Introduction
Ischemic heart disease (IHD), caused by myocardial
ischemic injuries, frequently presents as coronary heart disease
and acute myocardial infarction (1,2). IHD
was reported to cause 7.4 million mortalities in 2015, and is the
leading cause of mortality globally according to the World Health
Organization, representing a major socioeconomic burden. Therefore,
finding novel therapies to reduce the incidence of IHD is urgently
required.
Previous studies demonstrated that myocardial
hypoxia-ischemia is an important event during the entire process of
IHD (3–5), causing extensive cardiomyocyte death
(6,7). Evidence suggests that oxidative
stress is involved in myocardial ischemia and that antioxidant
treatment may be beneficial in cardiac damage during ischemia
(7–9). Markers of oxidative stress, including
reactive oxygen species (ROS), malondialdehyde (MDA) and superoxide
dismutase (SOD), are frequently used to monitor mitochondrial
oxidative damage (10,11). Furthermore, it has been
demonstrated that apoptosis serves an important role in
ischemic-hypoxic myocardial injury (12,13);
when the mitochondrial membrane potential is lost, the process of
apoptosis is activated, leading to cell death (14).
Garcinia mangostana is a tropical tree
commonly present in Southeast Asian countries, including Vietnam,
the Philippines and Thailand. The pericarp of mangosteen, the fruit
of G. mangostana, has been used as indigenous medicine for
the treatment of skin infections, wounds and diarrhea for a number
of years (15,16). Previously, α-mangostin (α-MG), a
major constituent extracted from the hull of mangosteen (17,18),
was demonstrated to possess a variety of pharmacological
properties, including anti-inflammatory (19), antitumor (20), cardioprotective (21–24),
antidiabetic (25), antibacterial
(26), antifungal (27), antioxidant (28) and antiobesity effects (29). α-MG has been demonstrated to arrest
the cell cycle and induce apoptosis in various cancer cells via the
mitochondrial pathway (20), and
its prevention of cisplatin-induced apoptotic death in LLC-PK1
porcine kidney cells has been associated with the inhibition of ROS
production (30). Previous in
vivo studies have additionally demonstrated the
cardioprotective effects of α-MG via the reduction of ROS
generation (22–24). However, the mechanism underlying
the protective effect of α-MG on cardiomyocytes remains to be fully
elucidated.
CoCl2, a chemical hypoxia-mimicking
agent, is able to simulate the effect of ischemic-hypoxic
myocardial injury (31). The H9C2
cell line is derived from rat embryonic cardiomyocytes, and may be
used to investigate the electrophysiological and biochemical
characteristics of myocardial tissue.
Myocardial cells frequently suffer from ischemic and
hypoxic death during the process of cardiac surgery; therefore, the
use of α-MG to potentially decrease the damage in cardiac surgery
may be beneficial. To investigate this, in the present study, α-MG
was added prior to CoCl2 to H9C2 cells.
CoCl2-treated H9C2 cells were used as a model to
evaluate the effects of α-MG on cardiomyoblasts exhibiting chemical
hypoxia-induced injury.
Materials and methods
Materials
A Cell Counting kit-8 (CCK-8, cat no. ck04) was
purchased from Dojindo Molecular Technologies, Inc., (Kumamoto,
Japan). α-MG and CoCl2 were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Antibodies against apoptosis
regulator Bcl-2 (rabbit anti-Bcl-2; cat no. 2870; 1:1,000),
apoptosis regulator BAX (rabbit anti-Bax; cat no. 2772s; 1:1,000),
rabbit anti-cleaved caspase-9 (cat no. 9507; 1:1,000), rabbit
anti-cleaved caspase-3 (cat no. 9662; 1:1,000) and mouse
anti-β-actin (cat. no. 3700) were purchased from Cell Signaling
Technology, Inc., (Danvers, MA, USA). Annexin V/propidium iodide
(PI) was purchased from BD Biosciences (Franklin Lakes, NJ, USA);
and the ROS detection kit was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Cell culture and treatment
H9C2 myocardial cells were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). Briefly, the H9C2 cells were cultured in high-glucose
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Invitrogen;
Thermo Fisher Scientific, Inc.) in 6-well plates with 5%
CO2 and 95% air at 37°C. The medium was changed every
2–3 days.
To determine the appropriate treatment conditions,
chemical hypoxia was achieved by adding different concentrations of
CoCl2 (50, 200, 400, 600 and 800 µM) to H9C2 cells for
24 h as the preliminary experiment (32). The proper concentration was
determined by the CCK-8 assay. In order to investigate the effects
of α-MG following CoCl2-induced cell injury,
CoCl2-treated H9C2 cells were maintained in complete
medium with 0.012, 0.06, 0.3, 0.6 or 1.2 mM α-MG. The control cells
were incubated without α-MG or CoCl2.
Experimental design
According to the results of the CCK-8 assay, H9C2
cells were randomly divided into the following groups: Group I
(Control), cells treated without α-MG or CoCl2; group
II, cells treated with CoCl2 alone; group III, cells
pretreated with 0.06 mM α-MG for 24 h and with CoCl2
over the next 24 h; and group IV, cells pretreated with 0.3 mM α-MG
for 24 h and with CoCl2 over the next 24 h.
Cell viability assay
CCK-8 was used to investigate the viability of H9C2
cells cultured in 96-well plates at a density of 5,000 cells/well.
When the cells had grown to 90% confluence, 600 µM CoCl2
was added into the plate treated for 24 h at 37°C, then cell injury
was induced. Thereafter, 10 µl CCK-8 solution was added to each
well and the cells were incubated for a further 2 h at 37°C.
Absorbance was measured at 450 nm with a microplate reader. The
mean optical density (OD) of four wells in each group was used to
calculate cell viability as follows: Cell viability
(%)=(ODtreatment group/ODcontrol group)
×100.
Determination of ROS, MDA and SOD
levels
The dichlorofluorescein diacetate (DCFH-DA) method
was used to detect the level of intracellular ROS (33). Intracellular ROS levels were
determined by measuring the oxidative conversion of cell permeable
2′,7′-DCFH-DA to fluorescent dichlorofluorescein (DCF) in a BioTek
Synergy 2 microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA). H9C2 cells were seeded (5×105 cells/well),
α-MG (0.06 or 0.3 mM) was added to the plates for 24 h and then the
CoCl2-induced cell injury protocol was performed. The
cells were incubated as previously described. The cells were washed
with D-Hank's (Thermo Fisher Scientific, Inc.) and incubated with
DCFH-DA at 37°C for 20 min. The DCF fluorescence distribution of
2×104 cells was detected by a microplate system at an
excitation wavelength of 488 nm and an emission wavelength of 535
nm. The MDA concentration was measured by Lipid Peroxidation MDA
Assay kit (Beyotime Institute of Biotechnology; cat no. S0131) and
SOD activity was measured using the Superoxide Dismutase Assay kit
(Beyotime Institute of Biotechnology; cat no. S0101), according to
the manufacturer's protocols.
