Introduction
Epilepsy, the 3rd most common chronic brain
disorder, is characterized by an enduring predisposition to
seizures, and by emotional and cognitive dysfunction (1). Microglia serve an important role in
epilepsy and the pathological hallmark of epilepsy is an increase
in the number of microglia and a decrease in neurons (2).
Major histocompatibility complex class II (MHC II)
expression serves a critical role in the induction of immune
responses through presentation of antigenic peptides to
CD4+ T lymphocytes (3).
Constitutive expression is restricted to a limited number of
professional antigen-presenting cells, although a variety of cell
types express MHC II following stimulation by interferon-γ
(4,5).
Microglia cells, as the resident innate immune cells
of the central nervous system (CNS), generally express low levels
of MHC II proteins; however, in inflammatory or neurodegenerative
conditions, activated microglial cells exhibit highly upregulated
MHC II expression (6–8). Microglia with high expression of MHC
II may activate CD4+ T lymphocytes, and induce immune
responses that may act by inducing neuronal cell death or the
degeneration of neuronal processes (9,10).
In epileptic rat models, MHC II-expressing microglia were
associated with neuronal death processes (11). Therefore, the inhibition of
microglial cell activation and MHC II expression may be
therapeutically significant in epilepsy.
Tripterygium wilfordii Hook F (TWHF), a
member of the Celastraceae plant family, has been identified to
have potent anti-inflammatory and immunosuppressive functions, and
is widely used in China for the treatment of rheumatoid arthritis
and systemic lupus erythematosus (12). Triptolide (designated as T10) is
one of the major active ingredients of TWHF that performs
anti-inflammatory and immunosuppressive functions (13,14).
Due to its small molecular size (molecular weight, 360 g/mol) and
lipophilic properties, T10 is able to penetrate the blood-brain
barrier readily, making it a potential neuroprotective drug for the
treatment of CNS inflammatory diseases. Previously, T10 has been
demonstrated to be beneficial in animal models of numerous CNS
disorders, including Alzheimer's and Parkinson's diseases (15,16).
A previous study demonstrated that the
administration of T10 markedly alleviated seizure behavior and
prevented damage to neurons in epileptic rat models. It was
additionally observed that the expression of MHC II on microglia in
the hippocampi of epileptic rats induced by kainic acid (KA) was
markedly decreased by treatment with T10 at a dose of 30 µg/kg
(17). However, the precise
molecular mechanisms through which T10 may affect MHC II expression
remain unknown. Understanding the mechanism of the repressive
effect of T10 on MHC II expression may elucidate important pathways
that may be targeted to treat epilepsy. Therefore, in the present
study, the molecular mechanisms underlying the effect of T10 on MHC
II expression in KA-activated microglia were investigated.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Fetal bovine serum (FBS) was purchased from Hyclone (GE
Healthcare Life Sciences, Logan, UT, USA). KA was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and T10 was
purchased from Shenyang Longpu Technology. Co., Ltd. (Shenyang,
China). TRIzol reagent was purchased from Invitrogen (Thermo Fisher
Scientific, Inc.). The PrimeScript Reverse Transcriptase kit and
RNA Polymerase Chain Reaction (PCR) kit version 3.0 were purchased
from Takara Biotechnology Co., Ltd. (Dalian, China). Rat monoclonal
anti-mouse MHC II antibody (cat. no. SC-59322) and rabbit
polyclonal anti-mouse CIITA antibody (cat. no. SC-48797) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit monoclonal anti-mouse phosphorylated c-Fos (cat. no. 5348),
rabbit polyclonal anti-mouse phosphorylated c-Jun (cat. no. 9164),
rabbit anti-mouse β-actin (cat. no. 4970), goat anti-rat IgG-HRP
second antibody (cat. no. 7077) and goat anti-rabbit IgG-HRP second
antibody (cat. no. 7074) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Radioimmunoprecipitation assay
(RIPA) lysis buffer was purchased from Nanjing KeyGen Biotech Co.,
Ltd. (Nanjing, China).
BV-2 microglia culture
The BV-2 microglia cell line was provided by
Professor Jinyan Wang (Chinese Medical University, Liaoning, China)
and were maintained in DMEM supplemented with 10% FBS, 100 U/ml
penicillin, and 100 µg/ml streptomycin. BV-2 microglia were kept in
a humidified incubator with 5% CO2 at 37°C. The cells
were passaged every 3 days while growing to 75–80% confluence.
