Introduction
The immune system defends against the high number of
non-self antigens, including pathogens that threaten host viability
(1). To achieve this, it uses a
remarkable array of immune processes that enable the efficient
recognition, initiation and elimination of harmful antigens to
maintain biological homeostasis (2). The immune response depends on
antigen-specific recognition and is typically classified into
innate and adaptive components, which are mediated by T and B
lymphocytes, and innate immune cells, respectively (3). There is synergy between these two
arms of immunity, mediated by immune components that involve
several different proteins and mediators, including cytokines and
the complement system (4).
The complement system is comprised of >30
proteins in the plasma and cell membrane, organized through a
hierarchy of proteolytic cascades that are triggered by initiators
(5). Following activation,
complement system components assemble the membrane attack complex
(MAC) to form pores in the cell membrane to destroy target cells.
During complement activation, several potent pro-inflammatory
mediators, including complement proteins C3a and C5a, are
simultaneously generated. These mediators further induce
anaphylaxis, recruit infiltrating inflammatory immune cells and
regulate T cell function (6,7). It
has also been reported that complement activation has a role in
reducing necrotic cell-induced inflammation by clearing dying cells
and thereby aiding in the prevention of autoimmune diseases
(8,9). Recently, Suresh et al
(10) demonstrated that MAC
formation can result in NACHT, LRR and PYD domains containing
protein 3 (NLRP3) inflammasome activation, which in turn activates
caspase-1 and promotes secretion of the inflammatory cytokines
interleukin (IL)-1β and IL-18. Thus, complement activation can
affect inflammation through multiple mechanisms.
In recent decades, the association between lipid
metabolism and inflammation or immunity has been widely explored.
Cumulative studies have revealed that a typically western diet rich
in saturated fatty acids and n-6 polyunsaturated fatty acids (n-6
PUFAs) induces obesity and causes immune cell infiltration into
adipose tissue (11,12). Thus, dietary fatty acids can act as
pro-inflammatory factors, promoting low-grade chronic inflammation
and insulin resistance in obesity (13–15).
Complement activation may have a key role in these high-fat
diet-induced metabolic diseases (16). Mice deficient in C1 activation or
treated with C1 or C2 receptor antagonists have reduced
inflammatory cytokine production and are protected from high-fat
diet-induced metabolic dysfunction (17,18).
Bavia et al (19)
demonstrated that C5 contributes to liver steatosis and
inflammation in the pathogenesis of non-alcoholic liver disease.
These data demonstrate the interplay between dietary fatty acids
and the complement system.
In contrast to saturated and n-6 PUFAs, n-3 PUFAs,
which are prevalent in fish oil (FO), are known to have beneficial
anti-inflammatory effects and can attenuate obesity-associated
diseases through various molecular mechanisms (20,21).
Although published studies suggest contradictory roles of dietary
FO in inflammatory disease intervention, the role of FO in the
function and development of immune cells has been confirmed
(22,23). High FO intake can affect
lymphocytes and promote myeloid cell differentiation by modifying
the bone marrow microenvironment (23). However, the precise mechanism of FO
in the complement system remains unknown. In the present study,
mice were fed a high FO diet in order to determine the effect of
high dietary FO on complement activation and inflammation in the
liver. The results indicated that a high FO diet may promote
inflammation and complement activation in the liver.
Materials and methods
Animals
C57BL/6 mice were purchased from the laboratory
animal facility of Yangzhou University (Yangzhou, China) and bred
in a specific-pathogen-free facility. As described in our previous
work (24), 10 6-week-old male
C57BL/6 mice (25 g) were fed a low-fat-diet (LFD; Teklad 2914;
Harlan Teklad Research Diets, Inc., Madison, WI, USA) or a high-FO
diet (59% fat, FO diet, D01112604; Research Diets, Inc., New
Brunswick, NJ, USA) for 4 weeks prior to experimentation (n=5 each
group). The mice were sacrificed using carbon dioxide (air
displacement rate, 15%/min) and tissues were subsequently
collected. Body, liver and spleen weight were recorded. All
protocols were approved by the Scientific Investigation Board of
Jiangsu University (Zhenjiang, China).
Preparation of single-cell suspensions
from mesenteric lymph nodes (MLNs) and spleens
MLNs and spleens were collected, cut into small
pieces and incubated in RPMI-1640 medium (PAA Laboratories; GE
Healthcare Life Sciences, Little Chalfont, UK) containing 10% fetal
bovine serum (FBS; PAA Laboratories; GE Healthcare Life Sciences),
PBS and collagenase IV (5 mg/ml; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with constant agitation for 20
min at 37°C. The tissues were washed in PBS and subsequently
homogenized by pipetting to produce a single-cell suspension. Cells
were passed through a steel mesh filter prior to centrifugation at
51 × g for 5 min at 4°C and re-suspension in PBS. For splenocyte
preparation, red blood cells were destroyed using an
ammonium-chloride-potassium lysis buffer (eBioscience; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocols.
Non-parenchymal cell (NPCs)
purification
Liver NPCs were prepared as previously described,
with minor modifications (25).
Livers collected from high-FO or LFD mice were cut into small
pieces and incubated in RPMI-1640 medium containing 10% FBS, PBS
and collagenase IV (5 mg/ml; Invitrogen; Thermo Fisher Scientific,
Inc.) with constant shaking for 20 min at 37°C. The tissues were
washed in PBS, homogenized by pipetting to produce a single-cell
suspension and were passed through a steel mesh filter prior to
centrifugation at 51 × g for 5 min at 4°C. Cells were resuspended
in 40% Percoll (GE Healthcare, Chicago, IL, USA) and subsequently
added to 70% Percoll. The NPCs were further purified by density
centrifugation at 321 × g for 20 min at 4°C. Cells from the buffy
coat layer between the 70 and 40% Percoll were collected and washed
with PBS.