Morphological assessment of apoptotic
cells by Annexin V-fluorescein isothiocyanate (FITC) and PI double
staining
Apoptosis in H9C2 cells was quantified using Annexin
V-FITC and PI double staining according to standard procedures
using the aforementioned kit. Cell apoptosis was analyzed using
CellQuest Pro software version 5.1 (BD Biosciences). Cellular
fluorescence was measured by flow cytometer (FACS
Calibur™, BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted by TRIzol™
(Invitrogen; Thermo Fisher Scientific, Inc.). A total of 100 ng
total RNA was used for cDNA synthesis using the Stratagene
AffinityScript qPCR cDNA Synthesis kit (Agilent Technologies, Inc.,
Santa Clara, CA, USA). The cDNA samples were diluted 10-fold with
nuclease-free H2O and 2 µl diluted template was combined
with Brilliant III Ultra-Fast SYBR® Green qPCR Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). β-actin
was used as an internal reference control. The relative expression
of target genes was determined by the 2−∆∆Cq method
(34). The qPCR cycling conditions
comprised initial denaturation for 3 min at 95°C, followed by 45
cycles at 95°C (10 sec) and 58°C (45 sec); data were acquired at
the end of the annealing/extension phase. Melt curve analysis was
performed at the end of each run between 58 and 95°C. The gene
primer sequences are exhibited in Table I. Data were analyzed using
Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA).
 | Table I.Primer sequences of Bcl-2, Bax,
caspase-3, caspase-9 and β-actin. |
Table I.
Primer sequences of Bcl-2, Bax,
caspase-3, caspase-9 and β-actin.
| Gene | Primer
sequences |
|---|
| Bcl-2 | FP:
5′-GGATGACTGAGTACCTGAA-3′ |
|
| RP:
5′-GCCATATAGTTCCACAAAGG-3′ |
| Bax | FP:
5′-GATGAACTGGACAACAACAT-3′ |
|
| RP:
5′-CACGGAAGAAGACCTCTC-3′ |
| Caspase-3 | FP:
5′-ATTATGGAATTGATGGATAGTGTT-3′ |
|
| RP:
5′-GTAGTCGCCTCTGAAGAA-3′ |
| Caspase-9 | FP:
5′-ACTGCCTCATCATCAACA-3′ |
|
| RP:
5′-GTTCTTCACCTCCACCAT-3′ |
| β-actin | FP:
5′-CGTAAAGACCTCTATGCCAACA-3′ |
|
| RP:
5′-AGCCACCAATCCACACAGAG-3′ |
Western blot analysis
Total proteins were extracted from cells using cell
lysis buffer [50 mM Tris-HCl, (pH 8.0) 120 mM NaCl; 0.5% NP-40; 1
mM phenylmethylsulfonyl fluoride] and determined using
bicinchoninic acid assay. A total of 40 µg protein extract was
separated by 12% SDS-PAGE, followed by transfer to a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.), blocking with 5%
non-fat milk in TBS-Tween buffer 7 (0.12 M Tris-base, 1.5 M NaCl,
0.1% Tween-20) for 1 h at room temperature, and incubation with the
appropriate antibodies including rabbit anti-Bcl-2 (cat no. 2870;
1:1,000), rabbit anti-Bax (cat no. 2772s; 1:1,000), rabbit
anti-cleaved caspase-9 (cat no. 9507; 1:1,000), rabbit anti-cleaved
caspase-3 (cat no. 9662; 1:1,000) and mouse anti-actin (cat no.
3700) were purchased from Cell Signaling Technology, Inc.,
(Danvers, MA, USA), overnight at 4°C. Subsequently, the blots were
incubated with horseradish peroxidase-conjugated anti-rabbit
secondary antibody (1:10,000; cat no. 111-035-003; Jackson
Immunoresearch Laboratories, Inc., West Grove, PA, USA) for 30 min
at room temperature. The bound antibodies were detected using a
chemiluminescence (ECL) system and autoradiography (cat. no.
PI32209; Pierce; Thermo Fisher Scientific, Inc.). Quantity One
analysis software version 4.6.9 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to quantify the relative band
intensities from western blot images.
Caspase-3 and caspase-9 activity
assay
The activity of caspase-3 and caspase-9 was
determined using a Caspase-3 Activity Assay kit and a Caspase-9
Activity Assay kit (Beyotime Institute of Biotechnology). This
assay is based on the detection of the chromophore p-nitroaniline
(pNA) by spectrophotometry following cleavage of pNA from the
substrate by caspase-3/caspase-9. Assays were performed in 96-well
plates (5×104 cells/well), with each well containing 10
µl protein cell lysate, 80 µl reaction buffer and 10 µl substrate
(Asp-Glu-Val-Asp-pNA for caspase-3 and Leu-Glu-His-Asp-pNA for
caspase-9). The results were quantified spectrophotometrically
using a BioTek Synergy 2 microplate reader (BioTek Instruments,
Inc.) at a wavelength of 405 nm. Caspase activity was presented as
a percentage relative to the control group.
Statistical analysis
The results are reported as the mean ± standard
error of the mean for at least three analyses for each sample.
Quantitative variables were compared using one-way analysis of
variance to compare differences in two or more groups, and the
Tukey test was performed for post-hoc subgroup analysis, as
appropriate. Statistical analysis was performed using the SPSS 19.0
software package (IBM Corp., Armonk, NY, USA) and GraphPad Prism
5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of α-MG on
CoCl2-induced cell viability
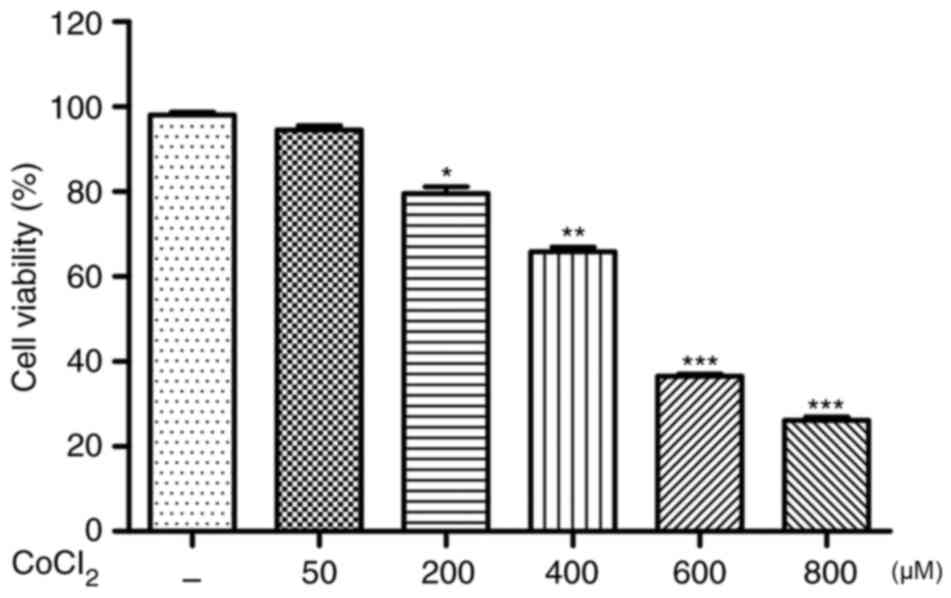
As exhibited in Fig.
