Introduction
Vascular smooth muscle cells (VSMCs) play an
important role in blood vessel tone regulation and in vascular
growth and response to injuries (1). In the media layer of mature blood
vessels, VSMCs exhibit extensive plasticity and can go through
phenotypic modulation from a quiescent contractile state to a
proliferative synthetic state in response to various environmental
stimuli, such as growth factors, reactive oxidative species, and
mechanical injury (2). It is
noteworthy that among abovementioned stimuli, platelet-derived
growth factor-BB (PDGF-BB) modulated phenotypic switch has been
thoroughly established and subsequently leads to the formation of
neointima in response to vascular injury (3,4).
This phenotypic modulation is characterized by the alteration of
proliferation and alterations in the expression of phenotypic
markers, such as alpha smooth muscle actin (α-SMA), calponin and
osteopontin (OPN) (5). Prevention
of PDGF-BB-induced VSMC phenotypic switch and proliferation leading
to the attenuated intimal hyperplasia and vascular remodeling
(6). On this basis, deeper
understanding of the mechanisms that may control VSMCs phenotype
modulation could be a critical therapeutic target in treatment of
atherosclerotic cardiovascular diseases.
Crocin is one of the major biologically active
substances of saffron. The well-known pharmacological effects of
crocin are anti-oxidant (7),
anti-cancer (8) and
neuroprotective activities (9).
Previously, crocin was shown with the ability to significantly
inhibit atheromatous plaque formation in atherosclerotic quails and
its mechanisms may through decreasing the EC apoptosis and
inhibiting the elevated calcium ion in oxidatively modified
low-density lipoprotein induced VSMCs (10). Recently, a study found crocin could
decrease blood lipid levels and inhibit lipogenesis by suppressing
the expression of lipogenesis-related proteins and elevating lipid
catabolism-related proteins (11).
Moreover, crocin could alleviate the inflammation in a VD3-induced
rat coronary atherosclerosis model by inhibiting NF-κB p65 nuclear
translocation (11). These all
suggested the potential protective effects of crocin in the
initiation and progression of atherosclerosis. Nevertheless, to
date, whether crocin could regulate VSMC phenotypic switch still
needs to be elucidated.
In the present study, we aimed to explore the role
of crocin in PDGF-BB-mediated phenotype switching of VSMCs. Primary
rats aortic VSMCs were treated with PDGF-BB followed by various
concentration of crocin. The proliferative rates and phenotypic
switch of VSMCs were then measured. Subsequently, the potent
mechanisms of the action were investigated.
Materials and methods
Cell and reagents
VSMCs were isolated from thoracic aortas of male SD
rats as previous indicated (12).
Animal studies conformed to the ARRIVE guidelines (2013) and were
approved by the Institutional Animal Care and Use Committee of the
First Hospital of China Medical University. The culture medium was
Dulbecco's modified Eagle's media (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 100 U/ml penicillin/100
µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.),
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.). Cells were maintained at 37°C and 5% CO2. VSMCs
of passages 5–9 were used for experiments. Crocin (cat. no.
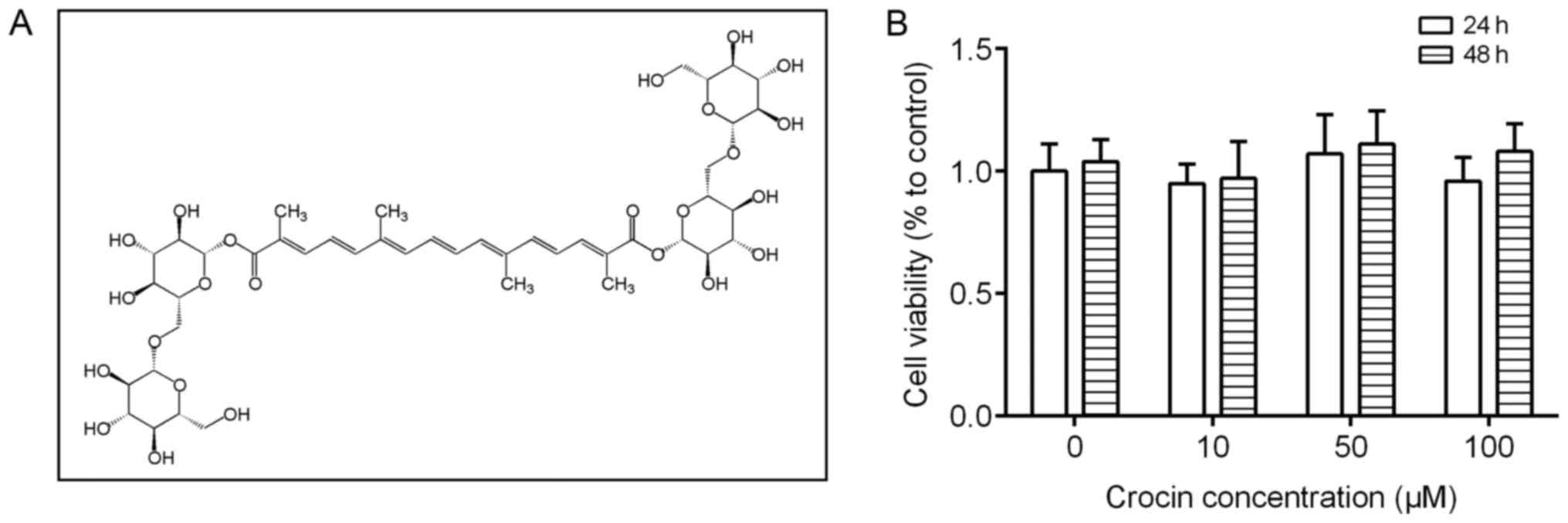
42553-65-1, purity >97%, structure presented in Fig. 1A) and PDGF-BB were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies against α-SMA, OPN,
calponin and kruppel-like factor 4 (KLF4) were obtained from Abcam
(Cambridge, MA, USA). Antibodies against phosphorylated (p-)JAK1,
JAK1, p-JAK2, JAK2, signal transducers and activators of
transcription (p-STAT3), STAT3, extracellular signal-regulated
kinase (p-ERK1/2), and ERK1/2 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against GAPDH was
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
AG490 and U0126 were acquired from MedChem Express (Monmouth
Junction, NJ, USA). The concentration of inhibitors was selected
according to previous studies (13,14).
