Introduction
Breast cancer is the most commonly diagnosed cancer
and principal cause of death among females worldwide (1). Despite enormous efforts towards
characterizing the underlying molecular mechanisms of breast
cancer, the potential mechanisms that regulate breast cancer
progression remain largely elusive and poorly defined. Clinically,
although advances have been made in the treatment of breast cancer
over the last decade, including the development of personalized
treatment based on four molecular types of breast cancer (luminal
A, luminal B, HER2-positive and triple-negative breast cancer), the
effective control of recurrence and metastasis remains the biggest
obstacle. Therefore, a better understanding of tumorigenesis and
the identification of novel therapeutic targets are urgently needed
for the treatment of patients with breast cancer.
Long non-coding RNAs (lncRNAs) are a class of RNA
transcripts over 200 nucleotides in length that lack protein-coding
capacity (2,3). Over the last few decades,
accumulating evidence has suggested the importance of lncRNAs in
the regulation of biological processes, such as cell
differentiation, development, and apoptosis (4). An increasing number of lncRNAs have
been shown to participate in many human diseases, including
malignancy (5,6). Aberrant lncRNA expression has been
reported in different types of cancers, exhibiting both oncogenic
and cancer-suppressive roles (7–9).
These findings suggest that the dysregulation of lncRNAs may be an
important cause of tumorigenesis and accelerate tumor
progression.
ZFAS1 (zinc finger antisense 1) is a newly
identified lncRNA that regulates alveolar development and
epithelial cell differentiation in the mouse mammary gland
(10). Recently, it was reported
that ZFAS1 is aberrantly expressed in cancer and is involved in the
progression of different types of tumors. It is upregulated in
gastric, colonic, glioma, and ovarian cancer and function as a
potential oncogene by promoting cell proliferation and metastasis
(11–14). In contrast, Askarian-Amiri et
al (10) found that ZFAS1 is
downregulated in invasive ductal breast carcinoma compared to
levels in normal breast tissue, a finding which leads the authors
to speculate that ZFAS1 serves as a tumor suppressor. However, the
biological role of ZFAS1 in breast cancer and its underlying
molecular mechanism are unclear and therefore served as the focus
of the present study.
In this study, we found that ZFAS1 expression was
downregulated in different human breast cancer cell lines, and
additional experiments further demonstrated that overexpression of
ZFAS1 inhibited breast cancer cell proliferation, migration, and
invasion in vitro. Mechanistically, we verified that ZFAS1
inhibited breast cancer cell migration and invasion by regulating
the epithelial-mesenchymal transition (EMT) process. Therefore, our
results suggest that ZFAS1 acts as a tumor suppressor in breast
cancer and may be an effective therapeutic target for patients with
breast cancer.
Materials and methods
Cell culture
Four human breast cancer cell lines (MDA-MB-231,
MCF-7, T-47D, and SK-BR-3) and a normal breast epithelial cell line
(MCF-10A) were obtained from the American Type Culture Collection
(ATCC; Rockefeller, MD, USA). Cells were cultured in RPMI-1640,
Dulbecco's modified Eagle's medium (DMEM), or DMEM/F12 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), containing
10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences),
and were incubated at 37°C in an incubator containing 5%
CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions. RNA
concentration and purity were determined using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA (500
ng) was reverse transcribed into cDNA using PrimeScript RT Master
Mix (Takara Biotechnology Co., Ltd., Dalian, China) following the
manufacturer's protocol. RT-qPCR was performed on an Eppendorf
Real-time PCR System (Eppendorf, Hamburg, Germany) using the SYBR
PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.) with
specific primers. GAPDH was used as an internal control. The
primers used for RT-qPCR were as follows: ZFAS1, forward:
5′-GAGGTTCAGGAAGCCATTCGTTCT-3′ and reverse:
5′-CCAGTGGTGACTCCCTCTTCCAAA-3′; GAPDH, forward:
5′-GAAGGCTGGGGCTCATTTGCAGGG-3′ and reverse:
5′-GGTGCAGGAGGCATTGCTGATGAT-3′. PCR reaction mixtures (25 µl) were
prepared, including 12.5 µl SYBR-Green permix, 2 µl cDNA, 0.75 µl
of each primer and 9 µl double-distilled water to a final volume of
25 µl. The thermocycling conditions were as follows: 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec,
annealing at 60°C for 30 sec, and extension at 72°C for 30 sec.
Relative expression fold-changes were calculated using the
2−∆∆Cq method. Each experiment was performed in
triplicate.
