Introduction
Breast cancer is the most common tumor diagnosed in
women and is one of the main causes of cancer-associated mortality
in women globally (1). Annually,
~1.3 million new breast cancer cases and 0.5 million breast
cancer-associated mortalities occur globally (2). There were 230,000 new breast cancer
cases and ~40,000 breast cancer-associated mortalities in the USA
in 2013 (3). The mortality among
patients with breast cancer has been decreasing in the USA since
1990 (3). The morbidity of breast
cancer among Chinese women has been rising recently (3). In China 169,000 new breast cancer
cases and ~45,000 mortalities are reported annually (4). Progress has been made in treatment
strategies, including adjuvant chemotherapy, radiotherapy,
endocrine therapy and targeted therapy (5). In addition, the detection rate of
early breast cancer with good prognosis is increasing (5). Therefore, the mortality of breast
cancer has been gradually decreasing (5). At present, indexes used for clinical
prognostic evaluation of breast cancer include consideration of the
number of metastatic lymph nodes, expression of estrogen receptor
(ER), progesterone receptor and herstatin, tumor diameter and
histological grade (6). These
parameters and indexes have been used to guide the systemic
treatment of breast cancer. Consequently, it is necessary to
continue to search for more effective drugs and therapeutic
strategies. Furthermore, researchers are trying to identify
biological targets that may predict breast cancer prognosis and
guide treatment (7).
Cyclooxygenase-2 (COX-2) is able to enhance the
invasive ability of tumor cells (8). It has been demonstrated that elevated
COX-2 expression may increase the activity of matrix
metalloproteinase-2 (8). As a
result, metalloproteinase-2 expression is upregulated, which
promotes tumor invasion of the lymph nodes and metastasis (8). Furthermore, cancer cells with
upregulated COX-2 expression can induce a paracrine effect
(9), which may induce adjacent
epithelial cells to express COX-2, which in turn may result in
malignant transformation, promoting tumor proliferation. Patients
with elevated COX-2 expression have low long-term disease-free
survival (10). In addition,
elevated COX-2 expression is associated with accelerated tumor
proliferation, negative ER and lymph node metastasis. COX-2 may
induce metastasis of breast cancer (10). It has been demonstrated that COX-2
expression is associated with breast cancer lymph node metastasis,
tumor differentiation, blood supply and negative ER (11).
Previous studies of breast cancer have focused on
its molecular mechanism. The aim of breast cancer research is to
identify pathogenic and diagnostic factors similar to alpha
fetoprotein in primary liver cancer (12). The arachidonic acid pathway is an
important molecular pathway in tumor research; it has been studied
in other tumors, especially gastrointestinal tumors (12). Prostaglandin E2 (PGE2) in this
pathway is closely associated with tumors. PGE2 may affect
tumorigenesis, development and transformation through multiple
downstream pathways (13).
A previous study reported that the mitogen-activated
protein kinase (MAPK) signaling pathway is associated with multiple
cellular biological behaviors (14). These include apoptosis,
differentiation, proliferation, cell cycle control, cell survival
and malignant transformation of cells (15). MAP kinase kinase kinase kinase 4
(MAP4K4) is an upstream kinase of the MAPK signaling system
(14). MAP4K4 has been
demonstrated to be upregulated in multiple tumor cells and may
accelerate cell transformation (16). Furthermore, it may enhance cell
invasion, reduce adhesion in cultured cells and affect tumor
prognosis (16).
The mechanism underlying breast cancer metastasis
remains to be elucidated (17).
Identification of breast cancer metastasis-associated microRNAs
(miRNAs) has provided a novel approach for research into breast
cancer metastasis (18). miRNAs
are involved in tumorigenesis and developmental processes,
including breast cancer cell growth, apoptosis, migration and
invasion. miRNAs may regulate breast cancer metastasis (18). Certain miRNAs promote breast cancer
metastasis, while other miRNAs serve inhibitory roles (17). Therefore, the present study aimed
to investigate the anti-cancer effect of miRNA-141 on the apoptosis
rate of breast cancer cells and the possible underlying
mechanism.
Materials and methods
Patients and ethical approval
A total of 56 patients with breast cancer (55–64
years old, female) and 6 healthy volunteers (58–62 years old,
female) included in the present study were admitted to the
Department of Breast Surgery, the First Affiliated Hospital of
Jinan University (Guangzhou, China) from May to October 2015.
Characteristics of patients with breast cancer and healthy controls
are presented in Table I. Blood
samples were obtained, and serum was collected by centrifugation at
1,000 × g for 20 min at 4°C and stored at −80°C. All human studies
were approved by the Ethics Committee of the First Affiliated
Hospital of Jinan University. All patients signed written informed
consent forms prior to the study. All animal experiments were
approved by the Laboratory Animal Ethics Committee of Jinan
University.
 | Table I.Characteristics of patients with
breast cancer and healthy controls. |
Table I.
Characteristics of patients with
breast cancer and healthy controls.
| Variable | Patients | Healthy
volunteers |
|---|
| Number | 56 | 6 |
| Age (years) | 55–64 | 58–62 |
| Female | 56 | 6 |
| Male | 0 | 0 |
| Tumor size, ≤3.0
cm | 31 | n/a |
| Tumor size, >3.0
cm | 25 | n/a |
| Edmondson grade
I–II | 23 | n/a |
| Edmondson grade
III–IV | 33 | n/a |
Isolation of CD4+ T
cells
C57BL/6 mice (6 weeks old, 19–20 g, n=8, male) were
purchased from Animal testing center of Jinan University
(Guangzhou, China) and housed at 22–23°C, 55–60% humidity, 12 h
light/dark cycle and had free access to food and water. C57BL/6
mice were anesthetized using 35 mg/kg pentobarbital sodium and
sacrificed by decollation. Splenocytes were collected and
homogenated using PBS. CD4+ T cells were isolated from
splenocytes of C57BL/6 mice using a CD4 isolation kit according to
the manufacturer's protocol (Miltenyi Biotec, Inc., Cambridge, MA,
USA).
Cell culture and transfection
Human breast cancer MCF7 cells were purchased from
the Cell Bank of the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China), and cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U penicillin/ml and 10 µg
streptomycin/ml at 37°C in a humidified atmosphere containing 5%
CO2. MAP4K4 plasmid (5′-GGCGAACGACTCCCCTGCAA-3′ and
5′-TGAGAGTTAGGGTTTTGCAT-3′), miRNA-141 (5′-CGGCCGGCCCTGGGTCCATC-3′
and 5′-CTCCCGGGTGGGTTC-3′), anti-miRNA-141 and negative control
mimics were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China). MAP4K4 plasmid (500 ng), miRNA-141 (200 ng), anti-miRNA-141
(200 ng) and negative control mimics (200 ng) were transfected into
MCF7 cells using 40 nM Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Following transfection for 4 h, new DMEM was added into
MCF7 cells and CD4+ T cells (1×106
cells/ml).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Mature mRNA was isolated using the miRNeasy Mini kit
(Qiagen GmbH, Hilden, Germany) from tissues or transfected cells
and reverse-transcribed using the a miRCURY LNA™ Universal RT miRNA
PCR kit (Exiqon A/S, Vedbaek, Denmark), in accordance with the
manufacturer's protocol. qPCR was performed using SYBR-Green master
mix (Exiqon A/S, Vedbaek, Denmark) using an ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the PCR: Initial
denaturation at 95°C for 5 min; 40 cycles of 95°C for 30 sec, 60°C
for 30 sec and 72°C for 30 sec; and 4°C for 10 min. The following
primers for miRNA-141 were used: Forward,
5′-CGCTAACACTGTCTGGTAAAG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′.
