Introduction
Nasopharyngeal carcinoma (NPC) frequently occurs in
the nasopharynx pharyngeal fossa and the anterior wall, which
originates from the nasopharyngeal mucosal epithelium (1). NPC is endemic in the southern
provinces of China and Southeast Asia (2). Due to the small size of the lesion
and the nonspecific symptoms in the early stages of NPC, there is a
risk of misdiagnosis and eventual distant metastasis. Radiation
therapy is the primary treatment for NPC at present (3). Nevertheless, distant metastasis is a
frequent cause of treatment failure in patients with NPC following
radiotherapy (4), and accounts in
a large part for the mortality rate of this type of cancer. In
addition, dissemination to distant organs and invasion of tumor
cells may be facilitated by epithelial-mesenchymal transition
(EMT), which facilitates cytoskeletal transfer into a more
migratory state (5). A decrease in
E-cadherin expression is a hallmark event in EMT (6). Therefore, there is a need to develop
novel strategies for metastasis prevention and to investigate the
underlying mechanisms in NPC.
MicroRNAs (miRNAs/miRs), as single-stranded,
non-coding small molecule RNAs, have been implicated in various
biological progresses, including the development and progression of
tumors, and are widely distributed in viruses, plants and higher
mammals (7–9). miRNAs bind to the 3′-untranslated
region (UTR) of target mRNAs, thereby regulating the expression of
target genes (10). miR-122 is
dysregulated in a number of types of cancer (10–12).
The tripartite motif-containing protein (TRIM) family contains
N-terminal RING finger, B-box and coiled-coil domains (13). It has been reported that certain
members of the TRIM family participate in multiple physiological
events, including cell cycle regulation, signal transduction and
oncogenic processes (14). TRIM29,
as a member of the TRIM family, serves important roles in many
tumors (15,16). Furthermore, it has been reported
that TRIM29 is involved in radioresistance in pancreatic cancer
(17). An abnormal activated state
of phosphatidylinositol 3-kinase (PI3K)/RAC-α
serine/threonine-protein kinase (AKT) is common in a variety of
types of cancer (18–20). In addition, it has been reported
that the abnormal activation of PI3K/AKT signaling by TRIM29 may
promote the progression of thyroid carcinoma (21). Accordingly, it may be speculated
that miR-122, TRIM29 and PI3K/AKT may have an unknown association
with the progression of NPC.
Therefore, the present study assessed the expression
of miR-122 and TRIM29 and their clinical significance in patients
with NPC. Furthermore, the potential functions of miR-122 and
possible associations with TRIM29 were examined in NPC cells. The
associated molecular mechanisms were additionally investigated. The
results revealed that miR-122 may exert its tumor suppressive role
by targeting TRIM29 and inhibiting PI3K/AKT signaling activity.
Materials and methods
Tissue specimens
The samples were collected from 30 patients with NPC
who received standard treatment between June 2014 and August 2015
in Jing Men No. 1 People's Hospital. No patient had a history of
cancer and other coexisting tumor. The adjacent tissues were
acquired at a site 5 cm from the tumor locus. Written informed
consent was obtained from all patients prior to enrolling in the
study. The clinical characteristics of the patients are presented
in Tables I–III. The protocols associated with the
use of patient tissues were approved by the ethics committee of
Jing Men No. 1 People's Hospital (Jingmen, China).
 | Table I.Correlation analysis between clinical
features and the expression of miR-122 in patients with NPC. |
Table I.
Correlation analysis between clinical
features and the expression of miR-122 in patients with NPC.
|
|
| miR-122
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. patients | Low group | High group | P-value |
|---|
| Age, years |
|
|
|
0.358 |
|
≤45 | 12 | 5 | 7 |
|
|
>45 | 18 | 10 | 8 |
|
| Sex |
|
|
|
0.961 |
|
Male | 17 | 9 | 8 |
|
|
Female | 13 | 7 | 6 |
|
| TNM stage |
|
|
|
0.002a |
|
I+II | 10 | 1 | 9 |
|
|
III+IV | 20 | 14 | 6 |
|
| Distant
metastasis |
|
|
|
<0.001a |
| No | 13 | 1 | 12 |
|
|
Yes | 17 | 14 | 3 |
|
 | Table III.Correlation analysis of expression
between miR-122 and TRIM29. |
Table III.
Correlation analysis of expression
between miR-122 and TRIM29.
|
| TRIM29 | P-value |
|---|
| miR-122 | − | + | −0.522 |
| − | 4 | 9 |
|
| + | 14 | 3 |
|
Cell culture and treatment
Human NPC cell lines 5-8F (CA-89) and 6-10B (CA-91)
were obtained from Changsha Axybio Biotechnology Co., Ltd.
(Changsha, China). The cells were maintained in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) and 50 µg/ml streptomycin in a 37°C incubator containing
5% CO2. An 8 MV linear accelerator (Elekta Instrument
AB, Stockholm, Sweden) was used for one-time exposure at room
temperature with the source-skin distance of 100 cm. The
irradiation dose was 3 Gy and the dose rate was 400 cGy/min. The
control group was not irradiated. The miR-122 mimics
(5′-UGGAGUGUGACAAUGGUGUUUG-3′) and corresponding negative control
(5′-UUCUCCGAACGUGUCACGUTT-3′) were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Cells
(1×104/well) were seeded into 6-well plates. A total of
12 h post-incubation at 37°C, either miR-122 mimics (75 nM) or
corresponding negative control (75 nM) were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Following a further 24 h of incubation at 37°C, the cells were
subjected to subsequent analysis.
Cell proliferation detection
The survival rate of transfected cells was
determined using a Cell Counting kit-8 (CCK-8) assay
(MedChemExpress, Monmouth Junction, NJ, USA). The cells
(2×103) were incubated in 96-well plates (100 µl/well).
A total of 10 µl CCK-8 solution was added and incubated for 4 h at
37°C. The absorbance was measured with microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 450 nm.
Cell migration and invasion
assays
Transwell assays were performed to observe the
migratory and invasive abilities of transfected cells. The cells
(1×104) in serum-free medium were placed onto the upper
Matrigel-coated insert (for the invasion assay) or insert without
Matrigel (for the migration assay) of Transwell chambers (Corning
Incorporated, Corning, NY, USA). Complete medium was added to the
lower chamber. A 24-well plate was used to hold the Transwell
inserts. Following 16 h, the cells that stayed on the upper surface
were removed, and the cells that had migrated through the pores
were collected. Another group was maintained at 37°C for 24 h to
allow the cells to invade through the Matrigel into the bottom
membrane. Subsequent to fixing with 4% paraformaldehyde for 15 min
at room temperature, the collected cells were stained with crystal
violet (0.1%) for 20 min at room temperature and quantified using a
light microscope (magnification, ×100; Nikon Corporation, Tokyo,
Japan).
