Introduction
Mesenchymal stem cells (MSCs) have been used as a
powerful stem cell source for cellular transplantation therapy, as
they exhibit numerous advantages (1–4).
MSCs may be easily obtained from various types of tissues, and
differentiate into different cell and tissue types with
immunomodulatory properties (5)
and homing capacity (6). MSCs
produce cytokines and chemokines that may promote cell
proliferative, anti-apoptotic, anti-inflammatory and cell homing
effects in wounded areas (7,8).
Brain-derived neurotrophic factor (BDNF) is a cytokine secreted by
MSCs and has neuroprotective, neurogenic and angiogenic effects,
thus promoting recovery after central nervous system (CNS) insult
(9,10). Modified MSCs overexpressing BDNF
may theoretically improve their therapeutic effect.
Although certain studies using genetically modified
techniques have exhibited promising results in cell culture and
small-animal CNS insult models, further preclinical research is
required in large animals due to numerous discordances between
laboratory and clinical research (11). Additionally, the labeling of
superparamagnetic iron oxide (SPIO) nanoparticles of cells is a way
of tracing the distribution and migration of transplanted cells in
large animals by magnetic resonance imaging (MRI) in vivo
with minor effects on cell viability (12,13).
However, few studies have reported the efficiency of genetically
modified MSCs after labeling with SPIO, especially for large
animals such as canines, which may be used as a suitable model of
human neurological disease and is evaluated by MRI (12,14).
Therefore, in this study, SPIO-labeled canine BMSCs
were used to evaluate the feasibility of BDNF gene lentiviral
transfection and the neural differentiation efficiency after gene
modification.
Materials and methods
Cell culture and identification
Bone marrow was extracted from the humerus of 20
healthy adult beagle dogs of either sex (sex ratio, males to
females 3:1) and 14.62±1.1 kg body weight (Laboratory Animal
Centre, Anhui, China) as previously described (12). All dogs were housed in a single
cages separately at well-ventilated facility with purified air and
12 h light/dark cycle at 24–28°C and fed twice a day with
commercial dry food and sterile water ad libitum. The National
Institutes of Health (Bethesda, MD, USA) and Institutional Animal
Care and Use Committee (IACUC) guidelines for use of animals in
research were followed, under an approved IACUC protocol of Nanjing
Medical University (Nanjing, China). MSCs were isolated and
purified from the bone marrow by density gradient centrifugation
and plastic wall adherence. Briefly, the bone marrow was layered
onto a density gradient solution at 1:1 volume ratio with equal
volume (Ficoll-Paque; Tianjin Haoyang Biological Products
Technology Co., Ltd., Tianjin, China), and centrifuged for 20 min
at 400 × g at 4°C. Enriched cells were obtained from the solution
interphase and washed twice with PBS, then cultivated in normal
culture medium: Low-glucose Dulbecco's modified Eagle medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin
at 37°C and 5% CO2. Primary isolated MSCs were defined
as passage (P)0. When 80–90% confluence was reached, the cells were
passaged (1:2 or 1:3 dilutions) with fresh medium.
To determine the expression of MSC markers [cluster
of differentiation (CD)34-, CD45-, CD44+ and CD90+], P2 BSMCs were
used for flow cytometry (BD FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA). In brief, BMSCs were collected in FACS tubes (BD
Biosciences) at 1×106 cells/tube and washed with PBS.
Cells were incubated with antibodies at room temperature for 1 h.
The antibodies included: anti-CD34 (cat. no. ab81289), CD45 (cat.
no. ab123522), CD44 (cat. no. ab157107), CD90 (cat. no. ab123511;
all Abcam, Cambridge, UK) all at 1:200. Labeled cells were washed
twice with PBS and re-suspender in 500 µl FACS buffer.
Subsequently, the cells were incubated with rabbit anti-mouse
immunoglobulin G secondary antibody labeled with phycoerythrin
(1:1,000; cat. no. ab7000; Abcam) of for 1 h. The expression of
these markers was evaluated using FlowJo software (version 7.6.3;
FlowJo LLC, Ashland, OR, USA) for data analysis.
To determine the multiple differentiation capacity,
P3 BMSCs were induced to osteoblastic and adipose differentiation
as previously reported (1). For
osteogenic differentiation, cells were seeded at a density of
4.5×104 cells/cm2 and cultured with induction
medium containing DMEM supplemented with 10% FBS (both Gibco;
Thermo Fisher Scientific, Inc.), 100 nM dexamethasone, 10 mM
β-glycerol-phosphate, and 100 µg/ml ascorbic acid (all purchased
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C and 5%
CO2. The induction medium was changed every 3 days.
After 14 days of induction, cells were fixed in 70% ethanol and
stained with 2% Alizarin Red at room temperature for 10 min. For
adipogenic differentiation, cells were plated at a density of
1.0×104 cells/cm2. Similarly, adipogenesis
was induced by culturing with induction medium containing DMEM
supplemented with 5% rabbit serum (Gibco; Thermo Fisher Scientific,
Inc.), 5 µg/ml insulin, 1 µM dexamethasone and 5 µg/ml
rosiglitazone (all obtained from Sigma-Aldrich; Merck KGaA) at 37°C
and 5% CO2. The induction medium was changed every 3
days. After 21 days of induction, cells were fixed in 70% ethanol
and stained with 0.3% Oil Red O at room temperature for 60 min.
SPIO labeling
BMSCs were cultured in 10 cm dish at
2×106 with fresh medium containing SPIO-poly-L-lysine
(Nanjing Nanoeast Biotech, Co., Ltd., Nanjing, China) at 20 µg/ml
for 24 h and then were washed three times in PBS to eliminate
extracellular SPIO. Efficiency of SPIO labeling was confirmed by
Prussian blue (PB) staining (12).
In brief, the cells were incubated with PB (2% potassium
ferrocyanide in 6% hydrogen chloride) for 15 min at room
temperature and then counterstained with nuclear fast red for 1 min
at room temperature. The percentage of iron-containing cells was
calculated according to the positively-stained cell numbers with
light microscope (IX71; Olympus Corp., Tokyo, Japan).
Lentivirus transfection
SPIO-BMSCs were seeded in 96-well plates at
1×104 cells per well (100 µl), cultured overnight and
transfected with enhanced green fluorescent protein
(eGFP)(+)-BDNF(−)-lentivirus (Shanghai GeneChem, Co., Ltd.,
Shanghai, China) at a multiplicity of infection (MOI) of 0, 1, 5,
10, 20, 50 and 100. After 12 h, transfection medium was changed to
normal culture medium without lentiviral particles, and cells were
cultured as routine. MSCs were used for analysis at day 3, 7 and
14. The percentage eGFP-positive cells was detected by Guava
Express analysis on a GuavaEasyCyte™ flow cytometer (Guava
Technologies; EMD Millipore, Billerica, MA, USA). At each time
point, the cellular viability was evaluated by using a Cell
Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). In brief, 10 µl CCK-8 was added into each well
and after 4 h incubation, and the optical density value was
measured at a wavelength of 450 nm using a microplate reader
(Thermo Fisher Scientific, Inc.). The optimal MOI was calculated by
multiplication of the percentage eGFP positive cells by the average
cellular survival rate.
