Introduction
Acute kidney injury (AKI) is a common problem in
critically ill patients, particularly in the intensive care unit
(ICU) (1). Sepsis and septic shock
are factors that contribute to the development of AKI, and ≥50%
mortality of sepsis patients in the ICU is associated with AKI
(2). Evidence suggests that
patients with non-severe pneumonia with sepsis have a significantly
higher incidence of AKI and increased inflammatory responses
(3,4). However, the underlying mechanisms are
not completely understood. A growing body of evidence has suggested
that sepsis-induced oxidative stress and inflammatory responses
contributed to sepsis-associated organ dysfunction, including AKI
(5–7).
Ursolic acid (UA) as a pentacyclic triterpenoid
compound is isolated from many plants, including Ligustrum
lucidum, Arctostaphylos uva-ursi and Eriobotrya
japonica, which are widely used as Chinese herbal medicines
(8). UA is readily absorbed from
daily foods, such as Fructus Crataegi (9), Fructus Chaenomelis Lagenariae
(10) and Fructus Mume (11). It is generally known that UA
exhibits multiple biological activities, such as anti-inflammatory
(12), anti-oxidant (13) and anti-tumor properties (14). Emerging evidence has demonstrated
that UA has a beneficial effect on hyperglycemia-induced renal
injury (15). In addition, UA
ameliorates carbon tetrachloride-induced oxidative DNA damage and
inflammation in kidneys of rats (16). Previous research has shown that UA
exerts protective effects on sepsis-induced organ damage (17,18).
However, the beneficial effect and underlying molecular mechanisms
of UA in sepsis-induced AKI are not clearly delineated. As sepsis
is an acute inflammatory and oxidative stress disorder involved in
AKI, inhibiting excessive inflammatory responses and oxidative
stress may be an effective therapeutic target.
In the present study, it was hypothesized that UA
may ameliorate sepsis-induced AKI via the inhibition of oxidative
stress and inflammatory responses. This study may further increase
understanding of organ failure prevention and the underlying
mechanisms of UA, which may be useful in managing sepsis-induced
AKI.
Materials and methods
Experimental animals
Ten-week-old male imprinting control region mice
(n=64) were purchased from Charles River Laboratories, Inc.,
(Wilmington, MA, USA) and were allowed to acclimate to the
environment for 1 week. The mice were fed under controlled
conditions: Temperature (25±2°C), humidity (55±5%) and 12-h
light/dark cycle, and the mice were given free access to food and
tap water.
Sepsis was induced in mice undergoing cecal ligation
and puncture (CLP) surgery (CLP Group, n=8). Mice were anesthetized
with 2% pentobarbital, and a 1- to 2-cm midline incision was made
along the linea-alba of the abdominal muscle to isolate and
exteriorize the cecum. A total of 75% of the cecum was ligated with
a 4-0 silk suture, and the cecum was punctured twice with a
21-gauge needle. A small amount (1 droplet) of feces was gently
extruded from the holes to ensure patency. The cecum was then
returned to the peritoneal cavity and the abdominal incision was
closed with 4-0 silk sutures. After the operation, 1 ml pre-warmed
normal saline was administered into the peritoneal cavity. The
survival rate was examined over the whole experiment (up to 4
days). For the UA (Aldrich Chemical Co., Milwaukee, WI, USA; high
performance liquid chromatography, 98%) treatment groups (2 mg/kg,
low-concentration, n=8 or 20 mg/kg, high-concentration, n=8), mice
received intraperitoneal administration of UA 2 weeks before CLP
surgery. In the sham-operated group (control group, n=8), mice
underwent the same procedure but were neither ligated nor
punctured, and the mice were intraperitoneally injected with 2 ml
PBS. In another experiment, the 96 h survival of CLP mice with or
without UA treatment was observed (n=8 in each group).
The animal study was approved by the Animal Care and
Use Committee at the Beijing Tsinghua Changgung Hospital (Beijing,
China; permit number: TCH-2015-0019).
Serum inflammatory cytokine
measurement
The effects of UA on serum cytokine levels were
analyzed by ELISA at 24 h after CLP, which was determined to be the
optimum measuring time in previous studies (17,19).
Serum levels of tumor necrosis factor-α (TNF-α; cat. no.
E-EL-M0049), interleukin (IL)-1β (cat. no. E-EL-M0037) and IL-6
(cat. no. E-EL-M0044) were detected using a mouse bioactive ELISA
assay, according to the manufacturer's protocol (Elabscience
Biotechnology Co., Ltd., Wuhan, China), there were three replicates
for each sample tested, n=8 in the control group; n=6 in CLP group
(2 mice succumbed within 24 h); n=7 in UA(L)+CLP group (1 mouse
succumbed within 24 h); n=8 in UA(H)+CLP group.
Measurement of serum creatinine (Scr)
and blood urea nitrogen (BUN)
Blood samples were collected at 24 h after CLP
surgery in each group and renal function was monitored by measuring
the concentration of BUN (cat. no. C013-2) and Scr (cat. no.
C011-1) in serum using an enzymatic kinetic methods and picric acid
methods detection kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China), according to the manufacturer's protocol. For BUN
and Scr, n=8 in the control group; n=6 in CLP group (2 mice
succumbed within 24 h); n=7 in UA(L)+CLP group (1 mouse succumbed
within 24 h); n=8 in UA(H)+CLP group.
Measurement of reactive oxygen species
(ROS)
The renal tissues were harvested and homogenized in
phosphate buffer at 24 h after CLP surgery in each group. ROS (ID:
GMS10016.3; Genmed Scientifics, Inc., Shanghai, China) levels were
measured using a dichloro-dihydro-fluorescein diacetate (DCFH-DA)
assay, according to the manufacturer's protocol. In brief, kidney
tissue whole homogenate (100 µl) was added to 1.4 ml 50 mM
phosphate buffer in dark-adapted counting vials. After dark
adaptation for 1 h at room temperature, the homogenate was
incubated with 10 µM DCFH-DA for 20 min at 37°C in the dark for the
detection of ROS. The samples were counted every 20 sec for 3 min
using a luminometer (Autolumat Plus LB953; Berthold Technologies
GmbH, Bad Wildbad, Germany).
