Introduction
Atherosclerosis (AS) is chronic vascular
inflammation (1) involving lumen
narrowing and rigidity due to cholesterol and lipid accumulation
(2,3). Additionally, AS is associated with
vascular endothelial damage, following which low-density
lipoprotein (LDL) enters the subendothelial layer where it is
oxidized (ox-LDL) and subsequently consumed by scavenger receptors.
Consequently, monocytes are recruited and infiltrate the artery
wall, where they differentiate into macrophages (4,5).
Thus, the ox-LDL-associated damage to vascular endothelial cells
(VECs) is directly associated with the initiation and development
of AS (6,7).
Traditional Chinese Medicine has widely employed
Epimedium brevicornum Maxim in ‘tonifying kidney and
strengthening bone’ in China, Korea and Japan (8–10).
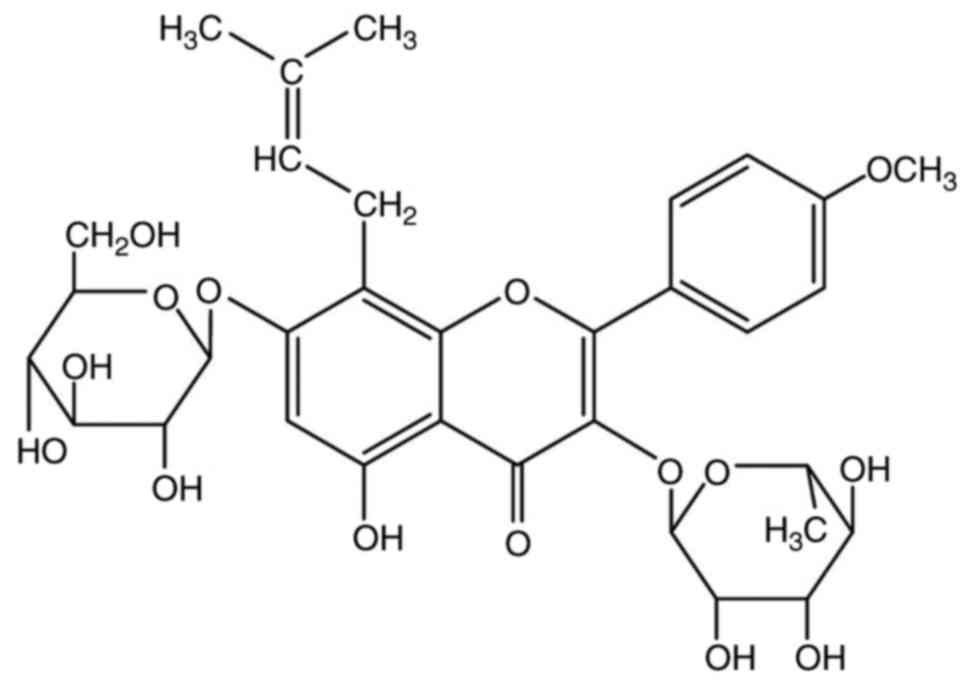
Icariin (C33H40O15; Fig. 1) is a pharmacologically active
flavonoid extracted from E. brevicornum Maxim (11,12),
with numerous pharmacological properties, including
antiosteoporosis (13), antitumor
(14), immunoregulation (15), anti-inflammation (5) and antioxidation (16). Icariin is additionally used to
treat cardiovascular diseases and exhibits anti-atherosclerotic
properties (17–21) that are associated with its
protective effects on endothelial cells (17); however, the underlying mechanisms
require further investigation. Thus, the present study analyzed the
effects of icariin on ox-LDL-induced injury and apoptosis in human
(HUVECs) by evaluating cell viability apoptosis and its associated
genes and proteins, including caspase-3 and apoptosis regulator
Bcl-2 (Bcl-2), in injured human HUVECs with or without treatment
with icariin.
Materials and methods
Cell culture and treatments
HUVECs were purchased from the Cell Bank of the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China), and were seeded into 96-well plates at a density of
1×104 cells/well and incubated for 12 h (37°C, 5%
CO2) in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Hangzhou Sijiqing Bioengineering Material Co.,
Ltd., Hangzhou, China), 1% penicillin and 1% streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following
pretreatment with 0, 10, 20 and 40 µM icariin (Sichuan Weike
Biotechnology Co., Ltd., Chengdu, China) for 24 h at 37°C, cells
were treated for 24 h with 100 µg/ml ox-LDL (Guangzhou Yiyuan
Biological Technology Co., Ltd., Guangzhou, China).