Plasmid construction and cell
transfection
Full-length human ZFAS1 was synthesized and
subcloned into a pcDNA3.1 vector (Invitrogen; Thermo Fisher
Scientific, Inc.), creating pcDNA-ZFAS1 for ZFAS1 overexpression
analysis. An empty pcDNA3.1 vector was used as a control. To
establish cell lines with stable overexpression of ZFAS1, cells
were grown on 6-well plates to 60–70% confluence and transfected
with 5 µg plasmid using Lipofectamine 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were incubated at 37°C in a CO2 incubator for 48 h prior
to evaluation for ZFAS1 expression and harvesting for further
analysis. The medium was replaced after 6 h post-transfection.
Cell proliferation assay
Cell proliferation was measured using the Cell
Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer's protocol. Cells
were seeded in 96-well plates (5×103 cells/well) and
grown in 100 µl culture medium. Cell viability was examined every
24 h for a total of five days. At each time point, 10 µl CCK-8
reagents was added to each well, followed by incubation for 2 h at
37°C. The absorbance of each well was then measured on a
spectrophotometer (BioTek, Winooski, VT, USA) at 450 nm. The assay
was performed in three replicate wells, and three parallel
experiments were performed for each sample.
Colony formation assay
Transfected cells were harvested after
trypsinization and seeded in 6-well plates (8×102
cells/well). The culture medium was refreshed every three days.
Once colony formation was visible, the cells were rinsed twice with
phosphate-buffered saline (PBS) followed by fixation with 4%
paraformaldehyde and staining with Giemsa for 20 min. The number of
colonies was counted under a light microscope (Nikon, Tokyo,
Japan), and each colony contained at least 50 cells.
5-Ethynyl-2-deoxyuridine (EdU)
assay
To intuitively observe cell proliferation, an EdU
incorporation assay was performed using an EdU Apollo in
vitro Imaging kit (Ribobio, Guangzhou, China) according to the
manufacturer's instructions. The transfected cells were cultured in
96-well plates at a density of 6×103 cells per well and
grown to 60–80% confluence. Next, EdU labeling medium (50 µΜ) was
added to each well, followed by incubation for 2 h at 37°C.
Subsequently, cells were stained with anti-EdU working solution and
Hoechst 33342 was used to label cell nuclei. EdU-positive cells
were visualized by a fluorescent microscope (Olympus, Tokyo, Japan)
and the percentage of EdU-positive cells was calculated.
Flow cytometry analysis
For the cell cycle assay, cells were harvested and
washed three times with ice cold PBS. Subsequently, cells were
fixed in 70% ethanol at 4°C for 24 h and then stained with
propidium iodide (PI) using the Cell Cycle Analysis kit (Beyotime
Institute of Biotechnology). The percentage of cells in each phase
of the cell cycle was calculated and compared using Modfit LT
software 3.1 (Verity Software House, Groton, MA, USA). To measure
the cell apoptosis rate, transfected cells were harvested and
washed using PBS. An Annexin V-FITC Apoptosis Detection kit
(Beyotime Institute of Biotechnology) was used to detect cell
apoptosis. According to the manufacturer's instructions, cells were
stained with fluorescein isothiocyanate (FITC)-Annexin V and PI,
and were incubated in the dark for 20 min at room temperature. Cell
apoptosis was analyzed using a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Cell invasion and migration assay
Migration and invasion assays were performed using a
24-well Transwell plate (8 µm pore size; Corning Incorporated,
Corning, NY, USA). For migration assays, the upper chamber of the
Transwell inserts was filled with 5×104 cells in 200 µl
serum-free DMEM. For invasion assays, cells (5×104) were
seeded in the upper chamber coated with Matrigel (Corning
Incorporated). Inserts were added to the bottom chamber wells
containing 600 µl of DMEM with 30% FBS. Next, the Transwell
chambers were incubated for 24 h at 37°C; then, the upper surface
of the chamber was wiped with cotton swabs, and the cells on the
filter surface were fixed with 4% paraformaldehyde and stained with
Giemsa. The number of migratory and invasive cells was counted and
photographed using a digital microscope (Nikon).
Western blotting
Cells were harvested after 48 h post-transfection
and lysed with RIPA buffer (Beyotime Institute of Biotechnology)
for protein extraction. Total protein concentration was quantified
by the BCA Protein Assay kit (Beyotime Institute of Biotechnology).
Equal amounts of protein (20 µg) for each sample were loaded and
separated by 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) gels and then transferred to
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% skim milk in Tris-buffered saline and Tween-20 at
room temperature for 1 h. The following primary antibodies were
added and the membranes were incubated with gentle shaking at 4°C
overnight: anti-E-cadherin (dilution, 1:200; cat. no. sc-21791;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-vimentin
(dilution, 1:200; cat. no. sc-6260; Santa Cruz Biotechnology,
Inc.), and anti-β-actin (dilution, 1:1,000; cat. no. 4970; Cell
Signaling Technology, Inc., Danvers, MA, USA). Then, the membranes
were incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (dilution, 1:200; cat. no. sc-2380; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. Finally, protein
bands were visualized using an enhanced chemiluminescence (ECL)
detection system (Thermo Fisher Scientific, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
21.0 (IBM Corp., Armonk, NY, USA). Data from the different groups
were compared and analyzed using two-way analysis of variance and
Bonferroni's post hoc test. Data are presented as the mean ±
standard deviation. Two-sided P-values were calculated and
P<0.05 was considered to indicate a statistically significant
difference.