Primers used for U6 were: Forward, 5′-ATTGGAACGATACAGAGAAGATT-3′
and reverse, 5′-GGAACGCTTCACGAATTTG-3′. The relative expression
levels of target genes were calculated with the 2−ΔΔCq
method (19).
Flow cytometry
A total of 5 ml peripheral blood was collected and
added into lymphocyte separation medium (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). Lymphocytes were
collected by centrifugation at 800 × g for 20 min at 4°C, fixed
with 4% paraformaldehyde for 15 min at room temperature, blocked
with 2% BSA (Beyotime Institute of Biotechnology, Haimen, China) in
PBS for 1 h at 37°C and incubated with anti-natural killer (NK)
cell (1:100, cat. no. 564537), anti-CD4-APC (1:100, cat. no.
560837), anti-CD8-PEcy5 (1:100, cat. no. 555367) at 37°C for 15
min, all from BD Biosciences (Franklin Lakes, NJ, USA). NK cell,
and CD4+ and CD8+ T cell numbers were
analyzed by a flow cytometer and analyzed using Flowjo 7.6.1
(FlowJo LLC, Ashland, OR, USA).
Additionally, MCF7 cells were seeded in 6-well
plates at a density of 2×106 cells/well, fixed with 4%
paraformaldehyde for 15 min at room temperature and resuspended
using a binding buffer (Nanjing KeyGen Biotech Co., Ltd.). Annexin
V-enhanced green fluorescent protein and propidium iodide (both
Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) were added into
each well and the solution was incubated for 30 min at room
temperature in the dark. Apoptosis rate was analyzed using a flow
cytometer as described above.
Cell proliferation and clonogenic
assay
CD4+ T cells from splenocytes of WT mice
and MCF7 cells following transfection were co-cultured for 24, 48
and 72 h. Cells transfected with negative control mimics served as
control. Viability of MCF7 cells was determined using an MTT assay.
MCF7 cells were seeded in 96-well plates at a density of
1×104 cells/well and 10 µl MTT was added into MCF7 cells
and incubated for 4 h at 37°C. Medium was subsequently removed and
samples were solubilized using 150 µl dimethyl sulfoxide (DMSO),
and the formazan was measured at a wavelength of 490 nm.
Determination of lactate dehydrogenase
(LDH) activity and TNF-α and interleukin (IL) −10 levels
CD4+ T cells and MCF7 cells following
transfection were co-cultured for 48 h. MCF7 cells were seeded in
96-well plates at a density of 1×104 cells/well and
cytotoxicity was detected with an LDH assay kit (Sigma-Aldrich;
Merck KGaA), according to the manufacturer's protocol.
The supernatant from CD4+ T cells and
MCF7 cells following transfection and co-culture for 48 h was
collected following centrifugation at 1,000 × g for 10 min at 4°C
and used to measure TNF-α (cat. no. H052) and IL-10 (cat. no. H009)
levels using ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Optical density was measured at a
wavelength of 450 nm.
Luciferase reporter assay
The 3′-untranslated region fragment of MAP4K4
containing miRNA-141 binding site was amplified by PCR and then
cloned into a pGL3 luciferase reporter vector (Promega Corporation,
Madison, WI, USA). The reporter vector was co-transfected with
miRNA-141 or scramble control using Lipofectamine® 3000
(Invitrogen; Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol provided. After 48 h, reporter
activity was quantified using a Dual-Luciferase Reporter Assay kit
(Promega Corporation) and normalized by comparison with
Renilla luciferase activity.
Western blot analysis
CD4+ T cells and MCF7 cells following
transfection were co-cultured for 48 h. MCF7 cells were seeded in
6-well plates at 2×106 cells/well, and lysed with
ice-cold lysis buffer (RIPA, Beyotime Institute of Biotechnology).
Protein concentration was determined using a bicinchoninic acid
protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Equivalent amount of proteins (50 µg/lane) were separated using
10–12% SDS-PAGE and transferred onto polyvinylidene fluoride
membranes. The membranes were blocked with 5% non-fat milk in TBS
containing 0.1% Tween-20 (TBST) for 1 h at 37°C and incubated with
anti-COX-2 (cat. no. 12282; 1:1,000; Cell Signaling Technology,
Inc.), anti-PGE2 (cat. no. sc-20676; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-TNF-α (cat. no.
sc-8301; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-IL-10 (cat.
no. sc-7888; 1:1,000; Santa Cruz Biotechnology, Inc.) and
anti-GAPDH (cat. no. sc-25778; 1:500; Santa Cruz Biotechnology,
Inc.) antibodies overnight at 4°C. The membrane was washed with
TBST for 1 h and incubated with a goat anti-rabbit horseradish
peroxidase conjugated IgG secondary antibody (cat. no. sc-2004;
1:5,000; Santa Cruz Biotechnology, Inc.) for 2 h at 37°C. The
analysis of protein expression was visualizated using BeyoECL Moon
(Beyotime Institute of Biotechnology) and resolved using
Image-ProPlus 6.0 software (Media Cybernetics, Inc., Rockville, MD,
USA) A total of 5 µg protein was used to measure the activity of
caspase-3/9 using a caspase-3 or caspase-9 assay kit (Beyotime
Institute of Biotechnology).
Statistical analysis
Results are presented as means ± standard error of
the mean (n=3). The data were analyzed using SPSS software (version
13.0; SPSS, Inc., Chicago, IL, USA). Differences between groups
were analyzed by one- or two-way analysis of variance followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
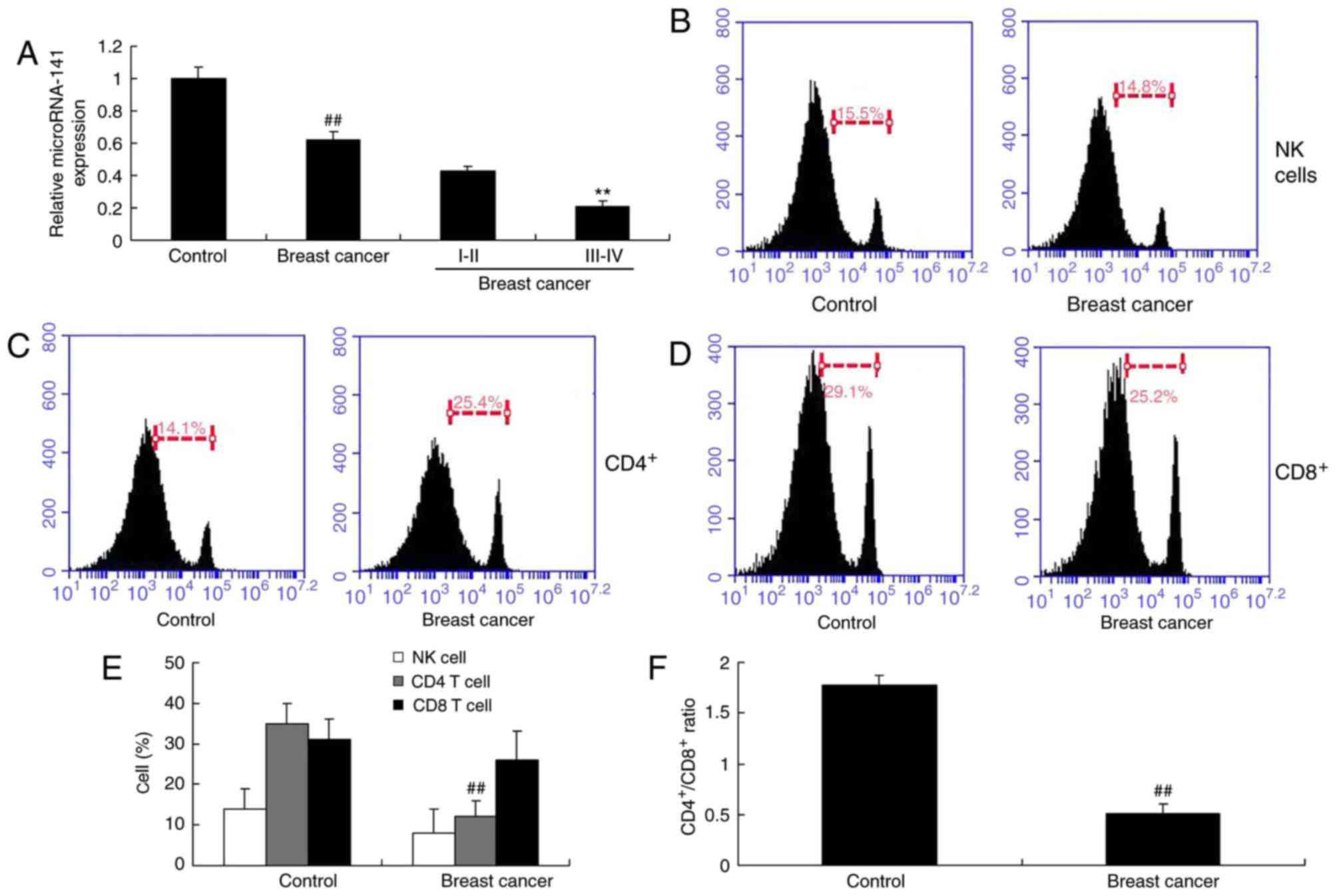
Expression of miRNA-141 in patients
with breast cancer and the abundance of NK cells, and
CD4+ and CD8+ T cells
In order to investigate the mRNA expression levels
of miRNA-141 in patient with breast cancer, RT-qPCR was performed
for the detection of miRNA-141 expression. The expression of
miRNA-141 in the serum of patients with breast cancer was
downregulated compared with the control group (Fig. 1A). The number of NK was not altered
in patients with breast cancer (Fig.