Viability of cells
Based on a previous report (17), the cells were maintained for 24 h
in serum-free DMEM to achieve cell cycle synchronization prior to
their treatments with icariin and ox-LDL. Following pretreatment
with icariin and ox-LDL, MTT reagent (0.5 mg/ml) was added to the
cells at a density of 1×104 cells/well and they were
incubated for 4 h at 37°C. Subsequently, the precipitate was
dissolved in 150 µl dimethyl sulfoxide, and the optical density of
the supernatant was measured at a wavelength of 490 nm.
Apoptosis
In the present study, the apoptotic ability was
evaluated using an Annexin V fluorescein isothiocyanate (FITC) kit
(Beijing Solarbio Science & Technology Co., Ltd., Beijing
China), FACSCalibur (BD Biosciences, San Jose, CA, USA) and ModFit
LT V3.3.11 software (Verity Software House Inc., Topsham, ME, USA).
Cells were washed with PBS and centrifuged for 5 min at 800 × g at
4°C, and the treated cells were resuspended in 200 ml 1X Annexin
binding buffer and harvested. Subsequently, cells were stained with
5 ml propidium iodide (PI; Sigma-Aldrich; Merck KGaA) and 2.5 ml
Annexin V/FITC, and protected from light for 15 min at 37°C.
Western blotting
HUVECs were seeded into 6-well plates at a density
of 1×104 cells/well, treated as aforementioned,
harvested and lysed for 30 min in ice-cold radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology, Jiangsu,
China). Following centrifugation for 20 min at 13,000 × g and 4°C,
the supernatants were analyzed using a bicinchoninic acid assay.
Equal amounts of protein samples (50 µg) were loaded onto a 15%
SDS-PAGE and electrotransferred to polyvinylidene difluoride
membranes, which were blocked with 5% fat-free milk for 1 h at room
temperature. At 4°C membranes were incubated with rabbit anti-human
monoclonal antibodies against caspase-3 (1:1,000; cat. no. ab23021;
Abcam, Cambridge, UK), anti-Bcl-2 (1:1,000; cat. no. ab47482;
Abcam) or anti-GAPDH (1:1,000; Sigma-Aldrich; Merck KGaA; cat. no.
sab4300645) overnight, followed by horseradish peroxidase-labeled
goat anti-rabbit secondary antibody (1:5,000; cat. no. sc45101;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at ambient
temperature. The bands were visualized by enhanced
chemiluminescence/X-ray films (GE Healthcare, Little Chalfont, UK)
and were analyzed using ImageJ version 1.46 (National Institutes of
Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HUVECs with
TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). A total of 5 µg total RNA was reverse transcribed using a
PrimeScript RT Master Mix kit (Takara Biotechnology Co., Ltd.,
Dalian, China), and RT-qPCR was performed using SYBR®
Premix Ex Taq (Beijing Transgen Biotech Co., Ltd., Beijing, China)
and the following program: Denaturing at 95°C for 10 sec, annealing
at 60°C for 15 sec and extension at 72°C for 30 sec (40 cycles).
GAPDH was used as a housekeeping gene. The following
oligonucleotide primers were used: Caspase-3 forward,
5′-GTGGAATTGATGCGTGATG-3′ and reverse, 5′-GGAATCTGTTTCTTTGCATG-3′;
Bcl-2 forward, 5′-GGTGCCACCTGTGGTCCACCT-3′ and reverse,
5′-CTTCACTTGTGGCCCAGATAGG-3′; and GAPDH forward,
5′-GTTACCAGGGCTGCCTTCTC-3′ and reverse, 5′-GATGGTGATGGGTTTCCCGT-3′.
Relative quantification was calculated using the 2−ΔΔCq
method (22) and the results were
normalized to those of GAPDH.
Statistical analysis
The SPSS 19.0 program (IBM, Corp., Armonk, NY, USA)
was used. Each independent experiment was performed in triplicate.
