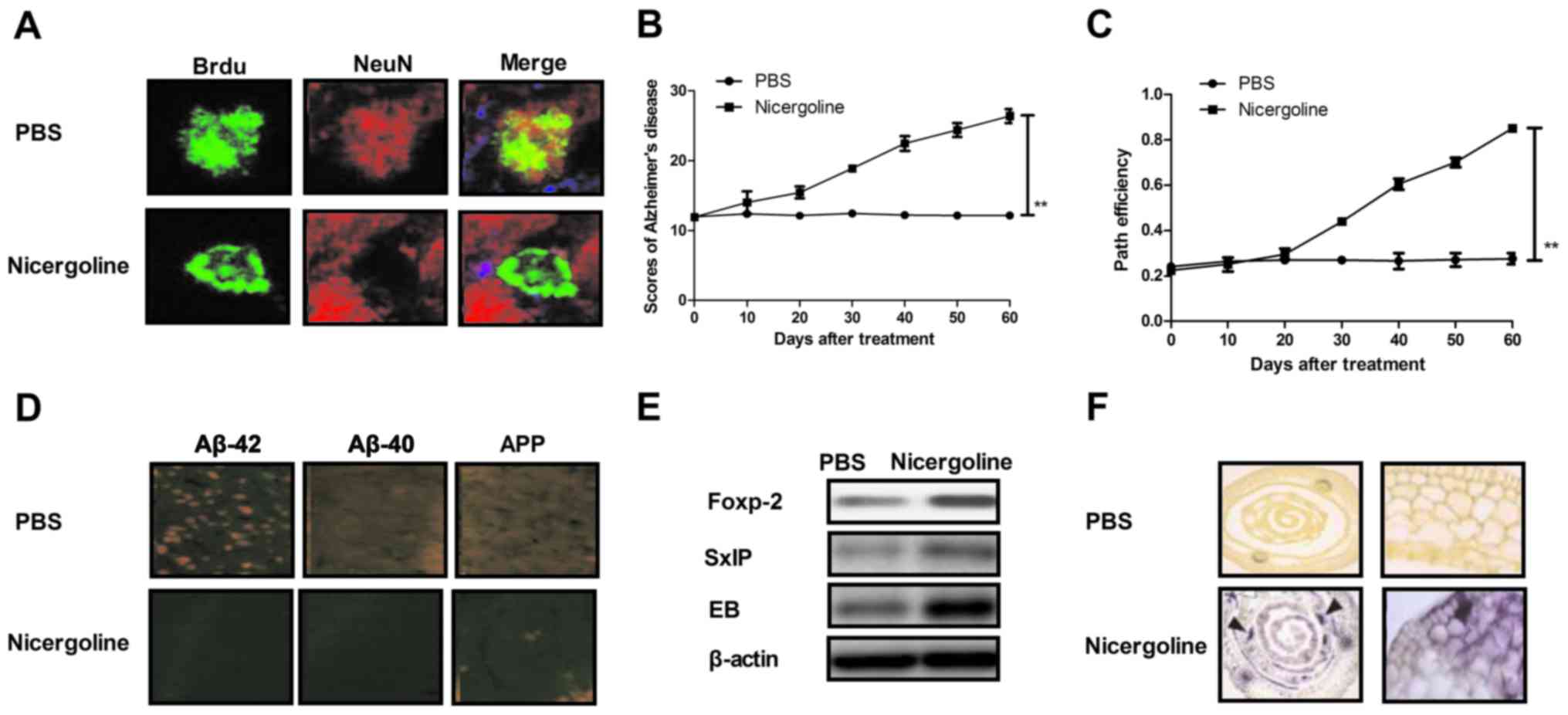
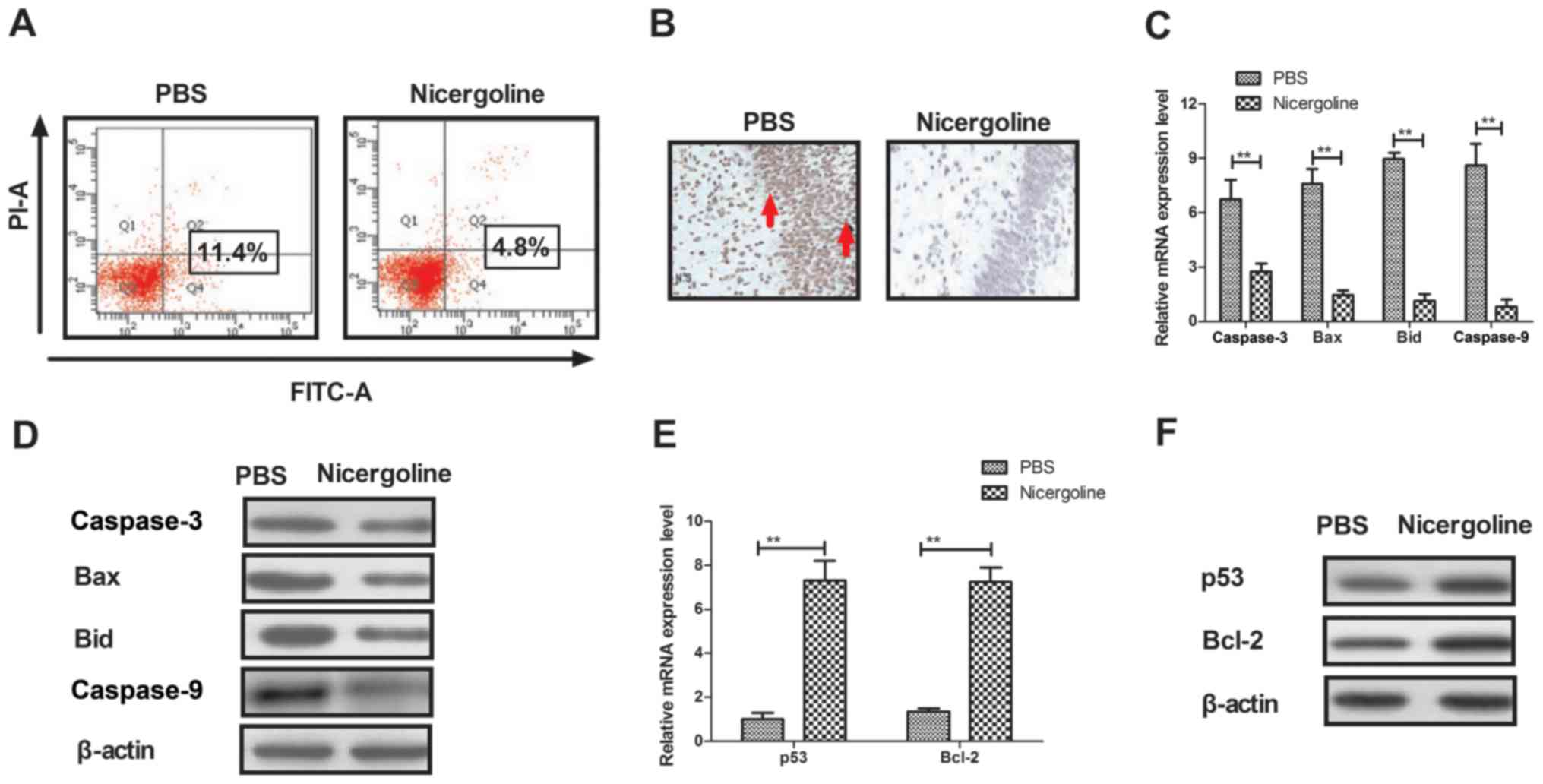
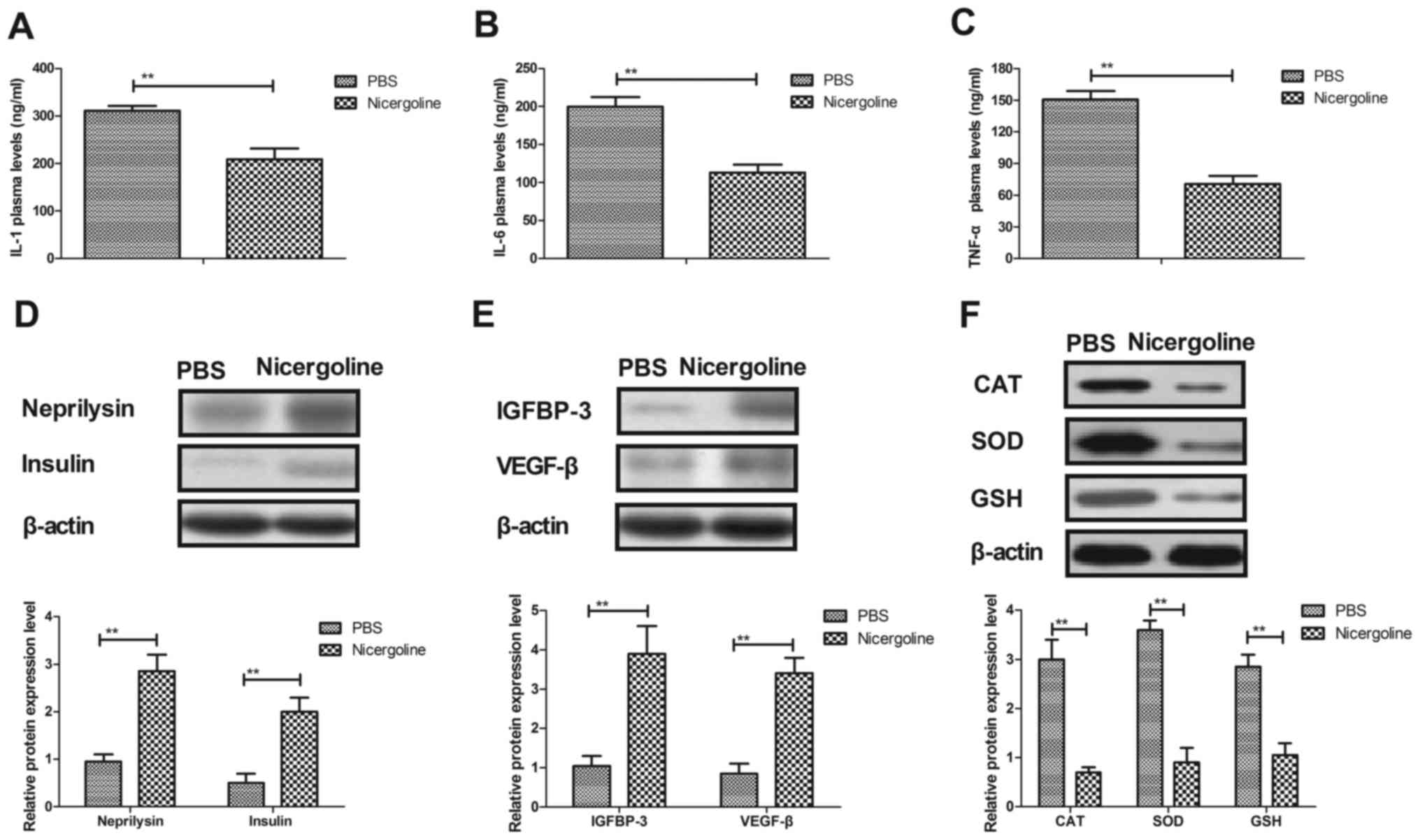
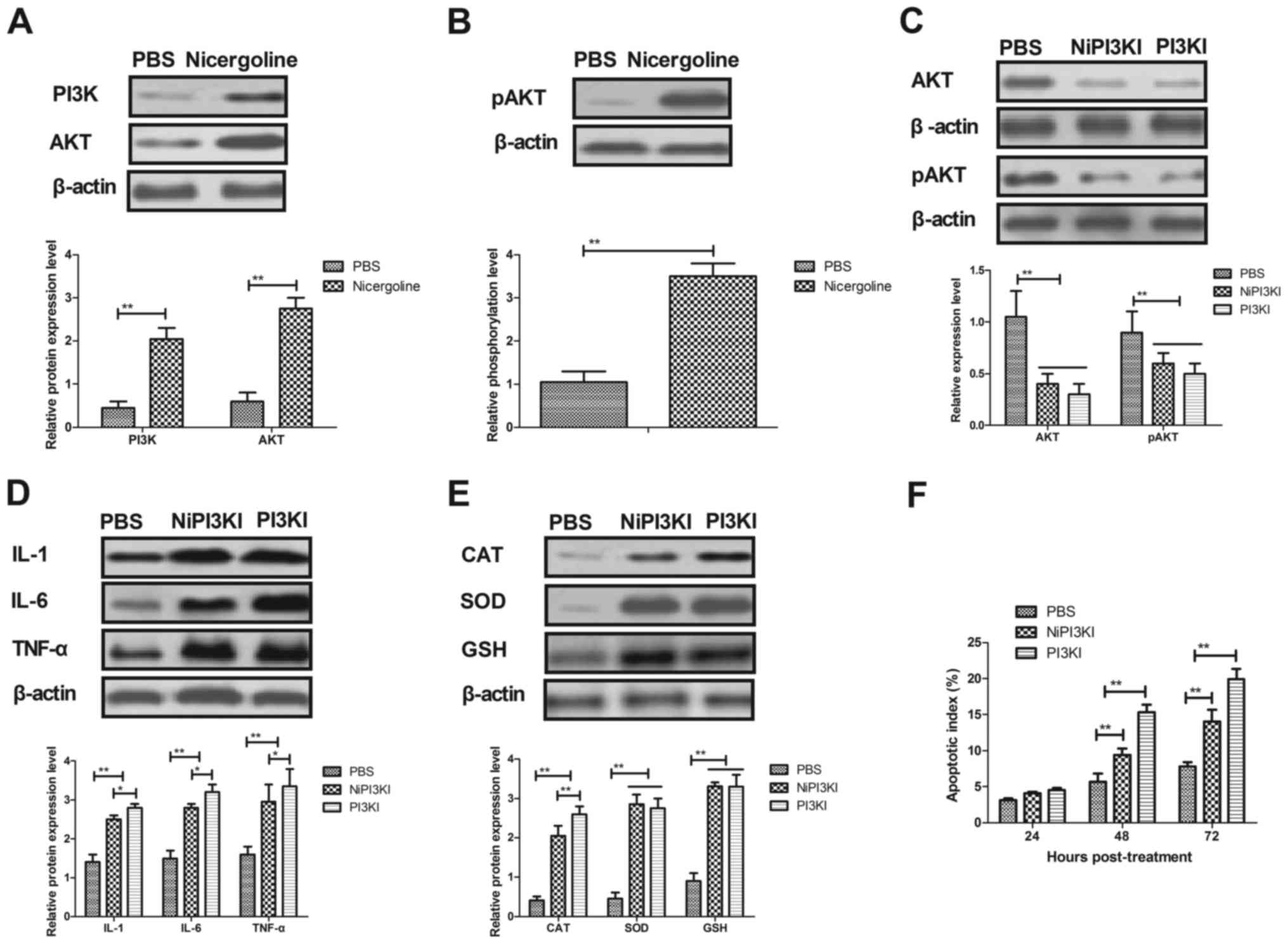
|
1
|
Armstrong RA: Survival in the pre-senile
dementia frontotemporal lobar degeneration with TDP-43
proteinopathy: Effects of genetic, demographic and
neuropathological variables. Folia Neuropathol. 54:137–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bando N and Nakamura Y: Preliminary
evidence that rivastigmine-induced inhibition of serum
butyrylcholinesterase activity improves behavioral symptoms in
Japanese patients with Alzheimer's disease. Geriatr Gerontol Int.