1B). The number of CD4+ T cells in patients with
breast cancer was markedly reduced, compared with the control group
(Fig. 1C). The number of
CD8+ cells was not altered in patients with breast
cancer (Fig. 1D). The
CD4+/CD8+ T cell ratio in patients with
breast cancer was lower compared with the control group (Fig. 1F).
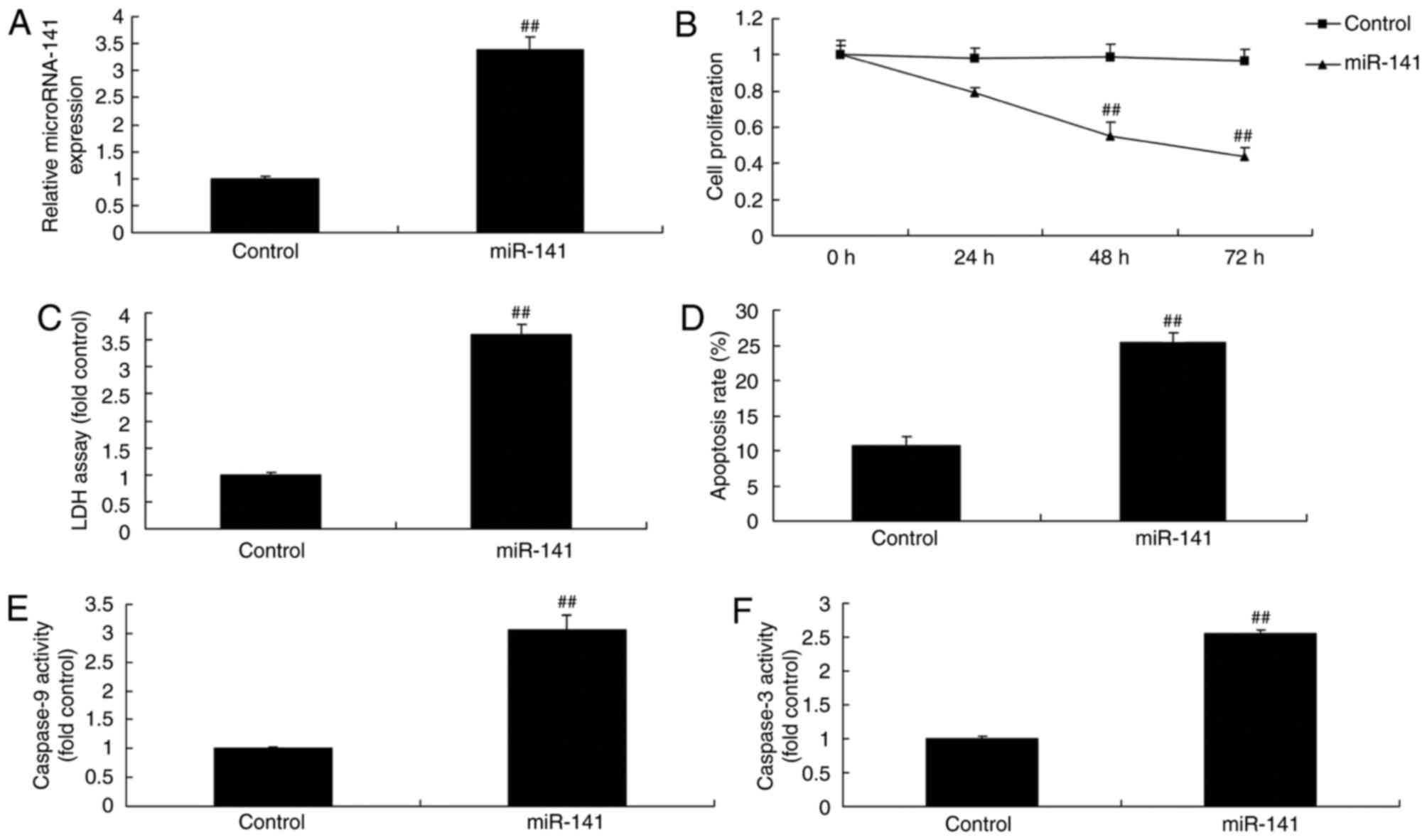
Overexpression of miRNA-141 affects
the toxicity of CD4+ T cells to breast cancer cells
Transfection with miRNA-141 mimics and co-culture
with CD4+ T cells increased the expression of miRNA-141,
inhibited cell proliferation, increased LDH activity and increased
the apoptosis rate of MCF-7 cells, compared with the control group
(Fig. 2A-D). The activity of
caspase-9 and −3 in MCF-7 cells transfected with miRNA-141 and
co-cultured with CD4+ cells increased compared with the
control group (Fig. 2E and F).