BV-2 microglia treatment
T10 was dissolved in dimethyl sulfoxide and mixed
with culture medium to a concentration of 10 nM. KA was diluted in
culture medium to a concentration of 100 µM. For treatment with KA,
BV-2 microglia cells were stimulated with KA for 2 h. For treatment
with T10, BV-2 microglia were pretreated with T10 for 16 h prior to
stimulation with KA. BV-2 microglia cultured in DMEM without any
treatment served as controls.
Immunocytochemistry
BV-2 microglia were treated as described above.
Cells were fixed with cold 4% paraformaldehyde for 15 min and
washed in PBS three times for 5 min followed by incubation with
normal goat serum (Beyotime Institute of Biotechnology, Shanghai,
China) for 20 min. Cells were subsequently incubated with MHC II
primary antibody (1:300) overnight at 4°C, followed by incubation
with goat anti-rat IgG-HRP secondary antibody (1:1,000) for 30 min
at room temperature. Color was developed with 3,3′-diaminobenzidine
for 15 min at room temperature and cells were observed with an
inverted microscope at ×200 magnification (CKX41; Olympus
Corporation, Tokyo, Japan).
Isolation of total RNA and reverse
transcription (RT)-PCR
Total RNA was prepared using TRIzol reagent and
primed with random hexamers for the synthesis of complementary DNA
using Avian Myeloblastosis Virus Reverse Transcriptase (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's instructions using DNAse-pretreated total mRNA.
Single-stranded cDNA was amplified via PCR with primers for MHC II,
CIITA and β-actin (Table I). The
product lengths for MHC II, CIITA and β-actin were 394, 489 and 349
bp, respectively. The following PCR conditions were applied: 35
cycles at 94°C for 30 sec; 54°C (MHC II), 58°C (CIITA) or 53°C
(β-actin) for 30 sec; and 72°C for 1 min. β-actin was used as an
internal control to evaluate the relative expression of MHC II and
CIITA. The PCR products were separated on a 1% agarose gel and
visualized under ultraviolet light following staining with
GoldViewand the results were analyzed by Quantity-One software
(version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
 | Table I.Sequences of primers used for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Sequences of primers used for reverse
transcription-polymerase chain reaction analysis.
| Genes | Primer sequence
(5′-3′) |
|---|
| MHC II | F:
GGACCCCACAGGACTTCACATACT |
|
| R:
GCCGTCTTCTCCTTGTTGCTGTGG |
| CIITA | F:
TGCAGGCGACCAGGAGAGACA |
|
| R:
GAAGCTGGGCACCTCAAAGAT |
| β-actin | F:
TGGAATCCTGTGGCATCCATGAAAC |
|
| R:
TAAAACGCAGCTCAGTAACAGTCCG |
Promoter prediction
The CIITA gene promoter was predicted by PROSCAN
version 1.7 software (https://www-bimas.cit.nih.gov/molbio/proscan/).
Western blotting
BV-2 microglia were washed with PBS three times,
placed at a temperature of 4°C, and lysed for 30 min in RIPA lysis
buffer. Lysates were subsequently centrifuged at 800 × g for 20 min
at 4°C. Protein concentration was quantified using the Bradford
assay with the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc.)
according to the manufacturer's instructions. Equal amounts of
protein (60–80 µg) were separated electrophoretically using
SDS-PAGE on a 10% gel; the gel was subsequently transferred to
0.45-µm polyvinylidene fluoride membranes. Membranes were soaked in
5% bovine serum albumin (Sigma Chemical Co., St. Louis, MO, USA)
for 2 h at room temperature, followed by incubation with the
appropriate primary antibodies (MHC II and CIITA at 1:500; p-c-Fos,
p-c-Jun and β-actin at 1:1,000) overnight at 4°C. Following washing
with TBS-Tween-20, the membranes were incubated with the
appropriate secondary peroxidase-conjugated antibodies (1:1,000)
for 2 h at room temperature, and visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology,).
Images were captured and analyzed using a ChemiDoc™ XRS+ imaging
system (Bio Rad Laboratories, Inc.).