Measurement of
8-hydroxy-2′-deoxyguanosine (8-OHdG)
Plasma levels of an oxidative stress marker, 8-OHdG,
was measured using a DNA damage ELISA kit (cat. no. ADI-EKS-350;
Enzo Life Sciences, Inc., Farmingdale, NY, USA), according to the
manufacturer's protocol.
Hematoxylin and eosin staining
Formalin-fixed (4% formalin at room temperature for
24 h) and paraffin-embedded kidney tissues were cut into ~5
µm-thick sections, which were stained with hematoxylin and eosin
(cat. no. C0105; Beyotime Institute of Biotechnology, Haimen,
China) at room temperature for 1–2 min, and visualized under a
light microscope (model DM 2500; Leica Microsystems, Inc., Buffalo
Grove, IL, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse kidney [n=8 in
the control group; n=6 in CLP group (2 mice succumbed within 24 h);
n=7 in UA(L)+CLP group (1 mouse succumbed within 24 h); n=8 in UA
(H) +CLP group] using TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and reverse transcribed into cDNA using a
SuperScriptIII reverse transcriptase kit (Invitrogen; Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol. RT-qPCR
was used to determine mRNA expression levels using SYBR-Green
Master mix (Invitrogen; Thermo Fisher Scientific, Inc.) on a
Stratagene MX3005P system (Agilent Technologies, Inc., Santa Clara,
CA, USA). The thermocycling conditions were as follows: 95°C for 10
min followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and
72°C for 30 sec. GAPDH served as an internal standard. Relative
gene expression was calculated using the 2−ΔΔCq method
(20). The following primers were
used: Forward, 5′-CACCACCATCAAGGACTCAA-3′ and reverse,
5′-GAGACAGAGGCAACCTGACC-3′ for TNF-α; forward,
5′-TCGCCAGTGAAATGATGGCTTA-3′ and reverse,
5′-GTCCATGGCCACAACAACTGA-3′ for IL-1β; forward,
5′-CGGAGAGGAGACTTCACAGAG-3′ and reverse, 5′-CATTTCCACGATTTCCCAGA-3′
for IL-6; forward, 5′-GGGTGAGAACGGGCCTGGAGGA-3′ and reverse,
5′-GTAGAAGCCCGGTGTGCCGTGA-3′ for superoxide dismutase (SOD)1;
forward, 5′-TCGGTAACGTGAGTGTGGCAAT-3′ and reverse,
5′-CGTGTTGGACCGGTGTGCACCGT-3′ for SOD2; forward,
5′-ACAGGGGAGGTGATAGCATT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTC-3′ for GAPDH. For PCR, n=8 in the
control group; n=6 in CLP group (2 mice death within 24 h); n=7 in
UA(L)+CLP group (1 mouse death within 24 h); n=8 in UA(H)+CLP
group. The experiment was repeated twice. Three replicates were
performed for each sample.
Western blotting
Kidneys were homogenized and lysed in NP-40 buffer
(Beyotime Institute of Biotechnology, Haimen, China). Following
5–10 min boiling, homogenates were centrifuged at 10,000 × g at 4°C
for 10 min to obtain the supernatant. Protein samples (50 µg) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked with 5% (w/v) non-fat milk powder in Tris-buffered
saline containing 0.1% (w/v) Tween 20 for 2 h at room temperature,
and subsequently incubated with the following primary antibodies:
TNF-α (cat. no. sc-52746), IL-1β (cat. no. sc-515598), IL-6 (cat.
no. sc-32296), NF-κB (cat. no. sc-114) and β-actin (cat. no.
sc-517582; all from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA; all 1: 1,000), at 4°C overnight. After being washed, the
membranes were incubated with horseradish peroxidase-conjugated
anti IgG (cat. no. sc-516102; 1:10,000; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h. Signal detection was carried out
with an enhanced chemiluminescence system (GE Healthcare, Chicago,
IL, USA), and protein bands were analyzed with Quantity
One® software version 4.5 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). For western blotting, n=8 in the control group;
n=6 in CLP group (2 mice succumbed within 24 h); n=7 in UA(L)+CLP
group (1 mouse succumbed within 24 h); n=8 in UA(H)+CLP group.
Statistical analysis
Data are presented as the mean ± standard deviation
for each group. Statistical analyses were performed by using
GraphPad Prism version 6.0 software (GraphPad Software, Inc., La
Jolla, CA, USA). Inter-group differences were analyzed by one-way
analysis of variance followed by Tukey's multiple comparison
post-hoc test. P<0.05 was used to indicate a statistically
significant difference.
Results
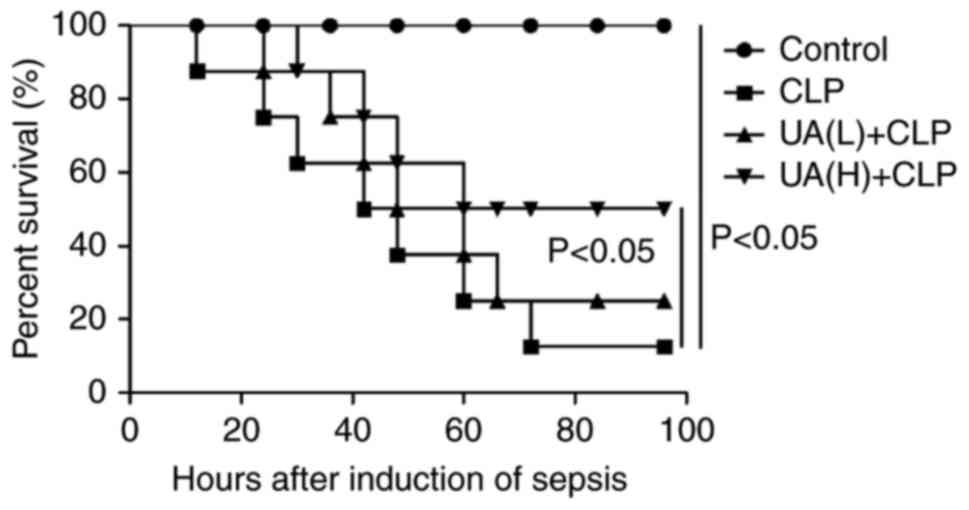
UA administration improves survival in
septic mice
A previous study demonstrated that UA administration
improves survival in CLP-induced lung injury septic mice (17). In line with these findings, the
present study revealed that UA pretreatment before the induction of
sepsis by CLP significantly improved the survival of mice
undergoing CLP compared with mice treated with CLP alone.