Luciferase assay
Bioinformatics analysis was performed using
TargetScan (http://www.targetscan.org/vert_71/) and microRNA.org (http://www.microrna.org.). The wild type (wt)
TRIM29-3′UTR reporter plasmid was purchased from Changsha Axybio
Biotechnology Co., Ltd. The mutant type (mut) TRIM29 3′UTR plasmid
was generated using a site-directed mutagenesis kit (Takara Bio,
Inc., Otsu, Japan) by mutating the site of the target region of
miR-122 (CACACUCC to GAGAGAGT), based on the wt TRIM29-3′UTR
reporter plasmid. The cells were seeded at a density of
5×105/well into a 6-well plate. After 24 h, the cells
were co-transfected with miR-122 mimics or miR-negative control,
wt/mut TRIM29 3′UTR reporter plasmid and a Renilla
luciferase pRL-TK vector (Promega Corporation, Madison, WI, USA)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h, according to the manufacturer's
protocol, a dual-luciferase assay system (Promega Corporation) was
employed to measure the luciferase activity. The luciferase
activity was normalized to Renilla luciferase activity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the extraction of total RNA and
miRNA. The cDNA was synthesized using total RNA, oligodT18 primers
(Takara Bio, Inc.) and M-MLV reverse transcriptase (Promega
Corporation). The temperature protocol used for RT was as follows:
25°C for 10 min, 42°C for 50 min and 70°C for 10 min. A TaqMan
miRNA reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and a TaqMan human miRNA assay kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used for the
amplification and quantification of miR-122, respectively. U6 was
adopted as the internal control for miR-122. qPCR was performed
with Fast SYBR Green Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on the ABI StepOne Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions used were as follows: 95°C for 10 min; followed by 40
cycles at 95°C for 15 sec and 60°C for 60 sec; and a final
extension step at 72°C for 7 min. Primers for miR-122 were
purchased from Changsha Axybio Biotechnology Co., Ltd. The primer
sequences used were as follows: miR-122 forward,
5′-GCCGTGGAGTGTGAT-3′; and reverse, 5′-GTGCAGGGTCCGAGGTCAATGG-3′.
The other primer sequences were as follows: U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
TRIM29 forward 5′-ACTCCTTTCCTGCCTTGTGA-3′ and reverse,
5′-GAGAGGCAGGCTGATACCAT-3′; E-cadherin forward
5′-TCACATCCTACACTGCCCAG-3′ and reverse, 5′-AGTGTCCCTGTTCCAGTAGC-3′;
metastatic tumor antigen 1 (MTA1) forward,
5′-ACAGACAAGCAGATCGACCA-3′ and reverse, 5′-GGCCTTGGAGATGTCGTAGA-3′;
matrix metalloproteinase-2 (MMP-2) forward,
5′-CGGTGCCCAAGAATAGATGC-3′ and reverse, 5′-AGACAGTCTCCATTGGCTCC-3′;
metalloproteinase inhibitor (TIMP2) forward,
5′-TGTGTTCCCTCAGTGTGGTT-3′ and reverse, 5′-TTCGGTTTCATTGCGTGTGT-3′.
The 2−ΔΔCq method was used to analysis the relative
expression level of target factors (22).
Western blot analysis
The extraction and separation of proteins was
performed using radioimmunoprecipitation assay lysis buffer (Thermo
Fisher Scientific, Inc.). Protein concentration was determined
using a bicinchoninic acid assay protein quantification kit (Thermo
Fisher Scientific, Inc.). Protein samples were then separated on
10% SDS-PAGE gels (20 µg/lane). The proteins were transferred onto
polyvinylidene fluoride membranes. The blots were blocked with 5%
bovine serum albumin (Beijing Solarbio Science & Technology,
Co., Ltd., Beijing, China) at room temperature for 2 h and then
incubated with primary antibodies at 4°C overnight. The next day,
the blots were maintained with horseradish peroxidase-conjugated
secondary antibodies at room temperature for 2 h. The blots were
determined with enhanced chemiluminescence reagents (Thermo Fisher
Scientific, Inc.). Densitometry was performed using Quantity One
software version 4.6.9 (Bio-Rad Laboratories, Inc.).
The primary antibodies used were as follows:
Anti-phosphorylated (p)-PI3K p85 (Tyr458)/p55 (Tyr199; 1:1,000;
cat. no. 4228), anti-p-AKT (Ser473; 1:2,000; cat. no. 4060),
anti-PI3K Class III (1:1,000; cat. no. 3358), anti-AKT (pan;
1:1,000; cat. no. 4685; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-TRIM29 (1:1,000; cat. no. ab108627; Abcam,
Cambridge, UK), anti-E-cadherin (1:1,000; cat. no. 3195), anti-MTA1
(1:1,000; cat. no. 5646; both Cell Signaling Technology, Inc.),
anti-MMP-2 (1:1,000; cat. no. sc13594; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and anti-TIMP2 (1:300; cat. no. 89025;
R&D Systems, Inc., Minneapolis, MN, USA), and GAPDH (1:5,000;
cat. no. ab8245; Abcam). The secondary antibodies were purchased
from Beijing CoWin Biotech Co., Ltd. (Beijing, China; cat. nos.
Cw0102s and Cw0103s; 1:2,000).
Statistical analysis
Data are presented as mean ± standard deviation. All
experiments were performed independently at least three times. A
χ2 test was used to analyze categorical variables. A
two-tailed Student's t-test or one-way analysis of variance
followed by Dunnett's post-hoc multiple comparison test was
employed to compare the differences between groups. Kaplan-Meier
analysis was used to draw the survival curves. Spearman's rank
correlation analysis was used to analyze bivariate correlations.
SPSS v.14.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.0
(GraphPad Software, Inc., La Jolla, CA, USA) software was used for
all the statistical analysis and graph-drawing. P<0.05 was
considered to indicate a statistically significant difference.
