Introduction
Diabetes is a common chronic disease worldwide.
Along with rapid economic development, the incidence of diabetes in
China has rapidly increased (1,2).
Type 1 diabetes is usually caused by damage to pancreatic β-cells
and insufficient secretion of insulin (3). To date, treatments targeting type 1
diabetes have not promised a complete cure. Advanced techniques
such as islet transplantation may be available in the near future
for treating type 1 diabetes (4–6).
However, a drawback of this technique is that a large number of
pancreatic β-cells undergo apoptosis owing to hypoxia (7,8). A
recent report indicated that diet cycles that mimic fasting in
animal models may successfully promote β-cell regeneration
(9). However, the effect of diet
therapy that mimics fasting in humans remains unknown. Reducing the
rate of apoptosis of pancreatic β-cells may be a primary target in
the treatment of patients with type 1 diabetes.
Appoptosin, encoded by SLC25A38, is a novel
proapoptotic protein located in the inner membrane of mitochondria
(10,11). It is strongly expressed in the
brain cells of patients with Alzheimer's disease, and it has been
identified to interact with the amyloid precursor protein (12). Studies have demonstrated that,
in vitro, the overexpression of appoptosin promotes
apoptosis in neuronal and 293T cells, accompanied by mitochondrial
fusion (10,13). In vivo murine studies have
also demonstrated that overexpression of appoptosin was detected in
the brains of ischemia-reperfused rats (10). To the best of our knowledge, no
previous studies have investigated the role of appoptosin in
diabetes and pancreatic β-cells. Therefore, the present study
investigated the role of appoptosin in MIN6 cells.
Cobalt chloride (CoCl2) has been
previously used to mimic hypoxia and induce cell apoptosis
(14,15). Cobalt inhibits prolyl hydrolase
domain (PHD) enzymes (oxygen sensors) by replacing iron, making
these enzymes unable to mark hypoxia inducible factor (HIF)-1α for
degradation (16).
Dimethyloxaloylglycine (DMOG) and
1,4-dihydrophenonthrolin-4-1-3-carboxylic acid (1,4-DPCA) are both
cell permeable, competitive inhibitors of PHDs and HIF-prolyl
hydroxylases (HIF-PHs). They are able to stabilize HIF-1α
efficiently at normal oxygen tensions in vitro (17–22).
In the present study, the results demonstrated that 400 µM
CoCl2 induced apoptosis in MIN6 cells and considerably
reduced cell viability. In addition, overexpression of appoptosin
in MIN6 cells increased caspase 3 activity and mitochondrial
damage. By contrast, inhibition of appoptosin by short hairpin
(sh)RNA partially restored the viability of MIN6 cells exposed to
hypoxia. Therefore, as overexpression of appoptosin increased
mitochondrial damage and cell apoptosis, inhibiting the expression
of appoptosin may reduce islet apoptosis during islet
transplantation and may provide a novel strategy for the care of
patients with diabetes.
Materials and methods
Cell culture and transfection
MIN6 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in high glucose Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 1% penicillin-streptomycin (GE
Healthcare, Chicago, IL, USA) and 15% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) (23). Detailed information regarding the
siRNA sequence has been described in a previous study (10). The siRNA and negative control siRNA
were synthesized and provided by Invitrogen (Thermo Fisher
Scientific, Inc.). The siRNA targeting sequence of appoptosin was
as follows: AGACGCTCATGTTACACCCAGTGAT (10). Overexpression appoptosin plasmids
were transfected into MIN6 cells by using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Overexpression
Appoptosin plasmids were constructed using pCMV-Myc as described
(11). Lipofectamine®
3000 was used according to the manufacturer's protocols. A brief
protocol for the transfection is as follows: Firstly, 4 µg plasmid
was addition to 500 µl DMEM. Then, 12 µl Lipofectamine®
3000 was added. Lastly, the mixture was kept at room temperature
for 10 min and then added to one well of a six-well plate.
Construction of the overexpression plasmids were conducted as
described (11). pCMV-Myc plasmids
served as the control.
Briefly, in the present study, 1×106 MIN6
cells were seeded in 6-well plates overnight prior to
CoCl2, DMOG, H2O2 and 1,4-DPCA
treatment. Then, 400 µΜ CoCl2 (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), 100 µM H2O2
(Sigma-Aldrich Merck KGaA), 1 mM DMOG and 100 µM 1,4-DPCA (both
Selleck Chemicals, Shanghai, China) were added to the plates
containing MIN6 cells (90% fusion). Finally, the cells were
collected and/or lysed according to the guidance of following
experiments. CoCl2 was dissolve in cell culture medium
(DMEM) to a concentration of 400 mM. H2O2
(100 µM) were diluted by the medium prior to experimentation. DMOG
(1 mM) and 1,4-DPCA (100 µM) were dissolved in 0.1% DMSO. 0.1% DMSO
served as the control in experiment containing DMOG and
1,4-DPCA.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay and cell viability
Cells (2×105) were plated in 24-well
plates overnight and cultured in an incubator at 37°C prior to the
experiment. MIN6 cells were washed in precooled 0.01 M PBS three
times. Fresh 4% paraformaldehyde was used to fix the cells for 10
min at room temperature, after which the cells were permeabilized
with 0.1% Triton-X 100 in 0.01 M PBS for 15 min at room
temperature. Subsequently, TUNEL (Roche Diagnostics, Indianapolis,
IN, USA) staining reagents were added and the cells were incubated
at 37°C in the dark for 1 h. Finally, the cell nuclei were stained
using DAPI (0.3 mM) for 3 min at room temperature and washed with
0.01 M PBS three times. The number of TUNEL-positive cells was
determined using ImageJ software (Java 1.8.0_112, National
Institutes of Health, Bethesda, MD, USA). A total of 5 fields per
view were observed with a fluorescent microscope; the excitation
wavelength applied was 555 nm. A Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
quantify viable MIN6 cells following 400 µM CoCl2
treatment with or without siRNA knockdown of appoptosin, according
to the manufacturer's protocol. MIN6 cells (1.5×104)
were seeded in 96-well plates and cultured in an incubator at 37°C
overnight.