1, H9C2 cells treated with CoCl2 (≥600 µM) for 24 h
exhibited significantly reduced cell viability (P<0.001). The
choice of 600 µM for the concentration of CoCl2 applied
to all experiments was based on these results from the CCK-8 assay.
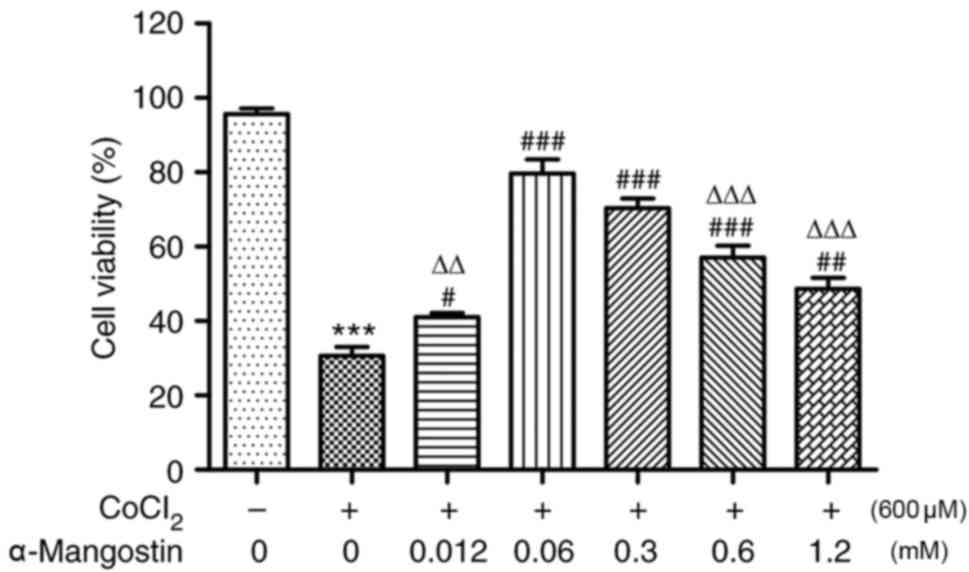
However, in H9C2 cells pretreated with various concentrations of
α-MG, CoCl2-induced hypoxic injury was significantly
reduced (P<0.05; Fig. 2). Cells
pretreated with α-MG at concentrations between 0 and 12 mM
presented as an ‘inverted U shape’ in the present study: At
concentrations of 0.012–0.06 mM, cell viability increased gradually
(P<0.05), reaching a maximum at 0.06 mM; at concentrations of
0.06–1.2 mM the curve exhibited a steep decline, indicating a
decrease in cell viability with increasing concentrations, although
cell viability remained higher compared with the group treated with
CoCl2 alone (P<0.05). Among the α-MG pretreatment
groups, all exhibited significant differences compared with the
0.06 mM group (P<0.01), apart from the 0.3 mM group
(P>0.05).
Effects of α-MG on
CoCl2-induced alterations in ROS generation, MDA
concentration and SOD activity
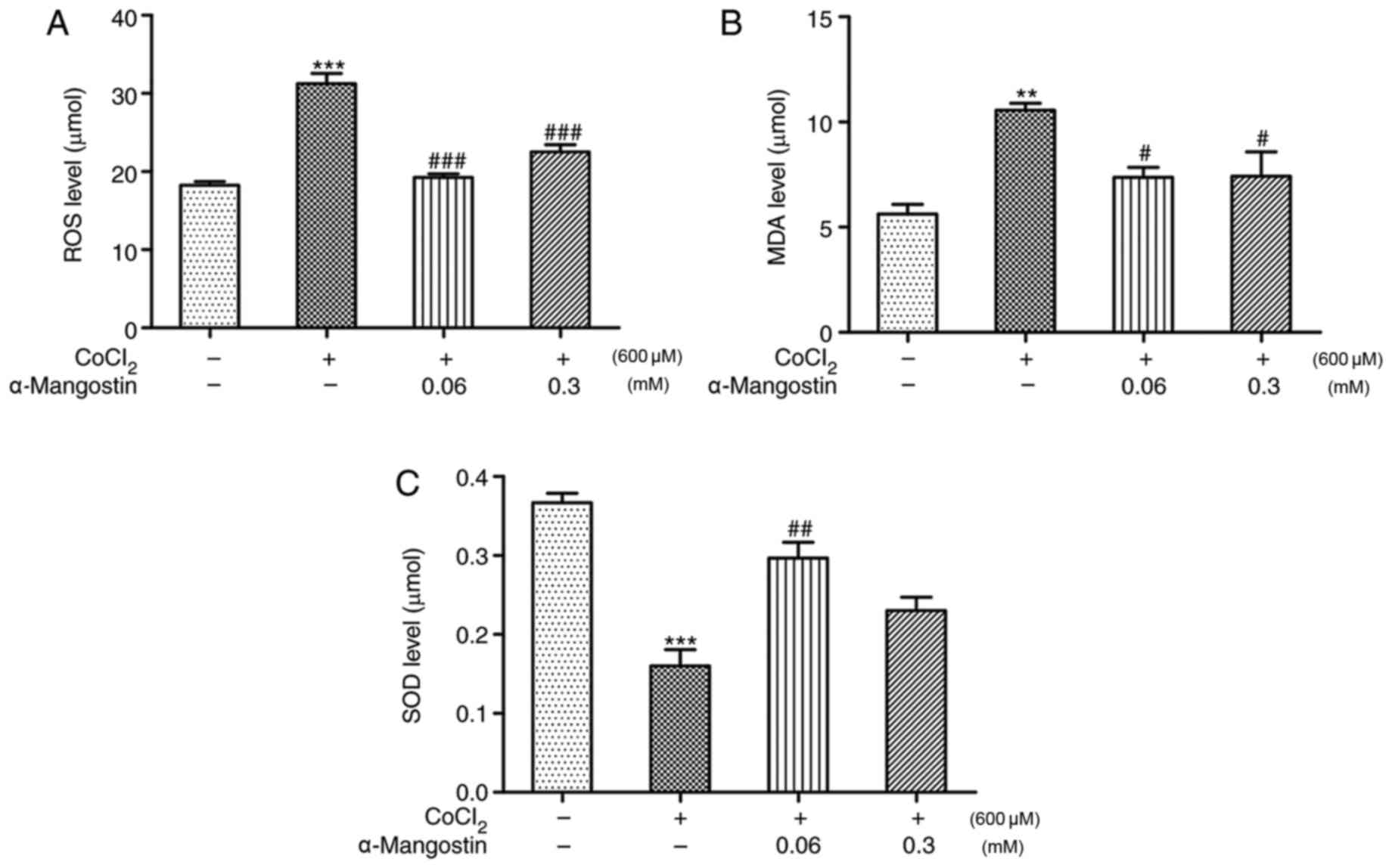
The involvement of ROS in the
CoCl2-induced apoptosis of H9C2 cells was evaluated by
measuring the level of ROS production. Following treatment of H9C2
cells with 600 µM CoCl2 for 24 h, the intracellular ROS
level increased significantly compared with that in the control
group (P<0.001). However, treatment with α-MG decreased the
intracellular ROS levels significantly (P<0.001), further
demonstrating its antioxidant effects (Fig. 3A). As exhibited in Fig. 3B and C, H9C2 cells treated with
CoCl2 exhibited significantly increased MDA
concentrations and reduced SOD concentration (P<0.01 and
P<0.001, respectively). The oxidative abnormalities were
ameliorated by α-MG at a concentration of 0.06 mM, as demonstrated
by the significant reduction in MDA concentration and the increase
in SOD activity (P<0.05).