ELISA
Blood samples were collected from the anesthetized
mice by cardiac puncture. Serum was prepared by spontaneous
coagulation followed by centrifugation at 900 × g for 20 min at
4°C. The serum was stored at −80°C and multiple freeze/thaw cycles
were avoided. Total hemolytic complement activity [50% total
hemolytic complement activity (CH50; cat. no. ml001989)], MAC (cat.
no. ml002054), C3 (cat. no. ml002033) and triglyceride (TG) (cat.
no. ml037783) serum levels were quantified by ELISA (Shanghai
Enzyme-linked Biotechnology Co., Ltd., Shanghai, China; http://www.mlbio.cn/) according to the manufacturer's
protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Liver tissues were harvested, snap-frozen in liquid
nitrogen, and stored at −80°C for further experimentation. The
tissue was homogenized in TRIzol reagent (Thermo Fisher Scientific,
Inc.) using a tissue homogenizer and total RNA was prepared
according to the manufacturer's protocols. cDNA was synthesized
using SuperScript™ III First-Strand Synthesis System
(cat. no. 18080051; Invitrogen; Thermo Fisher Scientific, Inc.).
qPCR was performed using SYBR Premix Ex Taq II™ (cat.
no. RR820; Takara Biotechnology Co., Ltd., Dalian, China) and
Bio-Rad (Bio-Rad Laboratories, Inc., Hercules, CA, USA) equipment
according to the manufacturer' protocols. Generally, qPCR cycling
was performed using the following amplification conditions: 95°C
for 30 sec, followed by 40 cycles of 95°C for 15 sec, 60°C for 20
sec, and 72°C for 30 sec. The relative mRNA levels of the target
genes were normalized to β-actin (26) (Table
I).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Sequence
(5′-3′) |
|---|
| C3 | F:
TGGTGGAGAAAGCAGTGATG |
|
| R:
ACGGGCAGTAGGTTGTTGTC |
| C4b | F:
CAGGACAGAACAGTGGAGCA |
|
| R:
CCCAAAGGAGCCATCATTC |
| C1qb | F:
CAACCTGTGTGTGAATCTCGTT |
|
| R:
GGTGAACAACCTCCTCTTGC |
| Factor B | F:
AAGTGTCAAGAAGGTGGCTCA |
|
| R:
TCAGGGAGGATAGGAATGCTT |
| Factor H | F:
ACCTCGCTGTGTTGGACTTC |
|
| R:
CCACTTTCCTCCTTCGCATA |
| MASP1 | F:
GCAAGGAGAGGGAAGATGAAG |
|
| R:
CCTGTTGTCTGTGTGGAGGA |
| IL-1β | F:
TGTGAAATGCCACCTTTTGA |
|
| R:
TGAGTGATACTGCCTGCCTG |
| MCP-1 | F:
ATGCAGGTCCCTGTCATG |
|
| R:
GCTTGAGGTGGTTGTGGA |
| β-actin | F:
TGGAATCCTGTGGCATCCATGAAAC |
|
| R:
TAAAACGCAGCTCAGTAACAGTCCG |
Flow cytometry
Cell suspensions were incubated with rat serum (cat.
no. 10710C; 1:50; Invitrogen; Thermo Fisher Scientific, Inc.) at
4°C for 10 min to prevent non-specific binding and were
subsequently stained with the following fluorescein-labeled
antibodies (1:200) at 4°C for 20 min: Anti-T-cell surface
glycoprotein CD3 (CD3; cat. no. 11-0032), anti-T-cell surface
glycoprotein CD4 (CD4; cat. no. 12-0043), anti-T-cell surface
glycoprotein CD8 (CD8; cat. no. 15-0081), anti-lymphocyte antigen 6
complex locus G (Gr-1; cat. no. 25-5931), anti-integrin α M (CD11b;
cat. no. 11-0112) and anti-receptor-type tyrosine-protein
phosphatase C (CD45; cat. no. 12-0451) All antibodies were
purchased from eBioscience (Thermo Fisher Scientific, Inc.). Cell
samples were washed with PBS three times prior to assessment by
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). Data were analyzed using CellQuest software (version 6.1, BD
Biosciences) or FlowJo (version 8.7, FlowJo LLC, Ashland, OR,
USA).
Histology
Liver samples were harvested, fixed in 4% buffered
formalin at room temperature overnight, dehydrated and embedded in
paraffin (27). Cross sections (5
µm thick) were stained with hematoxylin and eosin according to the
protocol (cat. no. 60524ES60; Yeasen Co., Ltd., Shanghai, China)
and observed under a light microscope (Axio Observer; Zeiss AG,
Oberkochen, Germany). Images were acquired using AxioVision
software (version 4.8.2, Zeiss AG). All images were captured at
magnification, ×400.