Immunofluorescence and JC-1
staining
Cells (2×105) were plated in 24-well
plates overnight and cultured in an incubator at 37°C prior to the
experiment. MIN6 cells were washed in precooled 0.01 M PBS three
times. Fresh 4% paraformaldehyde was used to fix the cells for 10
min at room temperature. Subsequently, the cells were permeabilized
with 0.1% Triton-X 100 in 0.01 M PBS for 15 min at room
temperature. Primary antibody appoptosin (cat. no. sc-515883; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and cleaved-caspase 3
(cat. no. 9661; Cell Signaling Technology, Inc., Danvers, MA, USA)
were diluted to one hundred. Goat anti-rabbit IgG (Alexa
Fluor® 488; cat. no. A-11034; Thermo Fisher Scientific,
Inc.) and Goat anti-mouse IgG (Alexa Fluor® 594; cat.
no. A-11020; Thermo Fisher Scientific, Inc.) were used as the
secondary antibodies, which were diluted to one thousand. The
detailed protocol of immunofluorescence has been described in our
previous study (24). In brief, 10
µM JC-1 (Invitrogen; Thermo Fisher Scientific, Inc.) and 2 µg/ml
Hoechst reagent (Guangzhou RiboBio Co., Ltd., Guangzhou, China)
were added to MIN6 cells (90% fusion) for 10 min at 37°C at the
same time. Cells were subsequently washed with warm DMEM. Finally,
the stained cells were cultured in warm DMEM containing 10% FBS for
live cell imaging (Confocal microscope FV1000; Olympus Corp.,
Tokyo, Japan).
Western blot analysis
MIN6 cells transfected with pCMV (empty vector) and
overexpression vector were lysed using radioimmunoprecipitation
assay buffer (EMD Millipore, Billerica, MA, USA). The concentration
of protein in each sample was determined using a bicinchoninic acid
kit (Thermo Fisher Scientific, Inc.). The lysates and loading
buffer were mixed and boiled for 10 min. Subsequently, lysates (30
µg total protein) were loaded onto 10% SDS-PAGE gels, which were
subjected to electrophoresis and the proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (EMD Millipore). The
PVDF membrane was blocked with 5% non-fat milk (Cell Signaling
Technology, Inc. USA) for 60 min at room temperature. The primary
antibodies against cleaved-caspase 3 (cat. no. 9661, Cell Signaling
Technology, Inc.), a-tubulin (cat. no. sc-5546, Santa Cruz
Biotechnology, Inc.), appoptosin (cat. no. PA5-42472, Thermo Fisher
Scientific, Inc.) and HIF-1a (cat. no. NB100-479, Novus
Biologicals, LLC, Littleton, CO, USA) were diluted to 1:1,000 and
incubated with membranes. The secondary antibodies (Sigma-Aldrich;
Merck KGaA) and enhanced chemiluminescence (ECL detection
substrate) reagent (Xiamen Lulong Biotech Co., Ltd., Xiamen, China)
were added to the membranes. Anti-rabbit IgG (1:5,000; cat. no.
31460; Thermo Fisher Scientific, Inc.) and anti-mouse IgG (1:5,000;
cat. no. 31430; Thermo Fisher Scientific, Inc.), horseradish
peroxidase (HRP) conjugated antibody was used as the secondary
antibody. The signal was captured on autoradiography film (Kodak,
Rochester, NY, USA). ImageJ (Java 1.8.0_112) was used for
densitometric analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in MIN6 cells was extracted using TRIzol
reagent (Tiangen Biotech, Beijing, China) and was reverse
transcribed into cDNA using Quant One Step RT-PCR kit (Tiangen
Biotech). FastFire qPCR PreMix kits (SYBR®-Green) were
purchased from Tiangen Biotech. The following thermocycling
conditions were used: 95°C for 3 min; 32 cycles of 95°C for 10 sec,
60°C for 10 sec, 72°C for 25 sec and 72°C for 5 min. Quantitative
PCR was performed on a Roche instrument (LightCycler®
480, Roche Diagnostics) and each experiments wererepeated in
triplicate. The primers used in the study were as follows:
Appoptosin forward, 5′-CGTCCCCAGTGATCGAGAAG-3′ and reverse,
5′-GCAGACGGGTTTTGAGGAGA-3′; and β-actin forward
5′-CCCAAAGCTAACCGGGAGAAG−3′ and reverse 5′-GACAGCACCGCCTGGATAG-3′
(25).