Results
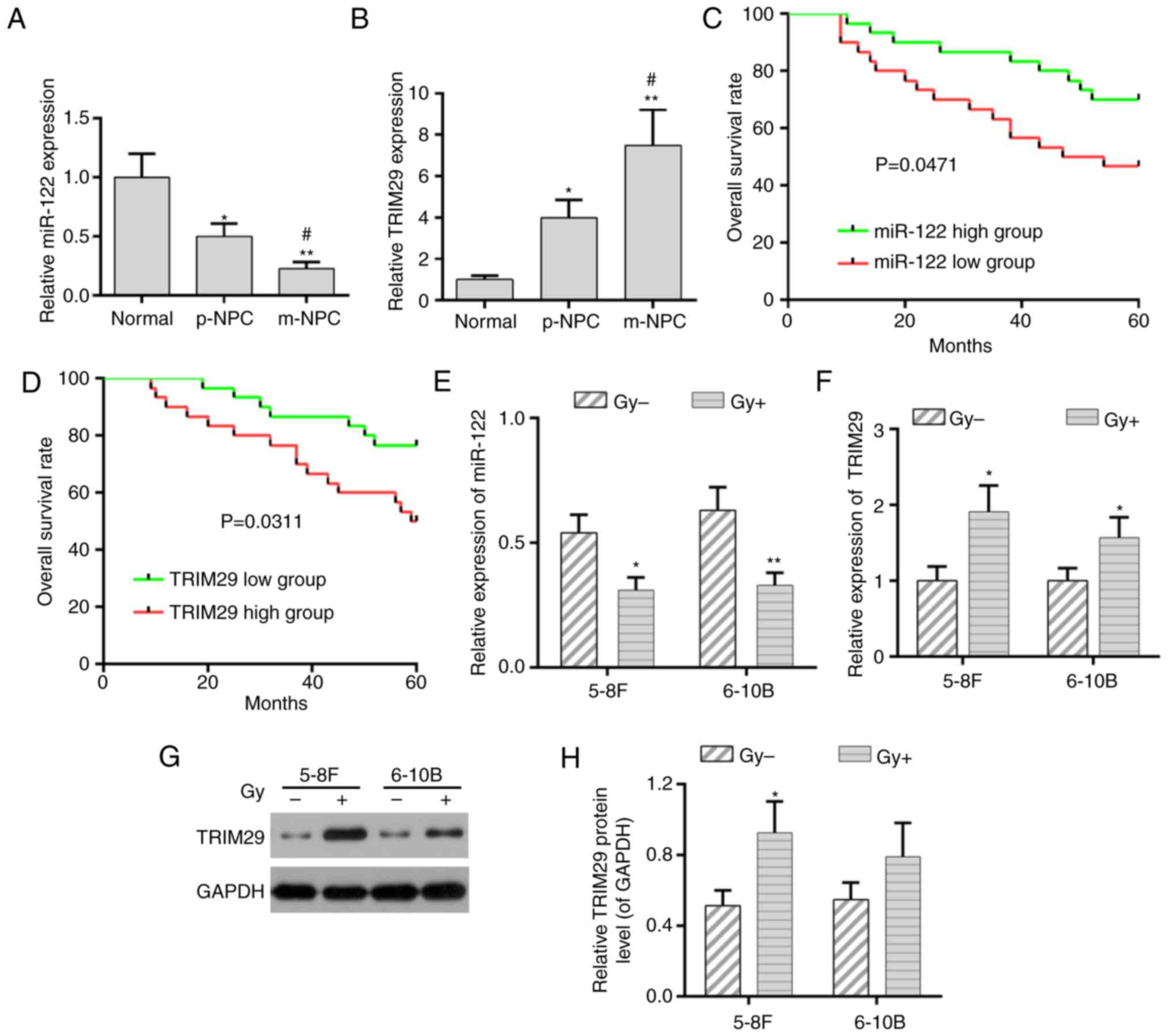
Expression of miR-122 and TRIM29 in
patients with NPC and cell lines
In order to determine the expression of miR-122 and
TRIM29 in patients with NPC, RT-qPCR was performed to analyze their
expression levels in cases with and without metastasis. It was
demonstrated that miR-122 expression was decreased in NPC cases
without metastasis (p-NPC), and more so in cases with metastasis
(m-NPC). By contrast, the expression of TRIM29 was increased in
p-NPC and further enhanced in m-NPC (Fig. 1A and B). Furthermore, the decreased
miR-122 expression and the increased TRIM29 expression were
significantly correlated with advanced TNM stage and distant
metastasis (Tables I and II). According to a previous study
(23), the NPC patients were
divided into two groups based on the median value of miR-122 or
TRIM29 expression: miR-122/TRIM29 high expression group (n=15), and
miR-122/TRIM29 low expression group (n=5). The overall survival
(OS) rate in the patients with low miR-122 expression was decreased
compared with those in the high miR-122 group (Fig. 1C; P<0.05). However, patients
with low TRIM29 expression had a higher OS rate compared with those
with high TRIM29 expression (Fig.
1D; P<0.05). In addition, the expression of miR-122 and
TRIM29 in patients with NPC was negatively correlated (Table III).
 | Table II.Correlation analysis between clinical
features and the expression of TRIM29 in patients with NPC. |
Table II.
Correlation analysis between clinical
features and the expression of TRIM29 in patients with NPC.
|
|
| TRIM29
expression |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. patients | Low group | High group | P-value |
|---|
| Age, years |
|
|
| 0.713 |
|
≤45 | 17 | 9 | 8 |
|
|
>45 | 13 | 6 | 7 |
|
| Sex |
|
|
| 0.409 |
|
Male | 22 | 12 | 10 |
|
|
Female | 8 | 3 | 5 |
|
| TNM stage |
|
|
| 0.002a |
|
I+II | 10 | 9 | 1 |
|
|
III+IV | 20 | 6 | 14 |
|
| Distant
metastasis |
|
|
| 0.010a |
| No | 13 | 10 | 3 |
|
|
Yes | 17 | 5 | 12 |
|
Subsequently, the expression of miR-122 and TRIM29
was detected in NPC cell lines, including 5-8F and 6-10B, following
radiation treatment. The RT-qPCR analysis demonstrated that, in all
NPC cell lines following radiation treatment, miR-122 and TRIM29
were downregulated and upregulated, respectively (Fig. 1E and F). The expression of TRIM29
was further confirmed by western blot analysis (Fig. 1G and H).