Statistical analysis
The data were statistically analyzed by a two-tailed
Student's t-test using GraphPad (version 7.0, GraphPad Software,
Inc., La Jolla, CA, USA) and are presented as the mean ± standard
error. Experiments were repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
General evaluation of mice fed high-FO
diet
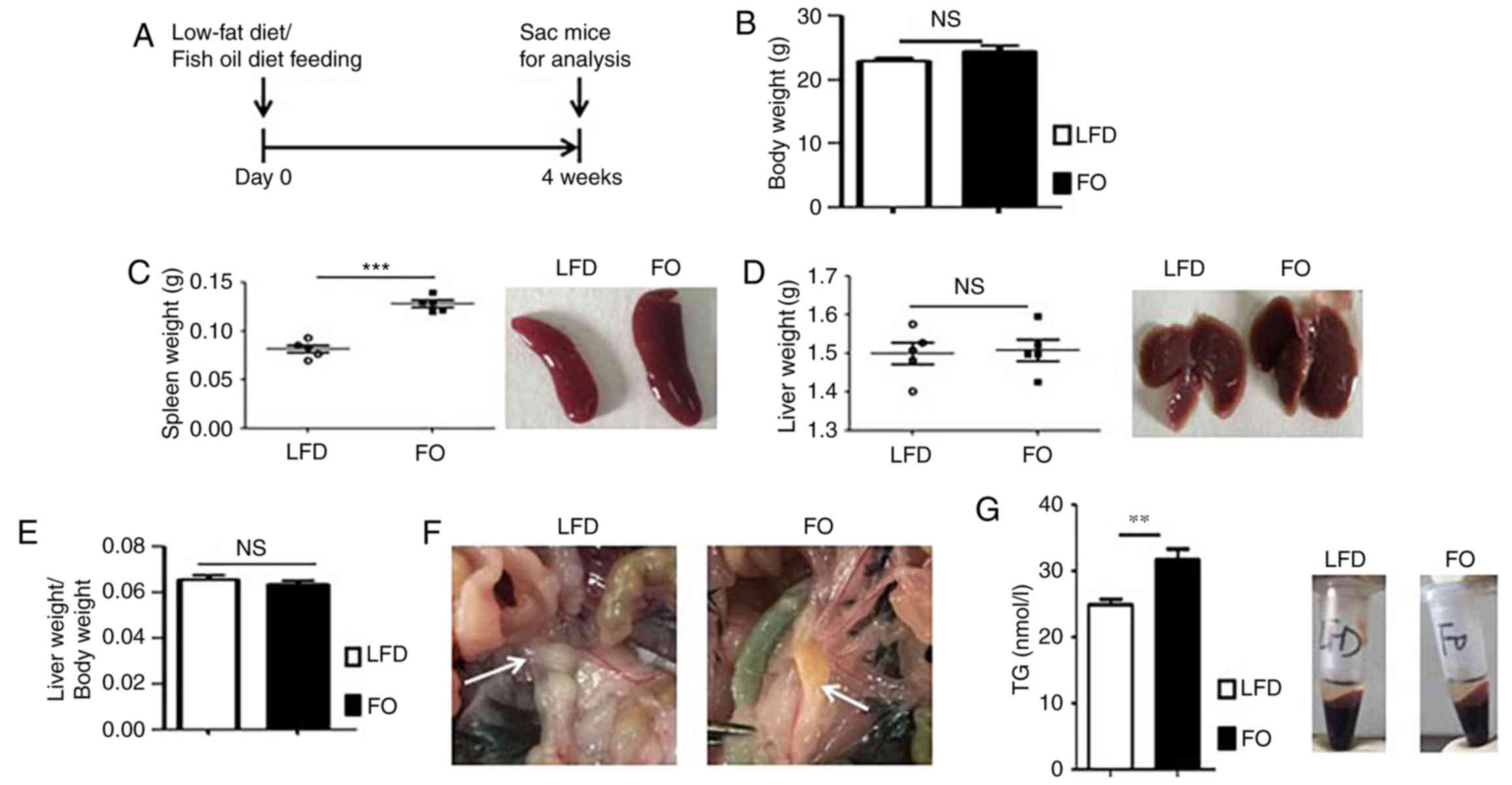
To investigate the effects of a FO on C57BL/6 mice,
male mice were fed a high-FO diet for 4 weeks (Fig. 1A). For each diet group, body, liver
and spleen weight was recorded. As we previously reported (25), the FO diet had no obvious effect on
body weight (Fig. 1B); however,
the spleen weight and size were markedly increased in the FO group
(Fig. 1C). There was no
significant difference in the liver weight (Fig. 1D) or the liver/body weight ratio
(Fig. 1E). Although there was no
difference in liver weight between the LFD-fed and FO-fed mice,
liver congestion was observed in the FO-fed cohort by visual
inspection (Fig. 1D). Similarly,
changes were observed in the secondary lymphoid organs of the gut
(Fig. 1F). Compared to MLNs
retrieved from the LFD cohort, which had a pearly white, smooth and
transparent surface, the MLNs of FO-fed mice were greasier, more
adhesive and light yellow in color (Fig. 1F). TG serum concentration of mice
fed high-FO or LFD diets were determined. As presented in Fig. 1G, the FO-diet significantly
increased the concentration of TG compared with LFD mice. These
results indicated that the high-FO diet may affect lipid metabolism
and impair peripheral immune organ homeostasis, including the
liver, spleen and MLNs.
High-FO diet induces liver
inflammation
As the liver has important roles in immunity and
metabolism, the effects of the FO diet on the liver were evaluated.
NPCs were isolated and various immune cell populations were
analyzed by flow cytometry. The percentage of CD4+ T cells and CD8+
T cells among the total population of CD3+ T cells was determined.
The percentage of CD8+ T cells in CD3+ cells was significantly
decreased in the high-FO diet group compared with the LFD group
(Fig. 2A and B). By contrast, the
percentage of CD4+ T cells in CD3+ cells significantly increased in
the high-FO diet group compared with the LFD group (Fig. 2A and B).