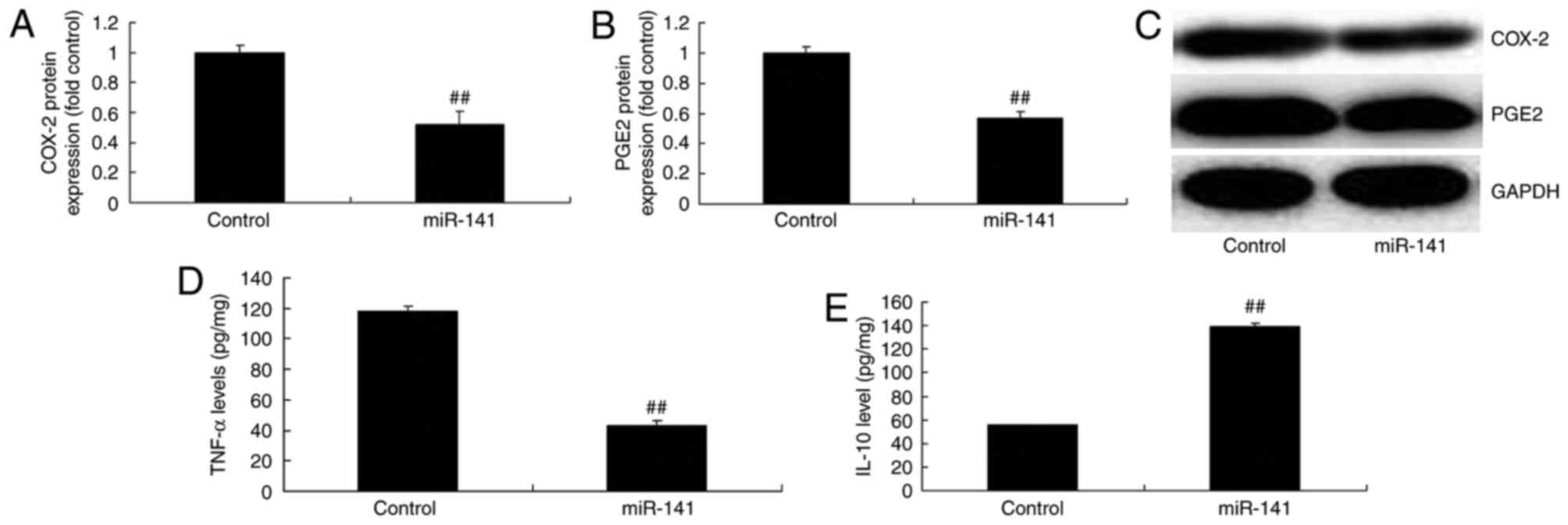
Overexpression of miRNA-141 affects
COX-2, PGE2, TNF-α and IL-10 expression levels
Overexpression of miRNA-141 suppressed COX-2 and
PGE2 protein expression in MCF-7 cells, compared with the control
group (Fig. 3A-C). Overexpression
of miRNA-141 significantly reduced TNF-α protein expression and
induced IL-10 protein expression in MCF-7 cells, compared with
control group (Fig. 3D-E).
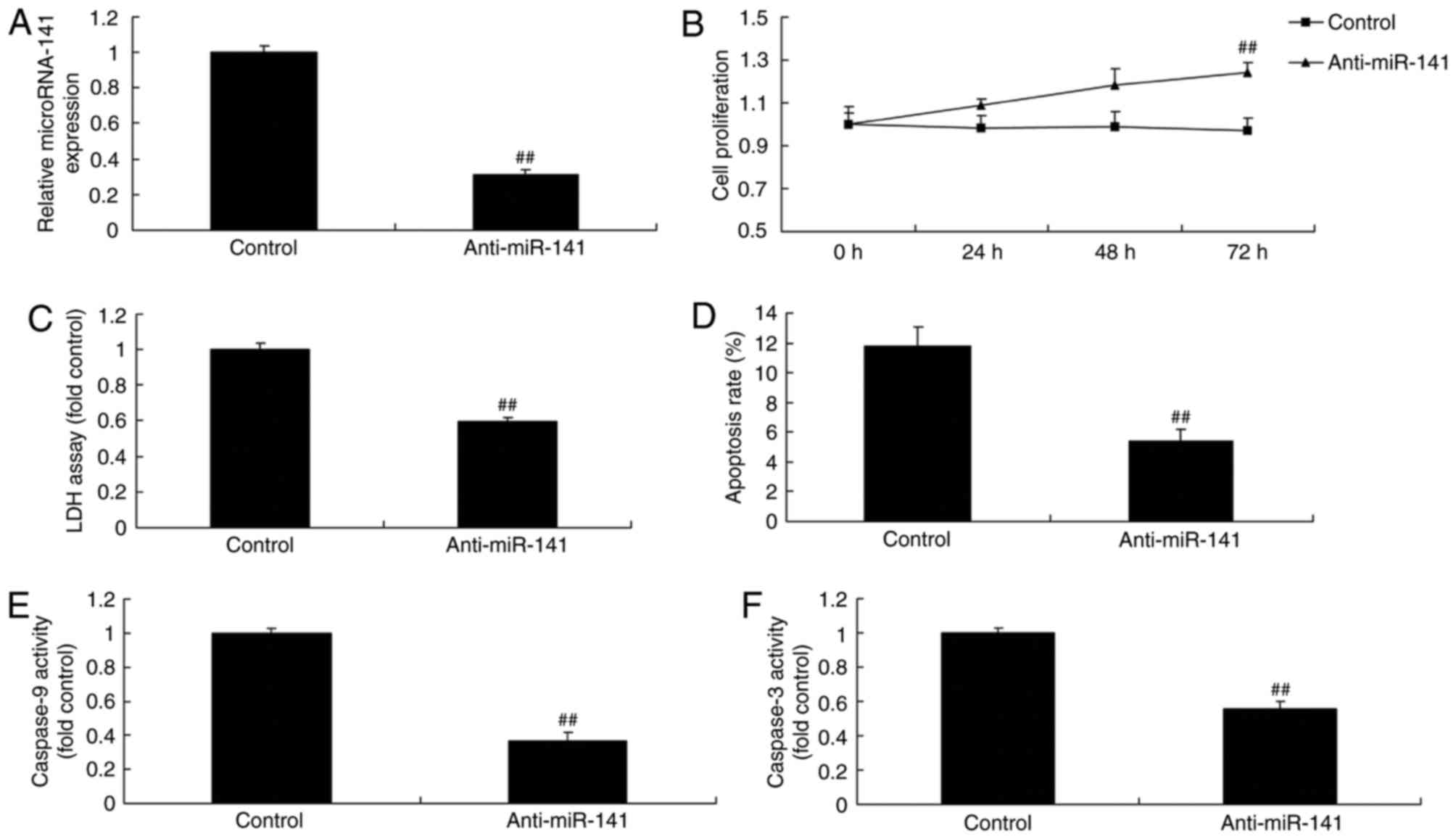
Downregulation of miRNA-141 affects
the toxicity of CD4+ T cells on breast cancer cells
The present study investigated the effect of
downregulation of miRNA-141 on the cytotoxicity of CD4+
T cells on breast cancer cells. Anti-miRNA-141 mimics effectively
reduced miRNA-141 expression, promoted cell proliferation,
inhibited LDH activity and apoptosis, and increased the
proliferation of MCF-7 cells, compared with the control group
(Fig. 4A-D). The activity of
caspase-9 and −3 in MCF-7 cells transfected with anti-miRNA-141 was
lower compared with the control group (Fig. 4E-F).