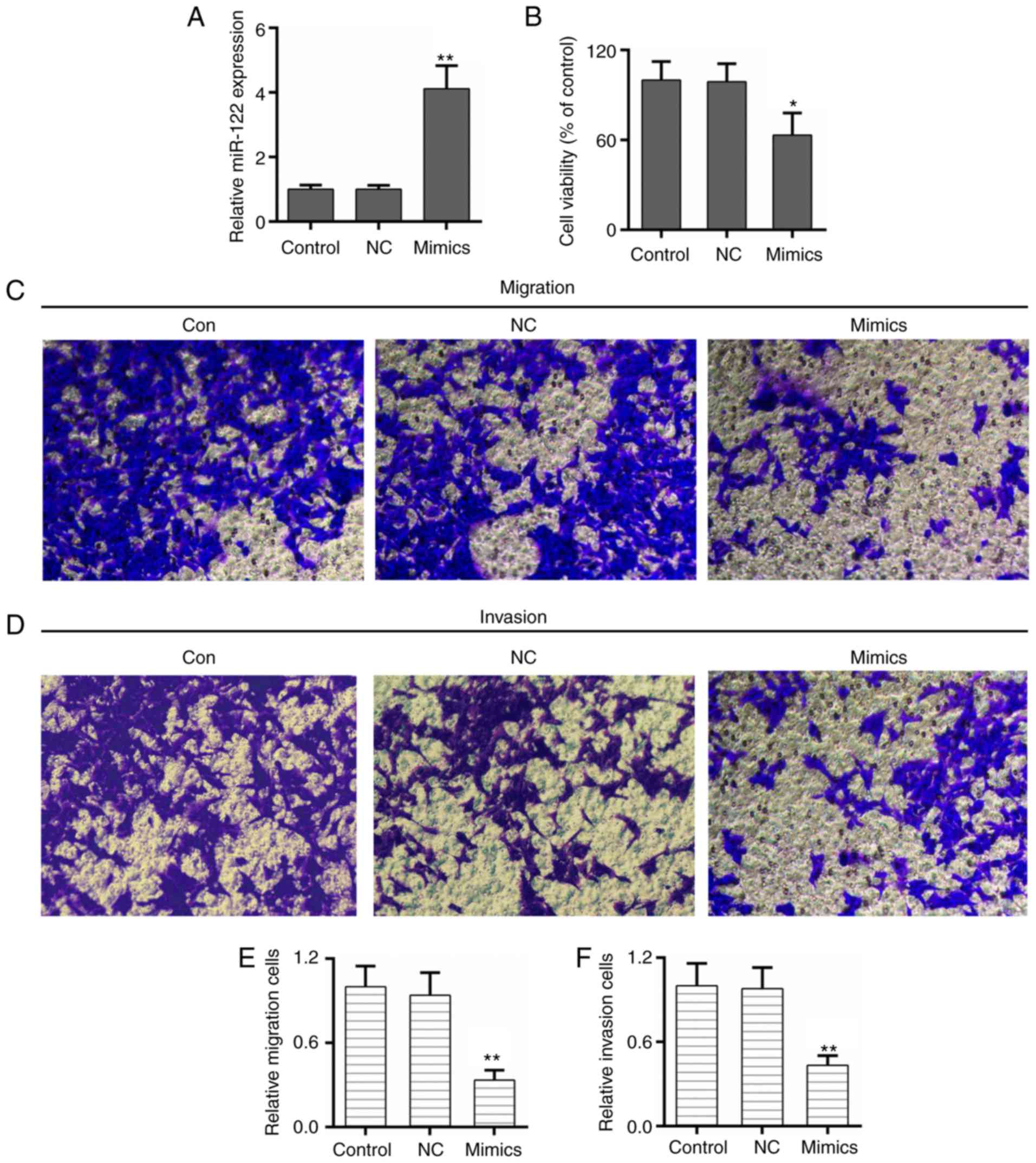
miR-122 inhibits the proliferation,
migration and invasion of NPC cells
The potential role of miR-122 and TRIM29 in the
progression and metastasis of NPC was determined. The results of
the present study demonstrated that the expression of miR-122 was
increased significantly in NPC cells that were transfected with
miR-122 mimics (Fig. 2A).
Furthermore, the cell survival rate was decreased in the miR-122
mimics group compared with the control and negative groups
(Fig. 2B). The Transwell assays
illustrated that the migratory and invasive abilities of NPC cells
were decreased by overexpression of miR-122 (Fig. 2C-F).
miR-122 regulates the expression of
metastasis-associated genes
The expression of metastasis-associated genes in the
miR-122 mimics group was assessed. MTA1 is associated with tumor
metastasis (24). As presented in
Fig. 3, the expression of
E-cadherin and MTA1 was increased and decreased in the miR-122
mimics group, respectively. The extracellular matrix may be
degraded by MMP-2 during the progression of cell invasion (25). It was noted that the expression of
MMP-2 was decreased in the miR-122 mimics group compared with the
control and negative control groups. Conversely, TIMP2, a
suppressor of MMP-2 (26), was
upregulated by the overexpression of miR-122 (Fig. 3A-C).
