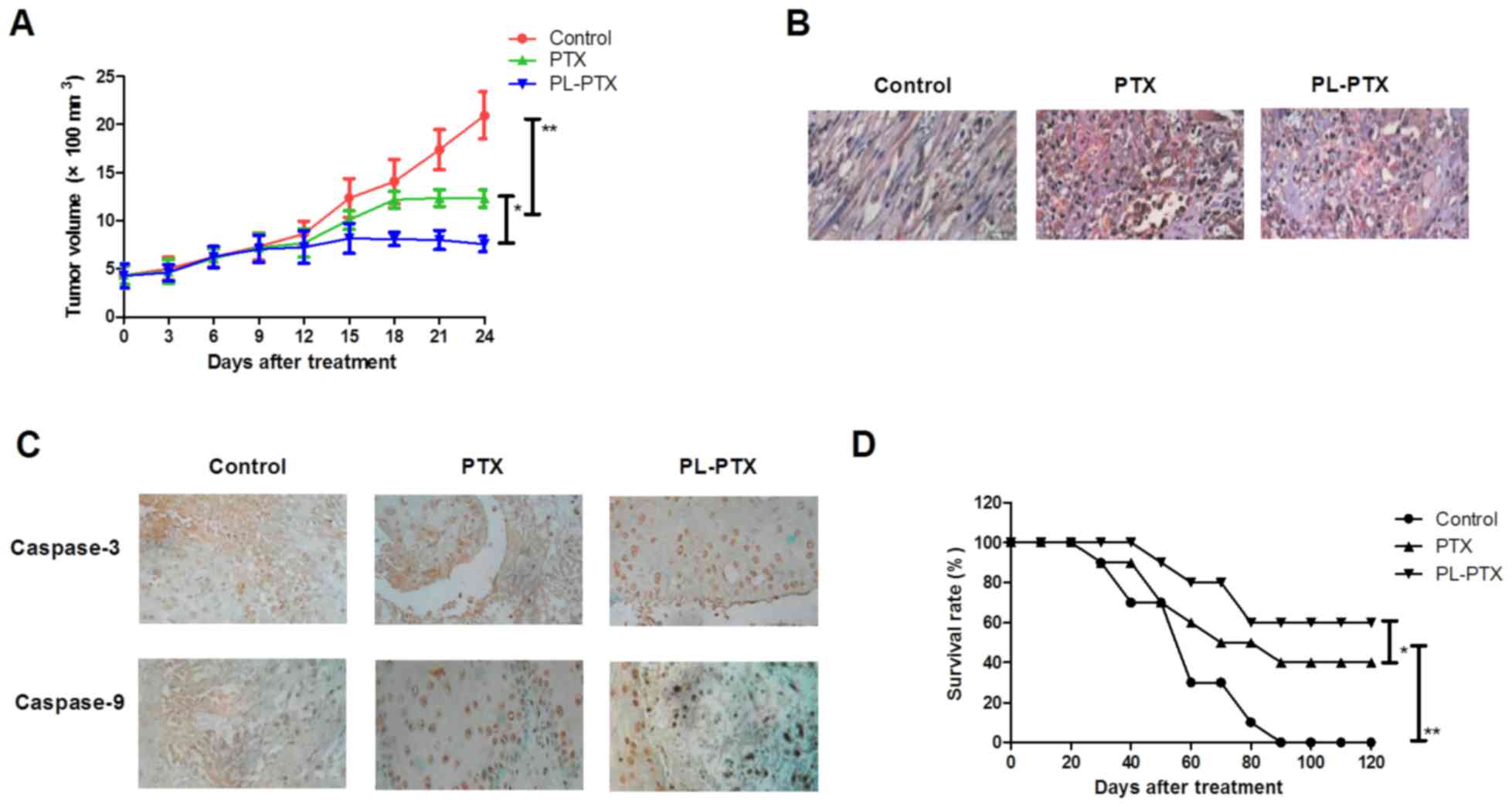
|
1
|
Tempfer CB, El Fizazi N, Ergonenc H and
Solass W: Metastasis of ovarian cancer to the breast: A report of
two cases and a review of the literature. Oncol Lett. 11:4008–4012.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shakeel S, Elit L, Akhtar-Danesh N,
Schneider L and Finley C: Care delivery patterns, processes, and
outcomes for primary ovarian cancer surgery: A Population-based
review using a national administrative database. J Obstet Gynaecol
Can. 39:25–33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto A, Miyasaka Y, Furuya K, Watanabe
H, Maruyama M, Nakada H, Takano A, Hada M, Nakagomi H, Omata M and
Oyama T: Pseudo-Meigs' syndrome due to ovarian metastases from
colon cancer: A case report and review of the literature. Surg Case
Rep. 2:1122016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pimentel C, Becquet M, Lavoue V, Henno S,
Leveque J and Ouldamer L: Ovarian metastases from breast cancer: A
series of 28 cases. Anticancer Res. 36:4195–4200. 2016.PubMed/NCBI
|
|
5
|
Lago V, Minig L and Fotopoulou C:
Incidence of lymph node metastases in apparent Early-stage
low-grade epithelial ovarian cancer: A comprehensive review. Int J
Gynecol Cancer. 26:1407–1414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raave R, de Vries RB, Massuger LF, van
Kuppevelt TH and Daamen WF: Drug delivery systems for ovarian
cancer treatment: A systematic review and meta-analysis of animal
studies. PeerJ. 3:e14892015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munhoz RR, Pereira AA, Sasse AD, Hoff PM,
Traina TA, Hudis CA and Marques RJ: Gonadotropin-releasing hormone
agonists for ovarian function preservation in premenopausal women
undergoing chemotherapy for Early-stage breast cancer: A systematic
review and Meta-analysis. JAMA Oncol. 2:65–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bian C, Yao K, Li L, Yi T and Zhao X:
Primary debulking surgery vs. neoadjuvant chemotherapy followed by
interval debulking surgery for patients with advanced ovarian
cancer. Arch Gynecol Obstet. 293:163–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ong BY, Ranganath SH, Lee LY, Lu F, Lee
HS, Sahinidis NV and Wang CH: Paclitaxel delivery from PLGA foams
for controlled release in post-surgical chemotherapy against
glioblastoma multiforme. Biomaterials. 30:3189–3196. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ansaloni L, Coccolini F, Morosi L,