Results
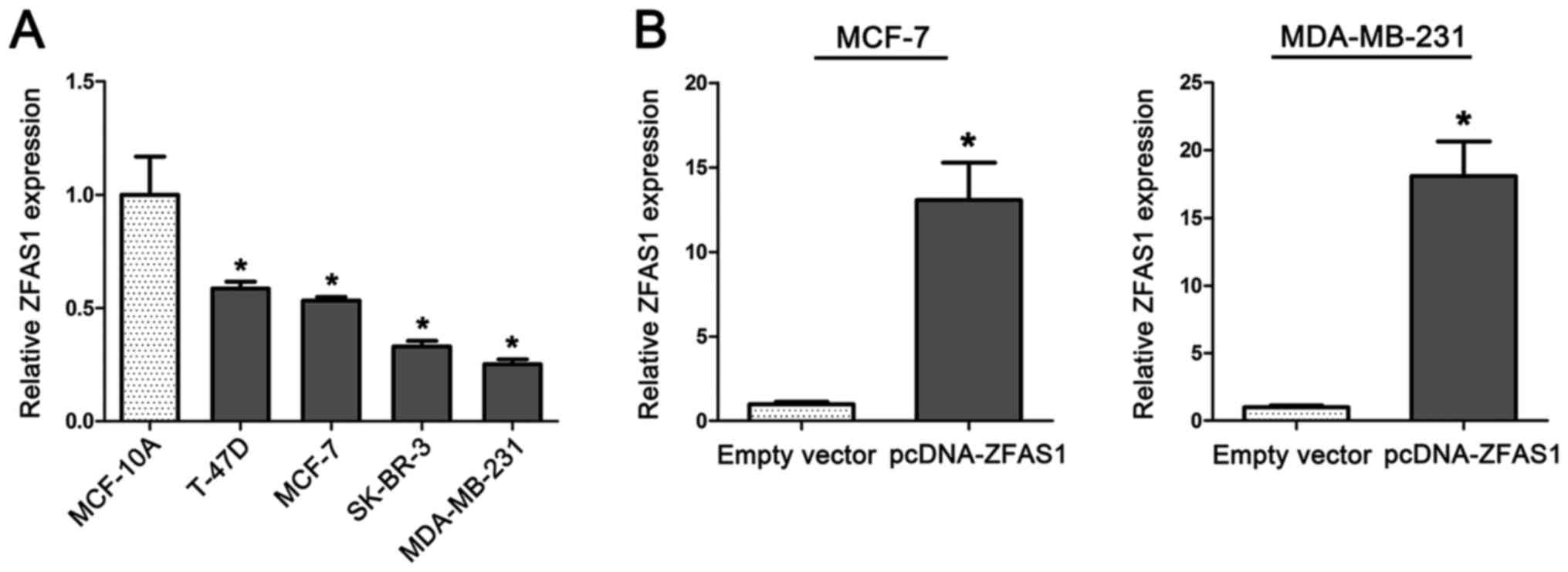
ZFAS1 is downregulated in breast
cancer cell lines
To investigate the biological function of ZFAS1 in
breast cancer tumorigenesis, we determined ZFAS1 expression levels
in four breast cancer cell lines (MCF-7, T-47D, MDA-MB-231, and
SK-BR-3) and a normal breast epithelial cell line (MCF-10A). We
found that expression of ZFAS1 was significantly downregulated in
cells from breast cancer cell lines compared to levels in cells
from the normal breast epithelial cell line MCF-10A (Fig. 1A).