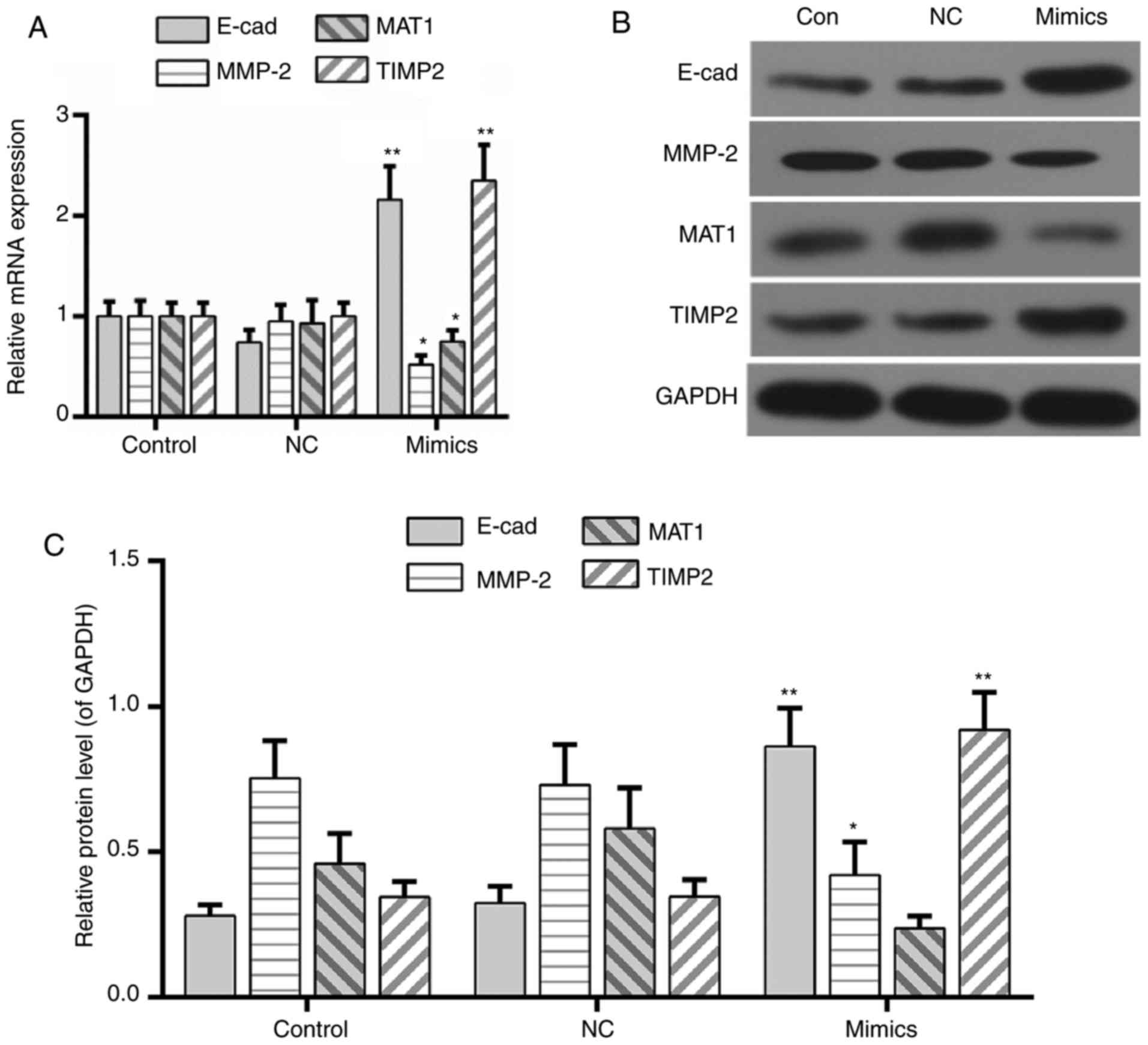 | Figure 3.MicroRNA-122 regulates the expression
of metastasis-associated genes. (A) Quantitative analysis of E-cad,
MTA1, MMP-2 and TIMP2. (B) Western blot assay and (C) determination
of the expression of E-cad, MTA1, MMP-2, and TIMP2. Data are
presented as the mean ± standard deviation, n=4. *P<0.05 and
**P<0.01 vs. control. E-cad, E-cadherin; MMP2, 72 kDa type IV
collagenase; MTA1, CDK-activating kinase assembly factor MTA1;
TIMP2, metalloproteinase inhibitor 2; NC, negative control. |
TRIM29 may be a direct target of
miR-122
The expression of TRIM29 was inhibited at the mRNA
and protein levels in the miR-122 mimics group (Fig. 4A-C). In addition, bioinformatics
analysis from available databases (TargetScan and microRNA.org) indicated that TRIM29 was a possible
target of miR-122 (Fig. 4D).
Meanwhile, the results of the luciferase reporter assay
demonstrated that the luciferase activity of the wild-type TRIM29
3′-UTR was inhibited by the excessive expression of miR-122,
whereas the luciferase activity of the mutant TRIM29 3′-UTR was not
affected (Fig. 4E). To confirm
this result, the luciferase activity reporter assay was performed
in another NPC cell line, 5-8F. The results were consistent between
the two NPC cell lines (Fig. 4F).
These data indicated that TRIM29 may be a direct target of
miR-122.
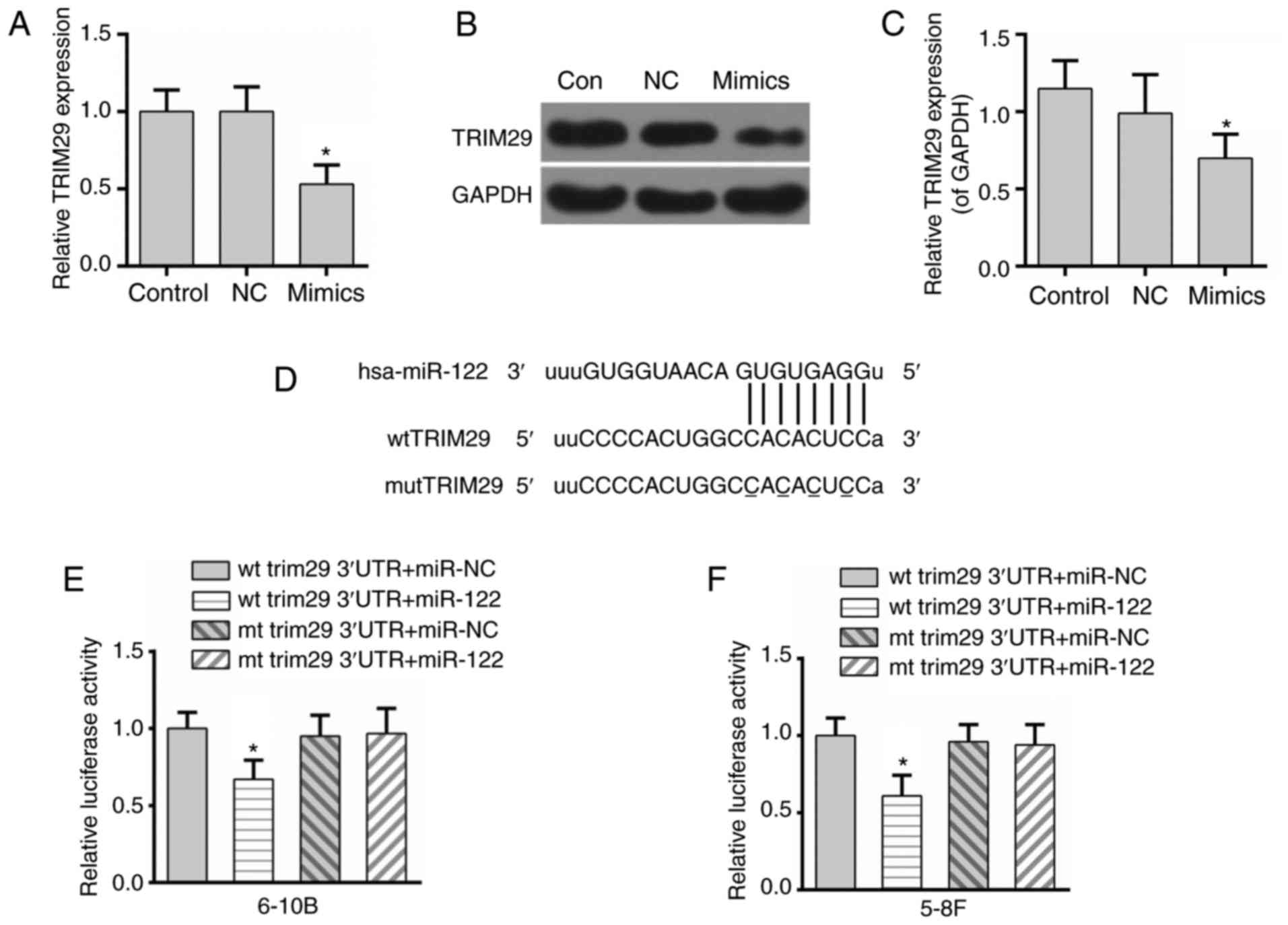 | Figure 4.TRIM29 may be a direct target of
miR-122. (A) miR-122 decreased the expression of TRIM29 at the mRNA
level. miR-122 decreased the expression of TRIM29 at the protein
level, as demonstrated by (B) western blotting and (C)
densitometry. (D) The potential complementary sequences of the wt
TRIM29 3′UTR and mut TRIM29 3′UTR, and the miR-122 binding
sequence. (E) miR-122 depressed the luciferase activity of the wt
3′-UTR of TRIM29 in 6-10B cells. (F) miR-122 depressed the
luciferase activity of the wt 3′-UTR of TRIM29 in 5-8F cells. Data
are presented as the mean ± standard deviation, n=3. *P<0.05 vs.