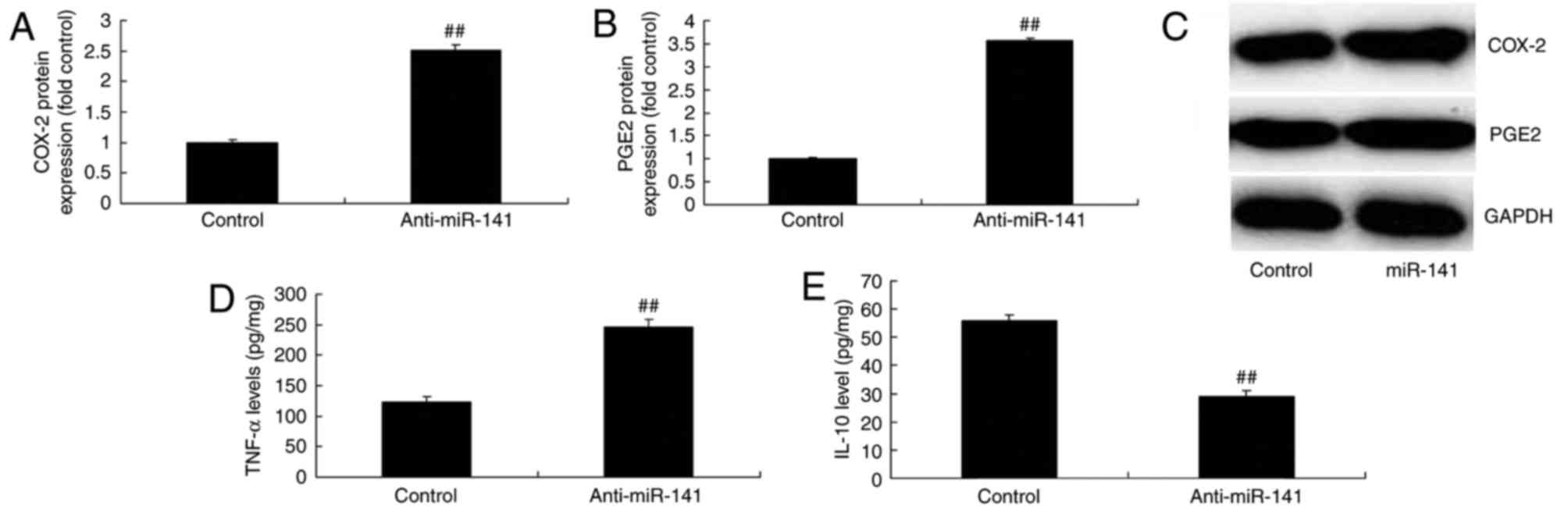
Downregulation of miRNA-141 affects
COX-2 and PGE2 production, and TNF-α and IL-10 expression
levels
Compared with the control group, overexpression of
miRNA-141 significantly induced COX-2 and PGE2 protein expression
in MCF-7 cells (Fig. 5A-C).
Downregulation of miRNA-141 significantly induced TNF-α protein
expression and suppressed IL-10 protein expression in MCF-7 cells,
compared with the control group (Fig.
5D and E).
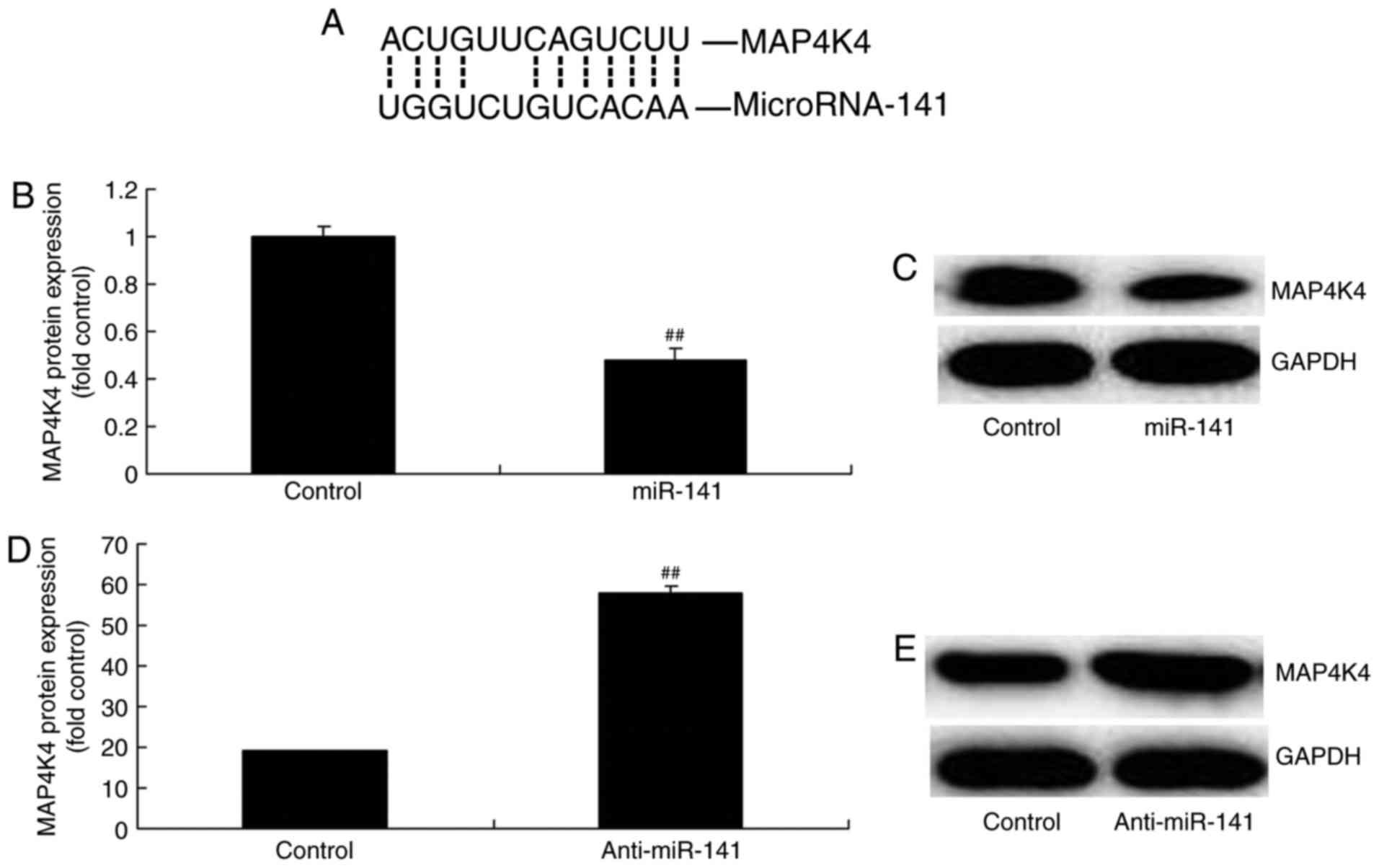
Effect of miRNA-141 on MAP4K4 protein
expression
Luciferase reporter was performed to further
investigate the underlying mechanism of action of miRNA-141 in
breast cancer and to elucidate whether MAP4K4 is modulated in MCF-7
cells. The results of the luciferase reporter revealed that MAP4K4
may be a target gene of miRNA-141 (Fig. 6A). Overexpression of miRNA-141
significantly suppressed MAP4K4 protein expression and
downregulation of miRNA-141 significantly increased MAP4K4 protein
expression in MCF-7 cells, compared with the control group
(Fig. 6B-E). The results suggest
that MAP4K4 may be involved in the pathogenesis of breast
cancer.