One-way analysis of variance followed by a Tukey's post-hoc
analysis was used, and the data are presented as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Protective effects of icariin
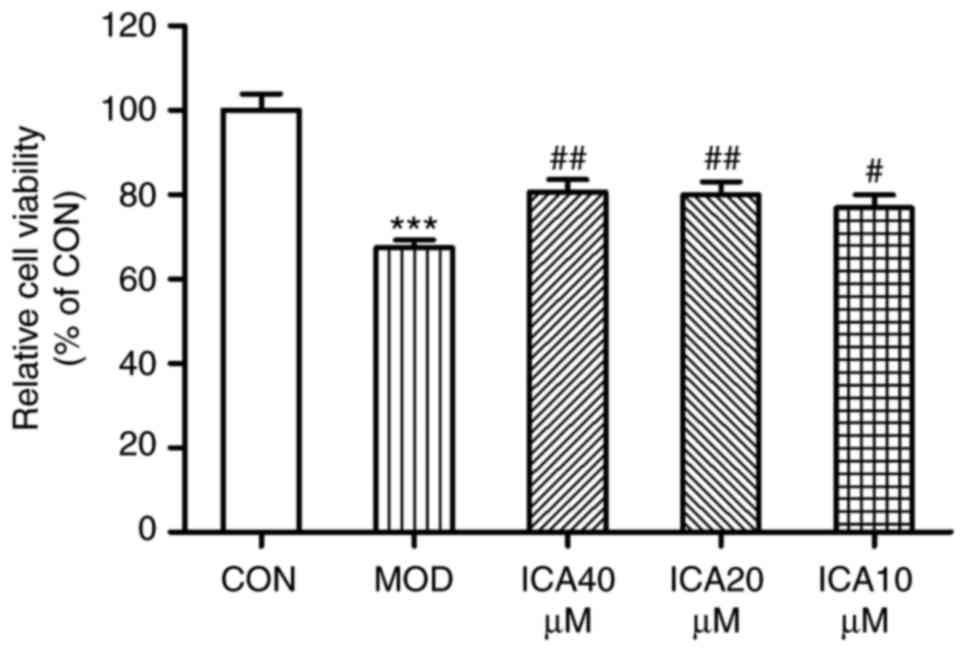
To investigate whether icariin exerts a protective
effect against injury, the present study analyzed the viability of
HUVECs treated with ox-LDL via an MTT assay. Treatment with ox-LDL
significantly decreased the viability of HUVECs compared with
control cells (Fig. 2), while
icariin mitigated this decrease. These findings suggested that
icariin may have exerted protective effects against injury within
HUVECs stimulated with ox-LDL.
Antiapoptotic effects of icariin
To determine the effect of icariin on cellular
apoptosis, Annexin-V and PI double staining was performed. The
present study investigated the apoptosis rate of HUVECs treated
with ox-LDL using flow cytometry. The apoptosis rate significantly
increased when HUVECs were treated with ox-LDL compared with the
control group (Fig. 3).
Additionally, pretreatment with icariin significantly mitigated
this effect, the improvement was more significant at 40 µM icariin
compared with the other assayed concentrations. These findings
indicated that icariin markedly inhibited apoptosis in HUVECs
stimulated with ox-LDL.
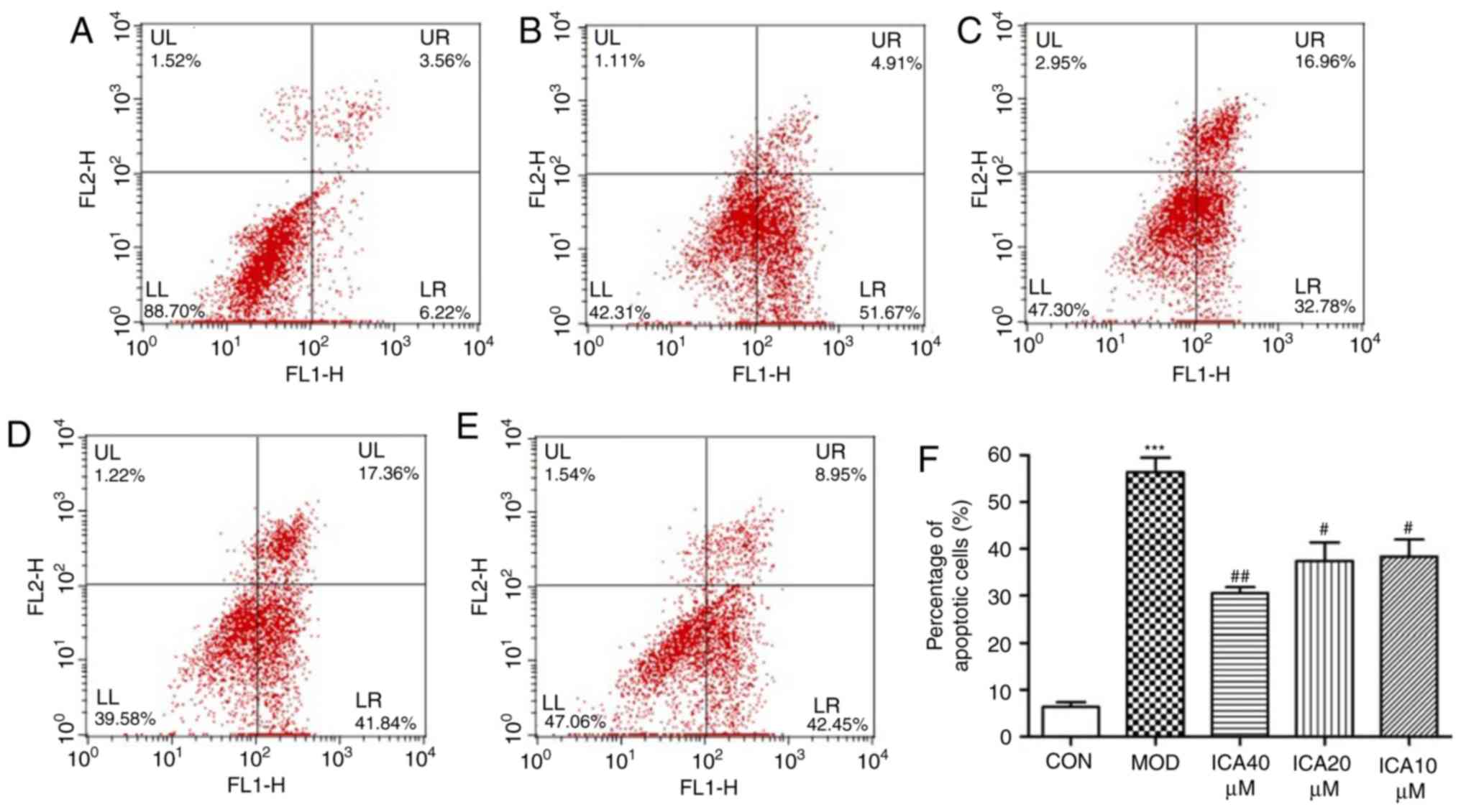 | Figure 3.Antiapoptotic effects of icariin on
HUVECs. Following 24 h pretreatment with icariin at different doses
(0, 10, 20 and 40 µM), cells were stimulated with ox-LDL (100
µg/ml), and flow cytometry was used to determine the rate of
apoptosis. (A) Representative results of control cells. (B)