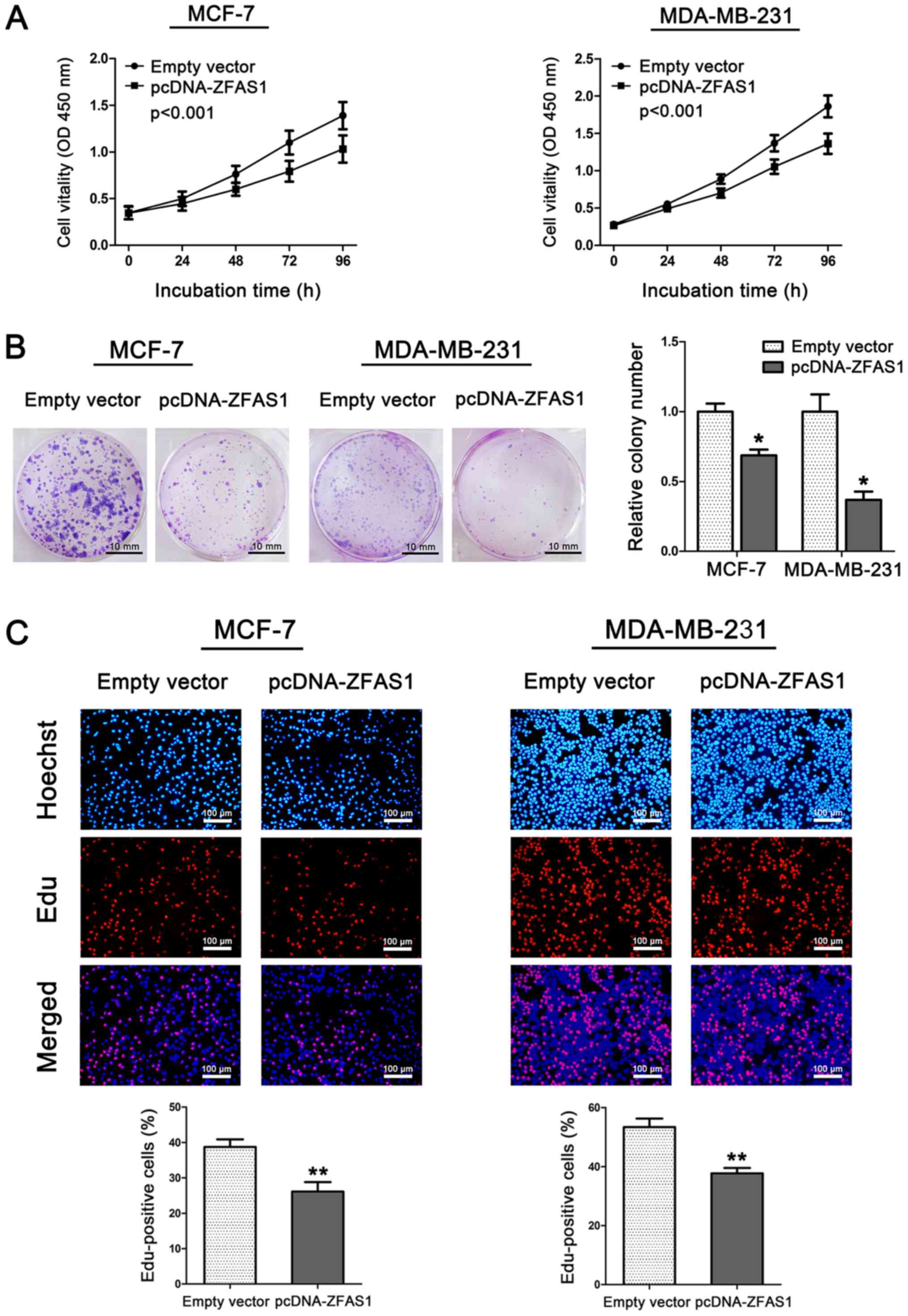
Overexpression of ZFAS1 inhibits
breast cancer cell proliferation by causing cell cycle arrest
Furthermore, we performed CCK-8, colony formation,
cell cycle, and EdU assays to evaluate the effect of ZFAS1 on cell
proliferation in two breast cancer cell lines, MCF-7 and
MDA-MB-231. Cells from the MCF-7 and MDA-MB-231 cell lines were
transfected with pCDNA-ZFAS1 to stably overexpress ZFAS1. ZFAS1
overexpression in these two cell lines was confirmed by RT-qPCR
(Fig. 1B). As shown in Fig. 2A, ZFAS1 overexpression
significantly inhibited cell viability both in MCF-7 and MDA-MB-231
cells compared to that found in control cells. In addition, ZFAS1
overexpression in MCF-7 and MDA-MB-231 cells resulted in
significantly decreased clonogenic survival (Fig. 2B). This finding was also confirmed
by EdU/Hoechst immunostaining. As shown in Fig. 2C, the percentage of EdU-positive
cells was significantly decreased after ZFAS1 overexpression.