Statistical analysis
All experimental results were analyzed using
Student's t-test or one-way analysis of variance followed by
Tukey's honest significant difference test. The results are
presented as the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference. All results were
analyzed using GraphPad Prism version 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
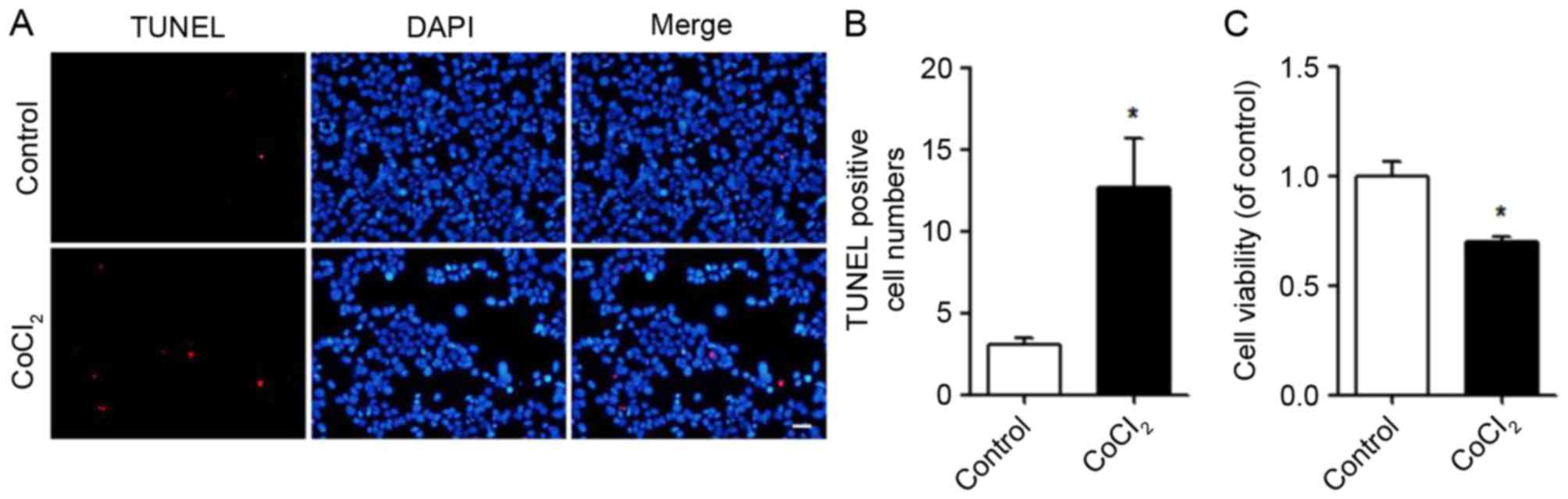
CoCl2 induces apoptosis in
MIN6 cells
Hypoxia is considered to be one of the leading
causes of apoptosis (26,27). In the present study, MIN6 cells
were cultured in 24- or 96-well plates. The next day, 400 µM
CoCl2 was added to induce cell apoptosis. The viability
of the MIN6 cells was measured using CCK-8 kits. Apoptosis was
detected using a TUNEL assay. Following CoCl2 treatment,
apoptosis was promoted (Fig. 1A)
and the number of TUNEL-positive cells was quantified (Fig. 1B). Compared with the control, the
viability of the MIN6 cells was significantly decreased following
CoCl2 treatment (Fig.
1C).
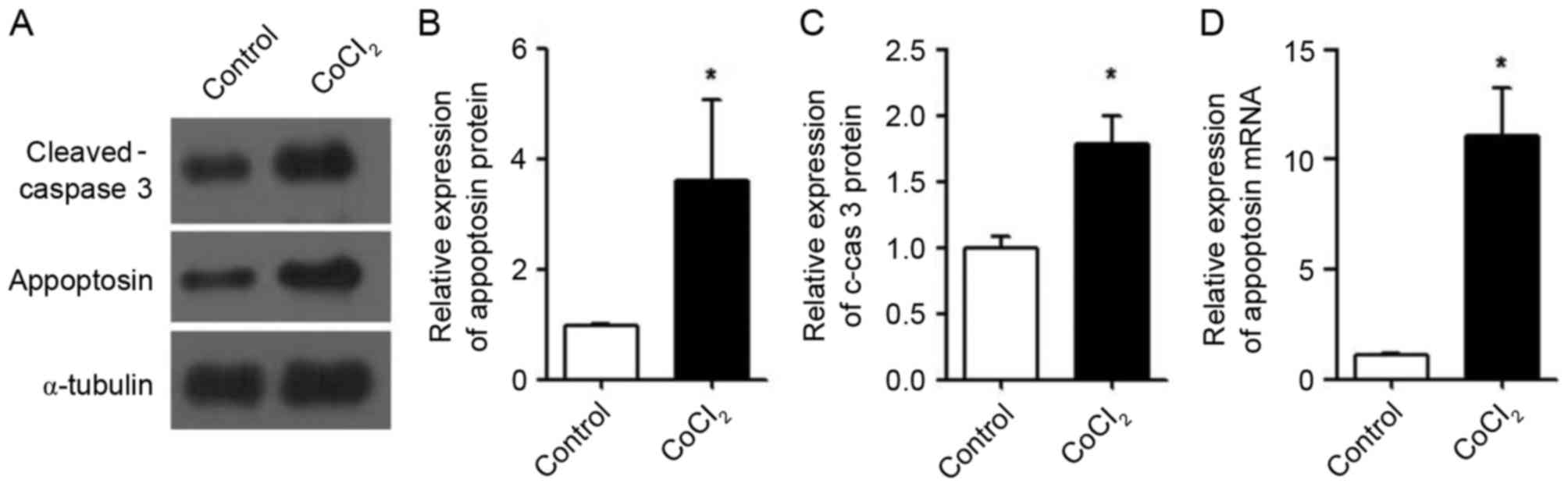
CoCl2 induces
overexpression of appoptosin and activation of caspase 3 in MIN6
cells
After confirming that CoCl2 induced
apoptosis in MIN6 cells, the expression levels of intracellular
appoptosin were determined. The results demonstrated that the
protein expression levels of appoptosin (Fig. 2A and B) and cleaved-caspase 3
(Fig. 2A and C) in MIN6 cells were
significantly increased following treatment with CoCl2.
Additionally, the mRNA expression levels of appoptosin were
increased by CoCl2 treatment in MIN6 cells compared with
the control (Fig. 2D).
HIF-1a and reactive oxygen species
(ROS) promote the expression of appoptosin
It is thought that the primary function of
CoCl2 in cells is the inhibition of HIF-1a degradation
via the inhibition of prolyl hydroxylases (28). Therefore, the present study
determined whether HIF-1a increased the expression levels of
appoptosin. DMOG and 1,4-DPCA were used to stabilize the cellular
HIF-1a protein (20), and they
significantly enhanced HIF-1a and appoptosin protein expression
levels in MIN6 cells compared with the control group (Fig. 3A and B). In addition,
CoCl2 is reported to induce the expression of cellular
ROS (29). Therefore, hydrogen
peroxide (H2O2) was used in the present study
to generate ROS in MIN6 cells. The results demonstrated that
concentrations of 25, 50 and 100 µM H2O2
significantly increased the expression levels of the appoptosin
protein compared with the 0 µM group (Fig. 3C and D). Therefore,
CoCl2 may increase appoptosin expression by inducing
HIF-1a and cellular ROS.