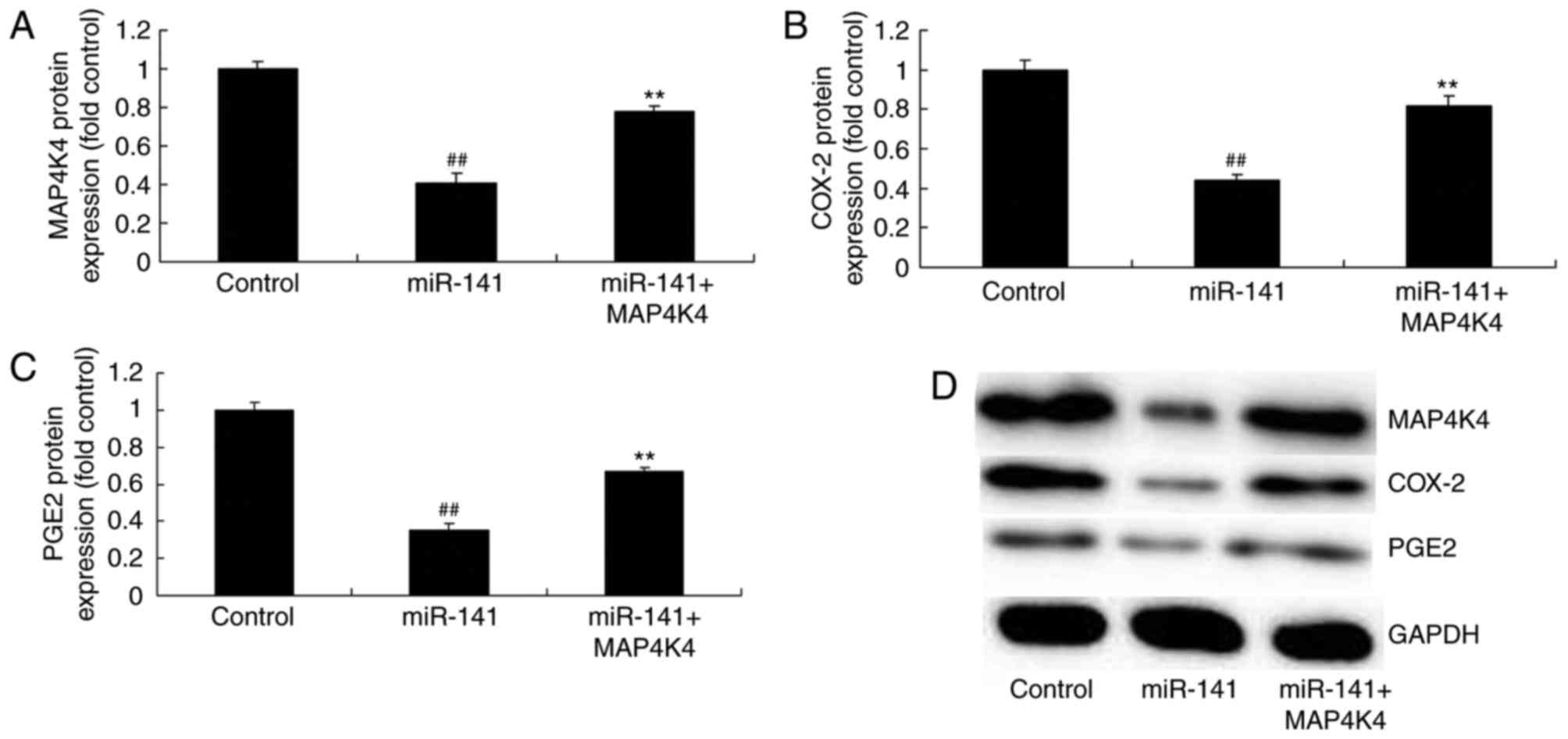
MAP4K4 plasmid induced the effects of
miRNA-141 on protein expression
Transfection with a MAP4K4 plasmid significantly
promoted MAP4K4 protein expression and induced COX-2 and PGE2
protein expression in MCF-7 cells transfected with miRNA-141,
compared with the miRNA-141 only group (Fig. 7).
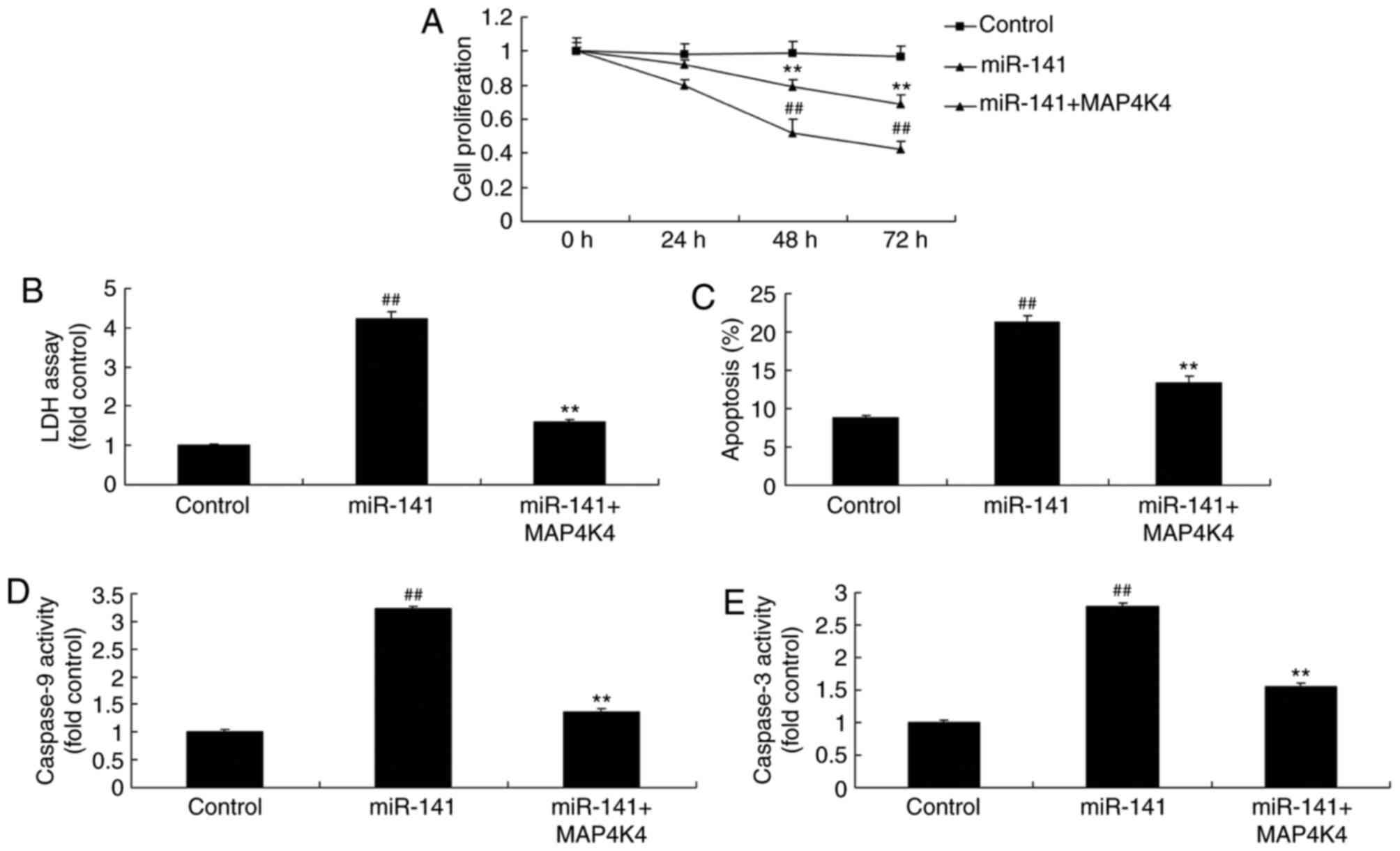
Promotion of MAP4K4 protein expression
reduced the effects of miRNA-141 on the toxicity of CD+
T cells on breast cancer cells
The results revealed that, compared with the
miRNA-141 group, the promotion of MAP4K4 protein expression
increased cell proliferation, inhibited LDH activity and apoptosis
rate, and reduced caspase-3 and caspase-9 activity in MCF-7 cells
(Fig. 8).
Discussion
Breast cancer is one of the most common malignancies
among women and its morbidity has been increasing in recent years
(1). The current methods of
treatment for breast cancer include surgery and chemotherapy.