Effects of α-MG on
CoCl2-induced cell apoptosis
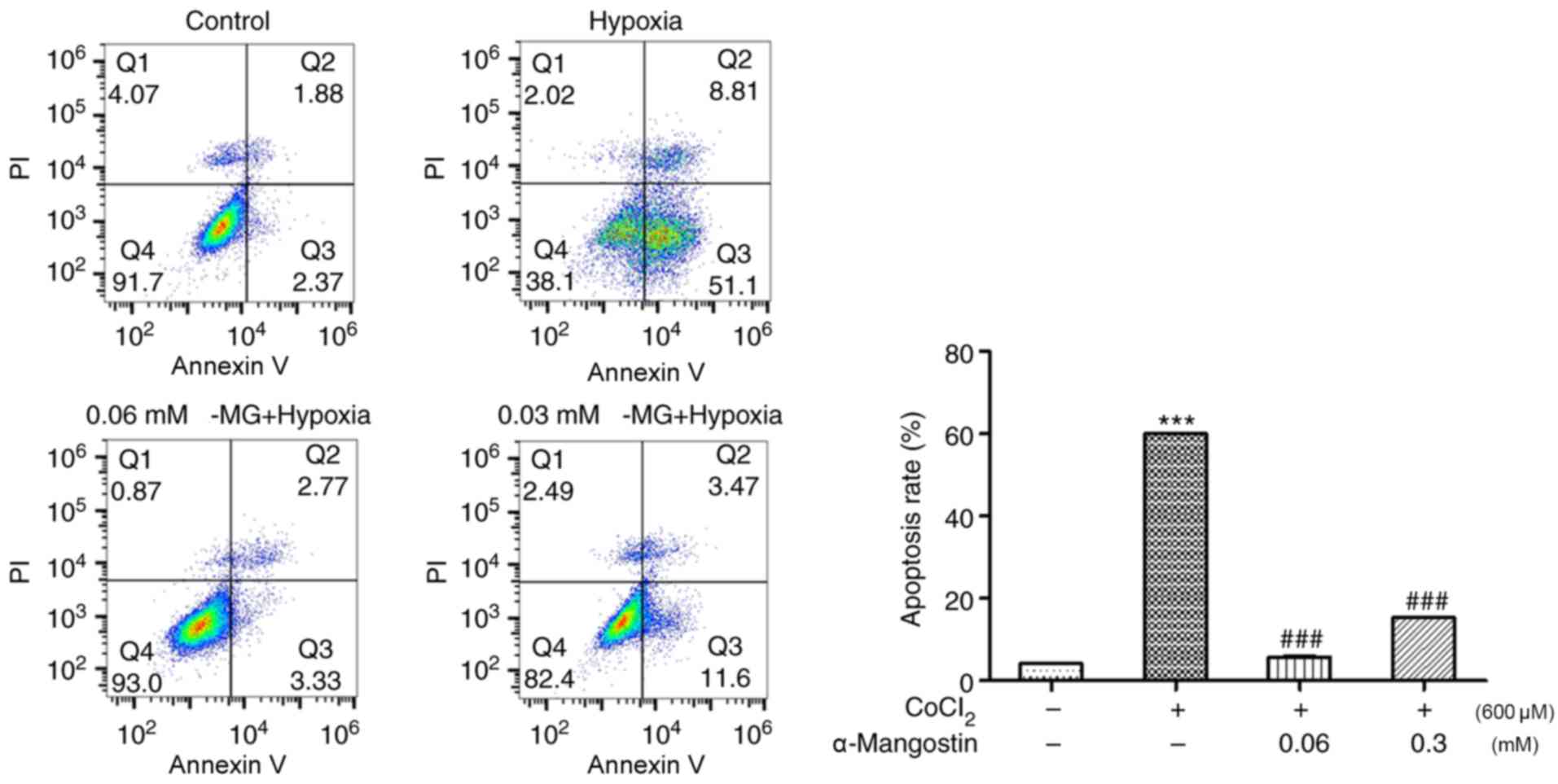
To further investigate the functional effect of α-MG
in H9C2 cells treated with CoCl2, cellular apoptosis in
H9C2 cells was assessed using the Annexin V-FITC/PI method with
flow cytometry. The results demonstrated that CoCl2
increased the percentage of apoptotic H9C2 cells to ~60%, which was
attenuated by treatment with 0.06 mM α-MG (reduced to ~5.8%) and
0.3 mM α-MG (P<0.001; reduced to ~15.34%), indicating the
cytoprotective effect of α-MG against chemical hypoxia-induced
apoptosis (Fig. 4).