Cell treatment
After an initial 24 h of culture in serum-free
medium, VSMCs were exposed to 20 ng/ml PDGF-BB for 24 h with or
without various concentration of crocin (10, 50, 100 µM). For
pathways inhibitors, VSMCs were pre-treatment for 1 h with AG490 or
U0126 at the final concentration of 10 and 50 µM, respectively.
Cell viability assay
VSMCs were cultured in 96-well plates
(5×103 cells/well). After reaching a confluence of 85%,
cells were treated with different concentration of crocin, and
stimulated with 20 ng/ml PDGF-BB for 24 h. Then, 10 µl Cell
Counting Kit-8 (CCK-8) reagent was added to each well followed by
incubation for an additional 2 h at 37°C. The absorbance of cells
was measured at 450 nm using a microplate reader.
Immunofluorescence staining
Following treatment of VSMCs with PDGF and crocin,
cells were fixed in 4% paraformaldehyde for 15 min and
permeabilized in 0.1% Triton X-100 for another 15 min at room
temperature. Subsequently, cells were blocked with 2% BSA for 30
min at room temperature followed by incubation with α-SMA antibody
(1:400) at 4°C overnight. After washes with PBS for three times,
cells were incubated with goat anti-rabbit IgG H&L Alexa
Fluor® 488 (1:500) at room temperature for another 1 h.
Finally, nuclei were stained with DAPI for 5 min. Images were taken
under a fluorescence microscope (IX73-A12FL/PH; Olympus, Tokyo,
Japan) at 200× magnification.
Western blot analysis
After treatment, the cells were lysed in RIPA lysis
buffer supplemented with protease/phosphatase inhibitor for 15 min
at 4°C and total protein concentrations were measured using the BCA
assay. Protein samples were loaded on 10–12% SDS-PAGE and
transferred to PVDF membranes followed by blocked with 5% skim milk
in TBST at room temperature for 1 h. After washing, the membranes
were incubated with the primary antibodies against α-SMA (1:1,000),
calponin (1:1,000), OPN (1:500), p-JAK1 (1:1,000), JAK1 (1:1,000),
p-JAK2 (1:1,000), JAK2 (1:1,000), p-STAT3 (1:1,000), STAT3
(1:1,000), KLF4 (1:1,000), p-ERK1/2 (1:10,000), ERK1/2 (1:10,000)
and GAPDH (1:1,000) overnight at 4°C. After three washes with TBST,
the blots were incubated for 1 h at room temperature with
HRP-conjugated goat anti-rabbit antibody/mouse antibodies at room
temperature for 1 h. Signals were visualized using an enhanced
chemiluminescence kit and densitometry analysis of the immunoblots
was carried out using Image J software (National Institute of
Health, Bethesda, MD, USA).
Data analysis
All data are expressed as mean ± SD and analyzed
using SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA). Statistical
analyses were performed using one-way ANOVA with Tukey's post hoc
test. The value of P<0.05 was statistically significant.
Results
Crocin inhibits PDGF-BB-induced VSMCs
proliferation and phenotypic switch
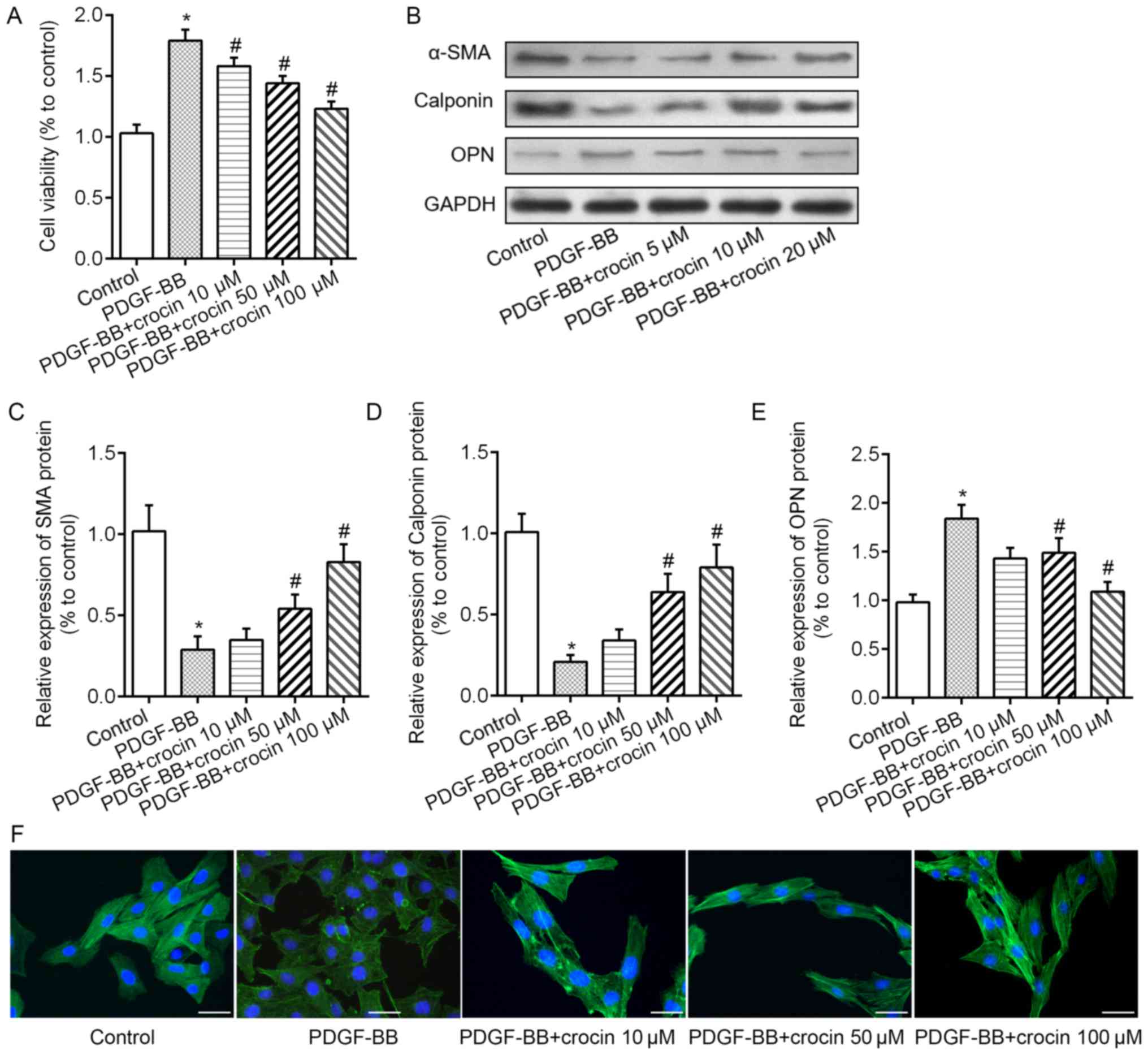
First, we assessed the effect of crocin on VSMCs
proliferation. As shown in Fig.