Ballerini A, Ceresoli M, Grosso G, Bertoli P, Busci LM, Lotti M,
Cambria F, et al: Pharmacokinetics of concomitant cisplatin and
paclitaxel administered by hyperthermic intraperitoneal
chemotherapy to patients with peritoneal carcinomatosis from
epithelial ovarian cancer. Br J Cancer. 112:306–312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karmakar S, Banik NL and Ray SK:
Combination of all-trans retinoic acid and paclitaxel-induced
differentiation and apoptosis in human glioblastoma U87MG
xenografts in nude mice. Cancer. 112:596–607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikanjam M, Gibbs AR, Hunt CA, Budinger TF
and Forte TM: Synthetic nano-LDL with paclitaxel oleate as a
targeted drug delivery vehicle for glioblastoma multiforme. J
Control Release. 124:163–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merighi S, Benini A, Mirandola P, Gessi S,
Varani K, Leung E, Maclennan S, Baraldi PG and Borea PA: Hypoxia
inhibits paclitaxel-induced apoptosis through adenosine-mediated
phosphorylation of bad in glioblastoma cells. Mol Pharmacol.
72:162–172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slaughter KN, Moore KN and Mannel RS:
Anti-angiogenic therapy versus dose-dense paclitaxel therapy for
frontline treatment of epithelial ovarian cancer: Review of phase
III randomized clinical trials. Curr Oncol Rep. 16:4122014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv W, Cheng L and Li B: Development and
evaluation of a novel TPGS-mediated paclitaxel-loaded PLGA-mPEG
nanoparticle for the treatment of ovarian cancer. Chem Pharm Bull
(Tokyo). 63:68–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai J, Cai Q, Biel MA, Wang C, Hu X, Wang
S and Lin J: Id1 and NF-kB promote the generation of CD133+ and
BMI-1+ keratinocytes and the growth of xenograft tumors in mice.
Int J Oncol. 44:1481–1489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griffith TS, Anderson RD, Davidson BL,
Williams RD and Ratliff TL: Adenoviral-mediated transfer of the
TNF-related apoptosis-inducing ligand/Apo-2 ligand gene induces
tumor cell apoptosis. J Immunol. 165:2886–2894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato T, Yamauchi N, Sasaki H, Takahashi M,
Okamoto T, Sakamaki S, Watanabe N and Niitsu Y: An
apoptosis-inducing gene therapy for pancreatic cancer with a
combination of 55-kDa tumor necrosis factor (TNF) receptor gene
transfection and mutein TNF administration. Cancer Res.