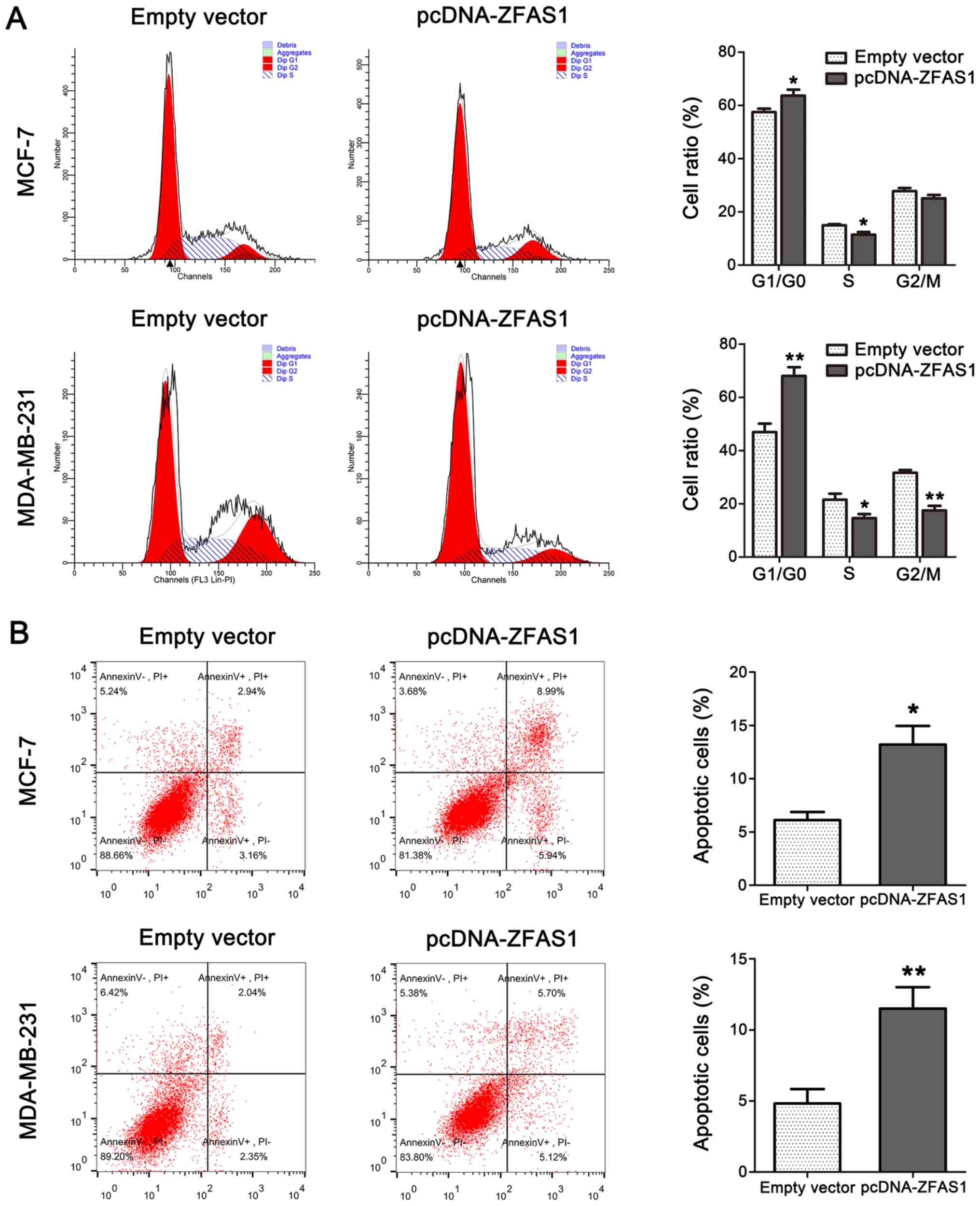
Overexpression of ZFAS1 promotes cell
cycle arrest and induces apoptosis in breast cancer cells
To determine the potential role of ZFAS1 in breast
cancer cell proliferation, we used flow cytometry to examine the
function of ZFAS1 in breast cancer cell cycle progression and
apoptosis. As shown in Fig. 3A,
cell cycle progression in ZFAS1-overexpressing cells was
significantly stalled at the G1/G0 phase compared to that in
control cells. Furthermore, cell apoptosis analysis by flow
cytometry demonstrated that ZFAS1 overexpression markedly increased
the percentage of apoptotic cells in both the MCF-7 and MDA-MB-231
cell lines compared to the percentage in control groups (Fig. 3B).