1B, treatment with different concentrations (10, 50, and 100
µM) of crocin for 24 or 48 h did not affect the proliferation of
the VSMCs, as detected by CCK-8 assay. Then, we investigated the
effects of crocin on PDGF-BB (20 ng/ml) treated VSMCs. The results
in Fig. 2A showed that cell
proliferation rate was dramatically increased in PDGF-BB treated
VSMCs and was decreased with additional treatment with crocin in a
concentration dependent manner. We hypothesized that the increased
VSMCs proliferation rate may largely due to the transition from a
contractile phenotype to a synthetic phenotype. Therefore, the
markers of VSMCs phenotypic switch were tested under crocin
treatment. Indicated by western blot analysis, PDGF-BB treatment
significantly decreased the expression levels of α-SMA and
calponin, whereas increased the expression levels of OPN.
Intriguing, these alterations were reversed by crocin in a
concentration-dependent manner (Fig.
2B-E). Moreover, the results of immunofluorescent staining with
α-SMA revealed that compared with control group, PDGF-BB not only
reduced the fluorescence intensity of α-SMA, but also perturbed
myofibrillar arrangement in the cytoplasm. After crocin treatment,
above changes were gradually abolished (Fig. 2F).
Crocin inhibits PDGF-BB-induced
activation of JAK/STAT3 and ERK/KLF4 signaling pathways in
VSMCs
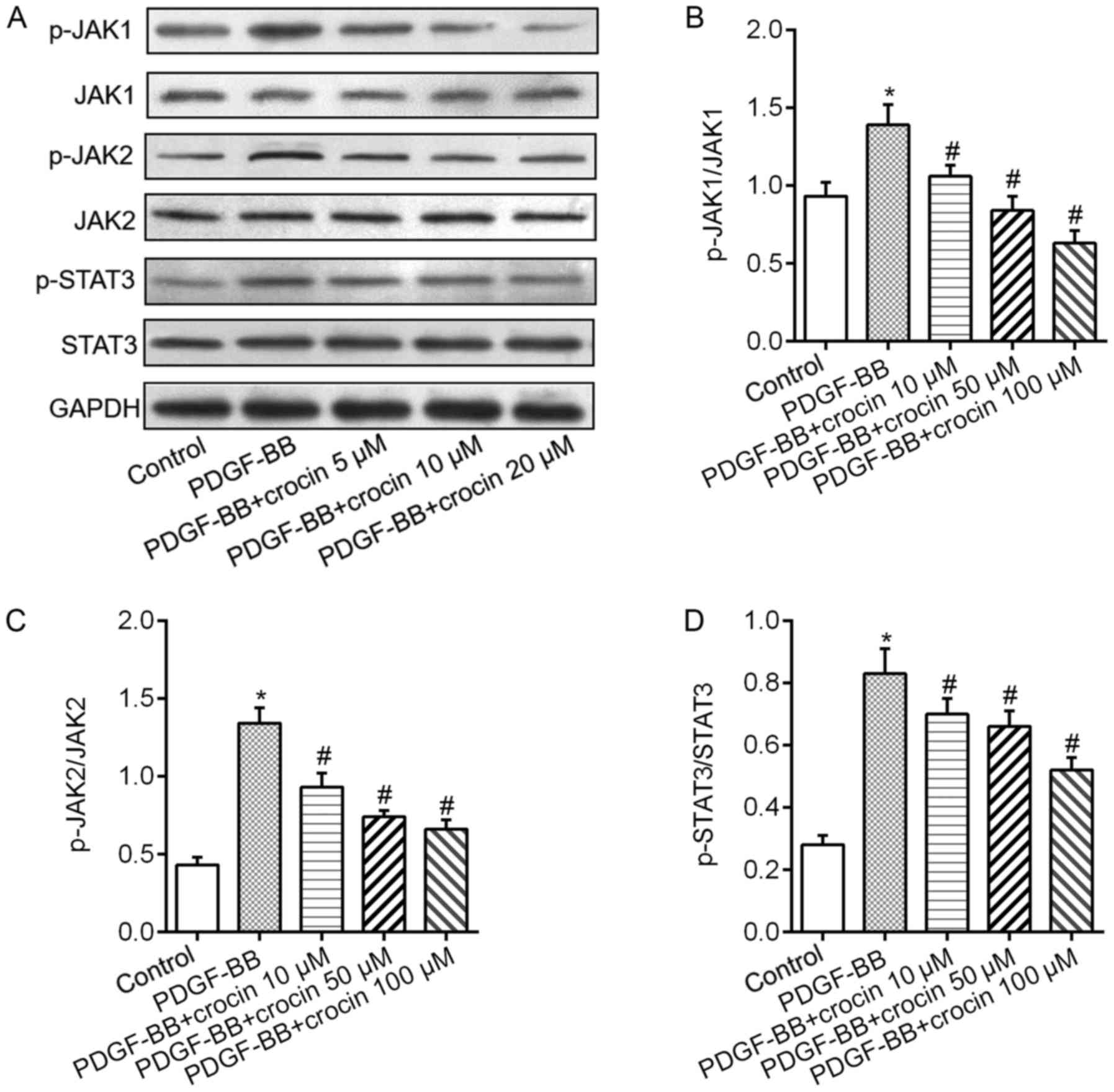
We then examined the underlying mechanism of the
inhibitory effect of crocin to VSMCs and JAK/STAT3 and ERK/KLF4
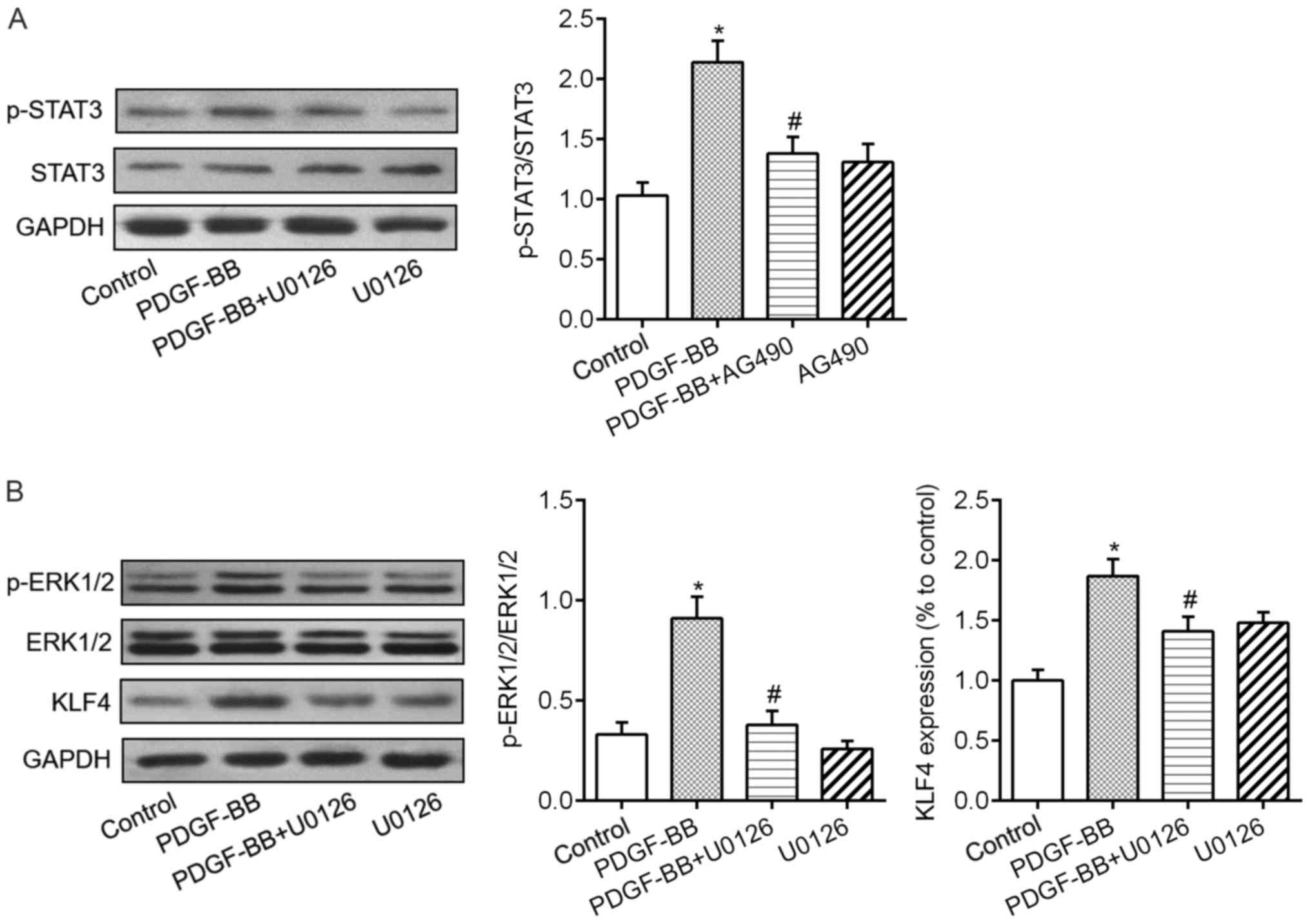
signaling pathways were investigated. Fig. 3A demonstrated that VSMCs exposure
to PDGF-BB resulted in significant increases in the phosphorylation
levels of JAK1, JAK2 and STAT3. Treatment of crocin dramatically
downregulated PDGF-BB-induced phosphorylation levels of JAK1, JAK2
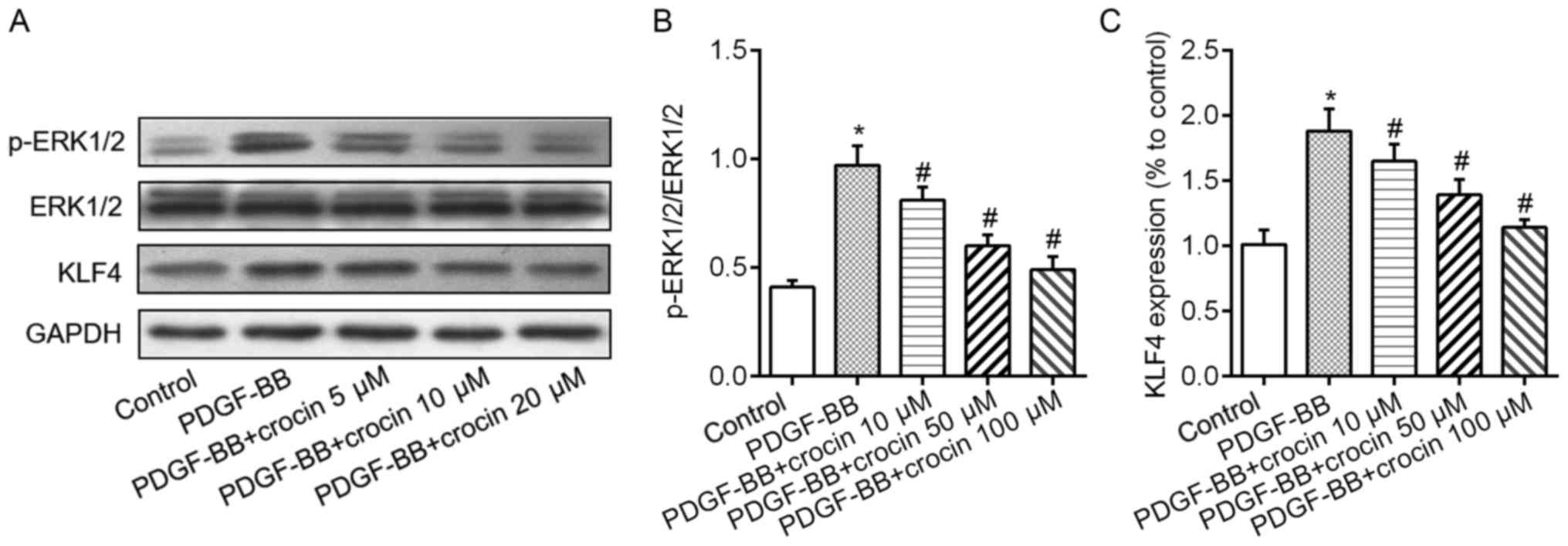
and STAT3 in a concentration-dependent fashion (Fig. 3B-D). Additionally, in cultured
VSMCs, exposure to PDGF-BB caused the elevated phosphorylation
levels of ERK1/2 and increased expression levels of KLF4 protein
(Fig. 4A). When the cells were
co-treated with various concentration of crocin, above changes were
abrogated in a concentration-dependent manner (Fig. 4B and C).