Statistical analysis
All statistics were calculated using SPSS 2.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance followed by
Fisher's least significant difference post hoc test was used to
calculate the statistical differences between the control and
treated samples. Values are expressed as the mean ± standard
deviation of three separate experiments. P<0.05 was considered
to indicate a statistically significant difference.
Results
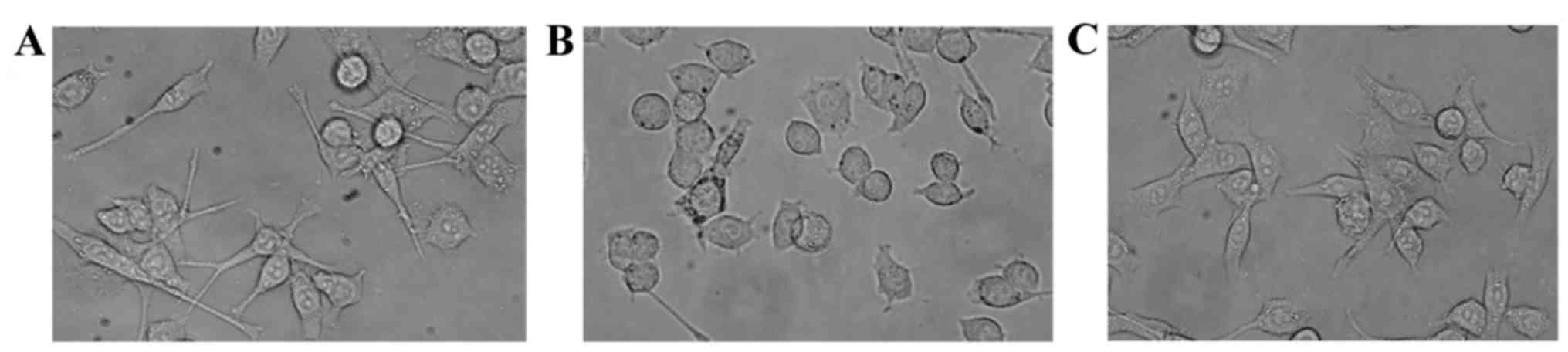
Effect of T10 on the morphology of
KA-activated microglia
Under normal conditions, BV-2 microglia remain in a
resting state and, morphologically, appear to be non-ramified cells
(Fig. 1A). Following treatment
with KA (100 µM) for 2 h, BV-2 microglia exhibited a highly
differentiated state with enlarged cell bodies (Fig. 1B). Treatment with T10 markedly
inhibited the activation of microglia (Fig. 1C).