BMSC neural differentiation
After determination of the optimal MOI, SPIO labeled
MSCs were divided into 4 groups: Transfection at optimized MOI with
eGFP(−)-BDNF(+)-lentivirus (BDNF+ group), transfection at optimized
MOI with eGFP(−)-BDNF(−)-lentivirus (BDNF-group) and
non-transfected group (mock group), for neural differentiation and
subsequent analysis. The non-transfected group without neural
differentiation was used as control group (control group). Neural
differentiation was induced as previously reported (15,16)
in BDNF+, BDNF- and mock group cells. Briefly, BMSCs were cultured
with DMEM + 10% FBS until sub-confluence. BMSCs were pre-induced
for 24 h with DMEM + 20% FBS + 1 mM β-mercaptoethanol, then were
induced for 8 h with DMEM + 100 mM butylated hydroxyanisole (BHA) +
2% dimethylsulfoxide (DMSO). Finally, of the media of the cells was
replaced with maintenance media containing DMEM + 100 mM BHA + 2%
DMSO + 25 mM KCl + 2 mM valproic acid + 10 µM forskolin +
N2 supplement for 1–3 days. BMSCs in the control group
were cultured with DMEM + 10% FBS.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Expression of cytokines [(BDNF, vascular endothelial
growth factor (VEGF) and chemokine receptor type 4 (CXCR-4)] and
neural precursor cells (indicated by nestin expression), neurons
[indicated by class 3 β tubulin (TUJ1) expression], neurogliocytes
[indicated by glial fibrillary acidic protein (GFAP) expression]
and oligodendrocytes (oligo1) were analyzed by RT-qPCR. Total RNA
was extracted from cultured cells using TRIzol reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
RNA was used for cDNA synthesis using a Prime Script RT Reagent kit
according to the manufacture's protocol and oligo dT primers
(Takara Biotechnology, Co., Ltd., Dalian, China). Briefly, the
first-strand cDNA was obtained by reverse transcription using 3 µl
of total RNA at 37°C for 15 min and 85°C for 5 sec. RT-qPCR was
performed at 95°C for 5 sec and 60°C for 34 sec in 20 µl buffer
containing SYBR premix ExTaq II and ROX Reference dye (Takara
Biotechnology, Co., Ltd.) and 0.2 µM each of the primers and SYBR
Premix DimerEraser (Takara Biotechnology, Co., Ltd.) on a 7900HT
system (ABI Prism® 7900HT; Thermo Fisher Scientific,
Inc). Glucuronidase β was used as an internal control. The relative
level of gene expression for each sample was calculated using the
2−ΔΔCq method (17).
Primer sequences are listed in Table
I.
 | Table I.Primers of canine specific
markers. |
Table I.
Primers of canine specific
markers.
| Gene | Primer
sequence | Marker |
|---|
| Nestin | F:
TGTAGGAGGTCCCTAAGCCC | Neural progenitor
cell |
|
| R:
CACATCCCTACACCACACCC |
|
| TUJ1 | F:
GCACACTGCTCATCAACAAG | Neuron |
|
| R:
TCTTGCTCTCCTTCATGGAC |
|
| GFAP | F:
TGAGATCGCCACCTACAGGA | Astrocyte |
|
| R:
ATCCAACACCTTGCCCACAA |
|
| BDNF | F:
CGCGGACTTGTATACCTCCC | Neurotrophin |
|
| R:
GGGACTTTTTCGAGGACGGT |
|
| VEGF | F:
CCCGGTATAAACCCTGGAGC | Angiogenesis |
|
| R:
ACGCGAGTCTGTGTTTTTGC |
|
| CXCR-4 | F:
CAGTTGAGGCTGTGGCAAAC | Chemokine |
|
| R:
GAGAGCAGGTATCCAGACGC |
|
| GUSB | F:
ACATCGACGACATCACCGTCA | Housekeeping
gene |
|
| R:
GGAAGTGTTCACTGCCCTGGA |
|
Immunofluorescence
To confirm the differentiation of the BMSCs,
neuron-like cells were analyzed by immunofluorescence using
antibodies against nestin, GFAP and TUJ1. The primary antibodies
included mouse anti-nestin (1:200; cat. no. ab22035), mouse
anti-GFAP (1:200; cat. no. ab53554), rabbit anti-nestin (1:200;
cat. no. ab105389) and rabbit anti-class 3 β tubulin (1:500; cat.
no. ab7751; all Abcam). Cells were fixed with 4% paraformaldehyde
at room temperature for 20 min. Cells were washed three times with
PBS and blocked with 0.4% Triton X-100 (Sigma-Aldrich; Merck KGaA)
at 4°C for 10 min. Then cells were washed three times with PBS and
immersed with 5% bovine serum albumin at 37°C for 30 min. Following
incubation with the primary antibodies overnight at 4°C, the cells
were washed three times with PBS, followed by incubation with
fluorescent goat anti-mouse immunoglobulin G (IgG) antibody (cat
no. ab150113), goat anti-mouse IgG antibody (cat no. ab97035), goat
anti-rabbit IgG (cat no. ab150079) and goat anti-rabbit IgG (cat
no. ab6787; all Abcam) at room temperature for 60 min, all at
1:200. Following washing, the cells were stained with 100 ng/ml
DAPI for 10 min at room temperature and mounted with anti-fade
mounting medium (both Sigma-Aldrich; Merck KGaA), and observed
under a fluorescent microscope. BMSCs that expressed neural (neuron
or glia) markers were considered to be immunopositive cells.
Statistical analysis
All analyses were performed in a double-blinded
manner. All values are expressed as the mean ± standard deviation.