JAK/STAT3 and ERK/KLF4 signaling
pathways mediate PDGF-BB-induced phenotype switching and VSMCs
proliferation
We subsequently tested whether inhibition of the
JAK/STAT3 and ERK/KLF4 signaling pathways were involved in
PDGF-BB-induced phenotypic switching and proliferation of VSMCs. We
found that administration of JAK inhibitor AG490 and ERK1/2
inhibitor U0126 significantly diminished PDGF-BB-induced STAT3
phosphorylation (Fig. 5A), ERK1/2
phosphorylation and increased KLF4 levels (Fig. 5B). Meanwhile, the results of CCK-8
assay showed that blockade of JAK/STAT3 and ERK/KLF4 signaling
pathways with AG490 or U0126 markedly attenuated PDGF-BB-induced
VSMCs proliferation (Fig. 6A).
Furthermore, the abnormal changes in phenotypic switching markers
including α-SMA, calponin and OPN were all rectified by AG490 or
U0126 pretreatment in VSMCs response to PDGF-BB (Fig. 6B-E). These findings suggested that
both JAK/STAT3 and ERK/KLF4 signaling pathways were required for
the role of crocin in VSMCs.
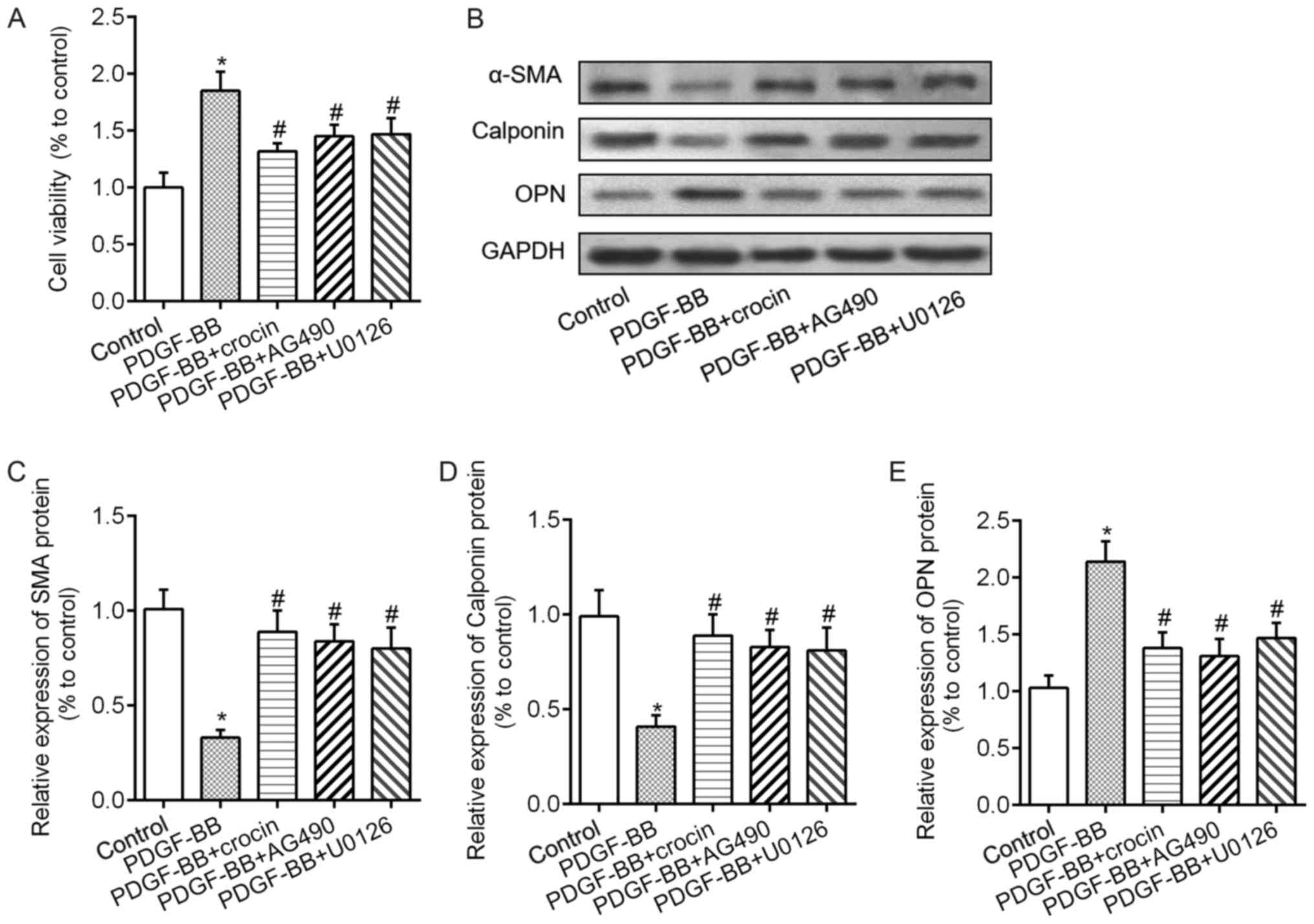 | Figure 6.Crocin repressed PDGF-BB-induced VSMCs
proliferation and phenotype switch via the JAK/signal transducers
and activators of transcription 3 and ERK/kruppel-like factor 4
signaling pathways. (A) Cell Counting Kit-8 assay revealed cell
proliferation in VSMCs treated with 20 ng/ml PDGF-BB, PDGF-BB +
crocin (100 µM), PDGF-BB + AG490 (10 µM, a JAK inhibitor), PDGF-BB
+ U0126 (50 µM, an ERK inhibitor). (B) Representative images showed
the expression levels of α-SMA, calponin and OPN protein. Bar graph
showing the relative protein levels of (C) α-SMA, (D) calponin and
(E) OPN in the groups. *P<0.05 vs. control;
#P<0.05 vs. PDGF-BB. VSMC, vascular smooth muscle
cell; PDGF-BB, platelet-derived growth factor-BB; ERK,
extracellular signal regulated kinase; JAK, Janus kinase. |
Discussion
The present findings revealed some novel findings
about crocin. Crocin effectively suppressed PDGF-BB-induced VSMCs
proliferation. In addition, crocin prevented PDGF-BB-induced the
VSMCs phenotype switch through inhibiting JAK/STAT3 and ERK/KLF4
signaling pathway.