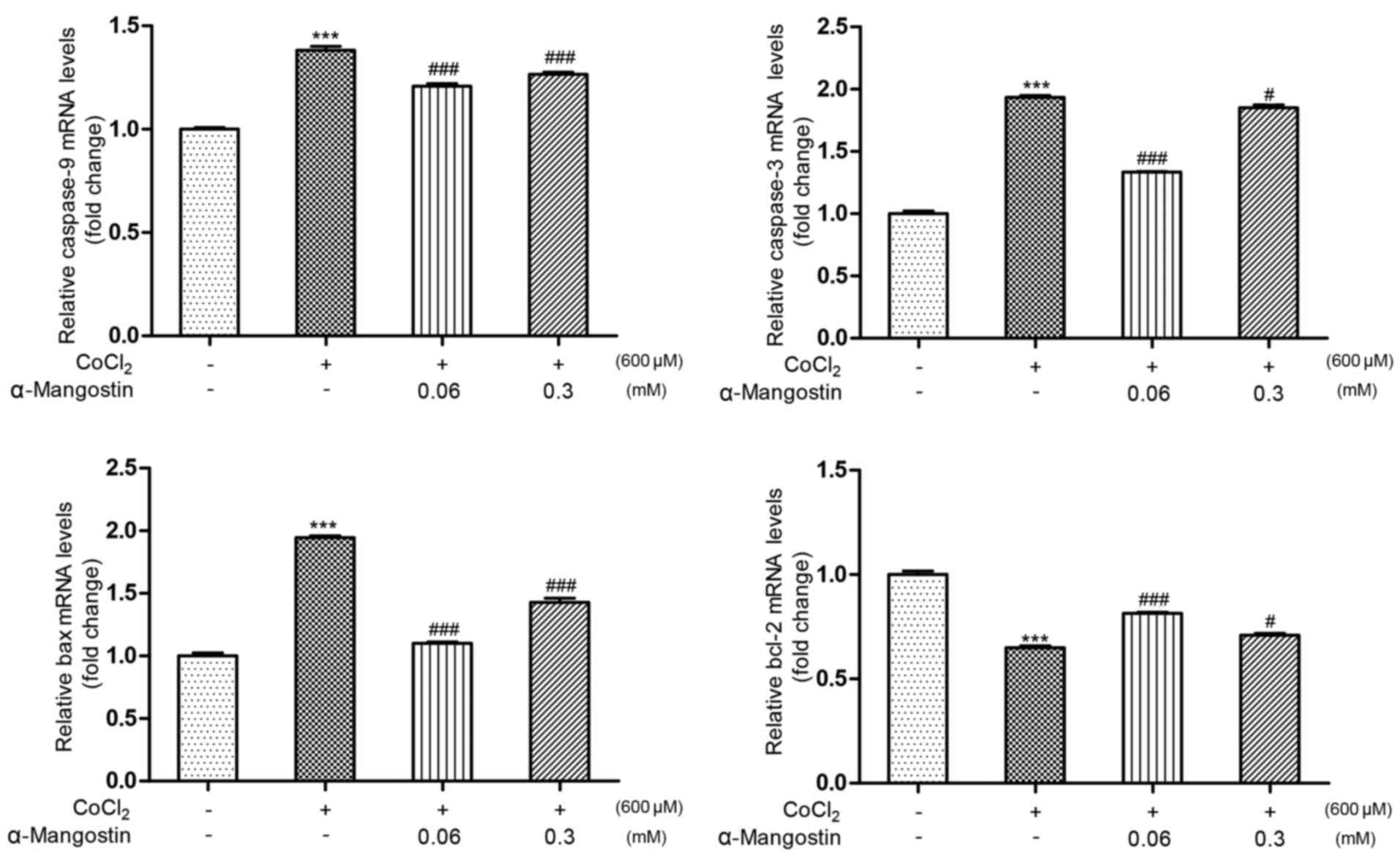
Effect of α-MG on cell
apoptosis-regulating genes
The results of the RT-qPCR (Fig. 5) revealed that treatment with
CoCl2 significantly decreased the expression level of
Bcl-2 to 64.9% and increased the expression levels of Bax,
caspase-9 and caspase-3 to 194, 138 and 193% relative to the
control group, respectively (P<0.001 vs. control group).
However, pretreatment with α-MG at a concentration of 0.06 mM
increased the expression level of Bcl-2 to 81.4% and attenuated the
expression levels of Bax, caspase-9 and caspase-3 to 110, 120 and
133% relative to the control group, respectively (P<0.001 vs.
CoCl2-induced injury group), while pretreatment with
α-MG at a concentration of 0.3 mM increased the expression level of
Bcl-2 to 70.8% and attenuated the expression levels of Bax,
caspase-9 and caspase-3 to 142, 126 and 182% relative to the
control group, respectively (P<0.05 vs. CoCl2-induced
injury group).
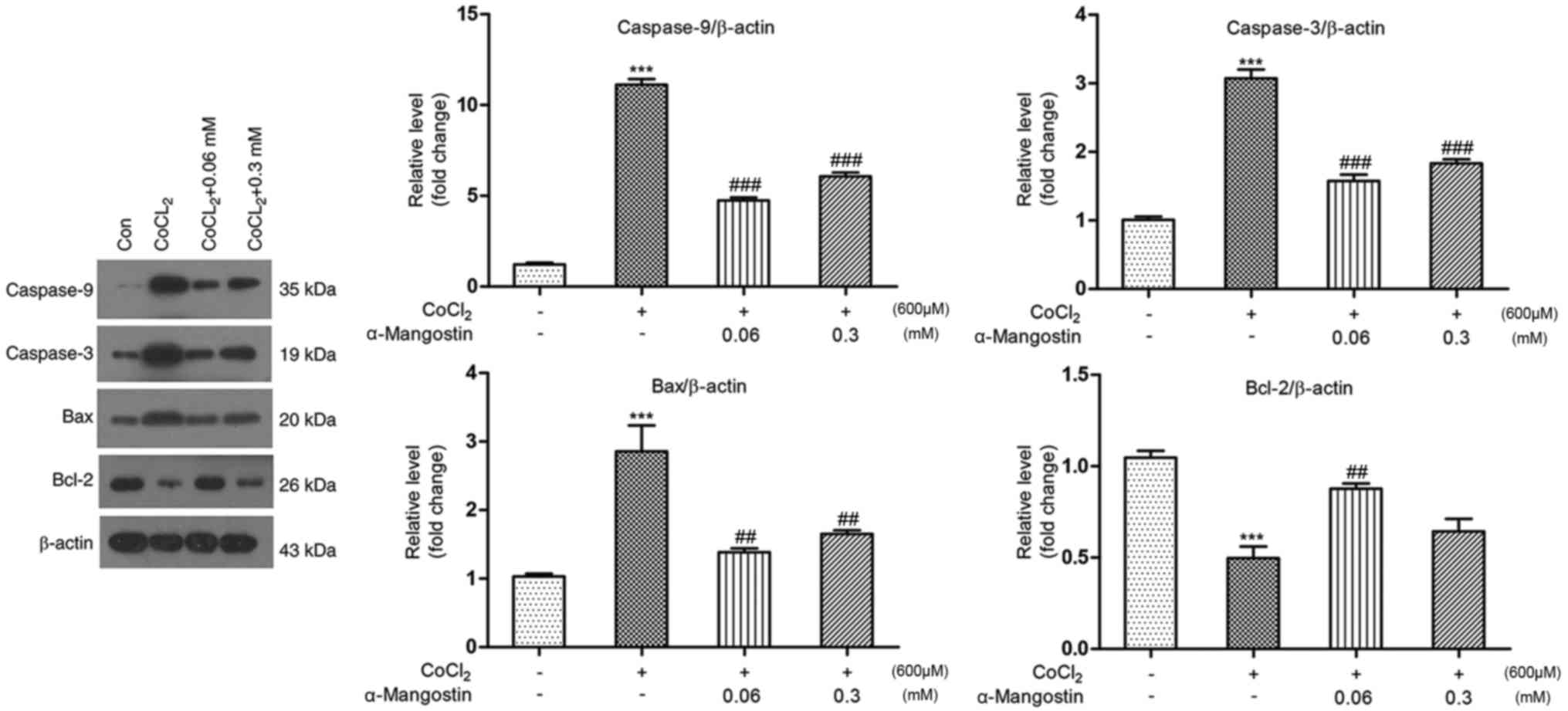
Effect of α-MG on cellular
apoptosis-regulating protein expression
As depicted in Fig.