control. TRIM29, tripartite motif-containing protein 29; NC,
negative control; wt, wild-type; mut, mutant; UTR, untranslated
region; miR, microRNA; Con, control. |
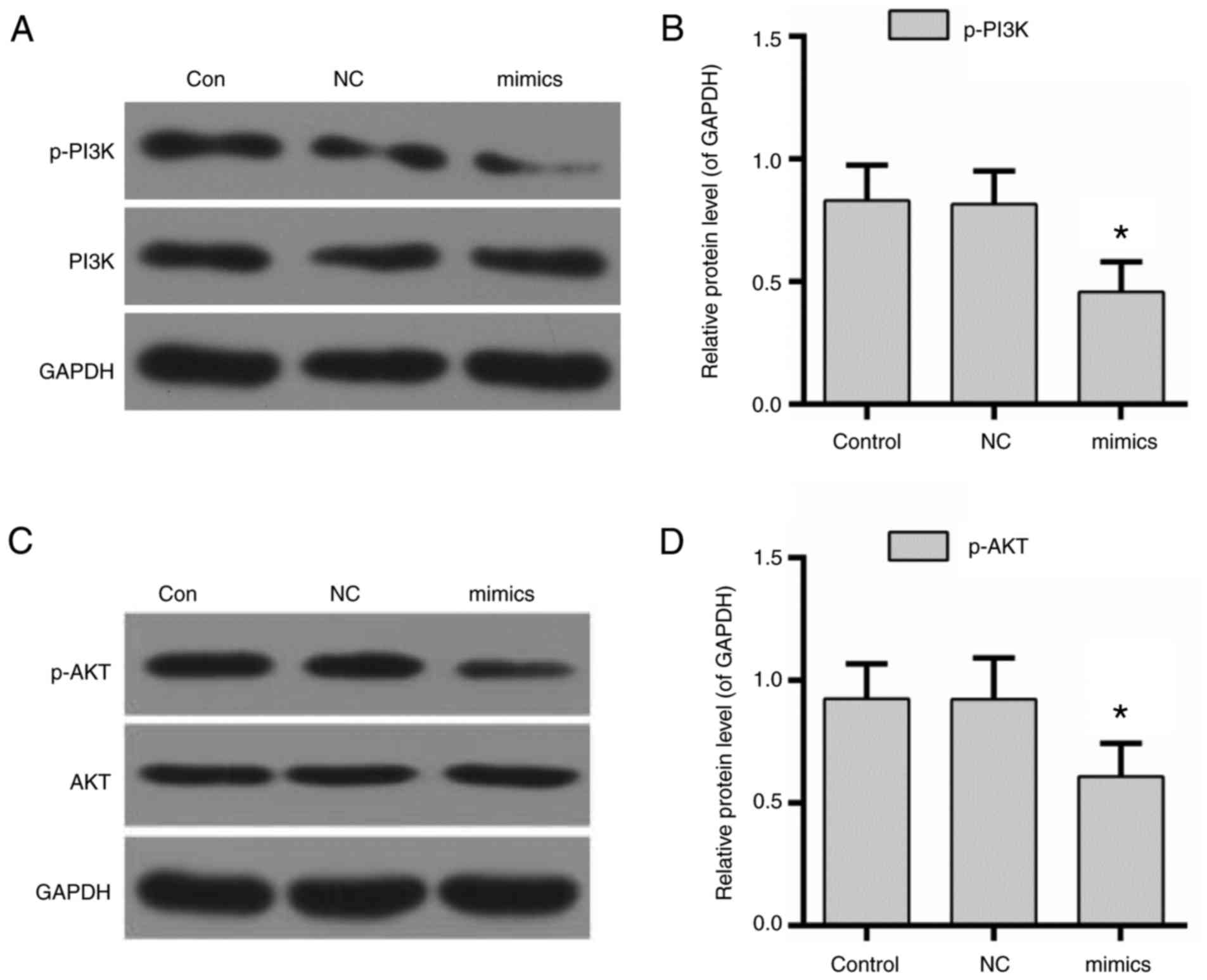
miR-122 decreases the expression of
p-PI3K and p-AKT
To further clarify the underlying molecular
mechanisms, the activity of PI3K/AKT was evaluated in NPC cells
transfected with miR-122. The results of the western blotting
demonstrated that the expression levels of p-PI3K and p-AKT were
decreased by the overexpression of miR-122 (Fig. 5).
Discussion
Recently, varieties of miRNAs have been demonstrated
to be the key regulators in tumor metastatic processes (27). Previous studies have reported that
miR-122 serves as a tumor suppressor and is suppressed in a number
of types of cancer, including gallbladder cancer, gastric cancer,
non-small-cell lung cancer and hepatocellular carcinoma (12,28,29).