Researches demonstrated that PDGF-BB plays a vital
role in the formation of neointima (15). In normal mature vessels, the
expression of PDGF and its receptors are very low or undetectable.
In contrast, its expression is dramatically increased at vascular
injury sites, followed by the increased activation of platelets and
the recruitment of monocytes (16). The expression of exogenous PDGF in
the arteries can induce intimal thickening through the stimulation
of VSMCs proliferation and migration (17). PDGF promoted VSMCs to switch from
the quiescent contractile phenotype to the synthetic phenotype by
inhibiting expression of α-SMA and calponin (18). Consistent with previous published
studies, we found that PDGF-BB decreased α-SMA and calponin
expression but increased OPN expression. The following experiments
found that crocin restored the expression of α-SMA and calponin in
a concentration dependent manner, accompanied by a decrease in cell
proliferation. These data suggested that crocin halts the change
toward a deleterious VSMCs phenotype induced by PDGF-BB. However,
the mechanisms through which crocin inhibited PDGF-BB-induced VSMCs
phenotypic switch remain unknown.
Janus kinase-signal transducer and activator of
transcription (JAK-STAT) pathway, which is one of major downstream
mediators of PDGF signaling, was activated in VSMCs exposed to
PDGF-BB in our experiments. Previous studies identified that STAT3
signaling pathway is required for PDGF-BB stimulated VSMC
proliferation (19). And
suppressing JAK/STAT3 signaling pathway leading to the inhibition
of proliferation and migration of smooth muscle cells (20). We revealed that crocin inhibited
the activation of JAK/STAT3 pathway induced by PDGF-BB in a
concentration dependent manner. Actually, Kim et al have
demonstrated that crocin effectively suppressed constitutive STAT3
activation, translocation of STAT3 to the nucleus through induction
of protein tyrosine phosphatase SHP-1 in multiple myeloma cells
(21). Accordingly, we identified
that suppression of STAT3 activation using 100 µM crocin or a
specific inhibitor resulted in VSMCs switching from the synthetic
phenotype to the contractile phenotype again. The role of STAT3 in
controlling VSMCs phenotypic switch was supported by Liao et
al study that activated STAT3 enhanced VSMCs proliferation and
suppressed the expression of contractile proteins, whereas
knockdown of endogenous STAT3 enhances VSMCs contractile phenotype
(22). Although should be further
validated in vivo, above data indicated that that JAK/STAT3
pathway was a potential therapeutic target for controlling
phenotypic switch of VSMCs.
Besides activation of STAT transcription factors,
PDGF-BB stimulation also leads to the initiation of the ERK
signaling pathway (23,24). Studies by several investigators
showed that activation of ERK1/2 signaling contributes to promote
VSMC proliferation (25,26). KLF4 is the downstream target of
ERK1/2 in the PDGF-BB-induced signaling pathway (27,28).
Consistent with another study (29), we noted that PDGF-BB induces
phosphorylation of ERK1/2 and elevated KLF4 expression in VSMCs.
Moreover, crocin treatment significantly inhibited PDGF-BB-induced
activation of ERK/KLF4 signaling pathway. It is noteworthy that
crocin can inhibit p-ERK1/2 in rat retina with ischemia/reperfusion
injury (30) and improve lipid
dysregulation in subacute diazinon exposure through inhibition of
ERK1/2 activation in rat liver (31). We for the first time showed that
crocin suppressed ERK1/2 pathway in cultured VSMCs. The role of
ERK1/2 and KLF4 in VSMCs phenotypic switch had been well
established (32–35). We also revealed that ERK1/2
inhibitor U0126 significantly reduced p-ERK1/2 and KLF4 levels.
Consistent with crocin, U0126 reversed PDGF-BB-induced VSMCs
phenotypic switch. Nevertheless, whether there excites another
pathway that control KLF4 expression and the exact upstream kinases
of ERK1/2 is still need to be identified. Collectively, our results
suggested that inhibiting PDGF-BB-induced activation of ERK/KLF4
signaling pathway may have contributed to the inhibition of VSMC
proliferation and phenotypic switch exerted by crocin. Of note,
studies found JAK/ERK/STAT pathway was associated with cell
proliferation, differentiation and survival (36,37).