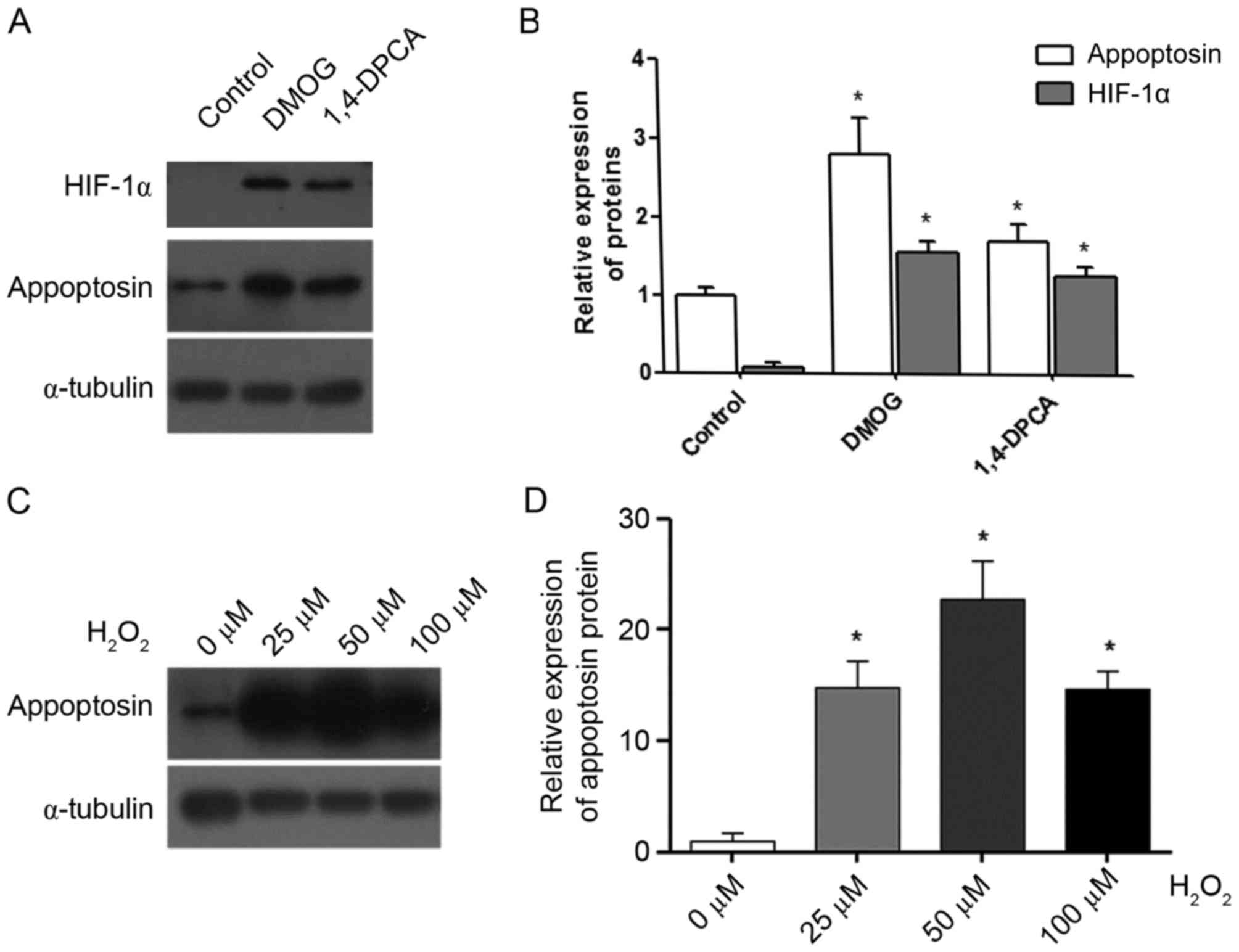 | Figure 3.HIF-1α and reactive oxygen species
promote appoptosin expression in MIN6 cells. DMOG and 1,4-DPCA were
used to treat MIN6 cells for 24 h. (A) Representative western blot
images for appoptosin and HIF-1α protein expression in control,
DMOG and 1,4-DPCA groups. (B) Protein expression levels of
appoptosin and HIF-1α in control, DMOG and 1,4-DPCA groups were
quantified by densitometric analysis. (C) Representative western
blot images for appoptosin protein expression in cells treated with
0–100 µM H2O2. (D) Protein expression levels
of appoptosin in cells treated with 0–100 µM
H2O2 were quantified by densitometric
analysis. Each experiment was repeated three times. *P<0.05 vs.