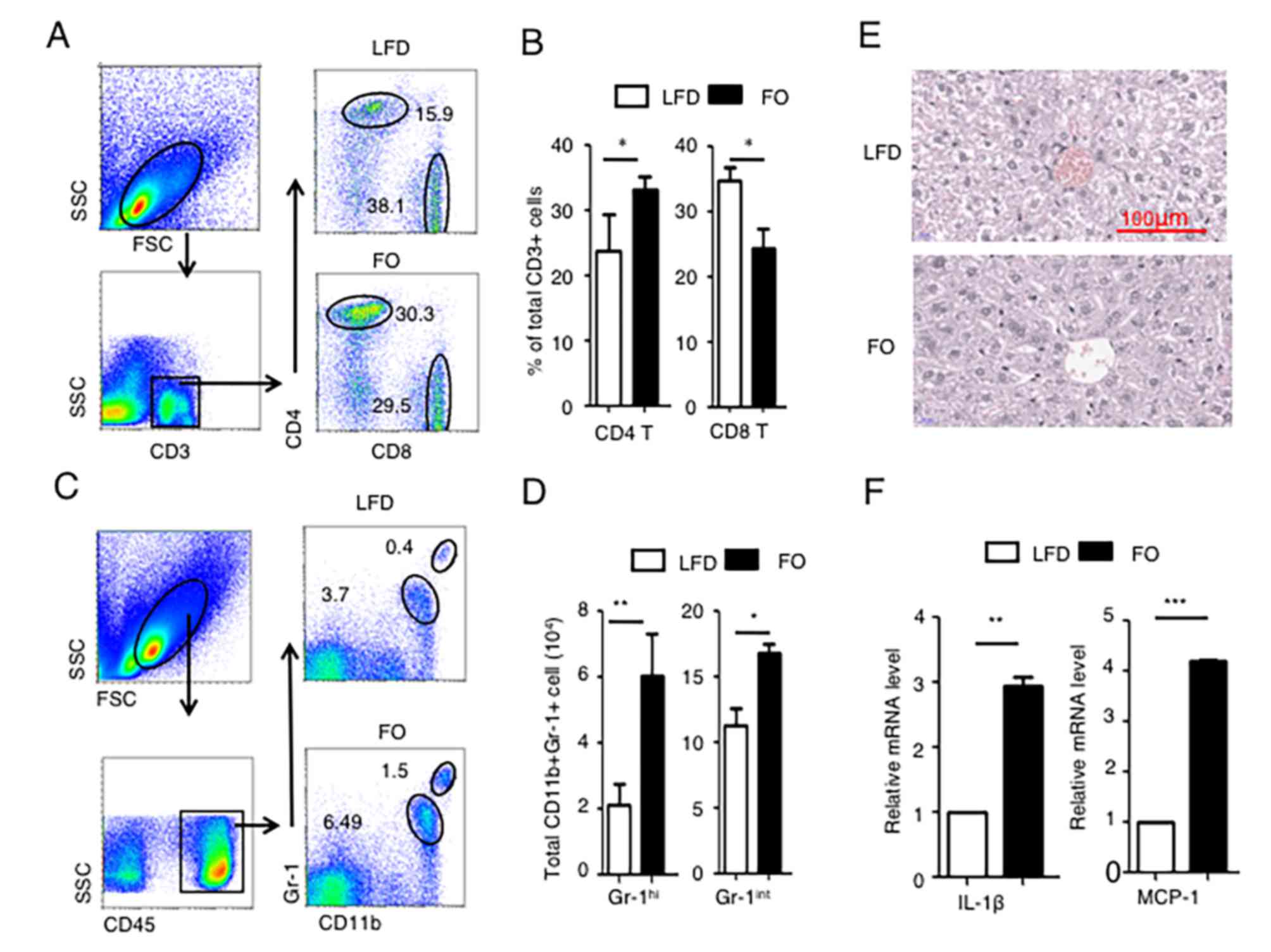 | Figure 2.A high-FO diet induces liver
inflammation. Livers from LFD or high-FO diet mice were collected
for analysis of immune cell populations, the inflammatory cytokine
profile and histology. Nonparenchymal hepatic cells were prepared
from liver tissue and the percentage of (A) number of CD4+ or CD8+
T cells among the CD3+ cells was analyzed by flow cytometry and (B)
the cell percentage calculated. (C) Number of Gr-1+cells among the
CD45+ cells was analyzed using flow cytometry and (D) the cell
percentage was calculated. (E) Representative image of liver
histology following hematoxylin and eosin staining. (F) IL-1β and
MCP-1 mRNA expression in liver tissue was determined by reverse
transcription-quantitative polymerase chain reaction. The data are
representative of three independent experiments and are presented
as the mean ± standard error. n=5 per group. *P<0.05,
**P<0.01, ***P<0.001. SSC, side scatter; FSC, forward
scatter; CD3, T-cell surface glycoprotein CD3; CD4, T-cell surface
glycoprotein CD4; CD8, T-cell surface glycoprotein CD8; LFD, low
fat diet; FO, fish oil; CD45, receptor-type tyrosine-protein
phosphatase C; CD11b, integrin α M; Gr-1, lymphocyte antigen 6
complex locus G; CD11b+ Gr-1hi, Gr-1-high neutrophils;
CD11b+ Gr-1int, Gr-1-moderate monocytes; IL-1β,
interleukin 1β; MCP-1, C-C motif chemokine 2. |
Gr-1+ myeloid cells are comprised of Gr-1-high
neutrophils (CD11b+ Gr-1hi) and Gr-1-moderate monocytes
(CD11b+ Gr-1int) (28).