Student's t-test and one-way analysis of variance followed by
Bonferroni's post hoc analysis were used for single and multiple
comparisons, respectively. Data was calculated using SPSS software
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology and phenotypes of cultured
BMSCs
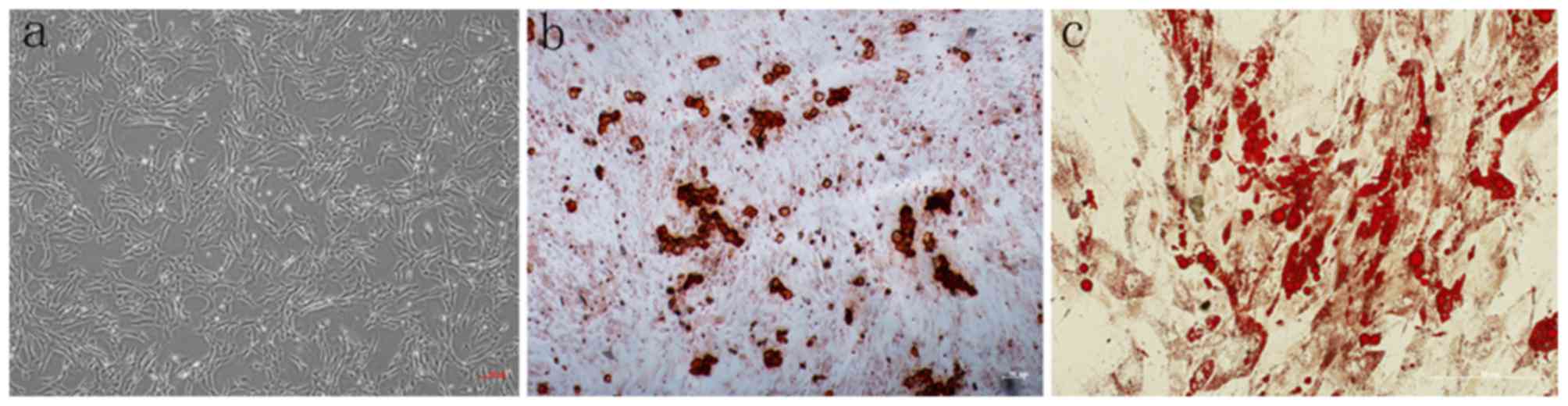
Following isolation by density gradient
centrifugation from canine bone marrow, BMSCs were cultured in
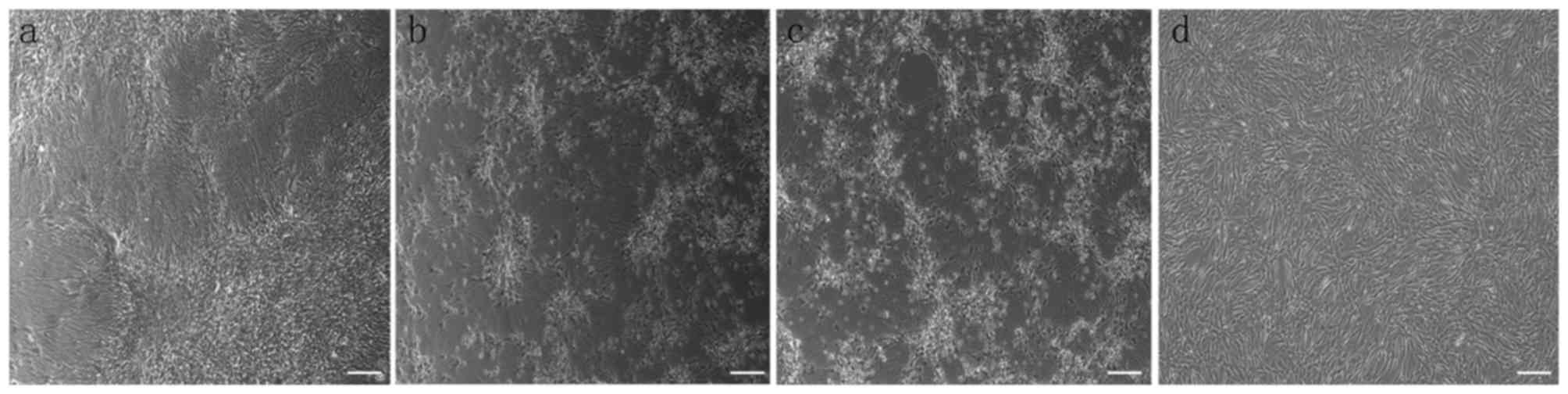
growth medium. After 14 days of culturing, the cell morphology was
altered from various shapes including discoidal flat, triangular
and elongated, to a spindle shape under a light microscope
(Fig. 1a). After 14 days of
osteogenic differentiation, the cells differentiated into the
osteogenic lineage, as demonstrated by slizarin red staining
(Fig. 1b). Additionally, after 21
days of adipogenic differentiation, these cells differentiate into
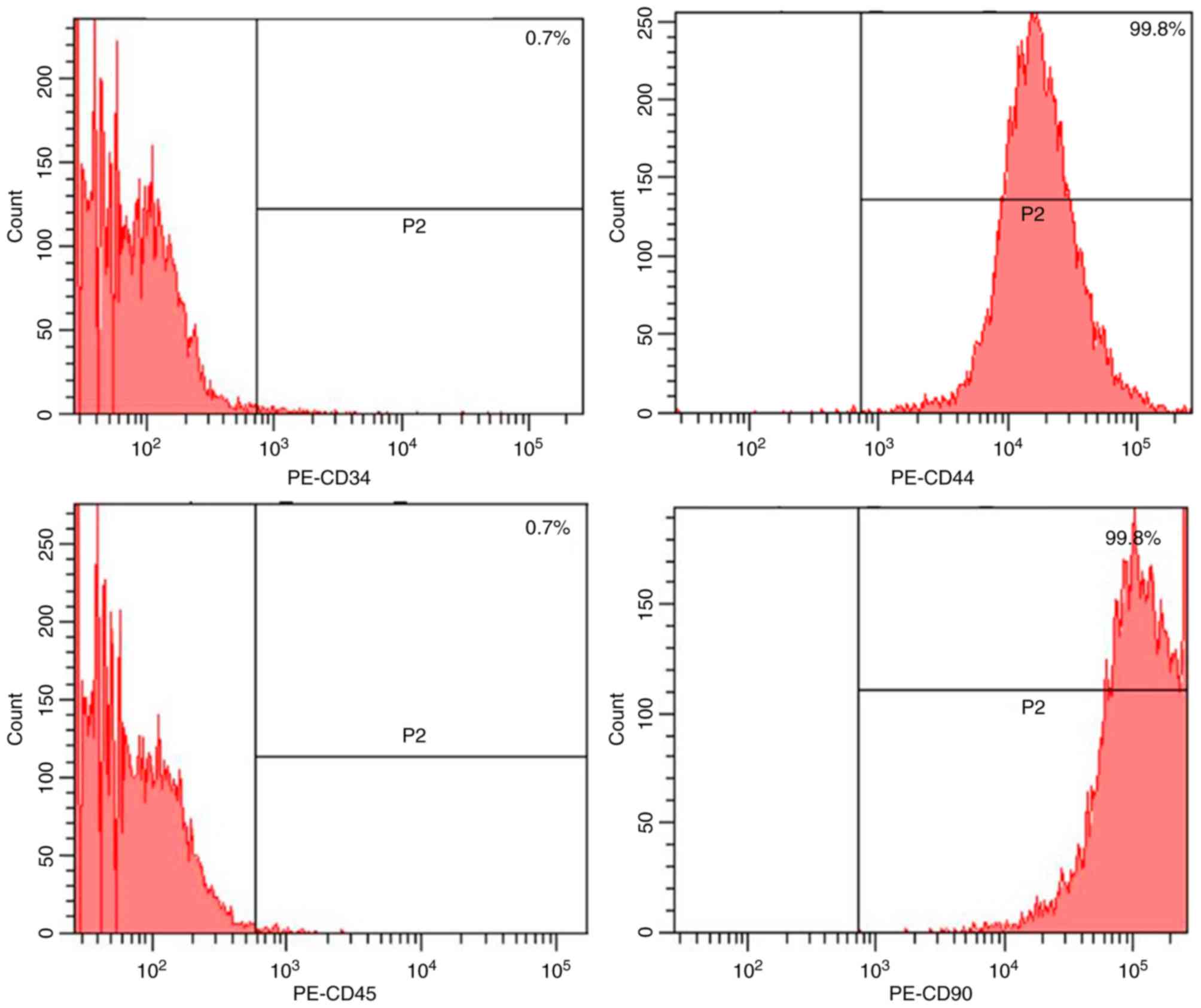
adipocytes with lipid droplets stained with Oil red O (Fig. 1c). Flow cytometry analysis
(Fig. 2) revealed that passage 2
BMSCs (7-day culture) were positive for CD44 and CD90 and negative
for CD34 and CD45.