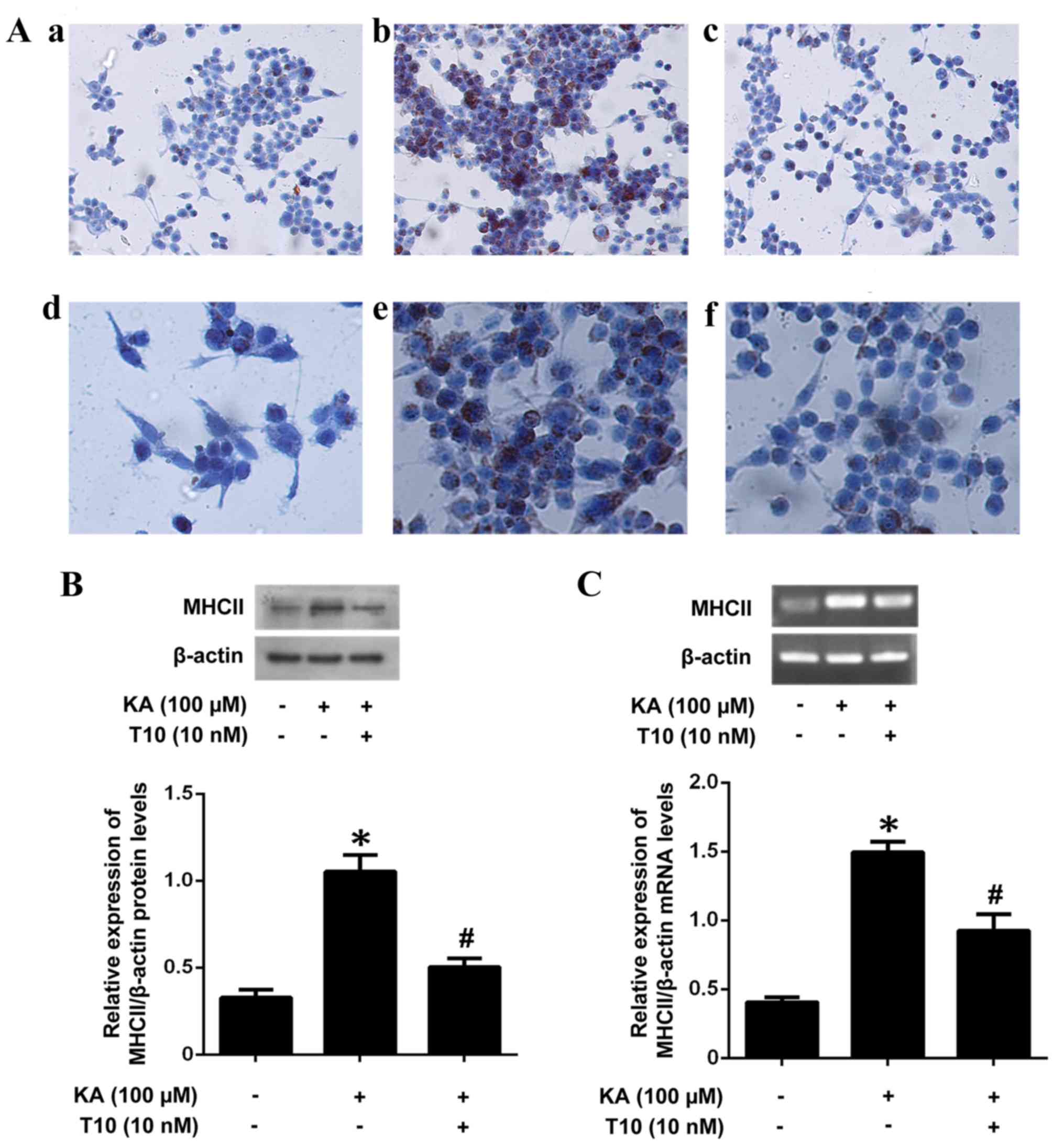
Effect of T10 on MHC II mRNA and
protein expression in KA-activated microglia
In order to investigate the inhibitory effect of T10
on MHC II mRNA and protein levels, BV-2 microglia were pretreated
with T10 (10 nM) for 16 h prior to stimulation with KA for 2 h. The
pretreatment with T10 significantly inhibited KA-induced MHC II
protein expression (Fig. 2A and
B). The results were additionally confirmed by RT-PCR (Fig. 2C), which illustrated an increased
expression level of MHC II in KA-treated microglia and a decreased
expression level of MHC II in microglia pretreated with 10 nM T10
for 16 h. The results indicated that T10 may significantly decrease
the mRNA and protein expression of MHC II in KA-activated
microglia.
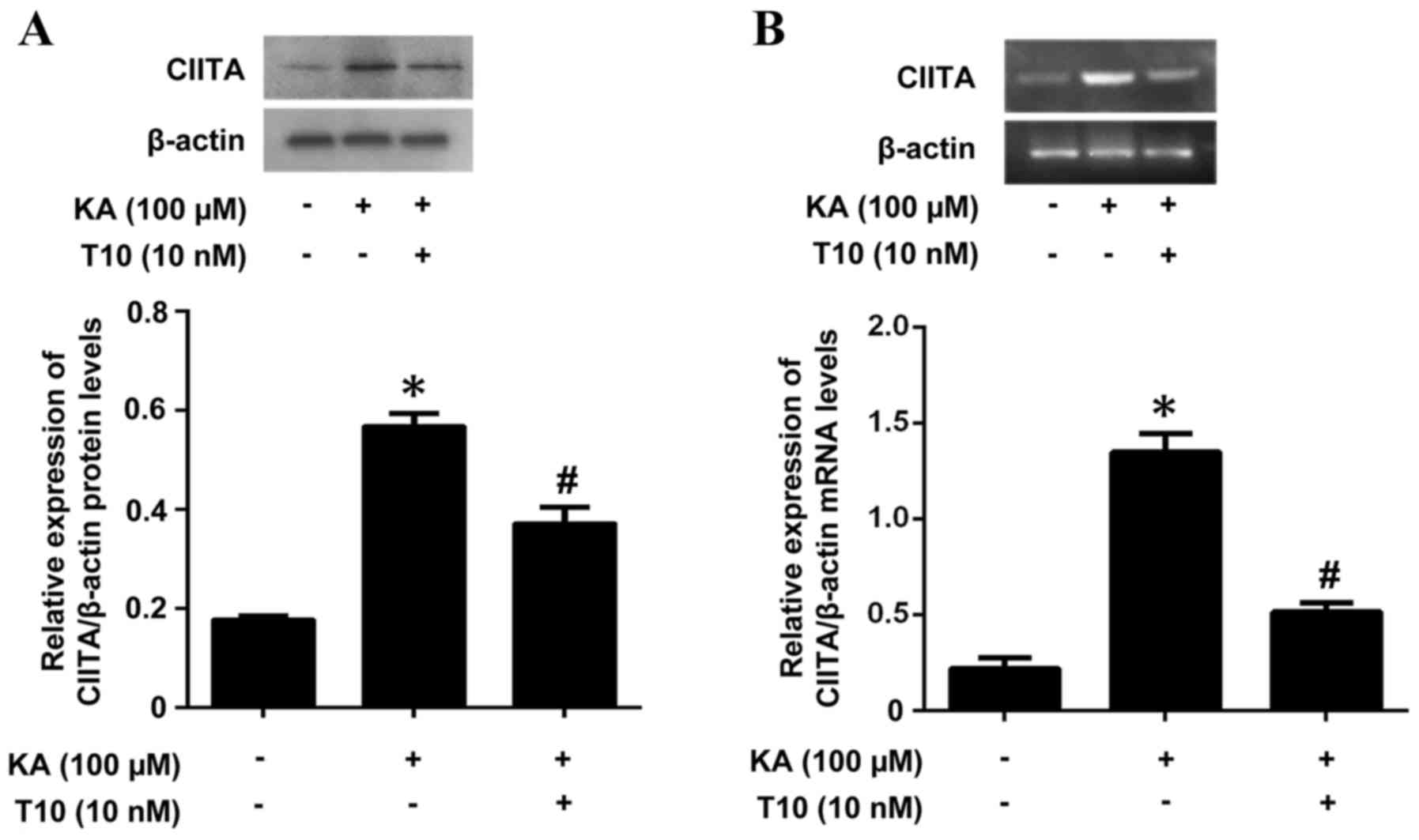
Effects of T10 on the expression of
CIITA mRNA and protein in KA-activated BV-2 microglia
As CIITA is a key factor in MHC II expression, the
present study investigated the ability of T10 to affect CIITA
expression as a potential mechanism of the inhibitory effects of
T10 on MHC II expression. The mRNA and protein levels of CIITA were
measured by RT-PCR and western blotting. Pretreatment with T10
significantly inhibited the KA-induced CIITA protein expression
(Fig. 3A). The results were
additionally confirmed by RT-PCR (Fig.
3B), which exhibited an increased level of CIITA in KA-treated
microglia, and a decreased level of CIITA in microglia pretreated
with 10 nM T10 for 16 h. The results suggested that T10 may target
signaling pathways involved in CIITA expression in KA-activated
BV-2 microglia.
AP-1 binding sites are present at the
CIITA promoter
PROSCAN prediction of the CIITA promoter highlighted
to the presence of AP-1 binding sites at positions −9113/-9105 from
the site of transcription initiation.