The fact that JAK/ERK/STAT signaling pathway is also involved in
attenuating cardiac ischemia/reperfusion injury (38) suggested an interaction between JAK
and ERK signaling. Since PDGF-BB treatment influences other MAPK
other MAPK pathways, such as JNK and p38 MAPK pathway (39), whether they participate in the
inhibitory effects of crocin still needs to be defined.
Taken together, our results disclose for the first
time that crocin inhibits PDGF-BB-induced VSMC phenotypic
alteration and subsequent proliferation through regulating
JAK/STAT3 and ERK/KLF4 signaling pathway. As VMSC proliferation is
one of the key mechanisms involved in the development and
progression of neointimal hyperplasia, which contributes to the
pathogenesis of atherosclerosis and restenosis, use of crocin may
be a potential way to restrain the progression of cardiovascular
disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GQ designed the study and performed the statistical
analysis; LT conducted all experiments and data correction; GQ and
LT wrote the manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Animal
Care and Use Committee of the First Hospital of China Medical
University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VSMCs
|
vascular smooth muscle cells
|
|
PDGF-BB
|
platelet-derived growth factor-BB
|
|
α-SMA
|
α-smooth muscle actin
|
|
OPN
|
osteopontin
|
|
STAT3
|
signal transducers and activators of
transcription
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
KLF4
|
kruppel-like factor 4
|
References
|
1
|
Ding Y, Zhang M, Zhang W, Lu Q, Cai Z,
Song P, Okon IS, Xiao L and Zou MH: AMP-activated protein kinase
alpha 2 deletion induces VSMC phenotypic switching and reduces
features of atherosclerotic plaque stability. Circ Res.
119:718–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang F, Chen Q, He S, Yang M, Maguire EM,
An W, Afzal TA, Luong LA, Zhang L and Xiao Q: miRNA-22 is a novel
mediator of vascular smooth muscle cell phenotypic modulation and
neointima formation. Circulation. pii: CIRCULATIONAHA.117.027799.
2017. View Article : Google Scholar
|
|
3
|
Yang X, Dong M, Wen H, Liu X, Zhang M, Ma
L, Zhang C, Luan X, Lu H and Zhang Y: MiR-26a contributes to the
PDGF-BB-induced phenotypic switch of vascular smooth muscle cells
by suppressing Smad1. Oncotarget. 8:75844–75853. 2017.PubMed/NCBI
|
|
4
|
Li G, Xie N, Yao Y, Zhang Y, Guo J, Feng
Y, Lv F, Xiao RP and Cao CM: Identification of PI3K regulatory
subunit p55γ as a novel inhibitor of vascular smooth muscle cell
proliferation and neointimal formation. Cardiovasc Res. 105:75–85.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaimoto T, Yasuda O, Ohishi M, Mogi M,
Takemura Y, Suhara T, Ogihara T, Fukuo K and Rakugi H: Nifedipine
inhibits vascular smooth muscle cell dedifferentiation via
downregulation of Akt signaling. Hypertension. 56:247–252. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song IS, Jeong YJ, Park JH, Shim S and
Jang SW: Chebulinic acid inhibits smooth muscle cell migration by
suppressing PDGF-Rβ phosphorylation and inhibiting matrix
metalloproteinase-2 expression. Sci Rep. 7:117972017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahaiee S, Moini S, Hashemi M and
Shojaosadati SA: Evaluation of antioxidant activities of bioactive
compounds and various extracts obtained from saffron (Crocus
sativus L.): A review. J Food Sci Technol. 52:1881–1888. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amin A, Hamza AA, Daoud S, Khazanehdari K,
Hrout AA, Baig B, Chaiboonchoe A, Adrian TE, Zaki N and
Salehi-Ashtiani K: Saffron-based crocin prevents early lesions of
liver cancer: In vivo, in vitro and network analyses. Recent Pat
Anticancer Drug Discov. 11:121–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mohammadzadeh L, Hosseinzadeh H, Abnous K
and Razavi BM: Neuroprotective potential of crocin against
malathion-induced motor deficit and neurochemical alterations in
rats. Environ Sci Pollut Res Int. 25:4904–4914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He SY, Qian ZY, Tang FT, Wen N, Xu GL and
Sheng L: Effect of crocin on experimental atherosclerosis in quails
and its mechanisms. Life Sci. 77:907–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Lei HT, Cao L, Mi YN, Li S and Cao
YX: Crocin alleviates coronary atherosclerosis via inhibiting lipid
synthesis and inducing M2 macrophage polarization. Int
Immunopharmacol. 55:120–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chi J, Meng L, Pan S, Lin H, Zhai X, Liu
L, Zhou C, Jiang C and Guo H: Primary culture of rat aortic
vascular smooth muscle cells: A new method. Med Sci Monit.
23:4014–4020. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao Y, Hu X, Guo X, Zhang B, Xu W and
Jiang H: Promoting effects of IL-23 on myocardial ischemia and
reperfusion are associated with increased expression of IL-17A and
upregulation of the JAK2-STAT3 signaling pathway. Mol Med Rep.
16:9309–9316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu N, Xu L, Shi Y, Fang L, Gu H, Wang H,
Ding X and Zhuang S: Pharmacologic targeting ERK1/2 attenuates the
development and progression of hyperuricemic nephropathy in rats.
Oncotarget. 8:33807–33826. 2017.PubMed/NCBI
|
|
15
|
Wu B, Zhang L, Zhu YH, Zhang YE, Zheng F,
Yang JY, Guo LY, Li XY, Wang L, Tang JM, et al: Mesoderm/mesenchyme
homeobox gene l promotes vascular smooth muscle cell phenotypic
modulation and vascular remodeling. Int J Cardiol. 251:82–89. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan G, Wang Q, Hu S, Wang D, Qiao Y, Ma G,
Tang C and Gu Y: Digoxin inhibits PDGF-BB-induced VSMC
proliferation and migration through an increase in ILK signaling
and attenuates neointima formation following carotid injury. Int J
Mol Med. 36:1001–1011. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park S, Kim JK, Oh CJ, Choi SH, Jeon JH
and Lee IK: Scoparone interferes with STAT3-induced proliferation
of vascular smooth muscle cells. Exp Mol Med. 47:e1452015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simon AR, Takahashi S, Severgnini M,
Fanburg BL and Cochran BH: Role of the JAK-STAT pathway in
PDGF-stimulated proliferation of human airway smooth muscle cells.