Approximately 90% of mice in the CLP group died at 72 h after
undergoing CLP surgery. Administration of UA (20 mg/kg) had a
protective effect against lethality induced by CLP (P<0.05), and
increased the animal survival rate from 12.5 to 50%. However, a low
concentration of UA (2 mg/kg) did not significantly improve the
survival of CLP-induced septic mice (Fig. 1).
UA improves sepsis-induced AKI in
mice
Previous studies have suggested that UA alleviates
sepsis-induced acute lung injury in rodent models (17,18).
However, there appears to be no associated reports on
sepsis-induced AKI. To determine the role of UA in sepsis-induced
AKI in mice, renal histological examination by hematoxylin and
eosin staining was implemented. In control mice, relatively intact
structures of the kidney tissues were observed. By contrast,
glomerular damage and vacuolization were observed in the proximal
tubules in the CLP group. Low concentrations of UA (2 mg/kg) had no
dramatic improvement in renal tissue damage in septic mice.
However, high concentrations of UA (20 mg/kg) ameliorated the renal
tissue damage in septic mice (Fig.
2A). It is well known that AKI is a serious complication in
septic rats, and BUN and Scr are frequently used as biomarkers in
the early stages of the development of AKI (21). In the present study, BUN and Scr
levels were measured at 24 h after CLP surgery to evaluate renal
function in septic mice. As shown in Fig. 2B and C, BUN and Scr were
significantly increased in mice undergoing CLP surgery compared
with the control group. Administration of high concentrations of UA
significantly reversed the CLP-induced upregulation of BUN and Scr
in septic mice (P<0.05). Low concentrations of UA did not
significantly reduce the levels of BUN and Scr.
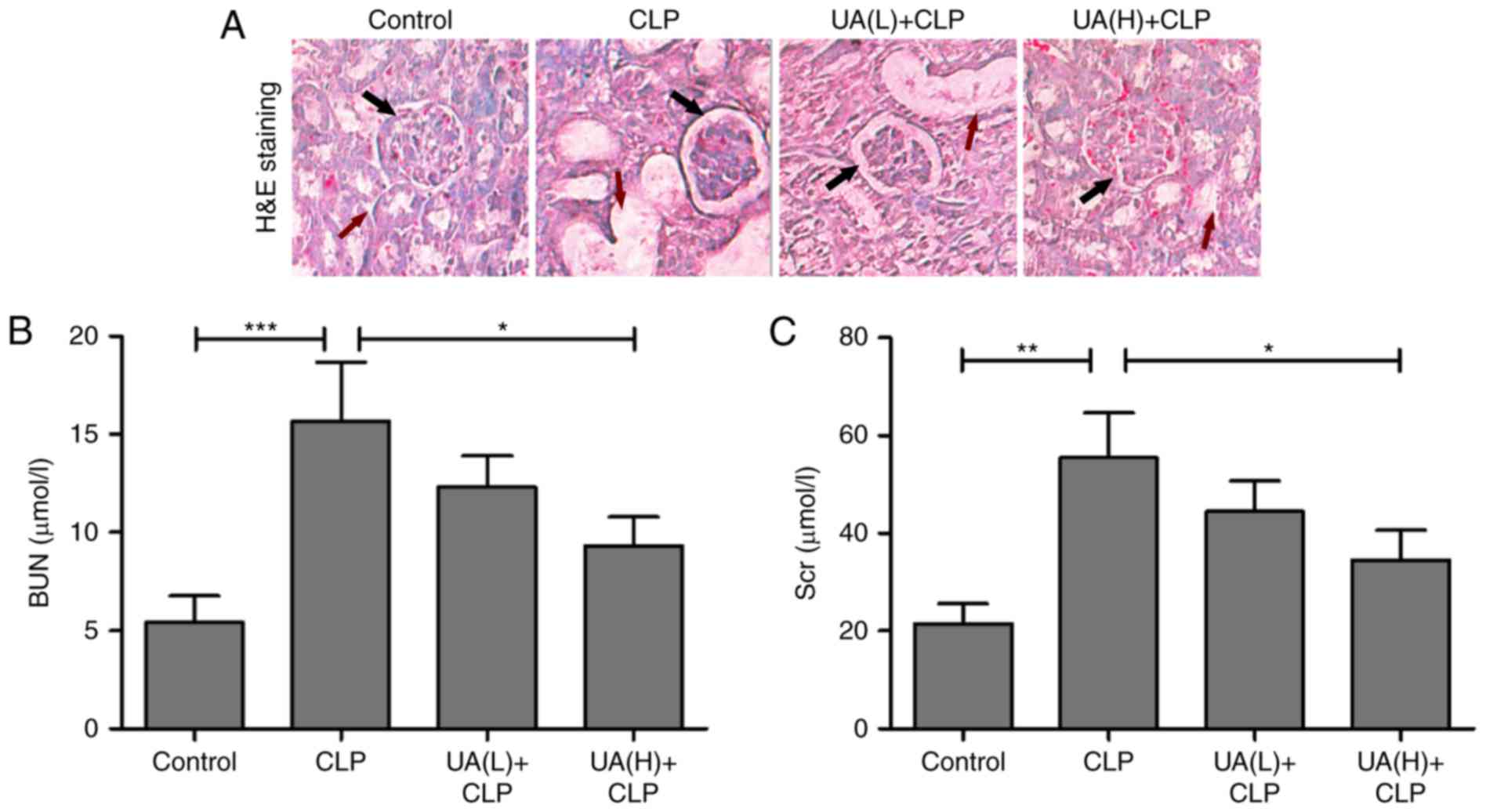 | Figure 2.UA improves sepsis-induced AKI. (A)