17:1306–1312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verkhratsky A, Rodriguez-Arellano JJ,
Parpura V and Zorec R: Astroglial calcium signalling in Alzheimer's
disease. Biochem Biophys Res Commun. 483:1005–1012. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortese GP and Burger C: Neuroinflammatory
challenges compromise neuronal function in the aging brain:
Postoperative cognitive delirium and Alzheimer's disease. Behav
Brain Res. 322:269–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pettigrew C, Soldan A, Zhu Y, Wang MC,
Brown T, Miller M and Albert M: BIOCARD Research Team: Cognitive
reserve and cortical thickness in preclinical Alzheimer's disease.
Brain Imaging Behav. 11:357–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fritz NE, Kegelmeyer DA, Kloos AD, Linder
S, Park A, Kataki M, Adeli A, Agrawal P, Scharre DW and Kostyk SK:
Motor performance differentiates individuals with Lewy body
dementia, Parkinson's and Alzheimer's disease. Gait Posture.
50:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stella F, Laks J, Govone JS, de Medeiros K
and Forlenza OV: Association of neuropsychiatric syndromes with
global clinical deterioration in Alzheimer's disease patients. Int
Psychogeriatr. 28:779–786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia KO, Ornellas FL, Martin PK, Patti
CL, Mello LE, Frussa-Filho R, Han SW and Longo BM: Therapeutic
effects of the transplantation of VEGF overexpressing bone marrow
mesenchymal stem cells in the hippocampus of murine model of
Alzheimer's disease. Front Aging Neurosci. 6:302014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramos B, Baglietto-Vargas D, del Rio JC,
Moreno-Gonzalez I, Santa-Maria C, Jimenez S, Caballero C,
Lopez-Tellez JF, Khan ZU, Ruano D, et al: Early neuropathology of
somatostatin/NPY GABAergic cells in the hippocampus of a PS1×APP
transgenic model of Alzheimer's disease. Neurobiol Aging.
27:1658–1672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Youn H, Ji I, Ji HP, Markesbery WR and Ji
TH: Under-expression of Kalirin-7 Increases iNOS activity in
cultured cells and correlates to elevated iNOS activity in
Alzheimer's disease hippocampus. J Alzheimers Dis. 12:271–281.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Namba T, Maekawa M, Yuasa S, Kohsaka S and
Uchino S: The Alzheimer's disease drug memantine increases the
number of radial glia-like progenitor cells in adult hippocampus.
Glia. 57:1082–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Shu H, Wang Z, Liu D, Shi Y, Xu L
and Zhang Z: Protective effect of APOE epsilon 2 on intrinsic
functional connectivity of the entorhinal cortex is associated with
better episodic memory in elderly individuals with risk factors for
Alzheimer's disease. Oncotarget. 7:58789–58801. 2016.PubMed/NCBI
|
|
13
|
Ando K, Oka M, Ohtake Y, Hayashishita M,
Shimizu S, Hisanaga S and Iijima KM: Tau phosphorylation at
Alzheimer's disease-related Ser356 contributes to tau stabilization
when PAR-1/MARK activity is elevated. Biochem Biophys Res Commun.