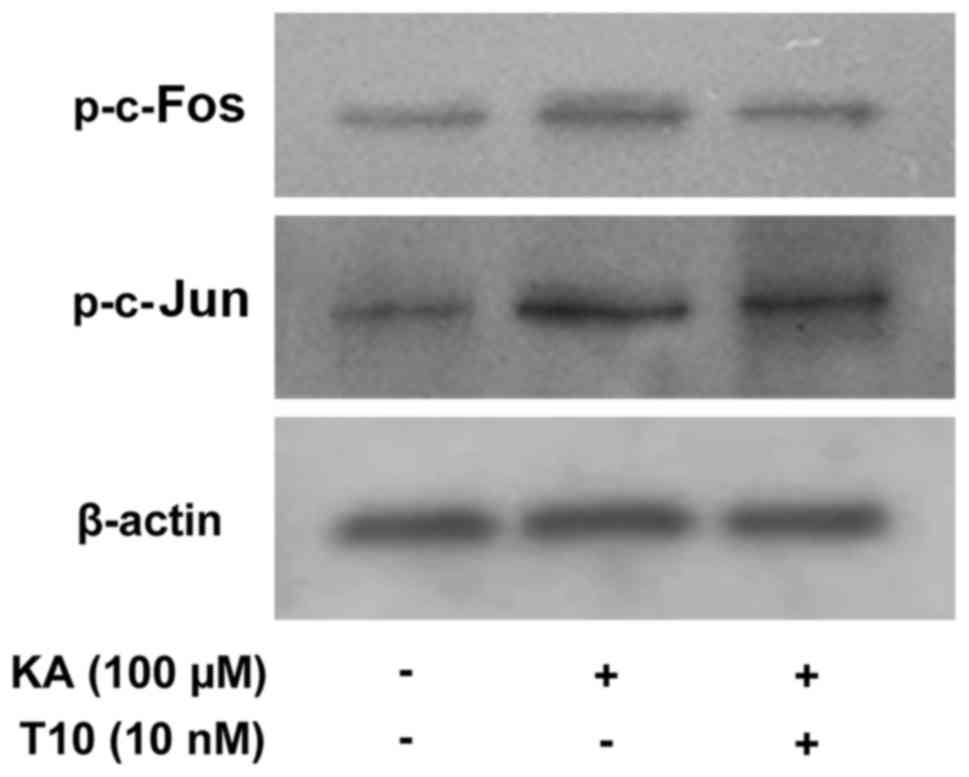
Effects of T10 on the phosphorylation
of c-Jun and c-Fos in KA-activated BV-2 microglia
In order to determine the effect of T10 on the
activation of AP-1, its effects on the phosphorylation of c-Jun and
c-Fos were investigated. The level of phosphorylated c-Jun and
c-Fos was measured by western blotting (Fig. 4). KA markedly increased the level
of phosphorylated c-Jun and c-Fos expression. Pretreatment with T10
decreased the increased expression of p-c-Jun and p-c-Fos in
KA-activated BV-2 microglia. The results indicated that T10 may
modulate AP-1 activity.
Discussion
Under normal conditions, microglia, as the resident
macrophages in the brain, remain in a less immunoreactive state and
perform immune surveillance functions (18). In response to abnormal stimulation,
microglia become activated very rapidly. The activated microglia
are differentiated into macrophage-like and dendritic-like cells,
exhibiting upregulated expression levels of MHC II and
co-stimulatory proteins that are required for antigen presentation
to T cells (19). In tetanus
toxin-induced epileptic rat models, MHC II-expressing microglia are
located in the dorsal hippocampi of rats exhibiting cell loss in
the CA1 region (11). In the
present study, BV-2 microglia exhibited morphological alterations
and became activated microglia following treatment with KA for 2 h.
The results demonstrated that the mRNA and protein expression
levels of MHC II were increased in KA-activated BV-2 microglia. It
has been reported that T10 possesses potent anti-inflammatory and
immunosuppressive effects. A previous study demonstrated that T10
significantly inhibited proinflammatory factor-induced
overexpression of MHC II and B7 molecules in renal tubular
epithelial cells (20). In the
present study, T10 markedly blocked the activation of microglia and
inhibited MHC II expression in KA-activated microglia.
The regulation of MHC II genes occurs primarily at
the transcriptional level, and the non-DNA-binding protein CIITA
has been demonstrated to be the master transactivator for class II
transcription. CIITA is essential, and is the major rate-limiting
factor for inducible and constitutive MHC II expression (21,22).
CIITA-deficient mice lack inducible MHC II expression and exhibit
sparse constitutive MHC II expression on subsets of thymic stromal
cells (23). Nikodemova et
al (24) reported that
minocycline was able to significantly decrease the severity of the
clinical course of experimental allergic encephalomyelitis; the
molecular mechanisms of action of minocycline may involve the
decreased expression of CIITA and result in a decrease in MHC II
expression in microglia. The results of the present study
demonstrated that T10 inhibited the expression of CIITA in
KA-activated microglia. The results of the present study indicated
that the inhibitory actions of T10 on MHC II overexpression occur
at the transcriptional level.