High expression of TRIM29 has been identified in a number of
aggressive diseases, and is an important oncogenic factor in cancer
(15). It has been reported that
poor prognosis may be associated with dysregulated miR-122 and
TRIM29 (30,31). Consistently, the results of the
present study demonstrated that miR-122 and TRIM29 expression was
downregulated and upregulated in NPC tissues, particularly in cases
with metastasis, respectively. Furthermore, low miR-122 expression
and high TRIM29 expression were significantly associated with
advanced TNM stage and distant metastasis in patients with NPC. A
poor patient overall survival rate was observed in the low miR-122
group and the high TRIM29 group. Additionally, a significant
negative correlation between the expression of miR-122 and TRIM29
was observed in patients with NPC. It was indicated that the
dysregulation of miR-122 and TRIM29 may serve as a prognostic
biomarker and optional target for NPC. Distant metastasis is the
primary cause of treatment failure in patients with NPC following
radiation therapy (32). The
prevention of metastasis in radiation therapy may be a promising
strategy for the treatment of NPC. Hence, the expression of miR-122
and TRIM29 was determined in NPC cell lines following radiation
treatment. As hypothesized, decreased miR-122 expression and
increased TRIM29 expression was identified in NPC cell lines with
radiation treatment.
Unlimited proliferation and metastasis are the
prominent properties of malignant tumors (33). The failure of radiation therapy is
primarily caused by distant metastasis. The present study further
clarified the potential effect of miR-122 on the metastasis of NPC
in vitro. It was observed that the overexpression of miR-122
was able to inhibit tumor progression by suppressing the viability,
migration and invasion of NPC cells, which indicated the antitumor
effects of miR-122. These data indicated that miR-122 acted as a
tumor suppressor in NPC cells, and were supported by previous
observations in multiple types of cancer (29,34,35).
However, it was likely that the decrease in cell viability may also
indirectly suppress the invasion and migration of NPC cells.
Therefore, the effect of miR-122 on the activity and expression of
metastasis-associated genes was assessed. It was revealed that the
expression of metastasis-associated genes, including E-cadherin,
MTA1, MMP-2 and TIMP2, was regulated by miR-122. However, due to
the complexity of tumorigenesis and the intracellular signal
transduction network, certain studies have claimed that the
overexpression of miR-122 may be associated with poor patient
prognosis in colorectal cancer (36) and renal cancer (37). In addition, the expression of
TRIM29 was downregulated in NPC cells that were transfected with
miR-122. Based on the above investigations, it was speculated that
miR-122 may exert its role by blocking the expression of TRIM29. To
verify this possibility, bioinformatics databases were searched to
predict the downstream targets of miR-122. The analysis results
demonstrated that possible miR-122 binding sites were located in
the 3′-UTR of TRIM29. Further evidence of this was provided by the
results of the luciferase reporter assay, in the 6-10B and 5-8F
cell lines, in the present study. It was therefore suggested that
TRIM29 may be a direct target of miR-122 and serve as an oncogene
in NPC. However, the tumor suppressor role of TRIM29 has
additionally been reported in certain studies (38,39).
Thus, the function of TRIM29 may be context-dependent in the
progression of different tumors. To illustrate the in-depth
molecular mechanisms, the activity of PI3K/AKT signaling, which is
dysregulated in a number of types of cancer, was detected (40). The results revealed that the
phosphorylation of PI3K and AKT was decreased by miR-122. A study
in thyroid cancer reported that the activation of the P13K/AKT
signaling pathway was inhibited by decreased TRIM29 expression
(21). However, it remains unclear
whether or not PI3K/AKT was modulated by TRIM29 in the present
study and thus requires further investigation.
The results of the present study suggested a
potential role of miR-122 and TRIM29 in metastatic progression
following radiation therapy. However, the present study did not
provide further evidence for the effect of miR-122 in the radiation
treatment of NPC. However, it was necessary to undertake in
vivo studies to assess the antitumor effect of miR-122 in
patients with NPC following radiation therapy. These investigations
implied that the antitumor effect of miR-122 in NPC was promising,
although this requires validation in future studies.
In conclusion, decreased expression of miR-122 and
increased expression of TRIM29 was significantly associated with
poor prognosis in patients with NPC. miR-122 may exert antitumor
effects through suppression of tumor proliferation, invasion and
migration, and modulation of the expression and activity of
metastasis-associated genes, including E-cadherin, MTA1, MMP-2 and
TIMP2. Notably, miR-122 may suppress NPC progression by targeting
TRIM29 and blocking the activity of PI3K/AKT signaling. Therefore,
miR-122 and TRIM29 may be developed as biomarkers and possible
molecular targets for the metastasis of NPC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LG designed the present study. QL and YY wrote the
manuscript. YY and QL performed the experiments. QL performed data
analysis. YY, QL and LG revised the manuscript for important
intellectual content.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients prior to enrolling in the present study. The protocols
associated with the use of patient tissues were approved by the
ethics committee of Jing Men No. 1 People's Hospital (Jingmen,
China).
Consent for publication
Written informed consent was obtained from all
patients prior to enrolling in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang DP, Ho JH, Poon YF, Chew EC, Saw D,
Lui M, Li CL, Mak LS, Lai SH and Lau WH: Establishment of a cell
line (NPC/HK1) from a differentiated squamous carcinoma of the
nasopharynx. Int J Cancer. 26:127–132. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee JH, Zhang J, Wei L and Yu SP:
Neurodevelopmental implications of the general anesthesia in
neonate and infants. Exp Neurol. 272:50–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee N, Harris J, Garden AS, Straube W,
Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, et
al: Intensity-modulated radiation therapy with or without
chemotherapy for nasopharyngeal carcinoma: Radiation therapy
oncology group phase II trial 0225. J Clin Oncol. 27:3684–3690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Mao YP, Xie FY, Liu LZ, Sun Y,
Tian L, Tang LL, Lin AH, Li L and Ma J: The seventh edition of the
UICC/AJCC staging system for nasopharyngeal carcinoma is
prognostically useful for patients treated with intensity-modulated
radiotherapy from an endemic area in China. Radiother Oncol.
104:331–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garzon R, Marcucci G and Croce CM:
Targeting microRNAs in cancer: Rationale, strategies and
challenges. Nat Rev Drug Discov. 9:775–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Nat
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ying SY, Chang DC and Lin SL: The microRNA
(miRNA): Overview of the RNA genes that modulate gene function. Mol
Biotechnol. 38:257–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Wang Q, Shen D, Sun X, Cao X and
Wu D: Downregulation of microRNA-122 promotes proliferation,
migration, and invasion of human hepatocellular carcinoma cells by
activating epithelial-mesenchymal transition. Onco Targets Ther.