corresponding control group. HIF-1α, hypoxia inducible factor-1α;
DMOG, dimethyloxaloylglycine; 1,4-DPCA,
1,4-dihydrophenonthrolin-4-1-3-carboxylic acid;
H2O2, hydrogen peroxide. |
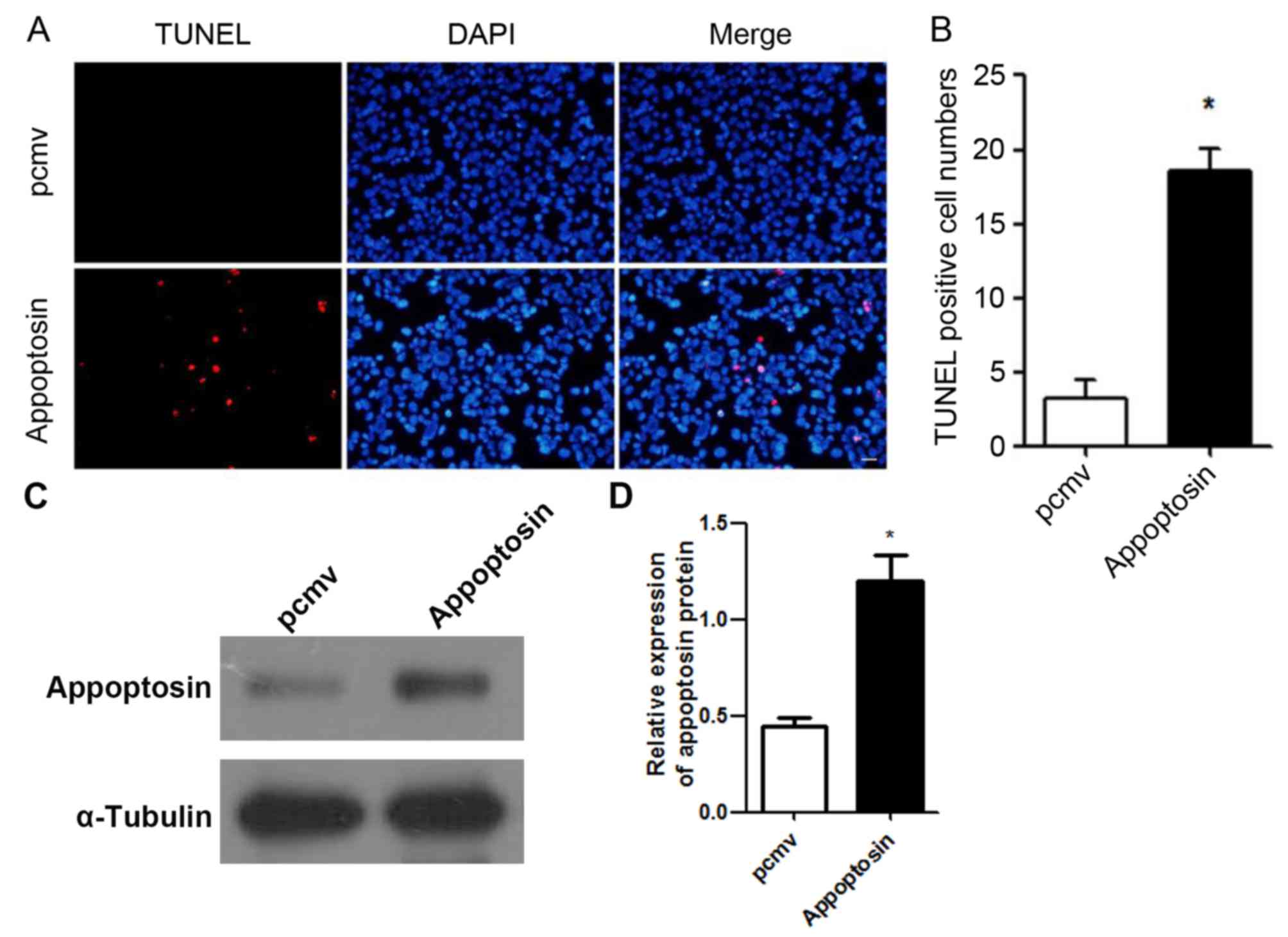
Overexpression of appoptosin induces
apoptosis in MIN6 cells
As the protein and mRNA expression levels of
appoptosin in MIN6 cells were determined following CoCl2
treatment, the present study evaluated whether overexpression of
appoptosin induced apoptosis in MIN6 cells. The results revealed
that high expression levels of appoptosin increased apoptosis in
MIN6 cells (Fig. 4A and B). These
results indicate that a high expression of appoptosin in MIN6 cells
may reduce cell viability. The expression of appoptosin in MIN6
cells was significantly increased after transfection (Fig. 4C and D).
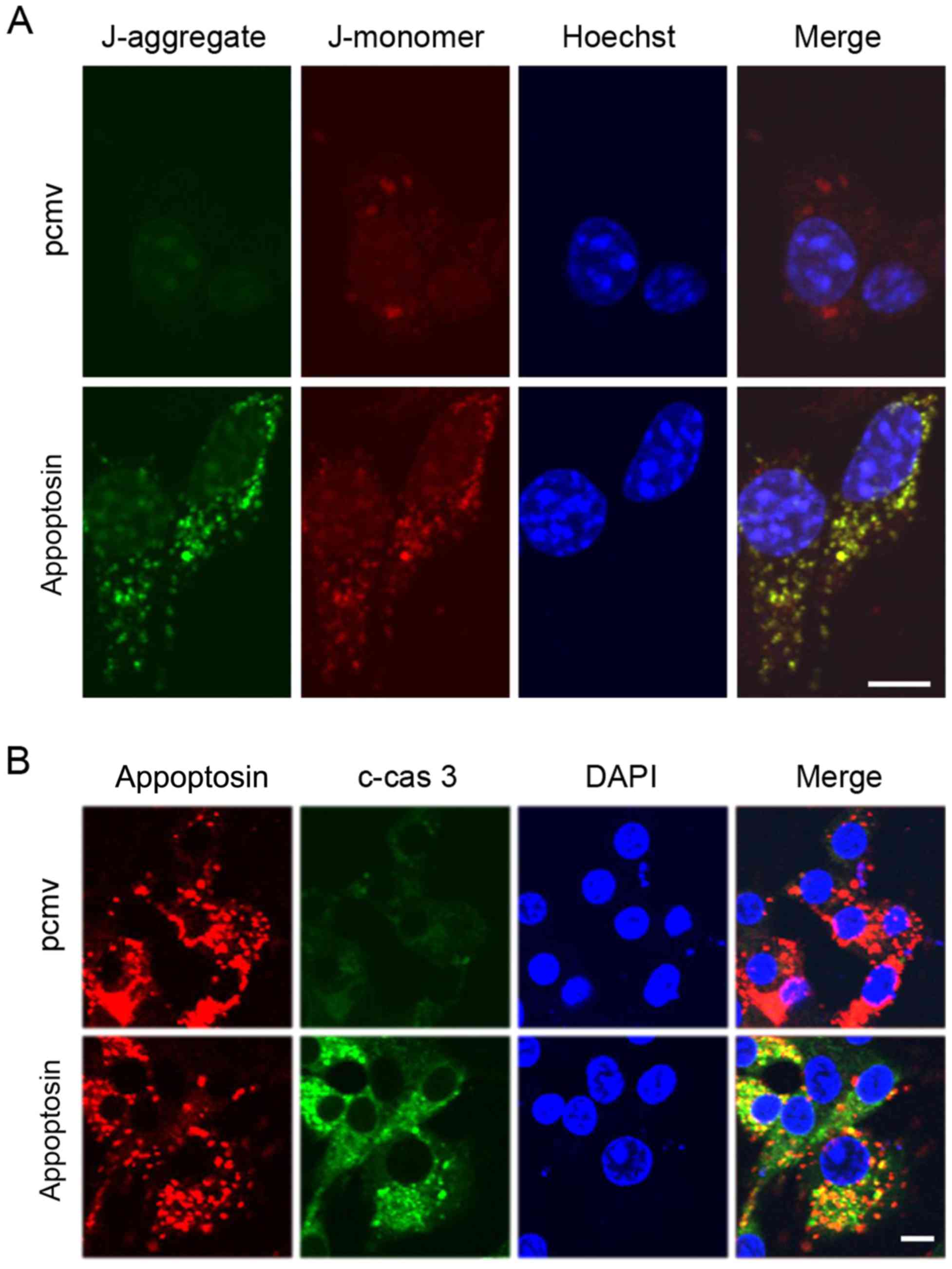
Overexpression of appoptosin induces
mitochondrial damage in MIN6 cells
As appoptosin is a mitochondrial protein, the
mitochondrial membrane potential was measured by using a JC-1
staining dye after overexpression of appoptosin in MIN6 cells. The
results demonstrated that high expression of appoptosin increased
mitochondrial damage (Fig. 5A).