Representative results of HUVECs stimulated with ox-LDL.
Representative results of icariin at (C) 10, (D) 20 and (E) 40 µM
in HUVECs stimulated with ox-LDL. (F) Percentage of early apoptotic
cells following the indicated treatments. ***P<0.001 vs. CON;
#P<0.05, ##P<0.01 vs. MOD. HUVECs,
human vascular endothelial cells; ICA, icariin-treated group;
ox-LDL, oxidized low-density lipoprotein; MOD, ox-LDL-simulated
group. |
Regulation of caspase-3 and Bcl-2 in
HUVECs by icariin
To investigate the mechanism underlying the
protective effects of icariin on ox-LDL-induced cellular apoptosis,
the relative gene and protein expression levels of Bcl-2 and
caspase-3 were quantified via RT-qPCR and western blot analysis,
respectively. Treatment of HUVECs with ox-LDL significantly
increased caspase-3 and decreased Bcl-2 expression at the protein
(Fig. 4) and mRNA level (Fig. 5). Conversely, icariin pretreatment
significantly suppressed these alterations. The results indicated
that icariin exerts antiapoptotic effects by downregulating
caspase-3 mRNA and protein expression levels while upregulating
those of Bcl-2.
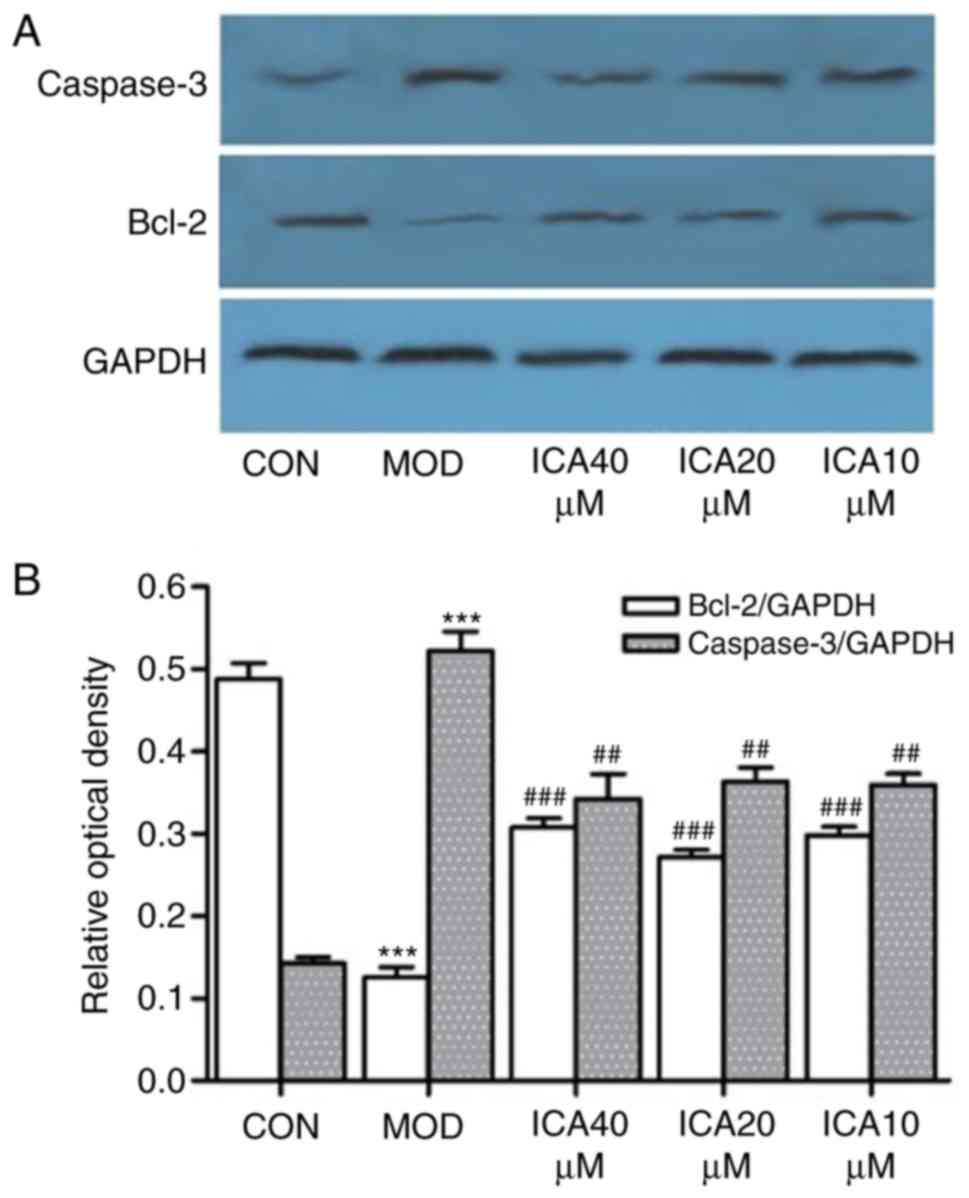 | Figure 4.Icariin-induced regulation of
caspase-3 and Bcl-2 protein expression in human vascular
endothelial cells. Following pretreatment with icariin (0, 10, 20
and 40 µM), cells were treated with ox-LDL (100 µg/ml) for 24 h,
and the protein expression levels of caspase-3 and Bcl-2 were
detected by western blot analysis; as a loading control, GAPDH was
used. (A) Expression of Bcl-2 and caspase-3. (B) Ratio of Bcl-2 and
caspase-3 expression levels to control levels. ***P<0.001 vs.