However, these approaches demonstrate poor efficacy for certain
patients (1). Abnormal cellular
apoptosis is one of the malignant manifestations of tumor cells
(3). In numerous malignancies,
tumor growth, invasion, metastasis and prognosis are closely
associated with the level of cellular apoptosis (3). Therefore, inducing tumor cell
apoptosis may inhibit tumor progression (4). Tumor metastasis is a complicated
multi-step process that involves multiple factors, including
oncogenes and tumor suppressor genes (4). interaction between oncogenes and
tumor suppressor genes serves a role in cancer cell apoptosis. To
the best of the authors' knowledge the present study was the first
to demonstrate that miRNA-141 expression in the serum of patients
with breast cancer was downregulated, compared with healthy
controls. However, only 56 patients with breast cancer and 6
healthy volunteers were included in the present study, which is a
limitation. The number of CD4+ T cells in patients with
breast cancer was markedly reduced.
T cells may be classified into helper T cells (Th),
cytotoxic T cells (Tc) and regulatory T cells (Treg) based on their
immunological effects. Th cells serve roles in the differentiation
of activated CD4+ T cells (20). By contrast, Tc cells serve a role
in the differentiation of activated CD8+ T cells, which
are cytotoxic (21). Forkhead box
protein 3 (FOXP3) is a specific biomarker of CD4+ Treg
cells (20). FOXP3 expression
reflects the number and functional activity of Treg cells. IL-10 is
a cytokine expressed by a number of immune cells. It may be
produced by T cells, B lymphocytes, mononuclear macrophages and
keratinocytes (21). The results
of the present study demonstrated that overexpression of miRNA-141
increased the toxicity of miRNA-141 on breast cancer cell growth
and caspase-3/9 activity. Feng et al (22) demonstrated that miRNA-141 induced
differentiation of CD4+ T cells to induce apoptosis in
colorectal cancer with lymph node metastasis.
COX-2 may be expressed in the endoplasmic reticulum
or the nuclear membrane (23).
Activated COX-2 is able to catalyze arachidonic acid to transport
more prostaglandins (PGs) into the nucleus, which regulates target
gene transcription (23).
Phospholipase A2 (PLA2) is activated by cytokines or inflammatory
mediators (24). Subsequently,
PLA2 is further oxidized into PGH2, the common precursor of all PGs
(23,24). It can be transformed by different
synthetases into bioactive end products, including prostaglandin D2
receptor 2, PGE2, SCF E3 ubiquitin ligase complex F-box protein
pof2, glucose-6-phosphate isomerase 2 (24). PGE2 can prevent antigen
presentation by dendritic cells. The above process allows tumors to
escape immune recognition, which contributes to tumor formation.
Recently, it was demonstrated that in human breast cancer
specimens, aromatase cytochrome P450 is positively associated with
COX-2 expression (25). T. PGE2
may increase aromatase activity, therefore elevating estrogen
synthesis and directly stimulating breast cancer proliferation
(26). In the present study,
overexpression of miRNA-141 suppressed COX-2 and PGE2 protein
expression in MCF-7 cells. Huang et al (27) reported that miR-141 regulates
colonic leukocyte trafficking by promoting the expression of IL-10
in murine colitis and human Crohn's disease.
COX-2 is the PG synthetase, excessive expression of
which may increase the levels of PG. Elevated COX-2 levels may be
detected in a number of tumors (26). This may increase PG synthesis and
promote tumor formation. PG is able to directly stimulate cell
growth. For instance, PGE2α and the PG F2-α receptor are able to
stimulate mitosis in Balb/C3T3 fibroblasts treated with
pro-epidermal growth factor (EGF) (28). PGE1 and PGE2 are able to stimulate
the proliferation of breast epithelial cells in the presence of EGF
(28). PGE2 is able to suppress T
and B cell proliferation, and cytokine synthesis, and reduce the
cytotoxicity of NK cells. PGE2 may inhibit TNF-α and IL-10
production (29). IL-10
demonstrates immune inhibitory effects and is expressed in a
variety of immune cells (30). T
cells, B lymphocytes, mononuclear macrophages and keratinocytes
secrete IL-10 (30). In addition,
IL-10 exhibits a dual role of promotion and inhibition of tumors
(31).
Inhibition of IL-10 increases the occurrence rate of
tumors and promotes cancer cell metastasis. IL-10 demonstrates
anticancer effects and suppresses breast cancer cell growth
(31). In the present study,
over-expression of miRNA-141 markedly reduced TNF-α protein
expression and induced IL-10 protein expression in MCF-7 cells.
Saito et al (32)
demonstrated that miRNA-141 may decrease myocardial
ischemia-reperfusion injury via suppression of TNF-α
expression.
MAP4K4 is upregulated in multiple tumors (15). Furthermore, it has been
demonstrated to serve a role in the acceleration of tumor cell
transformation, the promotion of cell invasion and the reduction of
cell adhesion (33). Upregulated
MAP4K4 expression in pancreatic cancer is positively associated
with postoperative recurrence, frequency of distant metastasis,
tumor size and the number of metastatic lymph nodes (34). The present study demonstrated that
overexpression of miRNA-141 markedly suppressed MAP4K4 protein
expression in MCF-7 cells, while the promotion of MAP4K4 protein
expression reduced the effects of miRNA-141 on the toxicity of
CD4+ T cells on breast cancer cells. Feng et al
(22) demonstrated that the
expression of miRNA-141 was downregulated in colorectal cancer and
that MAP4K4 protein expression was increased.
In conclusion, the present study demonstrated an
anti-cancer effect of microRNA-141 on breast cancer by cytotoxic
CD4+ T cells through MAP4K4 expression. The authors of
the present study hypothesize that miRNA-141 may be a novel target
for the therapy of breast cancer cells through cytotoxic
CD4+ T cells and COX-2, PGE2, TNF-α and IL-10 expression
by MAP4K4 expression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
QZ designed the experiments. HX and TF performed the
experiments. QZ and HX analysed the data and QZ wrote the
manuscript.
Ethics approval and consent to
participate
All human studies were approved by the Ethics
Committee of The First Affiliated Hospital of Jinan University. All
patients signed written informed consent forms prior to the study.
All animal experiments were approved by the Laboratory Animal
Ethics Committee of Jinan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pu Z, Zhang X, Chen Q, Yuan X and Xie H:
Establishment of an expression platform of OATP1B1 388GG and 521CC
genetic polymorphism and the therapeutic effect of tamoxifen in
MCF-7 cells. Oncol Rep. 33:2420–2428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Børøsund E, Cvancarova M, Moore SM,
Ekstedt M and Ruland CM: Comparing effects in regular practice of
e-communication and Web-based self-management support among breast
cancer patients: Preliminary results from a randomized controlled
trial. J Med Internet Res. 16:e2952014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maass N, Schem C, Bauerschlag DO, Tiemann
K, Schaefer FW, Hanson S, Muth M, Baier M, Weigel MT, Wenners AS,
et al: Final safety and efficacy analysis of a phase I/II trial
with imatinib and vinorelbine for patients with metastatic breast
cancer. Oncology. 87:300–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker EM, Rodriguez AI, Kohn B, Ball RM,
Pegg J, Pocock JR, Nunez R, Peterson E, Jakary S and Levine RA:
Acupuncture versus venlafaxine for the management of vasomotor
symptoms in patients with hormone receptor-positive breast cancer:
A randomized controlled trial. J Clin Oncol. 28:634–640. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohsumi S, Shimozuma K, Ohashi Y, Takeuchi
A, Suemasu K, Kuranami M, Ohno S and Watanabe T: Subjective and
objective assessment of edema during adjuvant chemotherapy for
breast cancer using taxane-containing regimens in a randomized
controlled trial: The National surgical adjuvant study of breast
cancer 02. Oncology. 82:131–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoffman CJ, Ersser SJ, Hopkinson JB,
Nicholls PG, Harrington JE and Thomas PW: Effectiveness of
mindfulness-based stress reduction in mood, breast- and
endocrine-related quality of life and well-being in stage 0 to III
breast cancer: A randomized, controlled trial. J Clin Oncol.