Stronger green staining (J-aggregate) indicated higher levels of
mitochondrial damage. Previous researchers reported that appoptosin
induced apoptosis via activation of the caspase pathway (10). The results of the current study
also demonstrated that overexpression of appoptosin increased the
levels of cellular cleaved-caspase 3 (Fig. 5B). Additionally, overexpressed
appoptosin co-localized with cleaved-caspase 3 in MIN6 cells
(Fig. 5B).
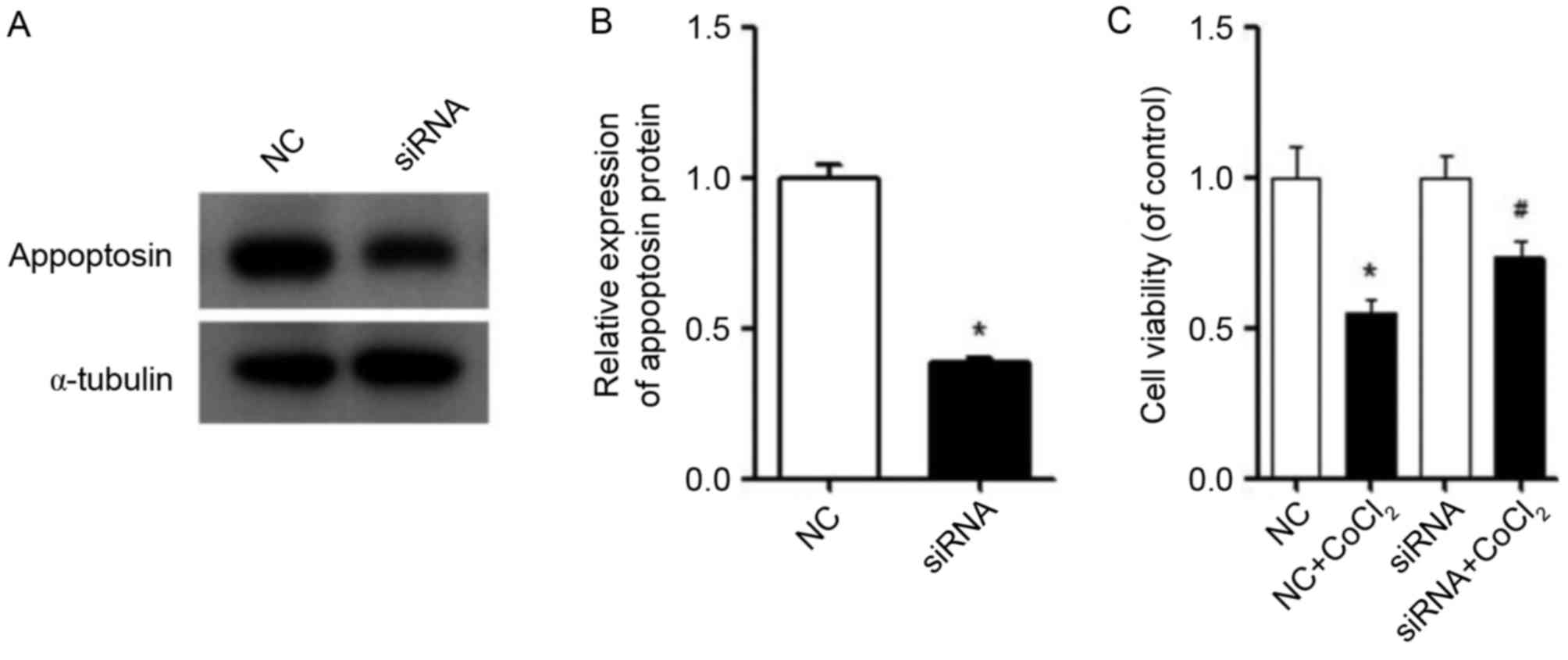
Inhibiting appoptosin partially
restores the viability of MIN6 cells
The expression of appoptosin protein was reduced by
siRNA in MIN6 cells (Fig. 6A and
B). The viability of MIN6 cells treated with CoCl2
after silencing of appoptosin was determined by a CCK-8 assay. The
results demonstrated that, in the presence of CoCl2,
silencing of appoptosin enhanced the cell viability of MIN6 cells
compared with the NC + CoCl2 group (Fig. 6C). In conclusion, these results
indicate that CoCl2 induced the expression of
appoptosin, and that overexpression of appoptosin led to
mitochondrial damage, while inhibition of appoptosin expression
improved the viability of MIN6 cells when exposed to
CoCl2.