The results revealed that CD11b+ Gr-1hi and CD11b+
Gr-1int myeloid cell populations were significantly
increased in the livers of the high-FO diet group compared with the
LFD group (Fig. 2C and D),
indicating that more inflammatory immune cells infiltrated the
liver following FO consumption, although no significant difference
was observed by histological examination (Fig. 2E). Pro-inflammatory cytokine
profiles in liver tissues were subsequently assessed. IL-1β and C-C
motif chemokine 2 (MCP-1) mRNA expression in the liver tissues of
the high-FO diet mice was significantly higher than those in the
LFD group (Fig. 2F). Collectively,
these data demonstrated that the FO diet induced liver
inflammation.
High-FO diet feeding promotes
complement system activation
As the liver is one of the major organs involved in
complement protein production, and complement activation is tightly
associated with inflammation and immunity (29), the effects of the high-FO diet on
the complement system were examined. CH50 and levels of MAC in the
serum were determined. Significantly higher CH50 (Fig. 3A) and MAC (Fig. 3B) production was detected in the
serum of high-FO diet mice. C3 is the most prevalent protein in the
complement system and is involved in all complement activation
pathways (30). ELISA and RT-qPCR
analyses demonstrated that the levels of C3 protein in the serum
(Fig. 3C) and mRNA in the liver
tissues (Fig. 3D) were
significantly increased in the high-FO diet group, compared with
the LFD group. Additionally, the mRNA expression of C4b and C 1qb
(Fig. 3E), two other important
activation components in the classical complement activation
pathway, were significantly increased in the liver of the high-FO
diet group, compared with the LFD group. The effects of the high-FO
diet on other pathways of the complement system were also
investigated. Significantly increased mRNA expression of complement
factor B, one component of the alternative pathway, was detected in
the liver (Fig. 3F). Additionally,
complement factor H, an inhibitory complement regulatory protein in
the alternative pathway, was significantly reduced in the high-FO
diet group compared with the LFD group (Fig. 3F). There was no significant
difference in the mRNA level of mannan-binding lectin serine
peptidase 1 (MASP1), a specific component of the lectin pathway
(Fig. 3G). Taken together, these
findings indicate that a high-FO diet may promote complement
activation.
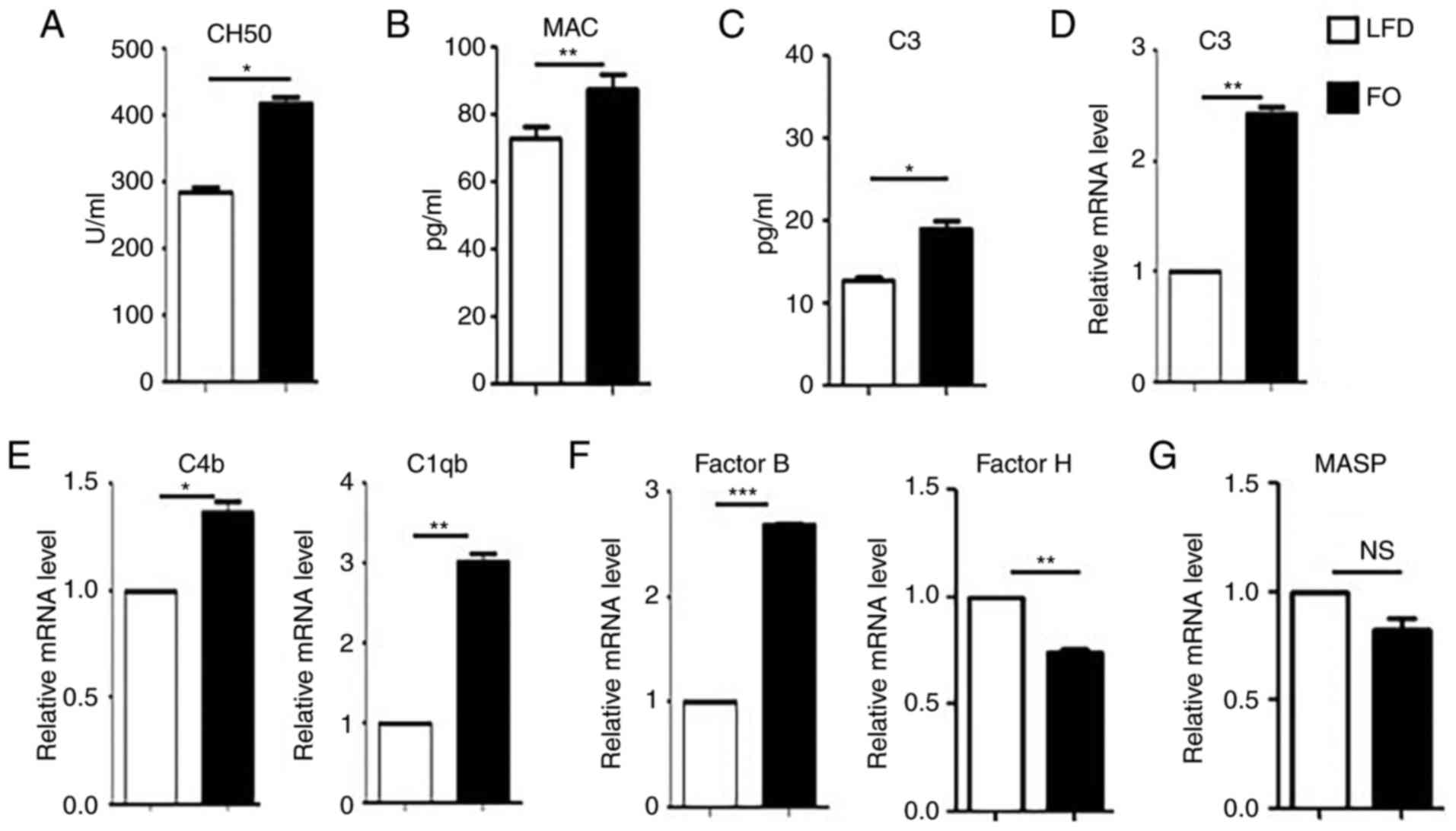 | Figure 3.A high-FO diet promotes complement
system activation. Serum and liver tissues were collected from LFD
and high-FO diet mice. (A) CH50 and (B) the levels of MAC in the
serum samples were detected using ELISA. (C) The protein levels of
C3 in the serum and (D) mRNA levels of C3 in the liver tissue were
analyzed by ELISA and RT-qPCR, respectively. The mRNA levels in the
liver of (E) complement proteins C4b and C1qb in the classical
pathway, (F) factor B and factor H in the alternative pathway and
(G) MASP1 in the lectin pathway were determined using RT-qPCR. The
relative mRNA levels of different target genes were normalized to
β-actin. The data are representative of three independent
experiments and are presented as the mean ± standard error. n=5 per
group. *P<0.05, **P<0.01, ***P<0.001 vs. LFD group.