9:2035–2047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin H, Sha J, Jiang C, Gao X, Qu L, Yan H,
Xu T, Jiang Q and Gao H: miR-122 inhibits metastasis and
epithelial-mesenchymal transition of non-small-cell lung cancer
cells. Onco Targets Ther. 8:3175–3184. 2015.PubMed/NCBI
|
|
13
|
Watanabe M and Hatakeyama S: TRIM proteins
and diseases. J Biochem. 161:135–144. 2017.PubMed/NCBI
|
|
14
|
Sato T, Takahashi H, Hatakeyama S, Iguchi
A and Ariga T: The TRIM-FLMN protein TRIM45 directly interacts with
RACK1 and negatively regulates PKC-mediated signaling pathway.
Oncogene. 34:1280–1291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosaka Y, Inoue H, Ohmachi T, Yokoe T,
Matsumoto T, Mimori K, Tanaka F, Watanabe M and Mori M: Tripartite
motif-containing 29 (TRIM29) is a novel marker for lymph node
metastasis in gastric cancer. Ann Surg Oncol. 14:2543–2549. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanno Y, Watanabe M, Kimura T, Nonomura K,
Tanaka S and Hatakeyama S: TRIM29 as a novel prostate basal cell
marker for diagnosis of prostate cancer. Acta Histochem.
116:708–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Yang H, Palmbos PL, Ney G, Detzler
TA, Coleman D, Leflein J, Davis M, Zhang M, Tang W, et al:
ATDC/TRIM29 phosphorylation by ATM/MAPKAP kinase 2 mediates
radioresistance in pancreatic cancer cells. Cancer Res.
74:1778–1788. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoki M and Fujishita T: Oncogenic Roles of
the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 407:153–189.
2017.PubMed/NCBI
|
|
20
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
21
|
Xu J, Li Z, Su Q, Zhao J and Ma J: TRIM29
promotes progression of thyroid carcinoma via activating P13K/AKT
signaling pathway. Oncol Rep. 37:1555–1564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang C, Wang H, Zhou L, Jiang T, Xu Y and
Xia L: MicroRNA-212 inhibits the metastasis of nasopharyngeal
carcinoma by targeting SOX4. Oncol Rep. 38:82–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toh Y and Nicolson GL: Identification and
characterization of metastasis-associated gene/protein 1 (MTA1).
Cancer Metastasis Rev. 33:837–842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Birkedal-Hansen H, Moore WG, Bodden MK,
Windsor LJ, Birkedal-Hansen B, DeCarlo A and Engler JA: Matrix
metalloproteinases: A review. Crit Rev Oral Biol Med. 4:197–250.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Musso O, Théret N, Campion JP, Turlin B,
Milani S, Grappone C and Clément B: In situ detection of matrix
metalloproteinase-2 (MMP2) and the metalloproteinase inhibitor
TIMP2 transcripts in human primary hepatocellular carcinoma and in
liver metastasis. J Hepatol. 26:593–605. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma L: MicroRNA and Metastasis. Adv Cancer
Res. 132:165–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ergün S, Ulasli M, Igci YZ, Igci M,
Kırkbes S, Borazan E, Balik A, Yumrutaş Ö, Camci C, Cakmak EA, et
al: The association of the expression of miR-122-5p and its target
ADAM10 with human breast cancer. Mol Biol Rep. 42:497–505. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Xing QF, Liu XQ, Guo ZJ, Li CY and
Sun G: MiR-122 targets VEGFC in bladder cancer to inhibit tumor
growth and angiogenesis. Am J Transl Res. 8:3056–3066.
2016.PubMed/NCBI
|
|
30
|
Fristrup N, Birkenkamp-Demtröder K,
Reinert T, Sanchez-Carbayo M, Segersten U, Malmström PU, Palou J,
Alvarez-Múgica M, Pan CC, Ulhøi BP, et al: Multicenter validation
of cyclin D1, MCM7, TRIM29, and UBE2C as prognostic protein markers
in non-muscle-invasive bladder cancer. Am J Pathol. 182:339–349.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Glebov OK, Rodriguez LM, Soballe P,
DeNobile J, Cliatt J, Nakahara K and Kirsch IR: Gene expression
patterns distinguish colonoscopically isolated human aberrant crypt
foci from normal colonic mucosa. Cancer Epidemiol Biomarkers Prev.
15:2253–2262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geara FB, Sanguineti G, Tucker SL, Garden
AS, Ang KK, Morrison WH and Peters LJ: Carcinoma of the nasopharynx
treated by radiotherapy alone: Determinants of distant metastasis
and survival. Radiother Oncol. 43:53–61. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schram FR and Ng PKL: What is Cancer? J
Crust Biol. 32:665–672. 2012. View Article : Google Scholar
|
|
34
|
Rao M, Zhu Y, Zhou Y, Cong X and Feng L:
MicroRNA-122 inhibits proliferation and invasion in gastric cancer
by targeting CREB1. Am J Cancer Res. 7:323–333. 2017.PubMed/NCBI
|
|
35
|
Qiao DD, Yang J, Lei XF, Mi GL, Li SL, Li
K, Xu CQ and Yang HL: Expression of microRNA-122 and microRNA-22 in
HBV-related liver cancer and the correlation with clinical
features. Eur Rev Med Pharmacol Sci. 21:742–747. 2017.PubMed/NCBI
|
|
36
|
Maierthaler M, Benner A, Hoffmeister M,
Surowy H, Jansen L, Knebel P, Chang-Claude J, Brenner H and
Burwinkel B: Plasma miR-122 and miR-200 family are prognostic
markers in colorectal cancer. Int J Cancer. 140:176–187. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jingushi K, Kashiwagi Y, Ueda Y, Kitae K,
Hase H, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
High miR-122 expression promotes malignant phenotypes in ccRCC by
targeting occludin. Int J Oncol. 51:289–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ai L, Kim WJ, Alpay M, Tang M, Pardo CE,
Hatakeyama S, May WS, Kladde MP, Heldermon CD, Siegel EM and Brown
KD: TRIM29 suppresses TWIST1 and invasive breast cancer behavior.
Cancer Res. 74:4875–4887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu J, Welm B, Boucher KM, Ebbert MT and
Bernard PS: TRIM29 functions as a tumor suppressor in
nontumorigenic breast cells and invasive ER+ breast cancer. Am J
Pathol. 180:839–847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|