Hematoxylin and eosin staining of kidney tissue from a
representative mouse in the control, CLP, UA(L)+CLP and UA(H)+CLP
groups. Magnification, ×100. Black arrows indicated glomerular
damage degree, and red arrows indicated the degree of vacuolization
in the proximal tubules. Concentration of (B) BUN and (C) Scr in
serum of mice of each group. UA, ursolic acid; CLP, cecal ligation
and puncture; UA(L)+CLP, low-dose ursolic acid + cecal ligation and
puncture; UA(H)+CLP, high-dose ursolic acid + cecal ligation and
puncture; BUN, blood urea nitrogen; Scr, serum creatinine; AKI,
acute kidney injury. *P<0.05; **P<0.001; ***P<0.001. |
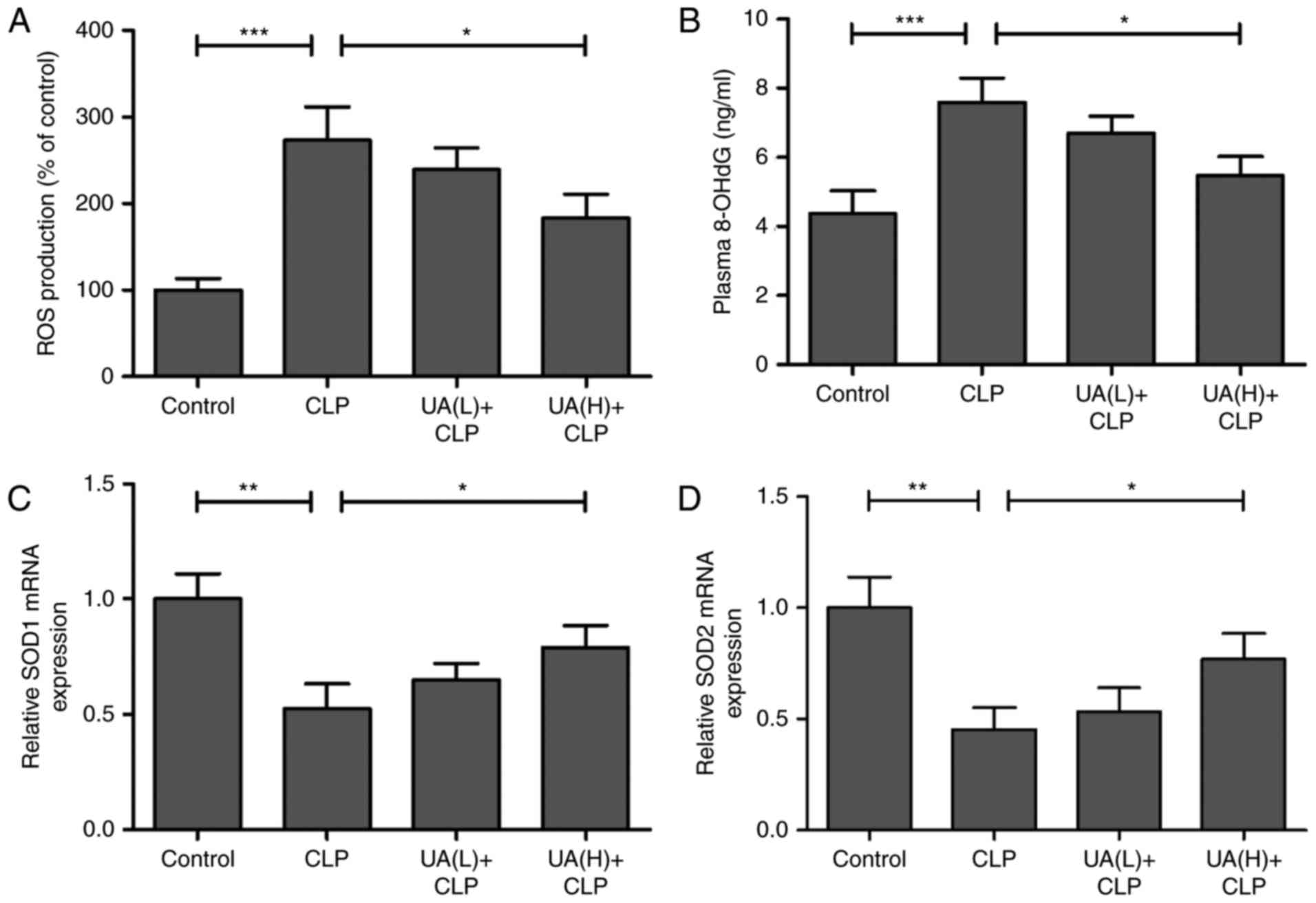
UA restrains CLP-induced oxidative
stress damage in septic mice
The effect of UA on CLP-induced ROS in the kidney of
septic mice was measured. Treatment with CLP surgery resulted in a
significant increase in ROS production compared with the control
group (P<0.001), whereas pretreatment with high concentrations
of UA significantly inhibited CLP-induced ROS production
(P<0.05; Fig. 3A). In addition,
plasma 8-OHdG levels, a measure of DNA oxidative damage, were
significantly higher in the CLP group compared with the control
group (P<0.001), whereas pretreatment with high concentrations
of UA significantly reversed CLP-induced 8-OhdG (P<0.05;
Fig. 3B). Furthermore, SOD1 and
SOD2, as antioxidant enzymes, serve crucial roles in the defense
against oxygen-free radicals (22). In the present study, the mRNA
expression levels of SOD1 and SOD2 were measured by RT-qPCR in
mouse kidney tissue, and the results demonstrated that SOD1 and
SOD2 were significantly decreased in the kidney from CLP septic
mice compared with that from control animals (P<0.01). High
concentrations of UA significantly reversed the CLP-induced
downregulation of SOD1 and SOD2 in CLP septic mice (P<0.05;
Fig. 3C and D).