CON; ##P<0.05, ###P<0.01 vs. respective
MOD group. Bcl-2, apoptosis regulator Bcl-2; CON, control; ICA,
icariin-treated group; ox-LDL, oxidized low-density lipoprotein;
MOD, ox-LDL-simulated group. |
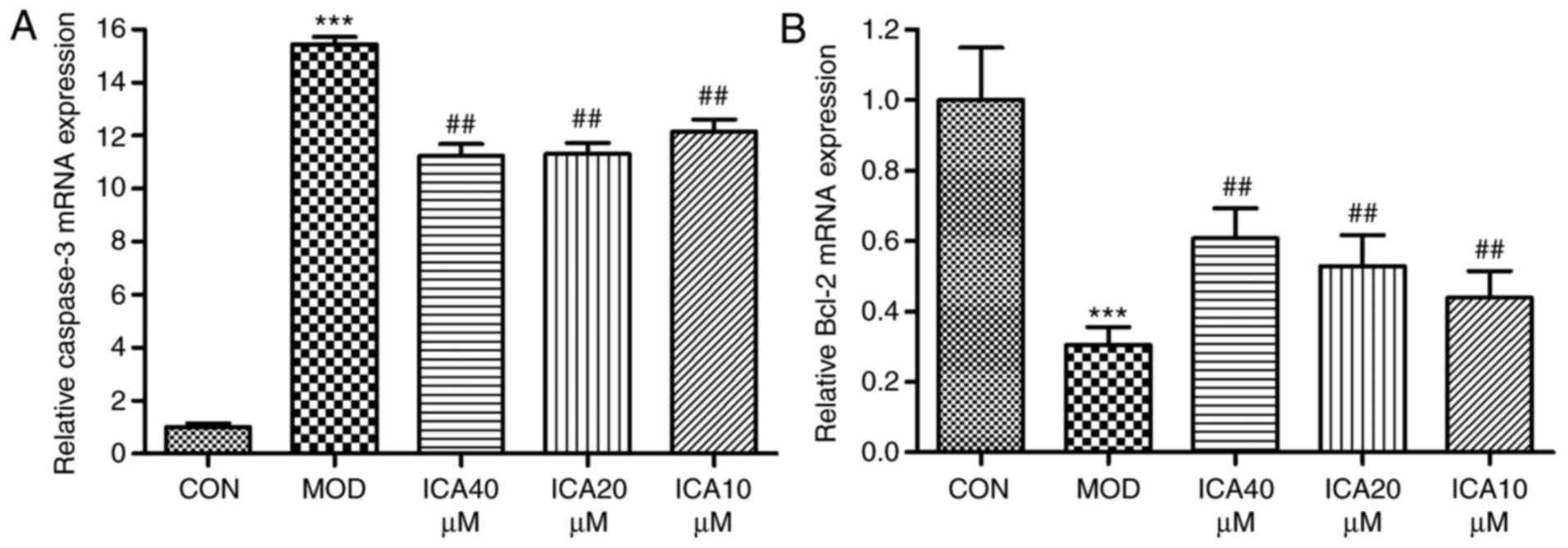 | Figure 5.Regulation of Bcl-2 and caspase-3 gene
expression in human vascular endothelial cells by icariin.
Following pretreatment with icariin (0, 10, 20 and 40 µM), cells
were treated for 24 h with ox-LDL (100 µg/ml). Subsequently, the
expression levels of Bcl-2 and caspase-3 mRNA were determined by
reverse transcription-quantitative polymerase chain reaction; as a
loading control, GAPDH was used. (A) Caspase-3 mRNA expression and
the ratio to the control level. (B) Bcl-2 mRNA expression and the
ratio to the control level. ***P<0.001 vs. CON;
##P<0.01 vs. MOD. Bcl-2, apoptosis regulator Bcl-2;
CON, controls; ICA, icariin-treated group; ox-LDL, oxidized
low-density lipoprotein; MOD, ox-LDL-simulated group. |
Discussion
Previous reports have demonstrated the harmful
effects of ox-LDL, which induced the apoptosis of endothelial cells
(23). Furthermore, ox-LDL is
involved in the pathogenesis of AS by injuring the vascular
endothelium (24,25). Previous studies have suggested that
ox-LDL may directly target VECs and induce apoptosis via the
mitochondrial apoptotic pathways (26–29).
In addition, ox-LDL promotes the recruitment of monocytes and
reactive oxygen species (30–32)
by upregulating (32,33) and binding to the lectin-like ox-LDL
receptor (23) on VECs. Caspases
have been suggested to be associated with the signaling pathways
underlying ox-LDL-induced apoptosis (23). Treatment with ox-LDL has been