6, protein expression was analyzed by western blotting.
Treatment with CoCl2 significantly downregulated the
expression level of Bcl-2 to 49.6% and upregulated the levels of
Bax, caspase-9 and caspase-3 to 285, 1,111 and 307% relative to the
control group, respectively (P<0.001). Pretreatment with α-MG at
a concentration of 0.06 mM upregulated the expression level of
Bcl-2 to 87.6% and downregulated the expression levels of Bax,
caspase-9 and caspase-3 to 138, 474 and 157%, respectively
(P<0.01), while pretreatment with α-MG at a concentration of 0.3
mM downregulated the expression levels of Bax, caspase-9 and
caspase-3 to 165, 650 and 183%, respectively (P<0.01), while
there was not significant difference with Bcl-2.
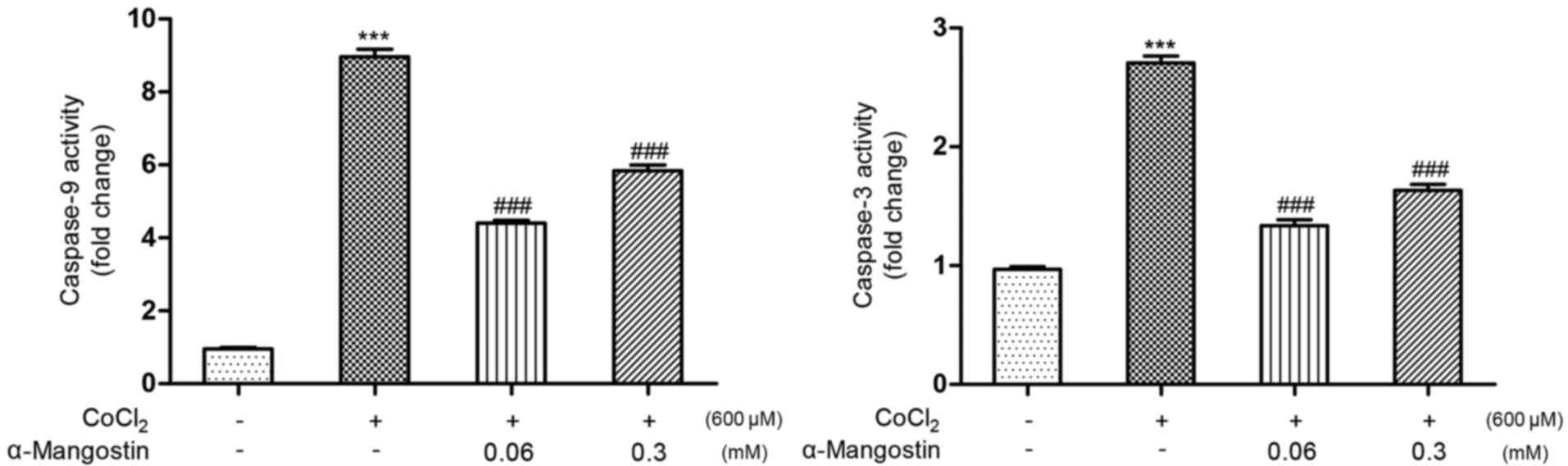
Effect of α-MG on the activation of
caspase-3 and caspase-9
The activation of caspase-3 and caspase-9 has
important influences on apoptosis. When compared with the control
group (Fig. 7), treatment with
CoCl2 significantly increased the activity of caspase-3
and caspase-9 to 270 and 906%, respectively (P<0.001). When
pretreated with α-MG (0.06 and 0.3 mM), the activities of caspase-3
(140 and 167%, respectively) and caspase-9 (447 and 573%,
respectively) were significantly attenuated (P<0.001 vs.
CoCl2-induced injury group).
Discussion
The findings of the present study revealed that
α-MG, a promising cardioprotective natural extract, was able to
suppress oxidative stress and inhibit apoptosis in H9C2 cells by
attenuating cellular oxidative damage. To the best of the authors'
knowledge, this is the first report describing the anti-apoptotic
effect of α-MG in H9C2 cells.
Mangosteen fruit has been used for centuries to
alleviate a number of pathological conditions in humans. In 1855,
α-MG was identified among the major xanthones isolated from the
pericarp of the mangosteen fruit (35). This compound is yellowish in color
and may additionally be obtained from other parts of the plant. The
structure of this xanthone was interpreted by Dragendorff [reviewed
in (36)]. The molecular formula,
type and position of the substituent groups of α-MG were
subsequently determined by Stout et al (37). Preclinical studies with purified
α-MG, the major constituent of the pericarp of mangosteen, have
demonstrated its beneficial effects in various diseases. However,
the cardioprotective effects of α-MG have not been extensively
investigated. In a study conducted by Devi Sampath and
Vijayaraghavan (22), rats were
administered oral α-MG at a dose of 200 mg/kg prior to the
induction of myocardial infarction by isoproterenol (ISO). On
comparing the experimental groups, the myocardial injury markers
and oxidation products were increased significantly in the blood
and myocardia of rats not treated with oral α-MG, whereas they were
significantly reduced in rats with myocardial injury that received
oral α-MG, demonstrating that α-MG exerts a protective effect
against lipid peroxidation and enhances the antioxidant tissue
defense system during ISO-induced myocardial infarction in rats. A
further study on the activity of rat myocardium mitochondrial
enzymes revealed significantly increased enzyme activity,
indicating mitochondrial damage, following treatment with ISO in
rats that did not receive oral α-MG, whereas oral α-MG was able to
reverse this result, suggesting that α-MG may reduce the occurrence
of ISO-induced myocardial infarction, mitochondrial dysfunction and
associated oxidative stress (23).