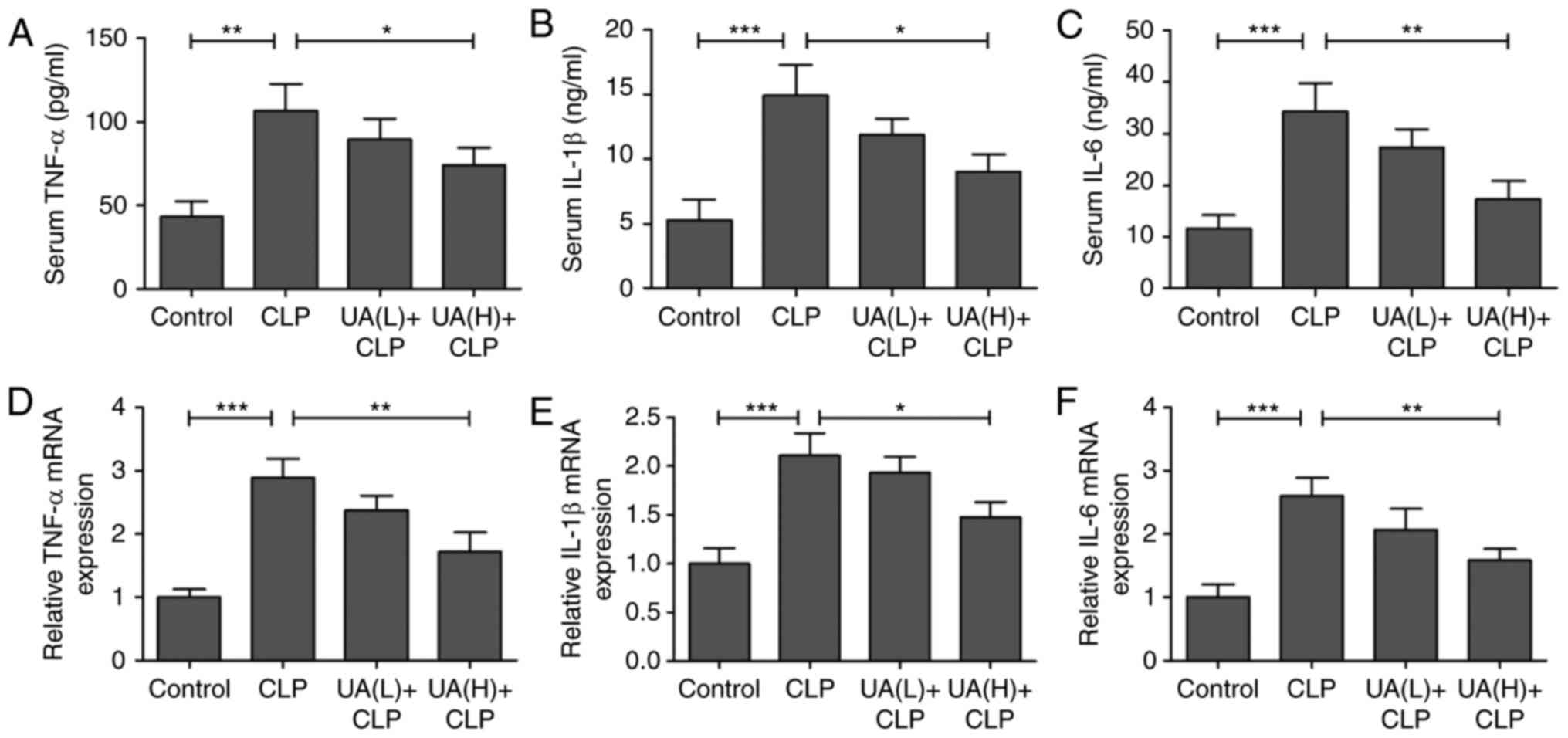
UA suppresses inflammatory responses
in septic mice
Previous studies have suggested that oxidative
stress directly or indirectly elevates inflammatory responses
(21,23,24).
Therefore, serum TNF-α, IL-1β and IL-6 levels were measured at 24 h
after CLP surgery. Serum TNF-α (Fig.
4A), IL-1β (Fig. 4B) and IL-6
(Fig. 4C) levels were
significantly increased in the CLP group compared with the control
group. However, treatment with high concentrations of UA
significantly decreased the levels of these inflammatory cytokines
in septic mice (P<0.05). In addition, the mRNA expression levels
of TNF-α (Fig. 4D), IL-1β
(Fig. 4E) and IL-6 (Fig. 4F) were measured using RT-qPCR in
kidney tissues, and the results demonstrated that treatment with
high concentrations of UA attenuated the CLP-activated increase in
TNF-α, IL-1β and IL-6 expression (P<0.05). As shown in Fig. 5A, B and C, it was found that
pretreatment with high concentrations of UA significantly
suppressed the CLP-induced increase in TNF-α, IL-1β and IL-6
protein expression in kidney tissues from septic mice (P<0.01).
Consequently, we examined whether the upstream NF-κB signal
transduction pathway was also involved in CLP-induced AKI. The
transcription factor NF-κB is considered to be the primary mediator
of the inflammatory response (25), and the inhibition of NF-κB has been
shown to alleviate sepsis-associated AKI in rats (5,26).
Therefore, the present study evaluated the effect of UA on the
nuclear translocation of NF-κB. The results demonstrated that the
expression of NF-κB was significantly increased in the CLP group
compared with control group (P<0.001). Conversely, the
administration of high concentrations of UA significantly reversed
the increase in NF-κB expression in the kidney from septic mice
(P<0.001; Fig. 5D).