RT-qPCR; reverse transcription-quantitative polymerase chain
reaction; CH50, 50% total hemolytic complement activity; MAC,
membrane attack complex; C3, complement C3; FO, fish oil; LFD, low
fat diet; C4b, complement component C4b; C1qb, complement component
1 q subcomponent β polypeptide; MASP1, mannan-binding lectin serine
peptidase 1; NS, no significance. |
Discussion
Our previous study demonstrated that a high-FO diet
promoted myeloid-derived suppressor cell differentiation,
suppressed T-cell-mediated adaptive immunity and promoted tumor
growth through multiple mechanisms, including the alteration of
hematopoiesis and the bone marrow microenvironment (24). However, the effects of a high-FO
diet on the complement system, a key effector and regulator of
innate and adaptive immunity, are not clear. The present study
provided evidence of complement activation and inflammation in the
liver tissue of mice fed a high-FO diet. After 4 weeks on a high-FO
diet, increased levels of CH50 and MAC were detected in the serum,
indicating that the FO diet increased complement activation in
vivo.
The liver is a unique organ that has important roles
in both the digestive and immune systems. In our previous study, it
was demonstrated that a saturated fatty acid and n-6 PUFA-rich
high-fat diet induced low-degree inflammation in the liver
(24). Nevertheless, in the
current study, an n-3 PUFA-rich FO diet promoted the infiltration
of CD11b+ Gr-1+ inflammatory myeloid cells into the liver,
suggesting that the induction of liver inflammation is mediated
specifically by n-3 PUFA-rich feeding, rather than by fatty acids
and n-6 PUFAs. We previously reported that FO feeding modified bone
marrow hematopoiesis and induced splenomegaly in mice (25). In the present study, a high-FO diet
not only increased the weight of the spleen, but also increased the
number of Gr-1+ myeloid cells in the liver. Considering the similar
roles of the spleen and liver in extramedullary hematopoiesis, the
increased myeloid cell infiltration into the liver was likely
closely associated with the effects of FO on bone marrow
hematopoiesis. Higher levels of MCP-1 in the liver may have also
contributed.
The liver contains specialized resident immune cells
and is also the predominant site of complement protein
biosynthesis. Although previous studies have demonstrated that most
mammalian cell types are capable of producing some or all of the
complement proteins (31), these
extrahepatic complement proteins are synthesized in a
tissue-specific manner and have biological functions predominantly
within the local environment (32). By contrast, systemic complement
proteins are synthesized in the liver by hepatocytes (33,34).
A previous study demonstrated that complement proteins C1q, C7 and
factor D are predominantly synthesized in extrahepatic sites
(31). However, the liver is the
main source of the major complement protein C3, although
monocyte/macrophage lineage cells also contribute to extrahepatic
C3 production. In the present study, increased protein and mRNA
levels of C3 were detected in the serum and liver tissue,
respectively. Considering the source and the role of C3 in the
complement system, these data suggest that the high-FO diet induced
increased liver cell synthesis of C3, which has the potential to
upregulate the biological effects of the complement system.
The function of the complement system depends on the
activation of an array of complement components, which subsequently
organize a hierarchy of proteolytic cascades. Based on their
differing initiation factors and complement components, three
different complement activation pathways have been identified; the
classical, alternative and lectin pathways (35). All pathways use the assembled MAC
to create a pore that mediates cytotoxicity in the target cell. In
the present study, increased levels of MAC were detected in the
serum of the high-FO diet mice, indicating that complement
activation had increased. In the classical pathway, following
initiation by immune complexes, C1q forms a complex with
subcomponents C1r and C1s to form serine proteases, which cleave C4
into large C4b and small C4a fragments. In the present study, the
mRNA levels of C1qb and C4b were increased in the high-FO diet
mice, suggesting that the high-FO diet promoted activation of the
classical complement pathway. This finding was further confirmed by
the CH50 analysis, in which increased CH50 was detected in the
high-FO group, compared with the LFD group. The high-FO
diet-induced activation of the classical complement pathway may be
initiated by C1q recognition of modified lipids in vivo
(36). Factor B interacts with C3
to activate the alternative complement pathway, which is negatively
regulated by factor H. The present study demonstrated that factor B
mRNA expression was increased, and the mRNA expression of negative
regulatory protein factor H was reduced in the liver of high-FO
diet mice, indicating that the alternative pathway was activated.
The association between the complement system, metabolism and
inflammasome activation has been extensively research.