Discussion
In the current study, it was demonstrated that the
expression levels of appoptosin in MIN6 cells was increased
following CoCl2 treatment. In addition, HIF-1α and ROS
increased appoptosin protein expression, and overexpression of
appoptosin induced mitochondrial damage and increased
cleaved-caspase 3 expression. Inhibition of appoptosin partially
recovered the viability of MIN6 cells. The results of the present
study indicated that high expression levels of appoptosin may
induce mitochondrial damage and apoptosis in pancreatic β-cells.
However, the expression of appoptosin in diabetes or islets remains
unknown. Further research is required to investigate the role of
appoptosin in diabetes, particularly in islet transplantation
research.
The primary role of pancreatic β-cells is the
secretion of insulin when glucose levels are high, which requires a
large amount of energy from oxidative phosphorylation; thus,
pancreatic β-cells are frequently exposed to hypoxia and oxidative
stress (30–32). In addition, high glucose levels
consume higher levels of oxygen in islets (33). Reducing the extent of damage to
pancreatic β-cells may help provide an improved treatment strategy
for type 1 and type 2 diabetes. In the present study, it was
observed that appoptosin was sensitive to hypoxia and cellular ROS.
The current study determined the effects of chemical induction of
HIF-1α expression on appoptosin expression. However, further
research is required to validate these findings using HIF-1α
overexpression or induction under actual hypoxic conditions
(O2 levels <1%). In addition, overexpression of
appoptosin induced mitochondrial damage and caspase 3 activation.
The results of the present study are consistent with those of
previous studies performed in neuronal and 293T cells (10,13).
Thus, it was hypothesized that the expression of appoptosin
increases during diabetes due to factors such as hypoxia and
oxidative stress. The present study indicated that inhibiting the
expression of appoptosin in pancreatic β-cells may promote cell
viability upon transplantation and provide a novel approach for
treating diabetes. A limitation of the present study is that only
one siRNA was used. Although its specificity has been confirmed in
a previous study (10), further
research is required to validate and exclude off-targeting effects
of the siRNA used.
Appoptosin is a major regulator in neuronal disease
and health. However, the role and involvement of appoptosin in
other diseases remains unclear. Although the present study
confirmed that the expression of appoptosin was increased in MIN6
cells following treatment with CoCl2 and
H2O2, the appoptosin promoter and the
proteins interacting with the promoter are yet to be identified,
and are the focus of future investigation. Identifying the promoter
and upstream regulators of appoptosin will aid in more effective
inhibition of its function and accelerating the degradation of
appoptosin may also protect pancreatic β-cells.
Appoptosin is an endometrial mitochondrial protein
that is closely associated with mitochondrial function. Insulin
secretion primarily depends on mitochondria for its energy
requirement. Thus, appoptosin may be associated with insulin
secretion in pancreatic β-cells. The association between appoptosin
and insulin secretion warrants further investigation.
Acknowledgements
The authors thank Professor Zhang (Institute of
Neuroscience, Xiamen University, Xiamen, China) for providing the
plasmids.
Funding
The present study was supported by grants from the
National Natural Science Foundation to SY (grant no. 30973912), XL
(grant no. 81570770), CH (grant no. 81673661) and SL (grant no.
81270901), the Key Project of Fujian Provincial Science and
Technology Planning programs (grant no. 2012D60) and the Xiamen
Innovation Program for Outstanding Youth Scientist (grant no.
2011S0446) to SL, Xiamen Science and Technology Bureau (Xiamen
Research Platform for Systems Biology of Metabolic Disease, grant
no. 3502Z20100001).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TW, XL, SL and SY conceived and designed the study.
TW, WW, HAAM, CH, LL, QY, HY and CY performed the experiments. TW
and HAAM wrote the paper. XL, SL and SY reviewed and edited the
manuscript. SY agrees to be accountable for all aspects of the
work. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chiang JL, Kirkman MS, Laffel LM and
Peters AL: Type 1 Diabetes Sourcebook Authors: Type 1 diabetes
through the life span: A position statement of the American
Diabetes Association. Diabetes care. 37:2034–2054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. Jama. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atkinson MA, Eisenbarth GS and Michels AW:
Type 1 diabetes. Lancet. 383:69–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ryan EA, Lakey JR, Rajotte RV, Korbutt GS,
Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, et
al: Clinical outcomes and insulin secretion after islet
transplantation with the Edmonton protocol. Diabetes. 50:710–719.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruni A, Gala-Lopez B, Pepper AR,
Abualhassan NS and Shapiro AJ: Islet cell transplantation for the
treatment of type 1 diabetes: Recent advances and future
challenges. Diabetes Metab Syndr Obes. 7:211–223. 2014.PubMed/NCBI
|
|
6
|
Shapiro AJ, Lakey JR, Ryan EA, Korbutt GS,
Toth E, Warnock GL, Kneteman NM and Rajotte RV: Islet
transplantation in seven patients with type 1 diabetes mellitus
using a glucocorticoid-free immunosuppressive regimen. N Engl J
Med. 343:230–238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludwig B, Rotem A, Schmid J, Weir GC,
Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen
A, et al: Improvement of islet function in a bioartificial pancreas
by enhanced oxygen supply and growth hormone releasing hormone
agonist. Proc Natl Acad Sci. 109:pp. 5022–5027. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryan EA, Paty BW, Senior PA, Bigam D,
Alfadhli E, Kneteman NM, Lakey JR and Shapiro AM: Five-year
follow-up after clinical islet transplantation. Diabetes.