Am J Physiol Lung Cell Mol Physiol. 282:L1296–L1304. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji Y, Yang X and Su H: Overexpression of
microRNA-375 impedes platelet-derived growth factor-induced
proliferation and migration of human fetal airway smooth muscle
cells by targeting Janus kinase 2. Biomed Pharmacother. 98:69–75.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim B, Lee KY and Park B: Crocin
suppresses constitutively active STAT3 through induction of protein
tyrosine phosphatase SHP-1. J Cell Biochem. 118:3290–3298. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao XH, Wang N, Zhao DW, Zheng DL, Zheng
L, Xing WJ, Ma WJ, Bao LY, Dong J and Zhang TC: STAT3 protein
regulates vascular smooth muscle cell phenotypic switch by
interaction with myocardin. J Biol Chem. 290:19641–19652. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang Y, Li JY, Jayakumar T, Hung SH, Lee
WC, Manubolu M, Sheu JR and Hsu MJ: Ketamine, a clinically used
anesthetic, inhibits vascular smooth muscle cell proliferation via
PP2A-activated PI3K/Akt/ERK inhibition. Int J Mol Sci. 18:pii:
E2545. 2017. View Article : Google Scholar
|
|
24
|
Chen Q, Chen L, Kong D, Shao J, Wu L and
Zheng S: Dihydroartemisinin alleviates bile duct ligation-induced
liver fibrosis and hepatic stellate cell activation by interfering
with the PDGF-βR/ERK signaling pathway. Int Immunopharmacol.
34:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gharibi B, Ghuman MS and Hughes FJ: Akt-
and Erk-mediated regulation of proliferation and differentiation
during PDGFRβ-induced MSC self-renewal. J Cell Mol Med.
16:2789–2801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JY, Kim KH, Lee WR, An HJ, Lee SJ, Han
SM, Lee KG, Park YY, Kim KS, Lee YS, et al: Apamin inhibits
PDGF-BB-induced vascular smooth muscle cell proliferation and
migration through suppressions of activated Akt and Erk signaling
pathway. Vascul Pharmacol. 70:8–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim MO, Kim SH, Cho YY, Nadas J, Jeong CH,
Yao K, Kim DJ, Yu DH, Keum YS, Lee KY, et al: ERK1 and ERK2
regulate embryonic stem cell self-renewal through phosphorylation
of Klf4. Nat Struct Mol Biol. 19:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawai-Kowase K and Owens GK: Multiple
repressor pathways contribute to phenotypic switching of vascular
smooth muscle cells. Am J Physiol Cell Physiol. 292:C59–C69. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen S, Dong S, Li Z, Guo X, Zhang N, Yu B
and Sun Y: Atorvastatin calcium inhibits PDGF-ββ-induced
proliferation and migration of VSMCs through the G0/G1 cell cycle
arrest and suppression of activated PDGFRβ-PI3K-Akt signaling
cascade. Cell Physiol Biochem. 44:215–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Qi Y and Yang X: Neuroprotective
effects of crocin against oxidative stress induced by
ischemia/reperfusion injury in rat retina. Ophthalmic Res.
54:157–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lari P, Rashedinia M, Abnous K and
Hosseinzadeh H: Crocin improves lipid dysregulation in subacute
diazinon exposure through ERK1/2 pathway in rat liver. Drug Res
(Stuttg). 64:301–305. 2014.PubMed/NCBI
|
|
32
|
Wang TM, Chen KC, Hsu PY, Lin HF, Wang YS,
Chen CY, Liao YC and Juo SH: microRNA let-7g suppresses
PDGF-induced conversion of vascular smooth muscle cell into the
synthetic phenotype. J Cell Mol Med. 21:3592–3601. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sunaga H, Matsui H, Anjo S, Syamsunarno
MR, Koitabashi N, Iso T, Matsuzaka T, Shimano H, Yokoyama T and
Kurabayashi M: Elongation of long-chain fatty acid family member 6
(Elovl6)-driven fatty acid metabolism regulates vascular smooth
muscle cell phenotype through AMP-activated protein
kinase/krüppel-like factor 4 (AMPK/KLF4) signaling. J Am Heart
Assoc. 5:pii: e004014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ouyang QF, Han Y, Lin ZH, Xie H, Xu CS and
Xie LD: Fluvastatin upregulates the α 1C subunit of CaV1.2 channel
expression in vascular smooth muscle cells via RhoA and ERK/p38
MAPK pathways. Dis Markers. 2014:2370672014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng J, Ge S, Zhang L, Che H and Liang C:
Aortic dissection is associated with reduced polycystin-1
expression, an abnormality that leads to increased ERK
phosphorylation in vascular smooth muscle cells. Eur J Histochem.
60:27112016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang C, Zhao Y, Song G and She K:
Resveratrol protects hippocampal neurons against cerebral
ischemia-reperfusion injury via modulating JAK/ERK/STAT signaling
pathway in rats. J Neuroimmunol. 315:9–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ottani A, Giuliani D, Neri L, Calevro A,
Canalini F, Vandini E, Cainazzo MM, Ruberto IA, Barbieri A, Rossi R
and Guarini S: NDP-alpha-MSH attenuates heart and liver responses
to myocardial reperfusion via the vagus nerve and JAK/ERK/STAT
signaling. Eur J Pharmacol. 769:22–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ottani A, Galantucci M, Ardimento E, Neri
L, Canalini F, Calevro A, Zaffe D, Novellino E, Grieco P, Giuliani
D and Guarini S: Modulation of the JAK/ERK/STAT signaling in
melanocortin-induced inhibition of local and systemic responses to
myocardial ischemia/reperfusion. Pharmacol Res. 72:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilson JL, Yu J, Taylor L and Polgar P:
Hyperplastic growth of pulmonary artery smooth muscle cells from
subjects with pulmonary arterial hypertension is activated through
JNK and p38 MAPK. PLoS One. 10:e01236622015. View Article : Google Scholar : PubMed/NCBI
|