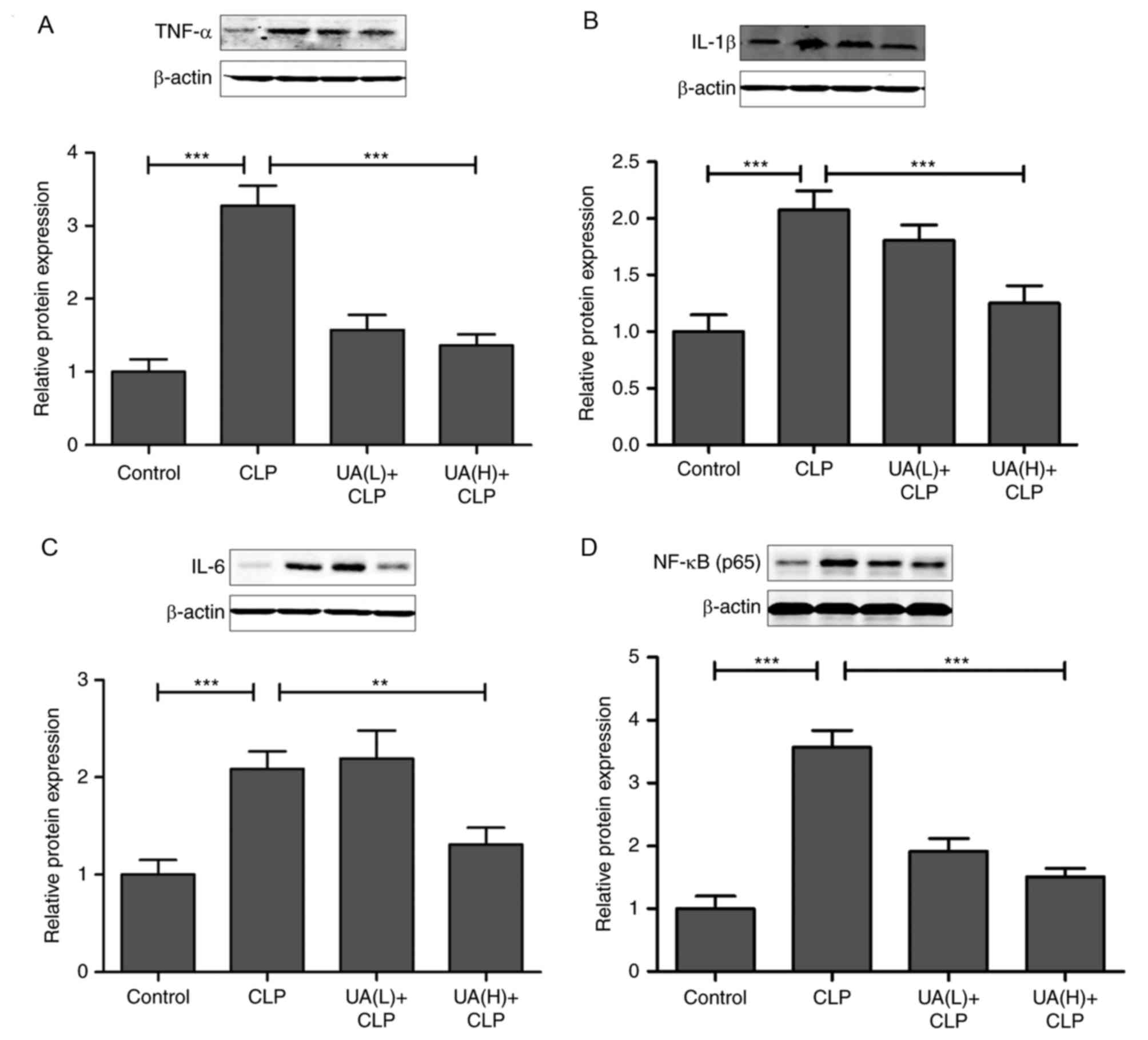 | Figure 5.UA inhibits NF-κB, TNF-α, IL-1β and
IL-6 protein expression in the kidney from septic mice. The levels
of (A) TNF-α, (B) IL-1β, (C) IL-6 and (D) NF-κB protein expression
were measured by western blotting and densitometric analysis in
each group. *P<0.05; **P<0.001; ***P<0.001. UA, ursolic
acid; CLP, cecal ligation and puncture; TNF-α, tumor necrosis
factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; NF-κB,
necrosis factor-κB. |
Discussion
The present study used the experimental model of
CLP-induced AKI to examine the effects of UA on sepsis-associated
kidney injury in mice. First, results demonstrated that UA
administration improved survival in septic mice induced by CLP
surgery. The treatment of UA revealed protection against AKI
induced by CLP surgery, including alleviation of glomerular damage
and vacuolization in the proximal tubules. Furthermore, the effects
of UA on oxidative stress and inflammation in septic mice were
determined. The findings suggested that the high concentrations of
UA (20 mg/kg) significantly protected against sepsis-induced AKI by
inhibiting oxidative stress and inflammatory cytokines in the
kidney from septic mice. It is well known that the concentration of
UA is flexible in different experimental research. Consistent with
previous reports, the dose of UA in rodent animal model is used at
the same magnitude (16).
Mounting evidence has demonstrated that high
free-radical concentrations are generated in patients with sepsis
syndrome, and the balance between oxidation and anti-oxidation is
markedly disturbed (5,27). Increased production of ROS is one
of the key features of sepsis, which may cause enhanced AKI
(5,21,28).
In the present study, the findings revealed that ROS production was
significantly increased by CLP surgery, whereas pretreatment with
high concentrations of UA significantly inhibited CLP-induced ROS
production in the kidney of septic mice, which suggested that UA
protects against sepsis-induced AKI by inhibiting oxidative stress
damage. A previous study indicated that SOD activity is
significantly downregulated in septic patients, which is negatively
associated with the severity of sepsis (29). In addition, the levels of the
antioxidant SOD notably decreased in septic rats in every region of
the brain (30). In the present
study, it was found that the mRNA expression levels of SOD1 and
SOD2 were significantly decreased in the kidney from septic mice,
and high concentrations of UA treatment significantly reversed
CLP-induced downregulation of SOD1 and SOD2. These results concur
with previous observations that an imbalance exists between
oxidants and antioxidants during sepsis (31). The findings of the present study
demonstrated an anti-oxidant role for UA in septic mice.