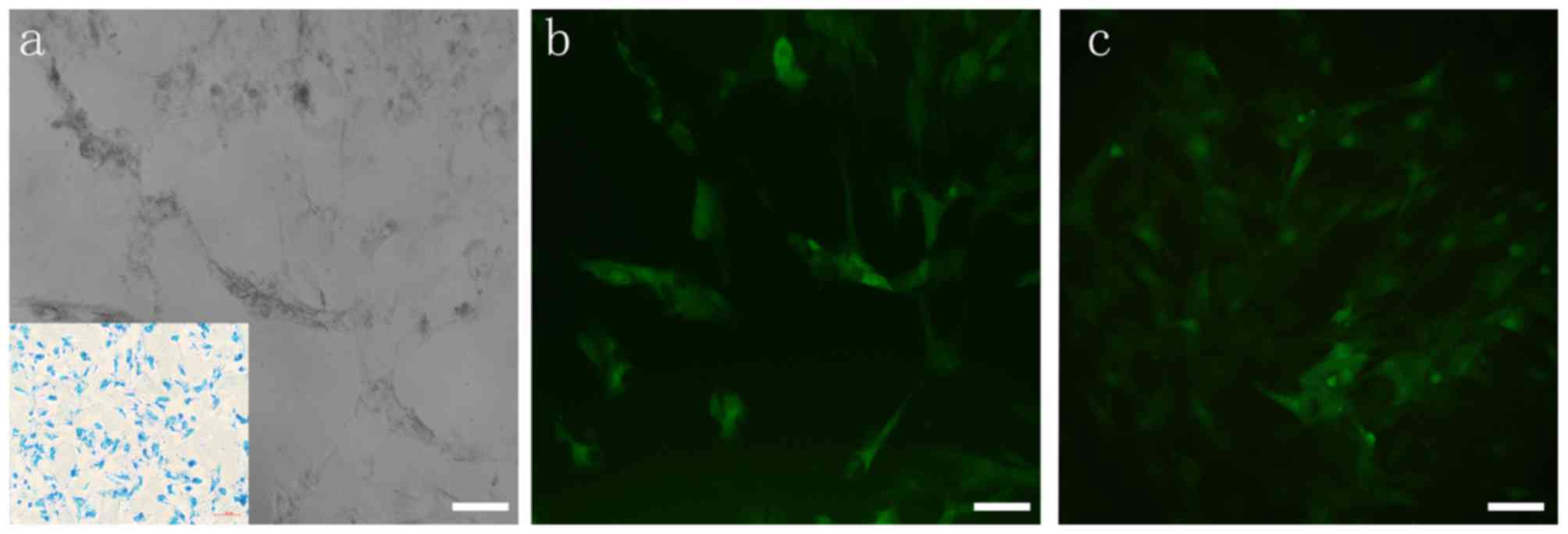
SPIO labeling
The uptake mechanism of SPIO particles by BMSCs is
mediated by endocytosis. In a previous study, 20 µg/ml was chosen
as the optimal concentration for high uptake efficiency and minimal
cytotoxicity (12). After
incubation with SPIO for 24 h, SPIO particles (blue staining;
Fig. 3a) were visible following PB
staining, and the labeling percentage of SPIO was ~100% (Fig. 3a).
Optimized MOI of lentivirus
transfection
After incubation with eGFP(+)-BDNF(−)-lentivirus for
12 h, SPIO-labeled BMSCs (Fig. 3a)
exhibited positive eGFP expression on day 3 (Fig. 3b), and proliferated with positive
eGFP expression on day 7 (Fig.
3c). As demonstrated in Fig.
4, the eGFP expression rate was MOI dose-dependent at day 3 and
exhibited a ≥80% positive rate when MOI ≥5 was used at day 14.
However, with the increasing of MOI, the cellular survival rate
decreased (MOI 50 and 100). Following combining the eGFP positive
rate and the average cellular survival rate at different MOIs, it
was revealed that at MOI 10, BMSCs exhibited higher eGFP expression
and proliferation capacity at day 7 and this was maintained at day
14, (% eGFP x% survival cells ×104 were 8752.37±242.11
and 8464.76±273.40 at day 7 and 14, respectively; P<0.0001).