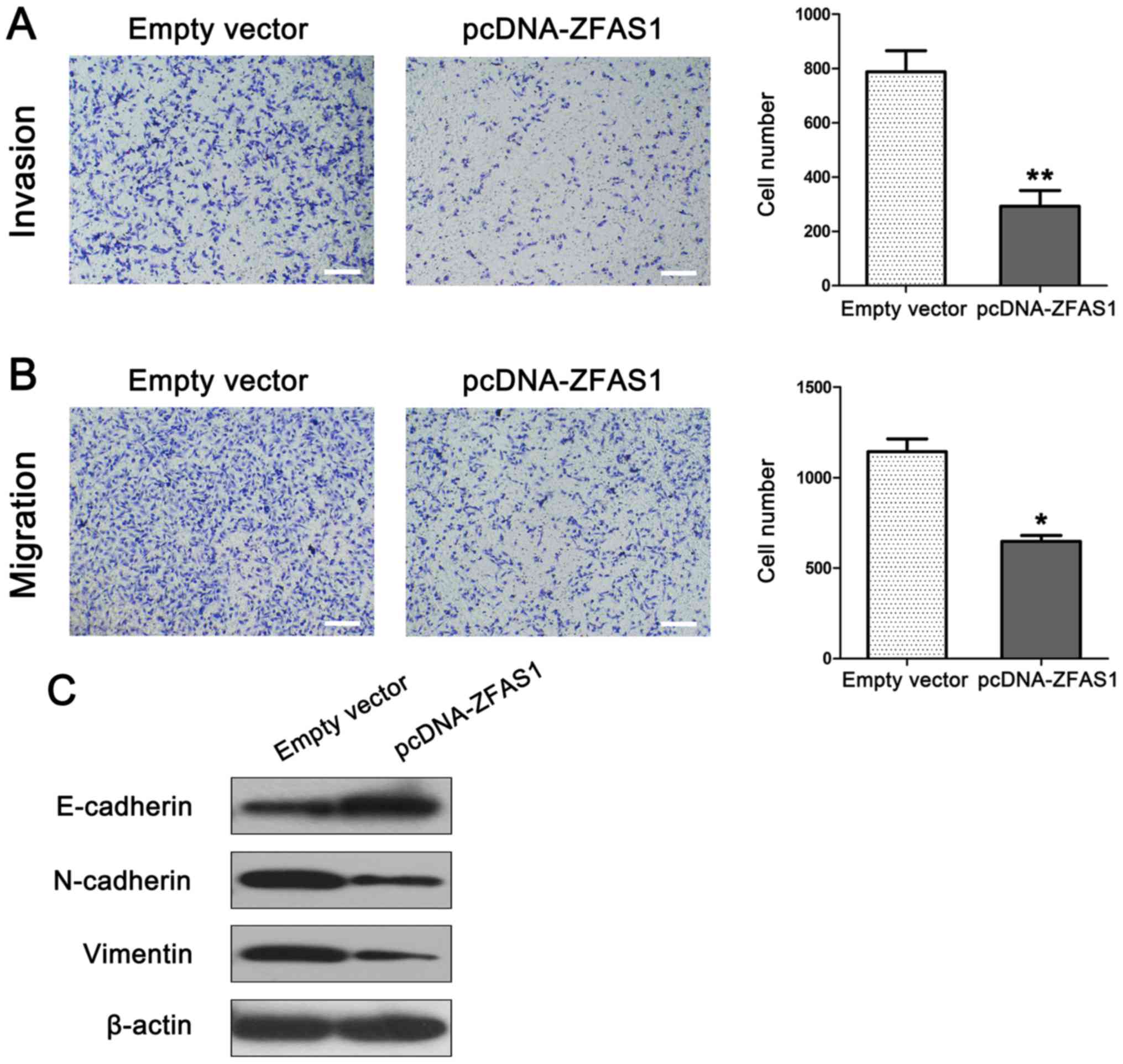
Overexpression of ZFAS1 inhibits
breast cancer cell migration and invasion by regulating EMT
To explore the potential role of ZFAS1 in breast
cancer metastasis, and investigate the effect of ZFAS1 on migration
and invasion capacity, we performed a Transwell assay using
MDA-MB-231 cells. MDA-MB-231 cells were transfected with
pCDNA-ZFAS1 or empty plasmid (negative control). As shown in
Fig. 4A, ZFAS1 overexpression
markedly suppressed cell migratory and invasion capacity compared
to that observed in the control group. As the EMT process is a
vital mechanism for cell metastasis, we next examined the effect of
ZFAS1 overexpression on EMT in breast cancer cells. Western
blotting was performed to examine the expression of EMT-related
markers in MDA-MB-231 cells. As expected, ZFAS1 overexpression
increased the expression of the epithelial marker E-cadherin while
decreasing the expression of the mesenchymal markers N-cadherin and
Vimentin in MDA-MB-231 cells (Fig.
4B).
Discussion
As the technologies for RNA profiling have improved
over the past few decades, genome-wide transcriptome projects have
detected large amounts of RNA that are transcribed but do not
encode proteins. In fact, in humans only approximately 2% of the
total genomic sequence is transcribed into protein-coding RNAs,
with a vast proportion considered to be non-coding RNAs (ncRNAs)
(15). The evolution and
development of complex organisms require powerful and sophisticated
regulatory systems, and the number of protein-coding genes alone
cannot explain organismal complexity. Alternatively, ncRNAs, which
account for a higher proportion of transcripts and exhibit complex
sequences, may act as regulators of biological functions (16).