478:929–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Zhao J and Lu G: miR-106b inhibits
tau phosphorylation at Tyr18 by targeting Fyn in a model of
Alzheimer's disease. Biochem Biophys Res Commun. 478:852–857. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Absalon S, Kochanek DM, Raghavan V and
Krichevsky AM: MiR-26b, upregulated in Alzheimer's disease,
activates cell cycle entry, tau-phosphorylation, and apoptosis in
postmitotic neurons. J Neurosci. 33:14645–14659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jahng GH, Oh J, Lee DW, Kim HG, Rhee HY,
Shin W, Paik JW, Lee KM, Park S, Choe BY and Ryu CW: Glutamine and
glutamate complex, as measured by functional magnetic resonance
spectroscopy, alters during face-name association task in patients
with mild cognitive impairment and Alzheimer's disease. J
Alzheimers Dis. 53:7452016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nesteruk T, Nesteruk M, Styczynska M,
Barcikowska-Kotowicz M and Walecki J: Radiological evaluation of
strategic structures in patients with mild cognitive impairment and
early Alzheimer's disease. Pol J Radiol. 81:288–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Minter MR, Zhang C, Leone V, Ringus DL,
Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM,
et al: Antibiotic-induced perturbations in gut microbial diversity
influences neuro-inflammation and amyloidosis in a murine model of
Alzheimer's disease. Sci Rep. 6:300282016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Zhao Z, Wei X, Zheng Y, Yu J,
Zheng J and Wang L: Long-term trihexyphenidyl exposure alters
neuroimmune response and inflammation in aging rat: Relevance to
age and Alzheimer's disease. J Neuroinflammation. 13:1752016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balez R, Steiner N, Engel M, Muñoz SS, Lum
JS, Wu Y, Wang D, Vallotton P, Sachdev P, O'Connor M, et al:
Neuroprotective effects of apigenin against inflammation, neuronal
excitability and apoptosis in an induced pluripotent stem cell
model of Alzheimer's disease. Sci Rep. 6:314502016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Justin Thenmozhi A, William Raja TR,
Manivasagam T, Janakiraman U and Essa M: Hesperidin ameliorates
cognitive dysfunction, oxidative stress and apoptosis against
aluminium chloride induced rat model of Alzheimer's disease. Nutr
Neurosci. 20:360–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SY, Yang J and Lee YC: The effects of
nicergoline on corneal nerve regeneration in rat corneas after
photorefractive keratectomy. Curr Eye Res. 36:29–33. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakashima T, Hattori N, Okimoto M,
Yanagida J and Kohno N: Nicergoline improves dysphagia by
upregulating substance P in the elderly. Medicine. 90:279–283.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pilkowska E, Jakubowska T, Witkowska K and
Kulczycki J: Nicergoline in the treatment of patients after a mild
ischemic stroke. Neurol Neurochir Pol. 36:1075–1085. 2002.(In
Polish). PubMed/NCBI
|
|
25
|
Felisati G, Pignataro O, Di Girolamo A,
Bruno E, Alessandrini M, Guidetti G, Monzani D, Beldi AM, Mira E,
Benazzo M, et al: Nicergoline in the treatment of dizziness in
elderly patients. A review. Arch Gerontol Geriatr Suppl. 163–170.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saletu B, Garg A and Shoeb A: Safety of
nicergoline as an agent for management of cognitive function
disorders. Biomed Res Int. 2014:6101032014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Caraci F, Chisari M, Frasca G, Canonico
PL, Battaglia A, Calafiore M, Battaglia G, Bosco P, Nicoletti F,
Copani A and Sortino MA: Nicergoline, a drug used for age-dependent
cognitive impairment, protects cultured neurons against
beta-amyloid toxicity. Brain Res. 1047:30–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fioravanti M and Flicker L: Efficacy of
nicergoline in dementia and other age associated forms of cognitive
impairment. Cochrane Database Syst Rev: CD003159. 2001. View Article : Google Scholar
|
|
29
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharma R, Ahmad G, Esteves SC and Agarwal
A: Terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay using bench top flow cytometer for evaluation of
sperm DNA fragmentation in fertility laboratories: Protocol,
reference values, and quality control. J Assist Reprod Genet.