TNF-α, IL-1β and IL-6 may be readily activated by
pathological conditions, and are biomarkers of early inflammatory
responses, as confirmed in previous studies (32–34).
Under septic conditions, the expression of inflammatory cytokines,
such as IL-1β, IL-6 and TNF-α, are significantly upregulated in
pathological tissues, including lung (17), cerebrum (18,30)
and kidney (3,5). Clinical studies have found that the
levels of TNF-α and IL-1β are increased in the serum of septic
patients (35). Inhibition of
TNF-α and IL-1β alleviates the progression of sepsis in animal
models (18,28). In the present study, it was
demonstrated that serum levels of TNF-α, IL-1β and IL-6 and mRNA
and protein expression levels of TNF-α, IL-1β and IL-6 in kidney
were significantly increased in septic mice; however, pretreatment
with UA attenuated the inflammatory response in septic mice,
resulting in the downregulation of TNF-α, IL-6 and IL-1β levels in
serum and kidney tissues. Inflammatory signals are known to merge
in the activation of the NF-κB signaling pathway, and NF-κB has
been shown to serve a critical role in modulating mortality in
experimental sepsis (36,37). The results of the present study
demonstrated that NF-κB was significantly increased in septic mice.
Consistent with several original studies (16,17),
the findings of the present study also revealed that UA has
anti-inflammatory properties by inhibiting NF-κB expression.
Previous studies indicated that targeted knockout of IL-17A
protects against sepsis-associated AKI and host defense (38,39).
The precise underlying pathogenic mechanism of TNF-α, IL-1β or IL-6
knockout in sepsis-induced AKI will be discussed in future studies.
In addition, a more in-depth kinetic analysis of the inflammatory
markers in septic mice is to be explored.
To the best of our knowledge, the present study
provides evidence for the first time that UA is a novel therapeutic
drug that protects against sepsis-induced AKI, and the underlying
mechanism was mediated, at least partially, by inhibiting oxidative
stress and inflammatory responses. The present study also provides
a possible method for therapy sepsis-induced AKI in clinical
practice. However, cross-sectional and longitudinal studies are
required to determine the proper concentration of UA, which will be
performed in future studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
Study design: ZW and ZZ; literature research, data
acquisition and data analysis: ZZ, RC, ZW and HZ; manuscript
preparation and manuscript editing: ZZ and RC; manuscript review:
ZW, ZZ, HZ and RC; final approval of the version of the manuscript
to be published: ZZ, HZ, RC and ZW; histological examination: RC;
establishment of animal model: HZ.
Ethics approval and consent to
participate
The animal study was approved by the Animal Care and
Use Committee at the Beijing Tsinghua Changgung Hospital (Beijing,
China; permit number: TCH-2015-0019).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Zhang A, Cai Y, Wang PF, Qu JN, Luo ZC,
Chen XD, Huang B, Liu Y, Huang WQ, Wu J and Yin YH: Diagnosis and
prognosis of neutrophil gelatinase-associated lipocalin for acute
kidney injury with sepsis: A systematic review and meta-analysis.
Crit Care. 20:412016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopes JA, Fernandes P, Jorge S, Resina C,
Santos C, Pereira A, Neves J, Antunes F and Gomes da Costa A:
Long-term risk of mortality after acute kidney injury in patients
with sepsis: A contemporary analysis. BMC Nephrol. 11:92010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zarjou A and Agarwal A: Sepsis and acute
kidney injury. J Am Soc Nephrol. 22:999–1006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murugan R, Karajala-Subramanyam V, Lee M,
Yende S, Kong L, Carter M, Angus DC and Kellum JA: Genetic and
Inflammatory Markers of Sepsis (GenIMS) Investigators: Acute kidney
injury in non-severe pneumonia is associated with an increased
immune response and lower survival. Kidney Int. 77:527–535. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li N, Xie H, Li L, Wang J, Fang M, Yang N
and Lin H: Effects of honokiol on sepsis-induced acute kidney
injury in an experimental model of sepsis in rats. Inflammation.
37:1191–1199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quoilin C, Mouithys-Mickalad A, Lécart S,
Fontaine-Aupart MP and Hoebeke M: Evidence of oxidative stress and
mitochondrial respiratory chain dysfunction in an in vitro model of
sepsis-induced kidney injury. Biochim Biophys Acta. 1837:1790–1800.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weng TI, Wu HY, Kuo CW and Liu SH:
Honokiol rescues sepsis-associated acute lung injury and lethality
via the inhibition of oxidative stress and inflammation. Intensive
Care Med. 37:533–541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin H, Pi J, Yang F, Jiang J, Wang X, Bai
H, Shao M, Huang L, Zhu H, Yang P, et al: Folate-chitosan
nanoparticles loaded with ursolic acid confer anti-breast cancer
activities in vitro and in vivo. Sci Rep. 6:307822016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu SM, Shih WT, Yang YH, Chen PC and Chu
YH: Use of traditional Chinese medicine in patients with
hyperlipidemia: A population-based study in Taiwan. J
Ethnopharmacol. 168:129–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Zhang L, Quan S, Hong Y, Sun L and
Liu M: Isolation and identification of Triterpenoid compounds in
the fruits of Chaenomeles lagenaria (Loisel.) Koidz. Zhongguo Zhong
Yao Za Zhi. 23(546–547): 5761998.(In Chinese).
|
|
11
|
Shen H, Cheng T, Qiao C, Su Z and Li C:
Antitumor effect in vitro and immuno-response in vivo of fructus
Mume. Zhongguo Zhong Yao Za Zhi. 20:365–368. 1995.(In Chinese).