58:1677–1683. 1998.PubMed/NCBI
|
|
19
|
Yang XJ, Zheng FY, Xu YS and Ou RY:
Ovarian cancer initially presenting with isolated ipsilateral
superficial inguinal lymph node metastasis: A case study and review
of the literature. J Ovarian Res. 7:202014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Debniak T, Gromowski T, Scott RJ, Gronwald
J, Huzarski T, Byrski T, Kurzawski G, Dymerska D, Górski B,
Paszkowska-Szczur K, et al: Management of ovarian and endometrial
cancers in women belonging to HNPCC carrier families: Review of the
literature and results of cancer risk assessment in Polish HNPCC
families. Hered Cancer Clin Pract. 13:32015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebell MH, Culp M, Lastinger K and Dasigi
T: A systematic review of the bimanual examination as a test for
ovarian cancer. Am J Prev Med. 48:350–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han X, Chen J, Jiang M, Zhang N, Na K, Luo
C, Zhang R, Sun M, Lin G, Zhang R, et al: Paclitaxel-paclitaxel
prodrug nanoassembly as a versatile nanoplatform for combinational
cancer therapy. ACS Appl Mater Interfaces. 8:33506–33513. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukuchi M, Mochiki E, Ishiguro T, Ogura T,
Sobajima J, Kumagai Y, Ishibashi K and Ishida H: Efficacy of
Nab-paclitaxel as Second-line chemotherapy for unresectable or
recurrent gastric cancer. Anticancer Res. 36:6699–6703. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Luo J, Fu Y, Li H, Ding R, Gong T
and Zhang Z: Novel oral administrated paclitaxel micelles with
enhanced bioavailability and antitumor efficacy for resistant
breast cancer. Colloids Surf B Biointerfaces. 150:89–97. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edwards SJ, Barton S, Thurgar E and Trevor
N: Topotecan, pegylated liposomal doxorubicin hydrochloride,
paclitaxel, trabectedin and gemcitabine for advanced recurrent or
refractory ovarian cancer: A systematic review and economic
evaluation. Health Technol Assess. 19:1–480. 2015. View Article : Google Scholar
|
|
26
|
Narod SA: Weekly vs. every-3-week
paclitaxel for ovarian cancer. N Engl J Med. 374:26022016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dessai SB, Chakraborty S, Babu TV, Nayanar
S, Bhattacharjee A, Jones J, Balasubramanian S and Patil VM:
Tolerance of weekly metronomic paclitaxel and carboplatin as
neoadjuvant chemotherapy in advanced ovarian cancer patients who
are unlikely to tolerate 3 weekly paclitaxel and carboplatin. South
Asian J Cancer. 5:63–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perkins CL, Fang G, Kim CN and Bhalla KN:
The role of Apaf-1, caspase-9, and bid proteins in etoposide- or
paclitaxel-induced mitochondrial events during apoptosis. Cancer
Res. 60:1645–1653. 2000.PubMed/NCBI
|
|
29
|
Pan J, Xu G and Yeung SC: Cytochrome c
release is upstream to activation of caspase-9, caspase-8, and
caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer
cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab.
86:4731–4740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oktem M, Dilek TU, Guner H and Tiras MB:
The effect of recombinant GM-CSF on IL-6 and TNF-alpha levels in
epithelial ovarian cancer patients who received paclitaxel and
cisplatinum: Preliminary results. Eur J Gynaecol Oncol. 25:478–480.
2004.PubMed/NCBI
|
|
31
|
Suyama H, Igishi T, Sano H, Matsumoto S,
Shigeoka Y, Nakanishi H, Endo M, Burioka N, Hitsuda Y and Shimizu
E: ERK activation and subsequent RB phosphorylation are important
determinants of the sensitivity to paclitaxel in lung
adenocarcinoma cells. Int J Oncol. 24:1499–1504. 2004.PubMed/NCBI
|
|
32
|
Atjanasuppat K, Lirdprapamongkol K,
Jantaree P and Svasti J: Non-adherent culture induces paclitaxel
resistance in H460 lung cancer cells via ERK-mediated up-regulation
of βIVa-tubulin. Biochem Biophys Res Commun. 466:493–498. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
34
|
Cheng YJ, Lee CH, Lin YP, Huang JY, Su CC,
Chang WT and Yang BC: Caspase-3 enhances lung metastasis and cell
migration in a protease-independent mechanism through the ERK
pathway. Int J Cancer. 123:1278–1285. 2008. View Article : Google Scholar : PubMed/NCBI
|