Mitogen-activated protein kinases (MAPKs) are the
most important signaling molecules with involvement in activated
microglia (25). MAPKs directly
regulate downstream targets via phosphorylation, and AP-1 is one of
the downstream targets. AP-1 is a transcription factor complex
composed of Jun family homodimers (c-Jun, Jun D and Jun B) or
heterodimers composed of Jun family members with any of the Fos
family members (c-Fos, Fos B, Fra1 and Fra2). Following
phosphorylation, AP-1 translocates to the nucleus and binds to
specific response elements on downstream target genes which, in
turn, promote immediate early gene expression. A previous study
established that MAPK-extracellular signal-regulated kinase and
MAPK8 are required for the regulation of CIITA and MHC II
expression in melanoma cell lines through an AP-1-responsive motif
in CIITA promoter III (26).
PROSCAN CIITA promoter analysis highlighted to the presence of AP-1
binding sites at positions −9113/-9105 from the site of
transcription initiation. Thus, it was hypothesized that AP-1 may
be involved in regulating CIITA and subsequent MHC II expression.
In the present study, T10 decreased the phosphorylation of c-Jun
and c-Fos, which may be the molecular mechanism through which T10
reduces the expression of CIITA.
In conclusion, the results of the present study
indicated that T10 significantly decreased the expression of MHC II
in KA-activated BV-2 microglia. The underlying mechanism may
involve the inhibition of CIITA expression via a mechanism
involving the inhibition of AP-1 activation. These results point to
a potential regulatory mechanism of MHC II expression in
KA-activated microglia, mediated by T10. T10 may be useful in the
treatment of epilepsy and other neurodegenerative diseases that are
characterized by overexpression of MHC II.
Acknowledgements
The authors of the present study would like to thank
Professor Jinyan Wang (Chinese Medical Sciences University,
Liaoning, China) for providing the BV-2 microglia.
References
|
1
|
Duncan JS, Sander JW, Sisodiya SM and
Walker MC: Adult epilepsy. Lancet. 367:1087–1100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tegelberg S, Kopra O, Joensuu T, Cooper JD
and Lehesjoki AE: Early microglial activation precedes neuronal
loss in the brain of the Cstb-/- mouse model of progressive
myoclonus epilepsy, EPM1. J Neuropathol Exp Neurol. 71:40–53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Graaf KL, Barth S, Herrmann MM, Storch
MK, Otto C, Olsson T, Melms A, Jung G, Wiesmüller KH and Weissert
R: MHC class II isotype- and allele-specific attenuation of
experimental autoimmune encephalomyelitis. J Immunol.
173:2792–2802. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casas C, Herrandograbulosa M, Manzano R,
Mancuso R, Osta R and Navarro X: Early presymptomatic cholinergic
dysfunction in a murine model of amyotrophic lateral sclerosis.
Brain Behav. 3:145–158. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vardjan N, Gabrijel M, Potokar M, Svajger
U, Kreft M, Jeras M, de Pablo Y, Faiz M, Pekny M and Zorec R:
IFN-γ-induced increase in the mobility of MHC class II compartments
in astrocytes depends on intermediate filaments. J
Neuroinflammation. 9:1442012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beach TG, Woodhurst WB, Macdonald DB and
Jones MW: Reactive microglia in hippocampal sclerosis associated
with human temporal lobe epilepsy. Neurosci Lett. 191:27–30. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Depboylu C, Stricker S, Ghobril JP, Oertel
WH, Priller J and Höglinger GU: Brain-resident microglia
predominate over infiltrating myeloid cells in activation,
phagocytosis and interaction with T-lymphocytes in the MPTP mouse
model of Parkinson disease. Exp Neurol. 238:183–191. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wojtera M, Sobów T, Kłoszewska I, Liberski
PP, Brown DR and Sikorska B: Expression of immunohistochemical
markers on microglia in Creutzfeldt-Jakob disease and Alzheimer's
disease: Morphometric study and review of the literature. Folia
Neuropathol. 50:74–84. 2012.PubMed/NCBI
|
|
9
|
Marinova-Mutafchieva L, Sadeghian M, Broom
L, Davis JB, Medhurst AD and Dexter DT: Relationship between
microglial activation and dopaminergic neuronal loss in the
substantia nigra: A time course study in a 6-hydroxydopamine model
of Parkinson's disease. J Neurochem. 110:966–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakahara H, Konishi Y, Beach TG, Yamada N,
Makino S and Tooyama I: Infiltration of T lymphocytes and
expression of icam-1 in the hippocampus of patients with
hippocampal sclerosis. Acta Histochem Cytochem. 43:157–162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaw JA, Perry VH and Mellanby J: MHC
class II expression by microglia in tetanus toxin-induced
experimental epilepsy in the rat. Neuropathol Appl Neurobiol.