PubMed/NCBI
|
|
12
|
Alvarado HL, Abrego G, Garduño-Ramirez ML,
Clares B, Calpena AC and García ML: Design and optimization of
oleanolic/ursolic acid-loaded nanoplatforms for ocular
anti-inflammatory applications. Nanomedicine. 11:521–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad S, Yadav VR, Sung B, Gupta SC,
Tyagi AK and Aggarwal BB: Ursolic acid inhibits the growth of human
pancreatic cancer and enhances the antitumor potential of
gemcitabine in an orthotopic mouse model through suppression of the
inflammatory microenvironment. Oncotarget. 7:13182–13196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang ZH, Hsu CC, Huang CN and Yin MC:
Anti-glycative effects of oleanolic acid and ursolic acid in kidney
of diabetic mice. Eur J Pharmacol. 628:255–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma JQ, Ding J, Xiao ZH and Liu CM: Ursolic
acid ameliorates carbon tetrachloride-induced oxidative DNA damage
and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB
activities. Int Immunopharmacol. 21:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu Z, Gu Z, Sun M, Zhang K, Gao P, Yang Q
and Yuan Y: Ursolic acid improves survival and attenuates lung
injury in septic rats induced by cecal ligation and puncture. J
Surg Res. 194:528–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Wan Y, Zhou T, Li J and Wei Y:
Ursolic acid attenuates lipopolysaccharide-induced acute lung
injury in a mouse model. Immunotherapy. 5:39–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh SJ, Kim JH and Chung DH: NOD2-mediated
suppression of CD55 on neutrophils enhances C5a generation during
polymicrobial sepsis. PLoS Pathog. 9:e10033512013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Huang J, Li Y, Chang R, Wu H, Lin
J and Huang Z: Exogenous carbon monoxide decreases sepsis-induced
acute kidney injury and inhibits NLRP3 inflammasome activation in
rats. Int J Mol Sci. 16:20595–20608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johns EJ, O'Shaughnessy B, O'Neill S, Lane
B and Healy V: Impact of elevated dietary sodium intake on NAD(P)H
oxidase and SOD in the cortex and medulla of the rat kidney. Am J
Physiol Regul Integr Comp Physiol. 299:R234–R240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Wang X, Zheng M and Luan QX:
Mitochondrial reactive oxygen species mediate the
lipopolysaccharide-induced pro-inflammatory response in human
gingival fibroblasts. Exp Cell Res. 347:212–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Closa D and Folch-Puy E: Oxygen free
radicals and the systemic inflammatory response. IUBMB Life.
56:185–191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leslie KL, Song GJ, Barrick S, Wehbi VL,
Vilardaga JP, Bauer PM and Bisello A: Ezrin-radixin-moesin-binding
phosphoprotein 50 (EBP50) and nuclear factor-κB (NF-κB): A
feed-forward loop for systemic and vascular inflammation. J Biol
Chem. 288:36426–36436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Souza AC, Volpini RA, Shimizu MH, Sanches
TR, Camara NO, Semedo P, Rodrigues CE, Seguro AC and Andrade L:
Erythropoietin prevents sepsis-related acute kidney injury in rats
by inhibiting NF-κB and upregulating endothelial nitric oxide
synthase. Am J Physiol Renal Physiol. 302:F1045–F1054. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galley HF, Davies MJ and Webster NR:
Xanthine oxidase activity and free radical generation in patients
with sepsis syndrome. Crit Care Med. 24:1649–1653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao L, Liu Z, Zhu J, Li B, Chai C and Tian
Y: Clinical evaluation of circulating microRNA-25 level change in
sepsis and its potential relationship with oxidative stress. Int J
Clin Exp Pathol. 8:7675–7684. 2015.PubMed/NCBI
|
|
30
|
Chen Q, Yu W, Shi J, Shen J, Gao T, Zhang
J, Xi F, Li J and Li N: Insulin alleviates the inflammatory
response and oxidative stress injury in cerebral tissues in septic
rats. J Inflamm (Lond). 11:182014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He L, Peng X, Zhu J, Chen X, Liu H, Tang
C, Dong Z, Liu F and Peng Y: Mangiferin attenuate sepsis-induced
acute kidney injury via antioxidant and anti-inflammatory effects.
Am J Nephrol. 40:441–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sedlár M, Kudrnová Z, Erhart D, Trca S,
Kvasnicka J, Krska Z, Mazoch J, Malíková I, Zeman M and Linhart A:
Older age and type of surgery predict the early inflammatory
response to hip trauma mediated by interleukin-6 (IL-6). Arch
Gerontol Geriatr. 51:e1–e6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Canault M, Peiretti F, Mueller C, Kopp F,
Morange P, Rihs S, Portugal H, Juhan-Vague I and Nalbone G:
Exclusive expression of transmembrane TNF-alpha in mice reduces the
inflammatory response in early lipid lesions of aortic sinus.
Atherosclerosis. 172:211–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kajahn J, Franz S, Rueckert E, Forstreuter
I, Hintze V, Moeller S and Simon JC: Artificial extracellular
matrices composed of collagen I and high sulfated hyaluronan
modulate monocyte to macrophage differentiation under conditions of
sterile inflammation. Biomatter. 2:226–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moldawer LL: Interleukin-1, TNF alpha and
their naturally occurring antagonists in sepsis. Blood Purif.
11:128–133. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Z, Wang Y, Ning Q, Gong C, Zhang Y,
Zhang L, Bu X and Jing G: The role of spleen in the treatment of
experimental lipopolysaccharide-induced sepsis with
dexmedetomidine. Springerplus. 4:8002015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo CJ, Luo F, Zhang L, Xu Y, Cai GY, Fu
B, Feng Z, Sun XF and Chen XM: Knockout of interleukin-17A protects
against sepsis-associated acute kidney injury. Ann Intensive Care.
6:562016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ogiku M, Kono H, Hara M, Tsuchiya M and
Fujii H: Interleukin-17A plays a pivotal role in polymicrobial
sepsis according to studies using IL-17A knockout mice. J Surg Res.
174:142–149. 2012. View Article : Google Scholar : PubMed/NCBI
|