The advent of next-generation RNA sequencing
technology, coupled with new computational methods for assembling
transcriptomes, has led to the identification of a considerable
number of lncRNAs. Transcript length (ranging from 200 to
>100,000 nt) and specific base pairing regions confer on lncRNAs
the ability to fold into complex secondary structures. These
secondary structures exert their characteristic functions by
interacting with specific proteins and acting as scaffolds to form
protein complexes (17). LncRNAs
are a highly heterogeneous group of transcripts that regulate the
expression of a wide variety of genes through diverse mechanisms
including epigenetic, transcriptional and post-transcriptional
regulation processes (18). In
addition, lncRNAs have emerged as important regulators of gene
expression in many cancers. For example, overexpression of the
lncRNA LUCAT1 overexpression is associated with poor prognosis of
non-small cell lung cancer and promotes cell proliferation through
epigenetically regulating p21 and p57 expression (19). Similarly the lncRNA TUG1 promote
gastric cancer cell proliferation by epigenetically silencing p57
expression (20). These findings
indicate that lncRNAs may represent a novel family of tumor
suppressor genes and oncogenes.
There is increasing evidence that aberrant
expression of ZFAS1 plays a crucial role in carcinogenesis. A
previous study revealed that ZFAS1 was highly expressed in gastric
cancer promoting tumor growth and metastasis by inducing EMT
(21). Another study showed that
ZFAS1 may destabilize p53 and interacts with the CDK1/cyclin B1
complex, which leads to cell cycle progression and inhibition of
apoptosis (22). Askarian-Amiri
et al (10) found that
ZFAS1 regulates epithelial cell proliferation and differentiation
in the developing mouse mammary gland and that ZFAS1 is
downregulated in human breast tumors compared to levels in normal
tissues. However, few studies have investigated the biological
roles of ZFAS1 in breast cancer.
To elucidate the role of ZFAS1 in the development of
breast cancer, we measured ZFAS1 expression in cells from several
breast cancer cell lines and a normal breast epithelial cell line,
and performed a series of in vitro assays. We discovered
that ZFAS1 expression in cells from breast cancer cell lines was
significantly decreased compared to that found in MCF-10A cells and
that increased expression of ZFAS1 inhibited breast cancer cell
proliferation, migration, and invasion. Moreover, the dysregulation
of cell cycle transitions is a hallmark of cancer cell (23). Thus, to investigate the possible
mechanisms responsible for the effect of ZFAS1 on breast cancer
cell proliferation, we used flow cytometry to determine that ZFAS1
overexpression inhibited cell proliferation by arresting the cell
cycle at the G0/G1 phase and promoting cell apoptosis. These
findings indicate that ZFAS1 may be a tumor suppressor in breast
cancer and that the influence of ZFAS1 on cell proliferation may be
associated with cell cycle and apoptosis control.
The migration and invasion of cancer cells are
common events that mediate changes in cellular behavior, and lead
to different steps in the metastatic process (24). EMT is a biological and pathological
process whereby epithelial cells lose their apical-basal polarity
and undergo a transition to a mesenchymal phenotype (25). It is considered to be a regulatory
mechanism of metastasis in many cancers including breast cancer
(26,27). To further investigate the molecular
mechanisms through which ZFAS1 inhibits the metastasis of breast
cancer cells, we examined the expression of EMT-related markers in
breast cancer cells with ZFAS1 overexpression. Our results showed
that E-cadherin expression was upregulated while N-cadherin and
Vimentin expression were downregulated in ZFAS1-overexpressing
cells, indicating that the effect of ZFAS1 on cell migration and
invasion was partially associated with EMT process.
In conclusion, our findings from the current study
demonstrate that ZFAS1 is downregulated in breast cancer cell
lines. In addition, the overexpression of ZFAS1 inhibited cell
proliferation, migration, invasion, and the EMT process. These
results suggest that ZFAS1 is a tumor suppressor and potential
therapeutic target for breast cancer, with further study required
to more fully elucidate the underlying molecular mechanisms of
ZFAS1 in breast cancer development.
Acknowledgements
Not applicable.
Funding
This study was supported by The National Natural
Science Foundation of China (grant nos. 21131002, 21427802 and
21671076) and The Science and Technology Development Project of
Jilin province (grant no. 20160101033JC).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF carried out the experimental work, and data
collection and interpretation. CF participated in the design and
coordination of experimental work, and the acquisition of data. NL
and KH participated in the study design, data collection, analysis
of data and preparation of the manuscript. XF and KW carried out
the study design, the analysis and interpretation of data, and
drafted the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Gesualdo F, Capaccioli S and Lulli M: A
pathophysiological view of the long non-coding RNA world.