Buelna-Chontal et al (24)
reported that α-MG exerts a protective effect in the post-ischemic
heart, which is associated with the prevention of oxidative stress
secondary to reperfusion injury. To date, studies have focused on
animal research, whereas a study in vitro has not been
reported.
Hypoxia may induce ROS generation and lipid
peroxidation (38). ROS, a series
of cellular molecules generated during oxygen metabolism, are
generally considered to be important mediators of oxidative stress
injury (39). The MDA
concentration frequently reflects the degree of lipid peroxidation,
indirectly reflecting the degree of cellular oxidative injury
(40). SOD decrease cellular free
oxygen radicals (41). The present
study demonstrated that the ROS level and MDA concentration were
increased in H9C2 cells treated with CoCl2, while the
activity of SOD was suppressed. In H9C2 cells treated with α-MG,
the results were reversed, demonstrating the antioxidant effects of
α-MG, which coincide with the observations in vivo.
It was previously reported that apoptosis is rare in
the healthy myocardium, with a percentage of 0.001–0.002% (42); however, when the cells become
damaged, they may undergo apoptosis. Oxidative stress usually
causes DNA damage, and instability of the membrane, cellular lipids
and proteins, leading to cellular dysfunction and apoptosis
(43). In the present study,
myocardial cell viability decreased following treatment with
CoCl2, and the apoptosis rate increased. α-MG-treated
groups (at concentrations of 0.06 and 0.3 mM) exhibited decreased
oxidative stress and apoptosis rates, indicating the protective
effect of α-MG against CoCl2-induced apoptosis.
The first limitation of the present study is
associated with the use of CoCl2, which is commonly used
for constructing hypoxic models in different cell lines (44,45).
CoCl2-treated cells were used as a model of chemical
hypoxia-induced injury; however, there are several other effects of
hypoxia that are not achieved by treatment with CoCl2.
For example, CoCl2 may stabilize the expression of
hypoxia-inducible factor 1α (HIF-1α) (46,47).
Second, the mechanisms underlying the antioxidant and
anti-apoptotic properties of α-MG are not fully elucidated, and
continued research is required in the future.
In conclusion, in the present study, a myocardial
cell model of hypoxia was successfully constructed and α-MG was
demonstrated to be effective in reducing apoptosis and oxidative
stress induced by CoCl2.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Celebrity
Award of Xiangya Hospital.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
FZ contributed to acquisition, analysis and
interpretation of data, writing the main manuscript text, LYL and
LWJ designed the study and contributed by revising the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
α-MG
|
α-mangostin
|
|
ROS
|
reactive oxygen species
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
IHD
|
ischemic heart disease
|
|
CCK-8
|
cell-counting kit 8
|
|
FACS
|
fluorescence activated cell
sorting
|
|
OD
|
optical density
|
|
PI
|
propidium iodide
|
References
|
1
|
Luo D, Yao YY, Li YF, Sheng ZL, Tang Y,
Fang F, Fang K, Ma GS and Teng GJ: Myocardial infarction
quantification with late gadolinium-enhanced magnetic resonance
imaging in rats using a 7-T scanner. Cardiovasc Pathol. 21:112–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shibata R, Sato K, Pimentel DR, Takemura
Y, Kihara S, Ohashi K, Funahashi T, Ouchi N and Walsh K:
Adiponectin protects against myocardial ischemia-reperfusion injury
through AMPK- and COX-2-dependent mechanisms. Nat Med.
11:1096–1103. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu LX, Gu XF, Zhu YC and Zhu YZ:
Protective effects of novel single compound, Hirsutine on hypoxic
neonatal rat cardiomyocytes. Eur J Pharmacol. 650:290–297. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Liu A, Hou R, Zhang J, Jia X,
Jiang W and Chen J: Salidroside protects cardiomyocyte against
hypoxia-induced death: A HIF-1alpha-activated and VEGF-mediated
pathway. Eur J Pharmacol. 607:6–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu HM and Deng L: Evaluation of
cardiomyocyte hypoxia injury models for the pharmacological study
in vitro. Pharm Biol. 50:167–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharov VG, Todor AV and Sabbah HN: Left
ventricular histomorphometric findings in dogs with heart failure
treated with the acorn cardiac support device. Heart Fail Rev.
10:141–147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ibáñez B, Heusch G, Ovize M and Van de
Werf F: Evolving therapies for myocardial ischemia/reperfusion
injury. J Am Coll Cardiol. 65:1454–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie Q, Li XX, Zhang P, Li JC, Cheng Y,
Feng YL, Huang BS, Zhuo YF and Xu GH: Hydrogen gas protects against
serum and glucose deprivation-induced myocardial injury in H9c2
cells through activation of the NF-E2-related factor 2/heme
oxygenase 1 signaling pathway. Mol Med Rep. 10:1143–1149. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Zhang P, Liu J, Zhou R, Li Q, You Z
and Dian K: Protective effects of hemoglobin-based oxygen carrier
given to isolated heart during ischemia via attenuation of
mitochondrial oxidative damage. Free Radic Biol Med. 48:1079–1089.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dou MM, Zhang ZH, Li ZB, Zhang J and Zhao
XY: Cardioprotective potential of Dendrobium officinale Kimura et
Migo against myocardial ischemia in mice. Mol Med Rep.
14:4407–4414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang
S, Wang N, Liang Z, Li Y, Chen W, et al: JAK2/STAT3 activation by
melatonin attenuates the mitochondrial oxidative damage induced by
myocardial ischemia/reperfusion injury. J Pineal Res. 55:275–286.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallo S, Gatti S, Sala V, Albano R,
Costelli P, Casanova E, Comoglio PM and Crepaldi T: Agonist
antibodies activating the Met receptor protect cardiomyoblasts from
cobalt chloride-induced apoptosis and autophagy. Cell Death Dis.
5:e11852014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pyo JO, Nah J, Kim HJ, Chang JW, Song YW,
Yang DK, Jo DG, Kim HR, Chae HJ, Chae SW, et al: Protection of
cardiomyocytes from ischemic/hypoxic cell death via Drbp1 and
pMe2GlyDH in cardio-specific ARC transgenic mice. J Biol Chem.
283:30707–30714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hausenloy DJ and Yellon DM: The
mitochondrial permeability transition pore: Its fundamental role in
mediating cell death during ischaemia and reperfusion. J Mol Cell
Cardiol. 35:339–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagy S and Shaw PE: Tropical and
subtropical fruits: Composition, properties and uses. Mol Nut.
25:5821980.
|
|
16
|
Gupta KK, Khandelwal G, Prasad G, Chopra
AK and Mishra A: A review on scientific technologies in practice to
innovate plant based molecules and to improve herbal drug quality
to overcome health problems. J App Nat Sci. 2:165–181. 2010.