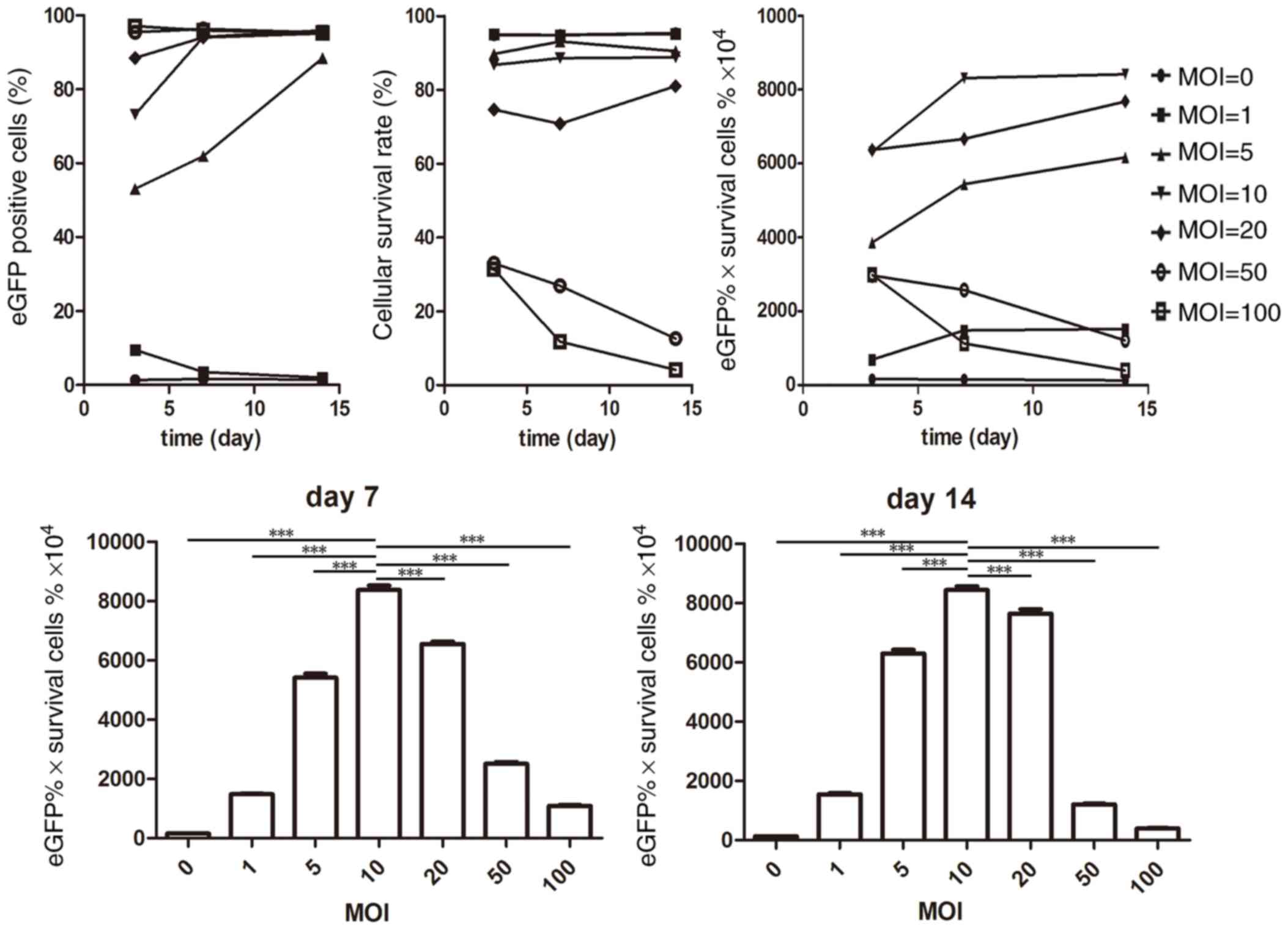 | Figure 4.Flow cytometry and Cell Counting kit-8
analysis of BMSCs following incubation with SPIO-PLL and
transfection with eGFP + lentivirus. The upper left panel presents
the dose-dependent effect on eGFP positive cells at day 3, and at
MOI 100, the majority of BMSCs were eGFP positive, whereas at MOI
0, eGFP was rarely expressed. The eGFP expression rate increased to
≥80% from day 7 to 14 at MOI ≥5. The upper middle graph presents
the cellular survival rate. MOI ≤10 maintained ≥80% survival rate
at day 3–14, whereas at MOI ≥50, the survival rate decreased from
day 3–14. The upper right graph shows the product of the eGFP
positive rate and the average cellular survival rate. The lower
graphs represent the significance of day 7 and day 14,
respectively, which indicated the optimal MOI was MOI 10. BMSCs,
bone marrow-derived mesenchymal stem cells; SPIO-PLL,
superparamagnetic iron oxide-poly-L-lysine; eGFP, enhanced green
fluorescent protein; MOI, multiplicity of infection;
***P<0.0001. |
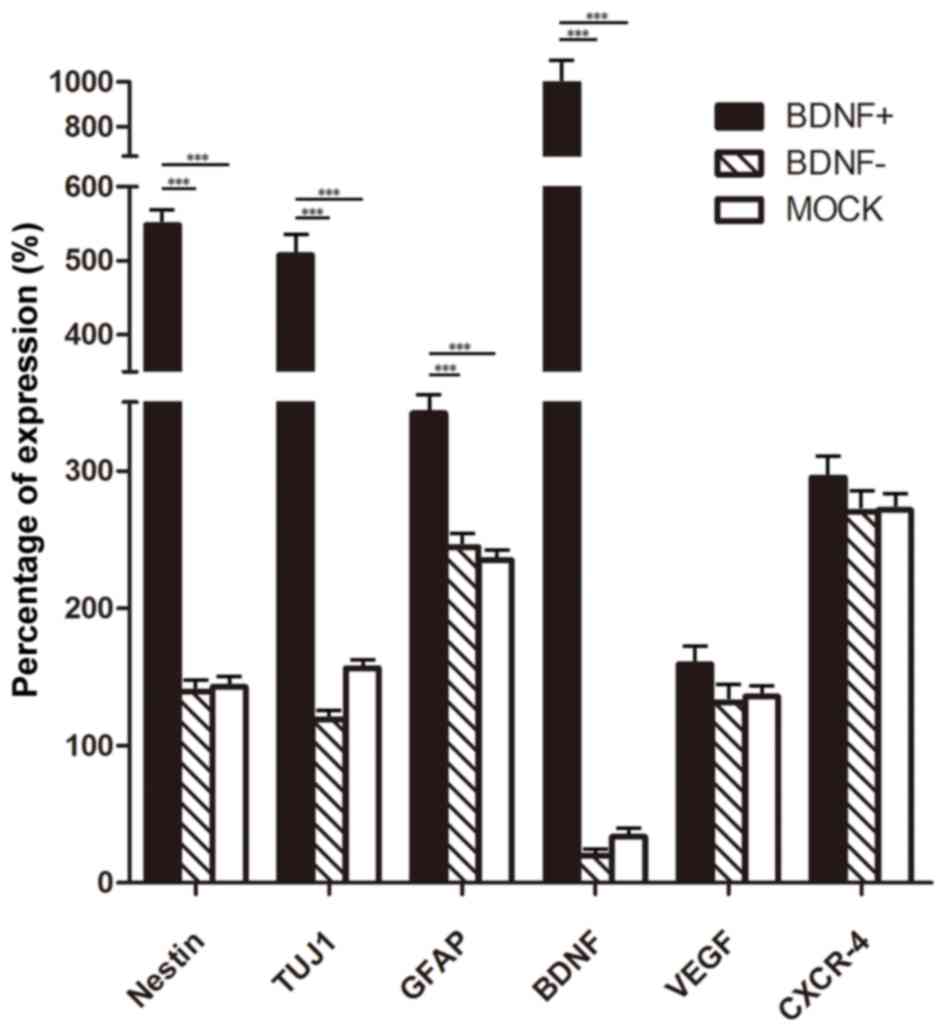
Expression of BDNF and neural
markers
To determine the expression of cytokines and neural
markers following SPIO-BMSCs neural differentiation, the expression
levels of cytokines (BDNF, VEGF and CXCR-4), and neural precursor
cells (nestin), neurons (TUJ1) and neurogliocytes (GFAP) were
detected. As exhibited in Fig. 5,
following neural differentiation, the neural markers (nestin, TUJ1
and GFAP) were significantly increased in the differentiation
groups compared with the control group. Additionally, VEGF and
CXCR-4 were also increased markedly in the differentiation groups
compared with the control, which are important for vascular
formation and cell homing, respectively. The expression of BDNF was
not increased following neural differentiation in the BDNF- and the
mock groups; however, in the BDNF+ group, the expression of BDNF
was significantly increased compared with the mock group, which
suggested that there was positive gene expression during neural
differentiation. Although not significant, the expression of VEGF
and CXCR-4 in the BDNF+ group was also higher than the other
differentiated groups. The relative percentages of expression of
neural markers and cytokines in the BDNF+, BDNF- and mock groups
were evaluated (Fig. 6). The
percentage of expression was calculated as follows: Expression in
differentiation groups - the expression in the control group)/the
expression in control group ×100. When comparing the BDNF+ group
with the other two groups, the percentage BDNF expression was ~10
fold higher. Expression of neural markers of nestin, TUJ1 and GFAP
also increased markedly in the BDNF+ group compared with BDNF- and
mock groups (approximately 3–6 fold). Furthermore, expression of
VEGF and CXCR-4 also increased in the BDNF+ group but the
difference was not significant compared with BDNF- and mock
groups.