Oncotarget. 5:10976–10996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Froberg JE and Lee JT: Long
noncoding RNAs: Fresh perspectives into the RNA world. Trends
Biochem Sci. 39:35–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang EB, Yin DD, Sun M, Kong R, Liu XH,
You LH, Han L, Xia R, Wang KM, Yang JS, et al: P53-regulated long
non-coding RNA TUG1 affects cell proliferation in human non-small
cell lung cancer, partly through epigenetically regulating HOXB7
expression. Cell Death Dis. 5:e12432014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou M, Zhang XY and Yu X: Overexpression
of the long non-coding RNA SPRY4-IT1 promotes tumor cell
proliferation and invasion by activating EZH2 in hepatocellular
carcinoma. Biomed Pharmacother. 85:348–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Huang H, Li Y, Li L, Hou W and You
Z: Decreased expression of long non-coding RNA GAS5 promotes cell
proliferation, migration and invasion and indicates a poor
prognosis in ovarian cancer. Oncol Rep. 36:3241–3250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Askarian-Amiri ME, Crawford J, French JD,
Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani
SR, et al: SNORD-host RNA Zfas1 is a regulator of mammary
development and a potential marker for breast cancer. RNA.
17:878–891. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nie F, Yu X, Huang M, Wang Y, Xie M, Ma H,
Wang Z, De W and Sun M: Long noncoding RNA ZFAS1 promotes gastric
cancer cells proliferation by epigenetically repressing KLF2 and
NKD2 expression. Oncotarget. 8:38227–38238. 2016.
|
|
12
|
Fang C, Zan J, Yue B, Liu C, He C and Yan
D: Long Noncoding RNA ZFAS1 promotes the progression of colonic
cancer by modulating ZEB1 expression. J Gastroenterol Hepatol.
32:1204–1211. 2016. View Article : Google Scholar
|
|
13
|
Gao K, Ji Z, She K, Yang Q and Shao L:
Long non-coding RNA ZFAS1 is an unfavourable prognostic factor and
promotes glioma cell progression by activation of the Notch
signaling pathway. Biomed Pharmacother. 87:555–560. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia B, Hou Y, Chen H, Yang S, Liu T, Lin M
and Lou G: Long non-coding RNA ZFAS1 interacts with miR-150-5p to
regulate Sp1 expression and ovarian cancer cell malignancy.
Oncotarget. 8:19534–19546. 2017.PubMed/NCBI
|
|
15
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mattick JS: RNA regulation: A new
genetics? Nat Rev Genet. 5:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonasio R and Shiekhattar R: Regulation of
transcription by long noncoding RNAs. Annu Rev Genet. 48:433–455.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y, Jin SD, Zhu Q, Han L, Feng J, Lu
XY, Wang W, Wang F and Guo RH: Long non-coding RNA LUCAT1 is
associated with poor prognosis in human non-small lung cancer and
regulates cell proliferation via epigenetically repressing p21 and
p57 expression. Oncotarget. 8:28297–28311. 2017.PubMed/NCBI
|
|
20
|
Zhang E, He X, Yin D, Han L, Qiu M, Xu T,
Xia R, Xu L, Yin R and De W: Increased expression of long noncoding
RNA TUG1 predicts a poor prognosis of gastric cancer and regulates
cell proliferation by epigenetically silencing of p57. Cell Death
Dis. 7:e21092016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Wang F, Chen H, Tan Q, Qiu S, Chen
S, Jing W, Yu M, Liang C, Ye S and Tu J: Increased expression of
long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal
transition of gastric cancer. Aging (Albany NY). 8:2023–2038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thorenoor N, Faltejskova-Vychytilova P,
Hombach S, Mlcochova J, Kretz M, Svoboda M and Slaby O: Long
non-coding RNA ZFAS1 interacts with CDK1 and is involved in
p53-dependent cell cycle control and apoptosis in colorectal
cancer. Oncotarget. 7:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahai E: Illuminating the metastatic
process. Nat Rev Cancer. 7:737–749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu Y, Sarkissyan M and Vadgama JV:
Epithelial-mesenchymal transition and breast cancer. J Clin Med.
5:2016. View Article : Google Scholar
|
|
27
|
Li W, Jia G, Qu Y, Du Q and Liu B and Liu
B: Long non-coding RNA (LncRNA) HOXA11-AS promotes breast cancer
invasion and metastasis by regulating epithelial-mesenchymal
transition. Med Sci Monit. 23:3393–3403. 2017. View Article : Google Scholar : PubMed/NCBI
|