30:1335–1342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan HM, Saxena A, Gabbidon K, Rana S and
Ahmed NU: Model-based survival estimates of female breast cancer
data. Asian Pac J Cancer Prev. 15:2893–2900. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueno T, Chow LW and Toi M: Increases in
circulating VEGF levels during COX-2 inhibitor treatment in breast
cancer patients. Biomed Pharmacother. 60:277–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Generali D, Buffa FM, Deb S, Cummings M,
Reid LE, Taylor M, Andreis D, Allevi G, Ferrero G, Byrne D, et al:
COX-2 expression is predictive for early relapse and aromatase
inhibitor resistance in patients with ductal carcinoma in situ of
the breast and is a target for treatment. Br J Cancer. 111:46–54.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brandao RD, Veeck J, Van de Vijver KK,
Lindsey P, de Vries B, van Elssen CH, Blok MJ, Keymeulen K, Ayoubi
T and Smeets HJ: A randomised controlled phase II trial of
pre-operative celecoxib treatment reveals anti-tumour
transcriptional response in primary breast cancer. Breast Cancer
Res. 15:R292013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chuah BY, Putti T, Salto-Tellez M,
Charlton A, Iau P, Buhari SA, Wong CI, Tan SH, Wong AL, Chan CW, et
al: Serial changes in the expression of breast cancer-related
proteins in response to neoadjuvant chemotherapy. Ann Oncol.
22:1748–1754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao J, Yang X, Li WT, Zhao CL and Lv SJ:
Silencing of COX-2 by RNAi modulates epithelial-mesenchymal
transition in breast cancer cells partially dependent on the PGE2
cascade. Asian Pac J Cancer Prev. 15:9967–9972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan T, Wang L and Wang B: Collagen and
prostaglandin E2 regulate aromatase expression through the
PI3K/AKT/IKK and the MAP kinase pathways in adipose stromal cells.
Mol Med Rep. 12:4766–4772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao X, Gao C, Liu G and Hu J: MAP4K4: An
emerging therapeutic target in cancer. Cell Biosci. 6:562016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YF, Qu GQ, Lu YM, Kong WM, Liu Y, Chen
WX and Liao XH: Silencing of MAP4K4 by short hairpin RNA suppresses
proliferation, induces G1 cell cycle arrest and induces apoptosis
in gastric cancer cells. Mol Med Rep. 13:41–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong T, Wang M, Zhao J, Liu Q, Yang C,
Luo W, Li X, Yang H, Kristiansen K, Roy B and Zhou Y: An esophageal
squamous cell carcinoma classification system that reveals
potential targets for therapy. Oncotarget. 8:49851–49860. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen H, Li L, Yang S, Wang D, Zhong S,
Zhao J and Tang J: MicroRNA-29a contributes to drug-resistance of
breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling
pathway. Gene. 593:84–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang X, Tang J, Liu X, Zeng L, Cheng C,
Luo Y, Li L, Qin SL, Sang Y, Deng LM and Lv XB: Downregulation of
miR-129-2 by promoter hypermethylation regulates breast cancer cell
proliferation and apoptosis. Oncol Rep. 35:2963–2969. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grimholt RM, Urdal P, Klingenberg O and
Piehler AP: Rapid and reliable detection of alpha-globin copy
number variations by quantitative real-time PCR. BMC Hematol.
14:42014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bos PD, Plitas G, Rudra D, Lee SY and
Rudensky AY: Transient regulatory T cell ablation deters
oncogene-driven breast cancer and enhances radiotherapy. J Exp Med.
210:2435–2466. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Datta J, Berk E, Xu S, Fitzpatrick E,
Rosemblit C, Lowenfeld L, Goodman N, Lewis DA, Zhang PJ, Fisher C,
et al: Anti-HER2 CD4(+) T-helper type 1 response is a novel immune
correlate to pathologic response following neoadjuvant therapy in
HER2-positive breast cancer. Breast Cancer Res. 17:712015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng L, Ma H, Chang L, Zhou X, Wang N,
Zhao L, Zuo J, Wang Y, Han J and Wang G: Role of microRNA-141 in
colorectal cancer with lymph node metastasis. Exp Ther Med.
12:3405–3410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Onguru O, Casey MB, Kajita S, Nakamura N
and Lloyd RV: Cyclooxygenase-2 and thromboxane synthase in
non-endocrine and endocrine tumors: A review. Endocr Pathol.
16:253–277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jana D, Sarkar DK, Maji A, Chikkala BR,
Hassanujjaman S, Mukhopadhyay M and Ganguly S: Can
cyclo-oxygenase-2 be a useful prognostic and risk stratification
marker in breast cancer? J Indian Med Assoc. 110:429–433.
2012.PubMed/NCBI
|
|
25
|
Olesch C, Sha W, Angioni C, Sha LK, Açaf
E, Patrignani P, Jakobsson PJ, Radeke HH, Grösch S, Geisslinger G,
et al: MPGES-1-derived PGE2 suppresses CD80 expression on
tumor-associated phagocytes to inhibit anti-tumor immune responses
in breast cancer. Oncotarget. 6:10284–10296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thakur A, Schalk D, Tomaszewski E,
Kondadasula SV, Yano H, Sarkar FH and Lum LG: Microenvironment
generated during EGFR targeted killing of pancreatic tumor cells by
ATC inhibits myeloid-derived suppressor cells through COX2 and PGE2
dependent pathway. J Transl Med. 11:352013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Shi T, Zhou Q, et al: miR-141
regulates colonic leukocytic trafficking by targeting CXCL12β
during murine colitis and human Crohn's disease. Gut. 63:1247–1257.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan L, Jiang R, Yang Y, Ding S and Deng
H: 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer
cell line MCF-7 and downregulates cytochrome P4501B1 through the
COX-2/PGE2 pathway. Oncol Rep. 28:2131–2137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Menetrier-Caux C, Bain C, Favrot MC, Duc A
and Blay JY: Renal cell carcinoma induces interleukin 10 and
prostaglandin E2 production by monocytes. Br J Cancer. 79:119–130.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Razmkhah M, Jaberipour M, Erfani N,
Habibagahi M, Talei AR and Ghaderi A: Adipose derived stem cells
(ASCs) isolated from breast cancer tissue express IL-4, IL-10 and
TGF-β1 and upregulate expression of regulatory molecules on T
cells: Do they protect breast cancer cells from the immune
response? Cell Immunol. 266:116–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang C, He L, He P, Liu Y, Wang W, He Y,
Du Y and Gao F: Increased drug resistance in breast cancer by
tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling
pathway. Med Oncol. 32:3522015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito S, Thuc LC, Teshima Y, et al:
Glucose fluctuations aggravate cardiac susceptibility to
ischemia/reperfusion injury by modulating microRNAs expression.
Circ J. 80:186–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma M and Baumgartner M: Morphed and
moving: TNFα-driven motility promotes cell dissemination through
MAP4K4-induced cytoskeleton remodeling. Microb Cell. 1:154–157.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S, Li X, Lu D, Xu Y, Mou W, Wang L,
Chen Y, Liu Y, Li X and Li LY: SOX2 regulates apoptosis through
MAP4K4-survivin signaling pathway in human lung cancer cells.
Carcinogenesis. 35:613–623. 2014. View Article : Google Scholar : PubMed/NCBI
|