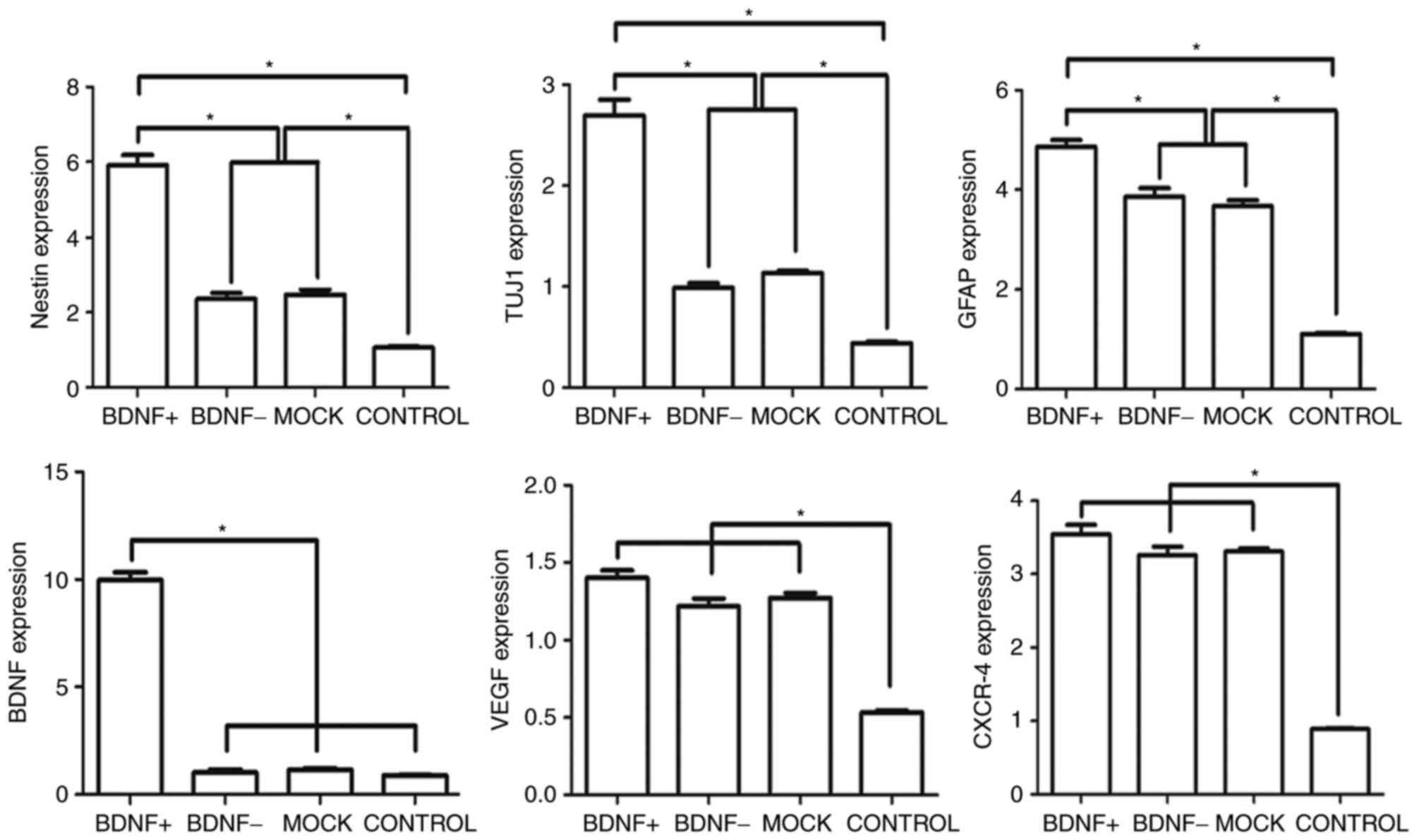 | Figure 5.Reverse transcription-quantitative
polymerase chain reaction analysis of BMSCs following neural
differentiation. Following neural differentiation, the BDNF+ group
exhibited significantly higher expression of neural markers,
nestin, TUJ1 and GFAP compared with the other groups, and the
expression of BDNF was also markedly higher in the BDNF+ group than
the other three groups. Compared with the control group, the neural
markers, VEGF and CXCR-4 expression were significantly higher in
the neural differentiation groups compared with the control group.