View Article : Google Scholar
|
|
17
|
Gopalakrishnan G and Balaganesan B: Two
novel xanthones from Garcinia mangostana. Fitoterapia.
71:607–609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suksamrarn S, Suwannapoch N, Ratananukul
P, Aroonlerk N and Suksamrarn A: Xanthones from the green fruit
hulls of Garcinia mangostana. J Nat Prod. 65:761–763. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chomnawang MT, Surassmo S, Nukoolkarn VS
and Gritsanapan W: Effect of Garcinia mangostana on
inflammation caused by Propionibacterium acnes. Fitoterapia.
78:401–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumoto K, Akao Y, Ohguchi K, Ito T,
Tanaka T, Iinuma M and Nozawa Y: Xanthones induce cell-cycle arrest
and apoptosis in human colon cancer DLD-1 cells. Bioorg Med Chem.
13:6064–6069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang DJ, Dai Z and Li YJ: Pharmacological
effects of Xanthones as cardiovascular protective agents.
Cardiovasc Drug Rev. 22:91–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devi Sampath P and Vijayaraghavan K:
Cardioprotective effect of alpha-mangostin, a xanthone derivative
from mangosteen on tissue defense system against
isoproterenol-induced myocardial infarction in rats. J Biochem Mol
Toxicol. 21:336–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sampath PD and Kannan V: Mitigation of
mitochondrial dysfunction and regulation of eNOS expression during
experimental myocardial necrosis by alpha-mangostin, a xanthonic
derivative from Garcinia mangostana. Drug Chem Toxicol.
32:344–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buelna-Chontal M, Correa F,
Hernández-Reséndiz S, Zazueta C and Pedraza-Chaverri J: Protective
effect of α-mangostin on cardiac reperfusion damage by attenuation
of oxidative stress. J Med Food. 14:1370–1374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumar V, Bhatt PC, Kaithwas G, Rashid M,
Al-abbasi FA, Khan JAJ, Anwar F and Verma A: α-mangostin mediated
pharmacological modulation of hepatic carbohydrate metabolism in
diabetes induced wistar rat. Beni-Suef Uni J Basic App Sci.
5:255–276. 2016.
|
|
26
|
Iinuma M, Tosa H, Tanaka T, Asai F,
Kobayashi Y, Shimano R and Miyauchi K: Antibacterial activity of
xanthones from guttiferaeous plants against methicillin-resistant
Staphylococcus aureus. J Pharm Pharmacol. 48:861–865. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaomongkolgit R, Jamdee K and Chaisomboon
N: Antifungal activity of alpha-mangostin against Candida
albicans. J Oral Sci. 51:401–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williams P, Ongsakul M, Proudfoot J, Croft
K and Beilin L: Mangostin inhibits the oxidative modification of
human low density lipoprotein. Free Radic Res. 23:175–184. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Quan X, Wang Y, Ma X, Liang Y, Tian W, Ma
Q, Jiang H and Zhao Y: α-Mangostin induces apoptosis and suppresses
differentiation of 3T3-L1 cells via inhibiting fatty acid synthase.
PLoS One. 7:e333762012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee D, Choi YO, Kim KH, Chin YW, Namgung
H, Yamabe N and Jung K: Protective effect of α-mangostin against
iodixanol-induced apoptotic damage in LLC-PK1 cells. Bioorg Med
Chem Lett. 26:3806–3809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldberg MA, Dunning SP and Bunn HF:
Regulation of the erythropoietin gene: Evidence that the oxygen
sensor is a heme protein. Science. 242:1412–1415. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park JK, Jeong JW, Kang MY, Baek JC, Shin
JK, Lee SA, Choi WS, Lee JH and Paik WY: Inhibition of the PI3K-Akt
pathway suppresses sFlt1 expression in human placental hypoxia
models in vitro. Placenta. 31:621–629. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia SJ, Jiang DJ, Hu CP, Zhang XH, Deng HW
and Li YJ: Lysophosphatidylcholine-induced elevation of asymmetric
dimethylarginine level by the NADPH oxidase pathway in endothelial
cells. Vascul Pharmacol. 44:143–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schmid W: Ueber das Mangostin. Eur J Org
Chem. 93:83–88. 1855.
|
|
36
|
Ibrahim MY, Hashim NM, Mariod AA, Mohan S,
Abdulla MA, Abdelwahab SI and Arbab IA: α-Mangostin from
Garcinia mangostana Linn: An updated review of its
pharmacological properties. Arabian J Chem. 9:317–329. 2016.
View Article : Google Scholar
|
|
37
|
Stout GH, Krahn MM, Yates P and Bhat HB:
The structure of mangostin. Chem Commun. 4:211–212. 1968.
|
|
38
|
Chang ST, Chung CM, Chu CM, Yang TY, Pan
KL, Hsu JT and Hsiao JF: Platelet glycoprotein IIb/IIIa inhibitor
tirofiban ameliorates cardiac reperfusion injury. Int Heart J.
56:335–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Hou J, Xia ZY, Zeng W, Wang X, Li
R, Ke C, Xu J, Lei S and Xia Z: Recombinant PTD-Cu/Zn SOD
attenuates hypoxia-reoxygenation injury in cardiomyocytes. Free
Radic Res. 47:386–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Gong GH, Xu YN, Yu LJ and Wei CX:
Sugemule-3 protects against isoprenaline-induced cardiotoxicity in
vitro. Pharmacogn Mag. 13:517–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moris D, Spartalis M, Tzatzaki E,
Spartalis E, Karachaliou GS, Triantafyllis AS, Karaolanis GI,
Tsilimigras DI and Theocharis S: The role of reactive oxygen
species in myocardial redox signaling and regulation. Ann Transl
Med. 5:3242017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dai ZJ, Gao J, Ma XB, Yan K, Liu XX, Kang
HF, Ji ZZ, Guan HT and Wang XJ: Up-regulation of hypoxia inducible
factor-1α by cobalt chloride correlates with proliferation and
apoptosis in PC-2 cells. J Exp Clin Cancer Res. 31:282012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grasselli F, Basini G, Bussolati S and
Bianco F: Cobalt chloride, a hypoxia-mimicking agent, modulates
redox status and functional parameters of cultured swine granulosa
cells. Reprod Fertil Dev. 17:715–720. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Srinivasan S and Dunn JF: Stabilization of
hypoxia-inducible factor-1α in buffer containing cobalt chloride
for Western blot analysis. Anal Biochem. 416:120–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang BW, Miyazawa M and Tsuji Y: Distinct
regulatory mechanisms of the human ferritin gene by hypoxia and
hypoxia mimetic cobalt chloride at the transcriptional and
post-transcriptional levels. Cell Signal. 26:2702–2709. 2014.
View Article : Google Scholar : PubMed/NCBI
|