reported to result in the activation of caspase-9, thus resulting
in the activation of caspase-3, the major effector caspase
responsible for the destruction of various substrates, including
the proteins involved in DNA repair, mRNA splicing, and DNA
replication (34).
Therefore, injury to endothelial cells in the
subendothelial space of the arterial wall is a critical
pathological cascade in the occurrence of AS. To the best of our
knowledge, the present study is the first to demonstrate that
icariin may significantly suppress injury induced by ox-LDL in
HUVECs. The effects of icariin were associated with increased
apoptosis. Treatment with ox-LDL notably reduced HUVEC viability,
increasing the apoptosis rate. Icariin significantly reversed
ox-LDL-mediated effects in HUVECs.
In order to elucidate the mechanism involved in the
protective influences of icariin in HUVECs, Bcl-2 and caspase-3 at
the gene and protein expression levels were investigated. It has
been reported that the caspase cascade served a pivotal role of in
apoptosis. Caspase-3 is activated during the final step of the
proapoptotic signaling pathway, while the suppression of caspase
activity attenuates injury and apoptosis in HUVECs (35). The Bcl-2 family of proteins is
considered to be an important family of apoptosis regulators, and
include anti- and pro-apoptotic molecules (36,37).
The results of the present study indicated significantly reduced
Bcl-2 mRNA and protein levels within ox-LDL-treated HUVECs compared
with the control. Conversely, the forced expression of Bcl-2 mRNA
and protein attenuated HUVEC apoptosis caused by ox-LDL, and
suppressed caspase-3 activity.
