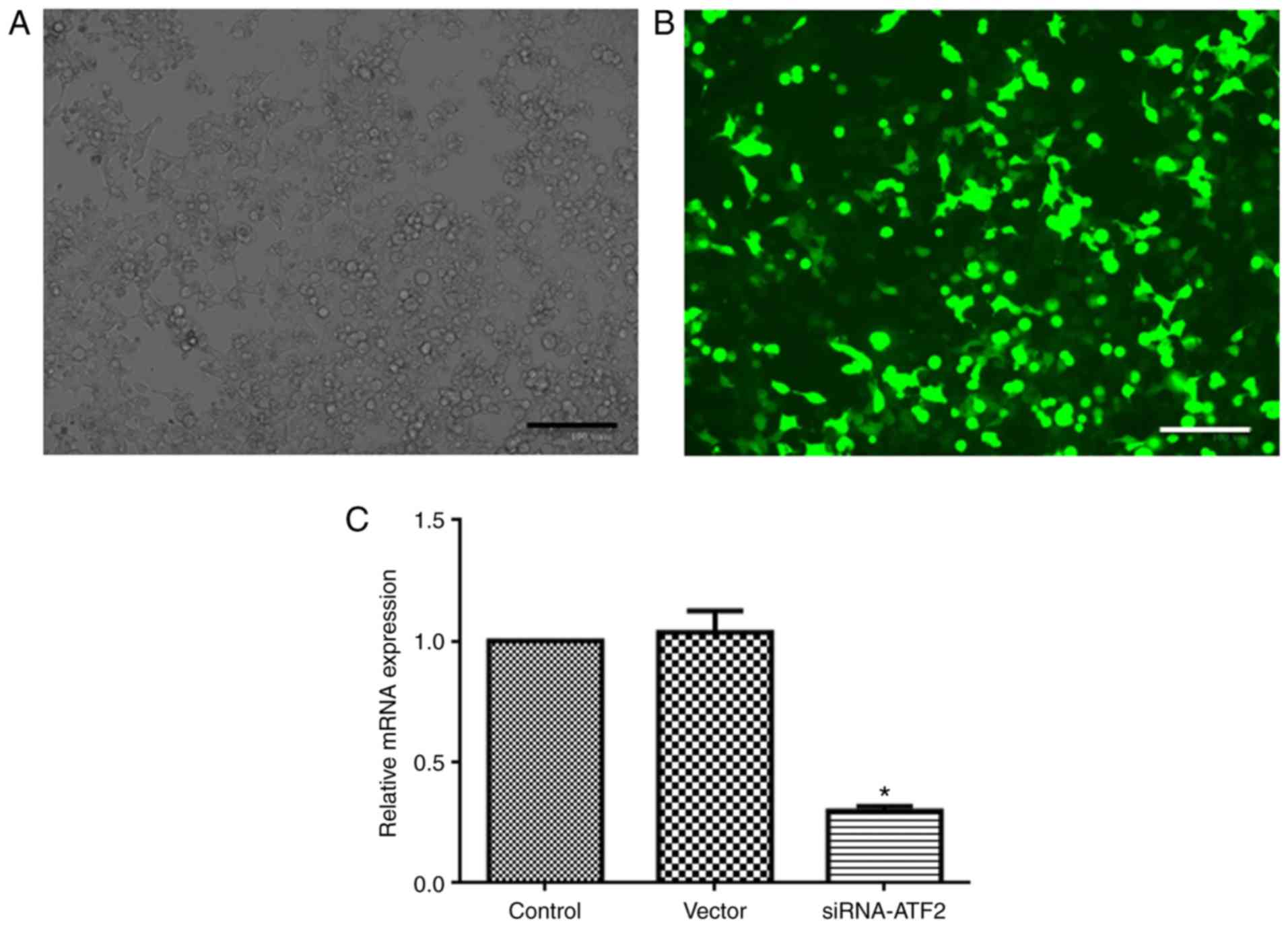
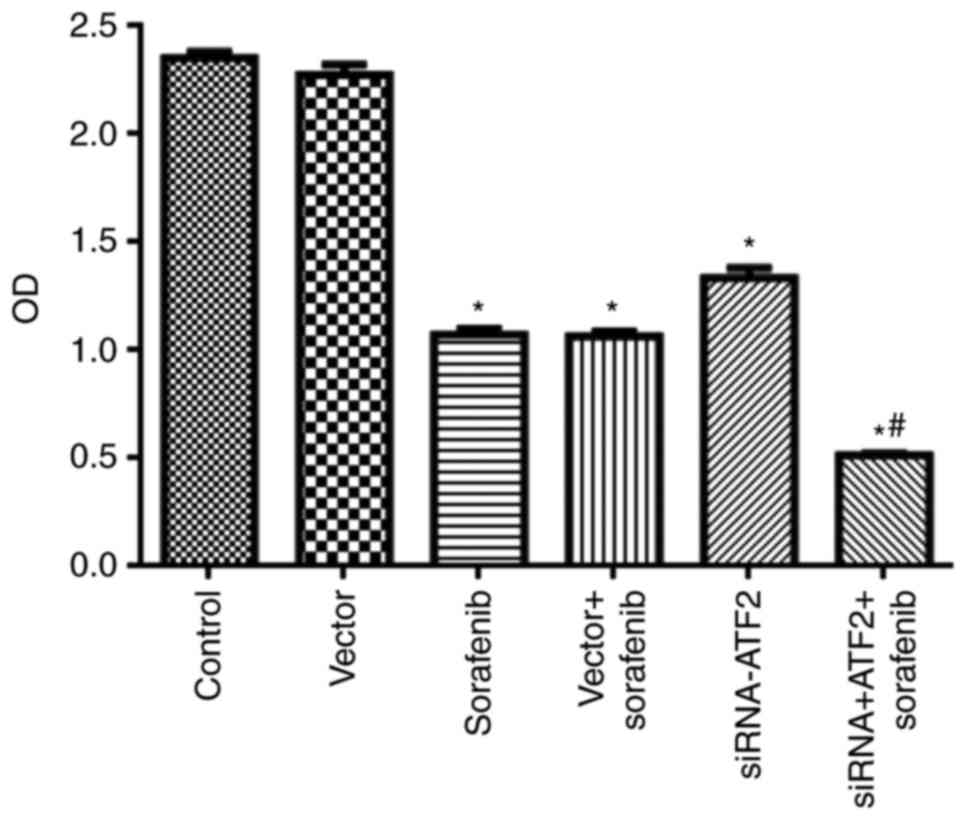