BMSCs, bone marrow-derived mesenchymal stem cells; BDNF,
brain-derived neurotrophic factor; TUJ1, class 3 β tubulin; GFAP,
glial fibrillary acidic protein; VEGF, vascular endothelial growth
factor; CXCR-4, chemokine receptor type 4. *P<0.05. |
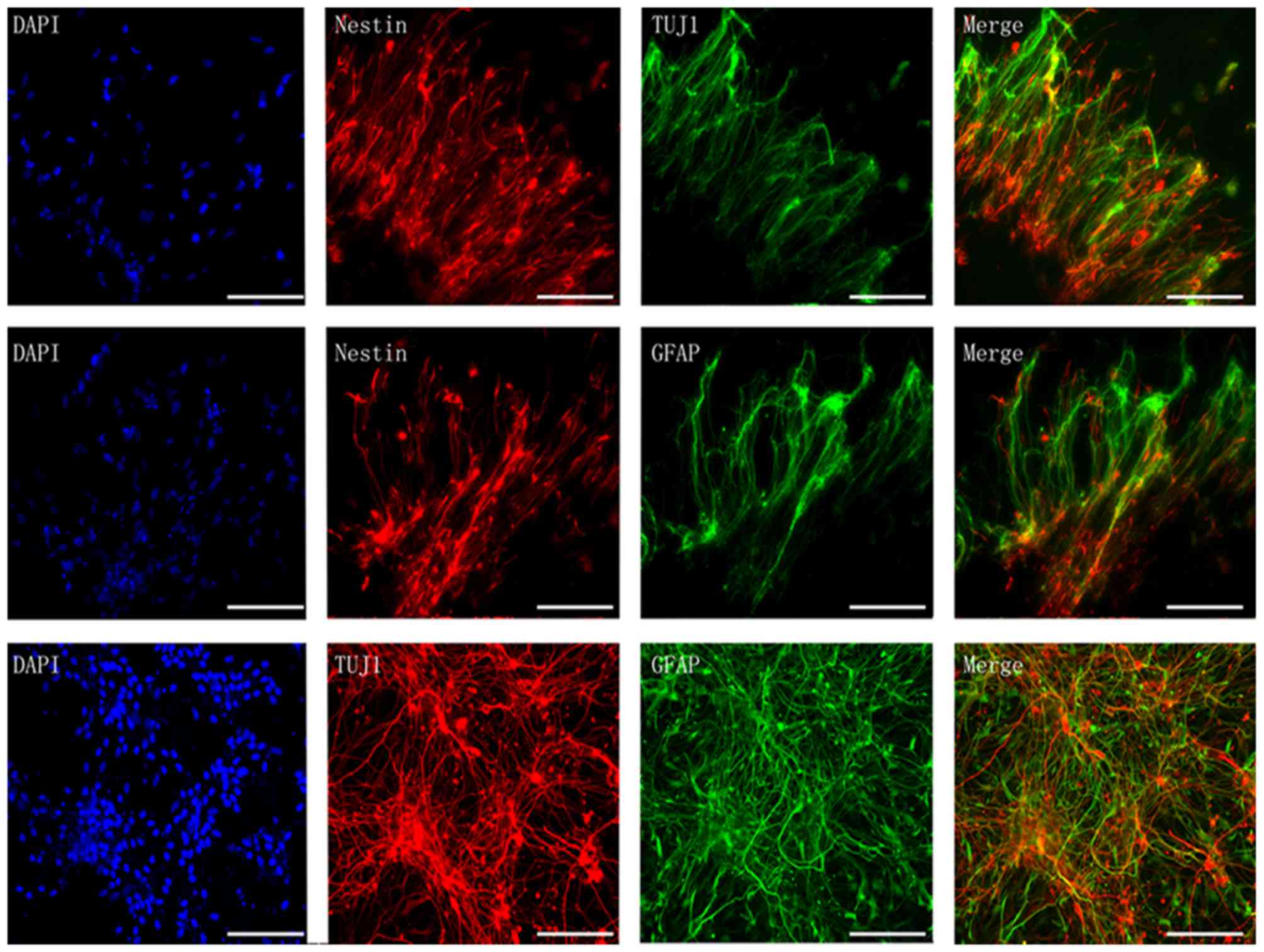
Immunofluorescence of neural-like
cells
As is presented in Fig.
7, following neural differentiation, the morphology of BMSCs
changed to neuron-like cells with comprehensive axon-like
connection with surrounding cells (Fig. 7). Additionally, the BDNF+ group
presented with much more neuron-like cells than the other groups.
Immunofluorescence revealed positive co-staining of nestin + TUJ1,
nestin + GFAP and TUJ1 + GFAP (Fig.
8), and double-positive cells in the BDNF+ group was higher
than the other groups, which is in accordance with the result of
RT-qPCR analysis.
Discussion
The present study revealed that canine-derived
SPIO-labeled and BDNF gene-modified BMSCs differentiated into
neuron-like cells in vitro. In addition, following neural
differentiation, the BDNF+ BMSCs had significantly increased
expression levels of nestin, TUJ1 and GFAP, which represent neural
precursor cells, neuron and neurogliocytes, respectively. These
results suggested that overexpression of BDNF promotes neural
differentiation, which may provide the foundation for cell
regenerative therapy and cell paracrine effect-based therapy.
Previous studies have utilized genetically
engineered MSCs modified using various gene therapy methods,
including delivery using adenoviral transfer (18,19),
Lipofectamine (20), physical cell
puncture (21), electroporation
(22), soundwaves (23) and lentiviruses (4,24).
The primary advantage of lentivirus transfection is the capacity of
gene integration with high efficiency, which establishes a new cell
line that expresses the target gene (25). Initial vector transfection
efficiency and optimization studies may provide the foundation of
future in vivo experiments, and therefore the efficiency of
vector transfection of MSCs may provide a pre- in vivo
evaluation (4,24,25).
In canine MSCs, lentivirus mediated gene overexpression has been
rarely reported (24). Previously,
Ahn et al (26) used a
lentivirus to overexpress interferon-β in MSCs to treat canine
melanoma, however the process of optimizing the MOI was not
reported. Lentiviruses can infect proliferating and
non-proliferating cells, which means that BMSCs can be eGFP
positive but not viable. Therefore, cellular viability analysis is
required to assess viral cytotoxicity and to optimize the MOI. In
this study, time points for transfection were set at day 3, 7 and
14, which is the duration usually required for expression of eGFP
(12). The results of the present
study demonstrated that, at day 7, lentivirus transfection at MOI
10 resulted in a marked increase in eGFP expression and
proliferation capacity, and this was maintained at day 14.
BDNF is an essential molecule for cerebral nerve
regeneration (27,28). However, the underlying mechanism is
not fully understood. A previous in vitro study demonstrated
that a BDNF overexpression plasmid promoted neural growth through
crosstalk with the Wnt/β-catenin signaling pathway via glycogen
synthase kinase-3β (29). Oh et
al (30) reported that,
following co-culture with MSCs, neuronal progenitor cells expressed
significantly higher levels of neural markers, including GFAP and
nestin, via activation of the Wnt signaling pathway. In this study,
the expression of neural markers GFAP and nestin were also
associated with neural differentiation, and BDNF-transfected MSCs
exhibited higher expression of these markers. Additionally, the
results also demonstrated increased expression of TUJ1 in cells
overexpressing BDNF, which may be associated phosphorylation of
protein kinase B (31). However,
further studies focusing on the quantification of specific proteins
that affect the signaling pathways are required.
Although not significant, the expression level of
VEGF, which is an essential molecule involved in angiogenesis, was
also increased by overexpression of BDNF. In addition, except for
the increased expression of neurotropic genes (Nestin, TUJ1, GFAP
and BDNF), the results demonstrated an increased gene expression of
CXCR-4. CXCR-4 and its ligand, chemokine stromal cell-derived
factor-1, have a critical role in MSC homing and recruitment at
injury sites (32). This
upregulation of CXCR-4 suggested that MSCs may actively migrate
following neural differentiation in vivo.
The main limitation of this study is that only in
vitro experiments were performed, and the neural
differentiation efficiency and therapeutic effects of BDNF-modified
BMSCs in vivo may be different due to the altered
microenvironment. However, the present study demonstrated that
lentivirus transfection is feasible for SPIO-labeled canine BMSCs,
and overexpression of BDNF may contribute to the neural
differentiation after successful transfection. This preliminary
data may be important for further in vivo studies in large
animals.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81571777, 81471764
and 81501565) and the Foundation of Research and Innovation Program
for Postgraduates in Jiangsu Province (grant no. KYLX15_0957).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and HBS conceived and designed the experiments.
XLL, QQZ and BW performed the experiments. BW, SSL and XQX analyzed
the data. XLL wrote the manuscript which was revised and reviewed
by QQZ, SSL and XQX, and the final version was approved by SL and
HBS.