Anaphylatoxins C3a and C5a, products of complement activation, have
been confirmed as important drivers of NLRP3 activation, through
induction of IL-1β gene transcription in monocytes (37–39).
Consistent with these findings, high mRNA levels of IL-1β were
detected in the liver of FO-fed mice in the present study,
indicating that IL-1β may be involved in high-FO diet-mediated
complement activation. The data of the present study is consistent
with the findings of previous studies (18,40)
that demonstrated that complement activation is closely associated
with lipid metabolism in the liver and contributes to liver
homeostasis and disease.
TG is the storage form of excess free fatty acids.
Generally, adipose tissues have the capacity to store excess free
fatty acids as TGs in lipid droplets; however, excess lipid
accumulation in non-adipose tissues can cause cell death or
dysfunction, which is termed lipotoxicity. It has been demonstrated
that unsaturated fatty acids have protective roles against
lipotoxicity in hyperlipidemic states through promoting TG
accumulation (41). Compared with
the LFD group, the high-FO diet group in the present study was fed
a variety of n-3 PUFAs, including eicosapentaenoic acid,
docosapentaenoic acid and docosahexaenoic acid. Unfortunately, this
FO diet also contained more saturated fatty acids and n-6 PUFAs
than the LFD, including palmitic acid (PA) and myristic acid (MA).
The concentration of PA and MA in the high-FO diet was 24.3 and
51.9 g/kg, respectively, but these saturated fatty acids were at
very low concentrations in the LFD (25). Thus, the high TG level in the serum
following FO feeding largely depended on the higher intake of
saturated fatty acids. Similarly, previous work demonstrated that
excess saturated fatty acids and n-6 PUFAs in the diet induced
complement activation and contributes to obesity-associated
metabolic diseases (16). Thus,
although the exact mechanism of the effects of a high-FO diet in
the activation of complement and liver inflammation was not been
clearly demonstrated in this study, saturated fatty acids and n-6
PUFAs in a high FO diet may partly contribute. In addition, the
exact role and potential mechanisms of n-3 PUFAs in the activation
of complement and liver inflammation should be further explored
using purified DHA and PEA. As fish oil is currently been used in
the prevention and therapy of cardiovascular diseases, the side
effects of the high-FO diet in complement activation need to be
avoided in this treatment.
Acknowledgements
The authors are very thankful to all members of the
Zhou laboratory and the Shao laboratory for helpful discussion.
Funding
The present study was funded by grants from the
National Natural Science Foundation of China (grant nos. 31570879,
31428006 and 81172834) and the Jiangsu Provincial Key Research and
Development Program (grant no. BE2017696).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Author's contributions
SX designed experiment, interpreted the data and
drafted the manuscript. HJ performed the majority of the
experiments and was a major contributor in writing the manuscript.
CY, TX and JX performed animal models preparation and flow
cytometer analysis. LZ and NY performed ELISA. XZ and QS analyzed
and interpreted the data and revised the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Scientific
Investigation Board of Jiangsu University (Zhenjiang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Austyn JM: Death, destruction, danger and
dendritic cells. Nat Med. 5:1232–1233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwasaki A and Medzhitov R: Regulation of
adaptive immunity by the innate immune system. Science.
327:291–295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cruvinel Wde M, Mesquita D Jr, Araújo JA,
Catelan TT, de Souza AW, da Silva NP and Andrade LE: Immune
system-part I. Fundamentals of innate immunity with emphasis on
molecular and cellular mechanisms of inflammatory response. Rev
Bras Reumatol. 50:434–461. 2010.(In English, Portuguese).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walport MJ: Complement. First of two
parts. N Engl J Med. 344:1058–1066. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arbore G, West EE, Spolski R, Robertson
AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O'Neill
LA, et al: T helper 1 immunity requires complement-driven NLRP3
inflammasome activity in CD4+ T cells. Science.
352:aad12102016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cravedi P, van der Touw W and Heeger PS:
Complement regulation of T-cell alloimmunity. Semin Nephrol.
33:565–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin M and Blom AM: Complement in
removal of the dead-balancing inflammation. Immunol Rev.
274:218–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricklin D, Hajishengallis G, Yang K and
Lambris JD: Complement: A key system for immune surveillance and
homeostasis. Nat Immunol. 11:785–797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suresh R, Chandrasekaran P, Sutterwala FS
and Mosser DM: Complement-mediated ‘bystander’ damage initiates
host NLRP3 inflammasome activation. J Cell Sci. 129:1928–1939.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu HQ, Qiu Y, Mu Y, Zhang XJ, Liu L, Hou
XH, Zhang L, Xu XN, Ji AL, Cao R, et al: A high ratio of dietary
n-3/n-6 polyunsaturated fatty acids improves obesity-linked
inflammation and insulin resistance through suppressing activation
of TLR4 in SD rats. Nutr Res. 33:849–858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Serini S, Piccioni E, Merendino N and
Calviello G: Dietary polyunsaturated fatty acids as inducers of
apoptosis: Implications for cancer. Apoptosis. 14:135–152. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shoelson SE, Lee J and Goldfine AB:
Inflammation and insulin resistance. J Clin Invest. 116:1793–1801.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalupahana NS, Claycombe KJ and
Moustaid-Moussa N: (n-3) Fatty acids alleviate adipose tissue
inflammation and insulin resistance: Mechanistic insights. Adv
Nutr. 2:304–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooke AA, Connaughton RM, Lyons CL,
McMorrow AM and Roche HM: Fatty acids and chronic low grade
inflammation associated with obesity and the metabolic syndrome.