In summary, the present study demonstrated that
icariin inhibited HUVEC damage and apoptosis induced by ox-LDL. The
antiapoptotic effects were associated with the downregulation of
caspase-3 and upregulation of Bcl-2. The results of the present
study provided additional evidence that icariin may prevent the
development of AS; however, further investigation into the
biological activity of icariin, including its effects on vascular
smooth muscle cells or foam cells, is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81773934)
and Natural Science of Jilin Province (grant no. 20150101221JC),
and the Applied Research Project of Tonghua Normal University
(grant no. 2014096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH performed the experiments and wrote the
manuscript, and KL, YZ performed the cell study. HL designed the
study, performed bibliographic research, drafted the manuscript and
provided comments. LR and ZF designed the study, analyzed the data
and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grundtman C and Wick G: The autoimmune
concept of atherosclerosis. Curr Opin Lipidol. 22:327–334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dell'omo G, Penno G, Pucci L, Lucchesi D,
Fotino C, Del Prato S and Pedrinelli R: ACE gene insertion/deletion
polymorphism modulates capillary permeability in hypertension. Clin
Sci (Lond). 111:357–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu F, Li C, Jin XP, Weng SX, Fan LL,
Zheng Z, Li WL, Wang F, Wang WF, Hu XF, et al: Celastrol may have
an anti-atherosclerosis effect in a rabbit experimental carotid
atherosclerosis model. Int J Clin Exp Med. 7:1684–1691.
2014.PubMed/NCBI
|
|
4
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang H, Yan L, Qian P, Duan H, Wu J, Li B
and Wang S: Icariin inhibits foam cell formation by down-regulating
the expression of CD36 and up-regulating the expression of SR-BI. J
Cell Biochem. 116:580–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruyndonckx L, Hoymans VY, Van
Craenenbroeck AH, Vissers DK, Vrints CJ, Ramet J and Conraads VM:
Assessment of endothelial dysfunction in childhood obesity and
clinical use. Oxid Med Cell Longev. 2013:1747822013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu YR, Chen JJ and Dai M: Paeonol
protects rat vascular endothelial cells from ox-LDL-induced injury
in vitro via downregulating microRNA-21 expression and TNF-α
release. Acta Pharmacol Sin. 35:483–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY,
Yao XS, Leung PC and Wong MS: The osteoprotective effect of Herba
epimedii (HEP) extract in vivo and in vitro. Evid Based Complement
Alternat Med. 2:353–361. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidaka S, Okamoto Y, Yamada Y, Kon Y and
Kimura T: A Japanese herbal medicine, Chujo-to, has a beneficial
effect on osteoporosis in rats. Phytother Res. 13:14–19. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakamoto S, Sassa S, Kudo H, Suzuki S,
Mitamura T and Shinoda H: Preventive effects of a herbal medicine
on bone loss in rats treated with a GnRH agonist. Eur J Endocrinol.
143:139–142. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao-Hong D, Chang-Qin X, Jian-Hua H,
Wen-Jiang Z and Bing S: Icariin delays homocysteine-induced
endothelial cellular senescence involving activation of the
PI3K/AKT-eNOS signaling pathway. Pharm Biol. 51:433–440. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng KW, Fu H, Liu GX and Wang XM: Icariin
attenuates lipopolysaccharide-induced microglial activation and
resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and
JNK/p38 MAPK pathways. Int Immunopharmacol. 10:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang G, Qin L, Sheng H, Yeung KW, Yeung
HY, Cheung WH, Griffith J, Chan CW, Lee KM and Leung KS:
Epimedium-derived phytoestrogen exert beneficial effect on
preventing steroid-associated osteonecrosis in rabbits with
inhibition of both thrombosis and lipid-deposition. Bone.
40:685–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang DC, Liu JL, Ding YB, Xia JG and Chen
GY: Icariin potentiates the antitumor activity of gemcitabine in
gallbladder cancer by suppressing NF-κB. Acta Pharmacol Sin.
34:301–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap SP, Shen P, Li J, Lee LS and Yong EL:
Molecular and pharmacodynamic properties of estrogenic extracts
from the traditional Chinese medicinal herb, Epimedium. J
Ethnopharmacol. 113:218–224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Huang JH, Ning Y and Shen ZY:
Icariin and its pharmaceutical efficacy: Research progress of
molecular mechanism. Zhong Xi Yi Jie He Xue Bao. 9:1179–1184.
2011.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Y, Liu K, Yan M, Zhang Y, Wang Y and
Ren L: Effects and mechanisms of icariin on atherosclerosis. Int J
Clin Exp Med. 8:3585–3589. 2015.PubMed/NCBI
|
|
18
|
Hu Y, Liu K, Yan M, Zhang Y, Wang Y and
Ren L: Icariin inhibits oxidized low-density lipoprotein-induced
proliferation of vascular smooth muscle cells by suppressing
activation of extracellular signal-regulated kinase 1/2 and
expression of proliferating cell nuclear antigen. Mol Med Rep.