20:392–398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tao X, Cush JJ, Garret M and Lipsky PE: A
phase I study of ethyl acetate extract of the chinese antirheumatic
herb Tripterygium wilfordii hook F in rheumatoid arthritis. J
Rheumatol. 28:2160–2167. 2001.PubMed/NCBI
|
|
13
|
Du F, Liu T, Liu T, Wang Y, Wan Y and Xing
J: Metabolite identification of triptolide by data-dependent
accurate mass spectrometric analysis in combination with online
hydrogen/deuterium exchange and multiple data-mining techniques.
Rapid Commun Mass Spectrom. 25:3167–3177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu WZ, Chen R, Brandwein S, Mcalpine J and
Burres N: Isolation, purification, and characterization of
immunosuppressive compounds from tripterygium: Triptolide and
tripdiolide. Int J Immunopharmacol. 17:351–356. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li FQ, Lu XZ, Liang XB, Zhou HF, Xue B,
Liu XY, Niu DB, Han JS and Wang XM: Triptolide, a Chinese herbal
extract, protects dopaminergic neurons from inflammation-mediated
damage through inhibition of microglial activation. J Neuroimmunol.
148:24–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao J, Xue B, Zhang L, Gong Y, Li K, Wang
H, Jing L, Xie J and Wang X: Triptolide inhibits
amyloid-beta1-42-induced TNF-alpha and IL-1beta production in
cultured rat microglia. J Neuroimmunol. 205:32–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu Y, Sun Z, Zeng CQ and Gao S: Effect of
triptolide on the expression of MHC molecules on microglia of
kainite-induced rat brain. J Liaoning Univ. 14:112–114. 2012.(In
Chinese).
|
|
18
|
Kreutzberg GW: Microglia: A sensor for
pathological events in the CNS. Trends Neurosci. 19:312–318. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponomarev ED, Novikova M, Maresz K,
Shriver LP and Dittel BN: Development of a culture system that
supports adult microglial cell proliferation and maintenance in the
resting state. J Immunol Methods. 300:32–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Liu ZH, Dai CS, Liu D and Li LS:
Triptolide inhibits proinflammatory factor-induced over-expression
of class II MHC and B7 molecules in renal tubular epithelial cells.
Acta Pharmacol Sin. 23:775–781. 2002.PubMed/NCBI
|
|
21
|
Boss JM and Jensen PE: Transcriptional
regulation of the MHC class II antigen presentation pathway. Curr
Opin Immunol. 15:105–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gongora C, Hose S, O'Brien TP and Sinha D:
Downregulation of class II transactivator (CIITA) expression by
synthetic cannabinoid CP55,940. Immunol Lett. 91:11–16. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang CH, Guerder S, Hong SC, van Ewijk W
and Flavell RA: Mice lacking the MHC class II transactivator
(CIITA) show tissue-specific impairment of MHC class II expression.
Immunity. 4:167–178. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nikodemova M, Watters JJ, Jackson SJ, Yang
SK and Duncan ID: Minocycline down-regulates MHC II expression in
microglia and macrophages through inhibition of IRF-1 and protein
kinase C (PKC)alpha/betaII. J Biol Chem. 282:15208–15216. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SH, Smith CJ and Van Eldik LJ:
Importance of MAPK pathways for microglial pro-inflammatory
cytokine IL-1 beta production. Neurobiol Aging. 25:431–439. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martins I, Deshayes F, Baton F, Forget A,
Ciechomska I, Sylla K, Aoudjit F, Charron D, Al-Daccak R and
Alcaide-Loridan C: Pathologic expression of MHC class II is driven
by mitogen-activated protein kinases. Eur J Immunol. 37:788–797.
2007. View Article : Google Scholar : PubMed/NCBI
|