways in
endothelial cells. Biol Cell. 101:525–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saadoun S, Papadopoulos MC, Hara-Chikuma M
and Verkman AS: Impairment of angiogenesis and cell migration by
targeted aquaporin-1 gene disruption. Nature. 434:786–792. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanada F, Taniyama Y, Iekushi K, Azuma J,
Okayama K, Kusunoki H, Koibuchi N, Doi T, Aizawa Y and Morishita R:
Negative action of hepatocyte growth factor/c-Met system on
angiotensin II signaling via ligand-dependent epithelial growth
factor receptor degradation mechanism in vascular smooth muscle
cells. Circ Res. 105:667–675. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanada F, Taniyama Y, Azuma J, Iekushi K,
Dosaka N, Yokoi T, Koibuchi N, Kusunoki H, Aizawa Y and Morishita
R: Hepatocyte growth factor, but not vascular endothelial growth
factor, attenuates angiotensin II-induced endothelial progenitor
cell senescence. Hypertension. 53:77–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang BW, Wu GJ, Cheng WP and Shyu KG:
Mechanical stretch via transforming growth factor-β1 activates
microRNA-208a to regulate hypertrophy in cultured rat cardiac
myocytes. J Formos Med Assoc. 112:635–643. 2013. View Article : Google Scholar : PubMed/NCBI
|