13:2899–2903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu Y, Sun B, Liu K, Yan M, Zhang Y, Miao C
and Ren L: Icariin attenuates high-cholesterol diet induced
atherosclerosis in rats by inhibition of inflammatory response and
p38 MAPK signaling pathway. Inflammation. 39:228–236. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu HB and Huang ZQ: Vasorelaxant effects
of icariin on isolated canine coronary artery. J Cardiovasc
Pharmacol. 49:207–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Mehta JL, Haider N, Zhang X,
Narula J and Li D: Role of caspases in Ox-LDL-induced apoptotic
cascade in human coronary artery endothelial cells. Circ Res.
94:370–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ehara S, Ueda M, Naruko T, Haze K, Itoh A,
Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, et al: Elevated
levels of oxidized low density lipoprotein show a positive
relationship with the severity of acute coronary syndromes.
Circulation. 103:1955–1960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li D, Yang B and Mehta JL: Ox-LDL induces
apoptosis in human coronary artery endothelial cells: Role of PKC,
PTK, bcl-2, and Fas. Am J Physiol. 275:H568–H576. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giovannini C, Matarrese P, Scazzocchio B,
Sanchez M, Masella R and Malorni W: Mitochondria hyperpolarization
is an early event in oxidized low-density lipoprotein-induced
apoptosis in Caco-2 intestinal cells. FEBS Lett. 523:200–206. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imanishi T, Hano T, Sawamura T, Takarada S
and Nishio I: Oxidized low density lipoprotein potentiation of
Fas-induced apoptosis through lectin-like oxidized-low density
lipoprotein receptor-1 in human umbilical vascular endothelial
cells. Circ J. 66:1060–1064. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salvayre R, Auge N, Benoist H and
Negre-Salvayre A: Oxidized low-density lipoprotein-induced
apoptosis. Biochim Biophys Acta. 1585:213–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suo J, Zhao L, Wang J, Zhu Z, Zhang H and
Gao R: Influenza virus aggravates the ox-LDL-induced apoptosis of
human endothelial cells via promoting p53 signaling. J Med Virol.
87:1113–1123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cominacini L, Pasini AF, Garbin U, Davoli
A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V
and Sawamura T: Oxidized low density lipoprotein (ox-LDL) binding
to ox-LDL receptor-1 in endothelial cells induces the activation of
NF-kappaB through an increased production of intracellular reactive
oxygen species. J Biol Chem. 275:12633–12638. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cominacini L, Rigoni A, Pasini AF, Garbin
U, Davoli A, Campagnola M, Pastorino AM, Lo Cascio V and Sawamura
T: The binding of oxidized low density lipoprotein (ox-LDL) to
ox-LDL receptor-1 reduces the intracellular concentration of nitric
oxide in endothelial cells through an increased production of
superoxide. J Biol Chem. 276:13750–13755. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D and Mehta JL: Antisense to LOX-1
inhibits oxidized LDL-mediated upregulation of monocyte
chemoattractant protein-1 and monocyte adhesion to human coronary
artery endothelial cells. Circulation. 101:2889–2895. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li D and Mehta JL: Upregulation of
endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and
implications in apoptosis of human coronary artery endothelial
cells: Evidence from use of antisense LOX-1 mRNA and chemical
inhibitors. Arterioscler Thromb Vasc Biol. 20:1116–1122. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morishima N, Nakanishi K, Takenouchi H,
Shibata T and Yasuhiko Y: An endoplasmic reticulum stress-specific
caspase cascade in apoptosis. Cytochrome c-independent activation
of caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo Y, Lu S, Dong X, Xu L, Sun G and Sun
X: Dihydromyricetin protects human umbilical vein endothelial cells
from injury through ERK and Akt mediated Nrf2/HO-1 signaling
pathway. Apoptosis. 22:1013–1024. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yin H, Zhou Y, Zhu M, Hou S, Li Z, Zhong
H, Lu J, Meng T, Wang J, Xia L, et al: Role of mitochondria in
programmed cell death mediated by arachidonic acid-derived
eicosanoids. Mitochondrion. 13:209–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu Y, Liu Q, Guo S, Zhang Q, Tang J, Liu
G, Kong D, Li J, Yan S, Wang R, et al: 2, 3, 7,
8-Tetrachlorodibenzo-p-dioxin promotes endothelial cell apoptosis
through activation of EP3/p38MAPK/Bcl-2 pathway. J Cell Mol Med.
21:3540–3551. 2017. View Article : Google Scholar : PubMed/NCBI
|