Eur J Pharmacol. 785:207–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vlaicu SI, Tatomir A, Boodhoo D, Vesa S,
Mircea PA and Rus H: The role of complement system in adipose
tissue-related inflammation. Immunol Res. 64:653–664. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim J, Iyer A, Suen JY, Seow V, Reid RC,
Brown L and Fairlie DP: C5aR and C3aR antagonists each inhibit
diet-induced obesity, metabolic dysfunction and adipocyte and
macrophage signaling. FASEB J. 27:822–831. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hillian AD, McMullen MR, Sebastian BM,
Roychowdhury S, Kashyap SR, Schauer PR, Kirwan JP, Feldstein AE and
Nagy LE: Mice lacking C1q are protected from high fat diet-induced
hepatic insulin resistance and impaired glucose homeostasis. J Biol
Chem. 288:22565–22575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bavia L, Cogliati B, Dettoni JB, Ferreira
Alves VA and Isaac L: The complement component C5 promotes liver
steatosis and inflammation in murine non-alcoholic liver disease
model. Immunol Lett. 177:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alvarez-Curto E and Milligan G: Metabolism
meets immunity: The role of free fatty acid receptors in the immune
system. Biochem Pharmacol. 114:3–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viggiano E, Mollica MP, Lionetti L,
Cavaliere G, Trinchese G, De Filippo C, Chieffi S, Gaita M,
Barletta A, De Luca B, et al: Effects of an high-fat diet enriched
in lard or in fish oil on the hypothalamic amp-activated protein
kinase and inflammatory mediators. Front Cell Neurosci. 10:1502016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poreba M, Mostowik M, Siniarski A,
Golebiowska-Wiatrak R, Malinowski KP, Haberka M, Konduracka E,
Nessler J, Undas A and Gajos G: Treatment with high-dose n-3 PUFAs
has no effect on platelet function, coagulation, metabolic status
or inflammation in patients with atherosclerosis and type 2
diabetes. Cardiovasc Diabetol. 16:502017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kremer JM: Fish oil and inflammation-A
fresh look. J Rheumatol. 44:713–716. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia S, Li X, Cheng L, Han M, Zhang M, Liu
X, Xu H, Zhang M, Shao Q and Qi L: Chronic intake of high fish oil
diet induces myeloid-derived suppressor cells to promote tumor
growth. Cancer Immunol Immunother. 63:663–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia S, Li XP, Cheng L, Han MT, Zhang MM,
Shao QX, Xu HX and Qi L: Fish oil-rich diet promotes hematopoiesis
and alters hematopoietic niche. Endocrinology. 156:2821–2830. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xia S, Guo Z, Xu X, Yi H, Wang Q and Cao
X: Hepatic microenvironment programs hematopoietic progenitor
differentiation into regulatory dendritic cells, maintaining liver
tolerance. Blood. 112:3175–3185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia S, Sha H, Yang L, Ji Y,
Ostrand-Rosenberg S and Qi L: Gr-1+ CD11b+ myeloid-derived
suppressor cells suppress inflammation and promote insulin
sensitivity in obesity. J Biol Chem. 286:23591–23599. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao B, Jeong WI and Tian Z: Liver: An
organ with predominant innate immunity. Hepatology. 47:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ricklin D, Reis ES, Mastellos DC, Gros P
and Lambris JD: Complement component C3-The ‘Swiss Army Knife’ of
innate immunity and host defense. Immunol Rev. 274:33–58. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morgan BP and Gasque P: Extrahepatic
complement biosynthesis: Where, when and why? Clin Exp Immunol.
107:1–7. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marsh JE, Zhou W and Sacks SH: Local
tissue complement synthesis-fine tuning a blunt instrument. Arch
Immunol Ther Exp (Warsz). 49 Suppl 1:S41–S46. 2001.PubMed/NCBI
|
|
33
|
Alper CA, Johnson AM, Birtch AG and Moore
FD: Human C′3: Evidence for the liver as the primary site of
synthesis. Science. 163:286–288. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morris KM, Aden DP, Knowles BB and Colten
HR: Complement biosynthesis by the human hepatoma-derived cell line
HepG2. J Clin Invest. 70:906–913. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walport MJ: Complement. Second of two
parts. N Engl J Med. 344:1140–1144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arlaud GJ, Biro A and Ling WL:
Enzymatically modified low-density lipoprotein is recognized by c1q
and activates the classical complement pathway. J Lipids.
2011:3760922011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arbore G and Kemper C: A novel
‘complement-metabolism-inflammasome axis’ as a key regulator of
immune cell effector function. Eur J Immunol. 46:1563–1573. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Samstad EO, Niyonzima N, Nymo S, Aune MH,
Ryan L, Bakke SS, Lappegård KT, Brekke OL, Lambris JD, Damås JK, et
al: Cholesterol crystals induce complement-dependent inflammasome
activation and cytokine release. J Immunol. 192:2837–2845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haeffner-Cavaillon N, Cavaillon JM, Laude
M and Kazatchkine MD: C3a(C3adesArg) induces production and release
of interleukin 1 by cultured human monocytes. J Immunol.
139:794–799. 1987.PubMed/NCBI
|
|
40
|
Bavia L, de Castro IA, Cogliati B, Dettoni
JB, Alves VA and Isaac L: Complement C5 controls liver lipid
profile, promotes liver homeostasis and inflammation in C57BL/6
genetic background. Immunobiology. 221:822–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Listenberger LL, Han X, Lewis SE, Cases S,
Farese RV Jr, Ory DS and Schaffer JE: Triglyceride accumulation
protects against fatty acid-induced lipotoxicity. Proc Natl Acad
Sci USA. 100:pp. 3077–3082. 2003; View Article : Google Scholar : PubMed/NCBI
|