54:2060–2069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng CW, Villani V, Buono R, Wei M, Kumar
S, Yilmaz OH, Cohen P, Sneddon JB, Perin L and Longo VD:
Fasting-mimicking diet promotes ngn3-driven β-cell regeneration to
reverse diabetes. Cell. 168:775–788.e712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Zhang YW, Chen Y, Huang X, Zhou
F, Wang W, Xian B, Zhang X, Masliah E, Chen Q, et al: Appoptosin is
a novel pro-apoptotic protein and mediates cell death in
neurodegeneration. J Neurosci. 32:15565–15576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Shi Z, Zhang L, Zhou Z, Zheng X,
Liu G, Bu G, Fraser PE, Xu H and Zhang YW: Appoptosin interacts
with mitochondrial outer-membrane fusion proteins and regulates
mitochondrial morphology. J Cell Sci. 129:994–1002. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Tseng IC, Heyser CJ, Rockenstein
E, Mante M, Adame A, Zheng Q, Huang T, Wang X, Arslan PE, et al:
Appoptosin-mediated caspase cleavage of tau contributes to
progressive supranuclear palsy pathogenesis. Neuron. 87:963–975.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng KM, Zhang J, Zhang Cl, Zhang YW and
Chen XC: Curcumin inhibits appoptosin-induced apoptosis via
upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta
Pharmacol Sin. 36:544–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piret JP, Mottet D, Raes M and Michiels C:
CoCl2, a chemical inducer of hypoxia-inducible factor-1 and hypoxia
reduce apoptotic cell death in hepatoma cell line HepG2. Ann N Y
Acad Sci. 973:443–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sano M, Minamino T, Toko H, Miyauchi H,
Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, et al:
p53-induced inhibition of Hif-1 causes cardiac dysfunction during
pressure overload. Nature. 446:4442007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jaakkola P, Mole DR, Tian YM, Wilson MI,
Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji
M, Schofield CJ, et al: Targeting of HIF-α to the von Hippel-Lindau
Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation.
Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bruick RK and Mcknight SL: A conserved
family of prolyl-4-Hydroxylases that modify HIF. Science.
294:1337–1340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin WG Jr: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
Implications for O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung EC, Ludwig RL and Vousden KH:
Mitochondrial localization of TIGAR under hypoxia stimulates HK2
and lowers ROS and cell death. Proc Natl Acad Sci USA. 109:pp.
20491–20496. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franklin TJ, Morris WP, Edwards PN, Large
MS and Stephenson R: Inhibition of prolyl 4-hydroxylase in vitro
and in vivo by members of a novel series of phenanthrolinones.
Biochem J. 353:333–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banerji B, Conejogarcia A, Mcneill LA,
McDonough A, Buck MR, Hewitson KS, Oldham NJ and Schofield CJ: The
inhibition of factor inhibiting hypoxia-inducible factor (FIH) by
beta-oxocarboxylic acids. Chem Commun (Camb). 5438–5440. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Litmanovitz I, Bar-Yoseph F, Lifshitz Y,
Davidson K, Eliakim A, Regev RH and Nemet D: Reduced crying in term
infants fed high beta-palmitate formula: A double-blind randomized
clinical trial. BMC Pediatr. 14:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Shangguan Z, Liu Y, Wang J, Li X,
Yang S and Liu S: Puerarin protects pancreatic β-cell survival via
PI3K/Akt signaling pathway. J Mol Endocrinol. 53:71–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng X, Zheng X, Wang X, Ma Z, Gupta
Sunkari V, Botusan I, Takeda T, Björklund A, Inoue M, Catrina SB,
et al: Acute hypoxia induces apoptosis of pancreatic β-cell by
activation of the unfolded protein response and upregulation of
CHOP. Cell Death Dis. 3:e3222012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang Y, Zhang Q, Tan J, Li L, An X and Lei
P: Intermittent hypoxia-induced rat pancreatic β-cell apoptosis and
protective effects of antioxidant intervention. Nutr Diabetes.
4:e1312014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yildirim Ö: The effect of vitamin C and
cobalt supplementation on antioxidant status in healthy and
diabetic rats. African J Biotechnol. 8:2009.
|
|
29
|
Leonard SS, Harris GK and Shi X:
Metal-induced oxidative stress and signal transduction. Free
Radical Biol Med. 37:1921–1942. 2004. View Article : Google Scholar
|
|
30
|
Sato Y, Endo H, Okuyama H, Takeda T,
Iwahashi H, Imagawa A, Yamagata K, Shimomura I and Inoue M:
Cellular hypoxia of pancreatic beta-cells due to high levels of
oxygen consumption for insulin secretion in vitro. J Biol Chem.
286:12524–12532. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carlsson PO, Liss P, Andersson A and
Jansson L: Measurements of oxygen tension in native and
transplanted rat pancreatic islets. Diabetes. 47:1027–1032. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cantley J, Selman C, Shukla D, Abramov AY,
Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten
SK, et al: Deletion of the von Hippel-Lindau gene in pancreatic
beta cells impairs glucose homeostasis in mice. J Clin Invest.
119:125–135. 2009.PubMed/NCBI
|
|
33
|
Wang W, Upshaw L, Strong DM, Robertson RP
and Reems J: Increased oxygen consumption rates in response to high
glucose detected by a novel oxygen biosensor system in non-human
primate and human islets. J Endocrinol. 185:445–455. 2005.
View Article : Google Scholar : PubMed/NCBI
|