Ethics approval and consent to
participate
All the animal experimental procedures were approved
by the local Animal Experiment Ethical Committee (Nanjing Medical
University, Nanjing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Volk SW, Wang Y and Hankenson KD: Effects
of donor characteristics and ex vivo expansion on canine
mesenchymal stem cell properties: Implications for MSC-based
therapies. Cell Transplant. 21:2189–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golpanian S, Wolf A, Hatzistergos KE and
Hare JM: Rebuilding the damaged heart: Mesenchymal stem cells,
cell-based therapy, and engineered heart tissue. Physiol Rev.
96:1127–1168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baligar P, Mukherjee S, Kochat V, Rastogi
A and Mukhopadhyay A: Molecular and cellular functions distinguish
superior therapeutic efficiency of bone marrow CD45 cells over
mesenchymal stem cells in liver cirrhosis. Stem Cells. 34:135–147.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tseng TC and Hsu SH: Substrate-mediated
nanoparticle/gene delivery to MSC spheroids and their applications
in peripheral nerve regeneration. Biomaterials. 35:2630–2641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: Strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012:3429682012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khubutiya MS, Vagabov AV, Temnov AA and
Sklifas AN: Paracrine mechanisms of proliferative, anti-apoptotic
and anti-inflammatory effects of mesenchymal stromal cells in
models of acute organ injury. Cytotherapy. 16:579–585. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burdon TJ, Paul A, Noiseux N, Prakash S
and Shum-Tim D: Bone marrow stem cell derived paracrine factors for
regenerative medicine: Current perspectives and therapeutic
potential. Bone Marrow Res. 2011:2073262011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagahara AH and Tuszynski MH: Potential
therapeutic uses of BDNF in neurological and psychiatric disorders.
Nat Rev Drug Discov. 10:209–219. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kermani P and Hempstead B: Brain-derived
neurotrophic factor: A newly described mediator of angiogenesis.
Trends Cardiovasc Med. 17:140–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rink C, Christoforidis G, Abduljalil A,
Kontzialis M, Bergdall V, Roy S, Khanna S, Slivka A, Knopp M and
Sen CK: Minimally invasive neuroradiologic model of preclinical
transient middle cerebral artery occlusion in canines. Proc Natl
Acad Sci USA. 105:14100–14105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW,
Zhang Y and Shi HB: In vivo MR imaging of intraarterially delivered
magnetically labeled mesenchymal stem cells in a canine stroke
model. PLoS One. 8:e549632013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Chen J, Liu X, Li H, Qi X and Guo X:
Autologous bone marrow stromal cell transplantation as a treatment
for acute radiation enteritis induced by a moderate dose of
radiation in dogs. Transl Res. 171:38–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Penha EM, Meira CS, Guimarães ET, Mendonça
MV, Gravely FA, Pinheiro CM, Pinheiro TM, Barrouin-Melo SM,
Ribeiro-Dos-Santos R and Soares MB: Use of autologous mesenchymal
stem cells derived from bone marrow for the treatment of naturally
injured spinal cord in dogs. Stem Cells Int. 2014:4375212014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. J Neurosci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammer K, Kazcorowski A, Liu L, Behr M,
Schemmer P, Herr I and Nettelbeck DM: Engineered adenoviruses
combine enhanced oncolysis with improved virus production by
mesenchymal stromal carrier cells. Int J Cancer. 137:978–990. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim MD, Kim SS, Cha HY, Jang SH, Chang DY,
Kim W, Suh-Kim H and Lee JH: Therapeutic effect of hepatocyte
growth factor-secreting mesenchymal stem cells in a rat model of
liver fibrosis. Exp Mol Med. 46:e1102014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ribeiro SC, Mendes R, Madeira C, Monteiro
GA, da Silva CL and Cabral JM: A quantitative method to evaluate
mesenchymal stem cell lipofection using real-time PCR. Biotechnol
Prog. 26:1501–1504. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han SW, Nakamura C, Kotobuki N, Obataya I,
Ohgushi H, Nagamune T and Miyake J: High-efficiency DNA injection
into a single human mesenchymal stem cell using a nanoneedle and
atomic force microscopy. Nanomedicine. 4:215–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liew A, André FM, Lesueur LL, De Ménorval
MA, O'Brien T and Mir LM: Robust, efficient, and practical
electrogene transfer method for human mesenchymal stem cells using
square electric pulses. Hum Gene Ther Methods. 24:289–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi Y, Park JE, Jeong JS, Park JK, Kim J
and Jeon S: Sound waves induce neural differentiation of human bone
marrow-derived mesenchymal stem cells via ryanodine
receptor-induced calcium release and Pyk2 activation. Appl Biochem
Biotechnol. 180:682–694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGinley L, McMahon J, Strappe P, Barry F,
Murphy M, O'Toole D and O'Brien T: Lentiviral vector mediated
modification of mesenchymal stem cells & enhanced survival in
an in vitro model of ischaemia. Stem Cell Res Ther. 2:122011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han SH, Jang G, Bae BK, Han SM, Koh YR,
Ahn JO, Jung WS, Kang SK, Ra JC, Lee HW and Youn HY: Effect of
ectopic OCT4 expression on canine adipose tissue-derived
mesenchymal stem cell proliferation. Cell Biol Int. 38:1163–1173.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn Jo, Lee Hw, Seo Kw, Kang Sk, Ra Jc and
Youn Hy: Anti-tumor effect of adipose tissue derived-mesenchymal
stem cells expressing interferon-β and treatment with cisplatin in
a xenograft mouse model for canine melanoma. PLoS One.
8:e748972013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fouda AY, Alhusban A, Ishrat T, Pillai B,
Eldahshan W, Waller JL, Ergul A and Fagan SC: Brain-derived
neurotrophic factor knockdown blocks the angiogenic and protective
effects of angiotensin modulation after experimental stroke. Mol
Neurobiol. 54:661–670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Helm EE, Tyrell CM, Pohlig RT, Brady LD
and Reisman DS: The presence of a single-nucleotide polymorphism in
the BDNF gene affects the rate of locomotor adaptation after
stroke. Exp Brain Res. 234:341–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang JW, Ru J, Ma W, Gao Y, Liang Z, Liu
J, Guo JH and Li LY: BDNF promotes the growth of human neurons
through crosstalk with the Wnt/β-catenin signaling pathway via
GSK-3β. Neuropeptides. 54:35–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh SH, Kim HN, Park HJ, Shin JY and Lee
PH: Mesenchymal stem cells increase hippocampal neurogenesis and
neuronal differentiation by enhancing the wnt signaling pathway in
an Alzheimer's disease model. Cell Transplant. 24:1097–1109. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahmani A, Kheradmand D, Keyhanvar P,
Shoae-Hassani A and Darbandi-Azar A: Neurogenesis and increase in
differentiated neural cell survival via phosphorylation of Akt1
after fluoxetine treatment of stem cells. Biomed Res Int.
2013:5825262013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Huang J, He X, Tang G, Tang YH, Liu
Y, Lin X, Lu Y, Yang GY and Wang Y: Postacute stromal cell-derived
factor-1α expression promotes neurovascular recovery in ischemic
mice. Stroke. 45:1822–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|