33:291–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wai-Hoe L, Wing-Seng L, Ismail Z and
Lay-Harn G: SDS-PAGE-based quantitative assay for screening of
kidney stone disease. Biol Proced Online. 11:145–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thinnes FP: Apoptogenic interactions of
plasmalemmal type-1 VDAC and Abeta peptides via GxxxG motifs induce
Alzheimer's disease-a basic model of apoptosis? Wien Med
Wochenschr. 161:274–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi LX, Wang JH and Shi XD: PI3K/AKT/mTOR
pathway and pediatric T acute lymphoblastic leukemia-review.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 24:1269–1274. 2016.(In
Chinese). PubMed/NCBI
|
|
35
|
Kong J, Ren G, Jia N, Wang Y, Zhang H,
Zhang W, Chen B and Cao Y: Effects of nicorandil in neuroprotective
activation of PI3K/AKT pathways in a cellular model of Alzheimer's
disease. Eur Neurol. 70:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kitagishi Y, Nakanishi A, Ogura Y and
Matsuda S: Dietary regulation of PI3K/AKT/GSK-3β pathway in
Alzheimer's disease. Alzheimers Res Ther. 6:352014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Kang T, Qi B, Kong L, Jiao Y, Cao Y,
Zhang J and Yang J: Neuroprotective effects of ginseng protein on
PI3K/Akt signaling pathway in the hippocampus of D-galactose/AlCl3
inducing rats model of Alzheimer's disease. J Ethnopharmacol.
179:162–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang B, Wang Y, Li H, Xiong R, Zhao Z,
Chu X, Li Q, Sun S and Chen S: Neuroprotective effects of
salidroside through PI3K/Akt pathway activation in Alzheimer's
disease models. Drug Des Devel Ther. 10:1335–1343. 2016.PubMed/NCBI
|
|
39
|
Ferencik M, Novak M, Rovensky J and Rybar
I: Alzheimer's disease, inflammation and non-steroidal
anti-inflammatory drugs. Bratisl Lek Listy. 102:123–132.
2001.PubMed/NCBI
|
|
40
|
McGeer EG and McGeer PL: Chronic
inflammation in Alzheimer's disease offers therapeutic
opportunities. Expert Rev Neurother. 1:53–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quinn J, Montine T, Morrow J, Woodward WR,
Kulhanek D and Eckenstein F: Inflammation and cerebral amyloidosis
are disconnected in an animal model of Alzheimer's disease. J
Neuroimmunol. 137:32–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Verdile G, Keane KN, Cruzat VF, Medic S,
Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE and
Newsholme P: Inflammation and oxidative stress: The molecular
connectivity between insulin resistance, obesity, and alzheimer's
disease. Mediators Inflamm. 2015:1058282015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang L, Tang Y and Huang X: Brain cell
apoptosis after cerebral hypoxia-ischemia in neonatal rat. Zhonghua
YI XUE ZA ZHI. 78:567–569. 1998.(In Chinese). PubMed/NCBI
|
|
44
|
Zhang XQ, Zhang ZM, Yin XL, Zhang K, Cai H
and Ling F: Exploring the optimal operation time for patients with
hypertensive intracerebral hemorrhage: Tracking the expression and
progress of cell apoptosis of prehematomal brain tissues. Chin Med
J (Engl). 123:1246–1250. 2010.PubMed/NCBI
|
|
45
|
Johnson S, Tazik S, Lu D, Johnson C,
Youdim MB, Wang J, Rajkowska G and Ou XM: The new inhibitor of
monoamine oxidase, M30, has a Neuroprotective effect against
dexamethasone-induced brain cell apoptosis. Front Neurosci.
4:1802010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang S, Zhou G, Liu H, Zhang B, Li J, Cui
R and Du Y: Protective effects of p38 MAPK inhibitor SB202190
against hippocampal apoptosis and spatial learning and memory
deficits in a rat model of vascular dementia. Biomed Res Int.
2013:2157982013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yürüker V, Nazıroğlu M and Şenol N:
Reduction in traumatic brain injury-induced oxidative stress,
apoptosis, and calcium entry in rat hippocampus by melatonin:
Possible involvement of TRPM2 channels. Metab Brain Dis.
30:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chatterjee A, Chattopadhyay D and
Chakrabarti G: MiR-16 targets Bcl-2 in paclitaxel-resistant lung
cancer cells and overexpression of miR-16 along with miR-17 causes
unprecedented sensitivity by simultaneously modulating autophagy
and apoptosis. Cell Signal. 27:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|