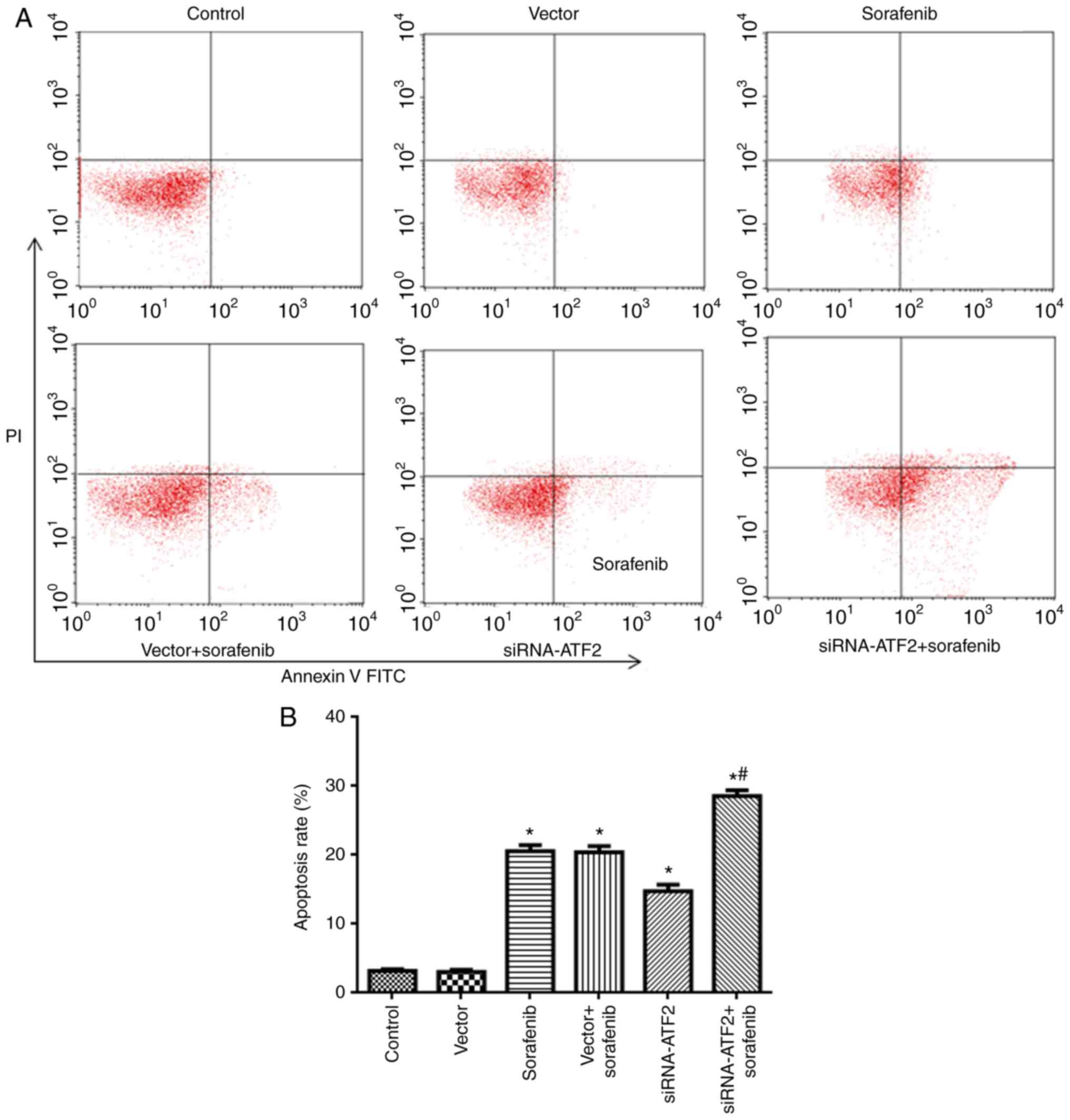
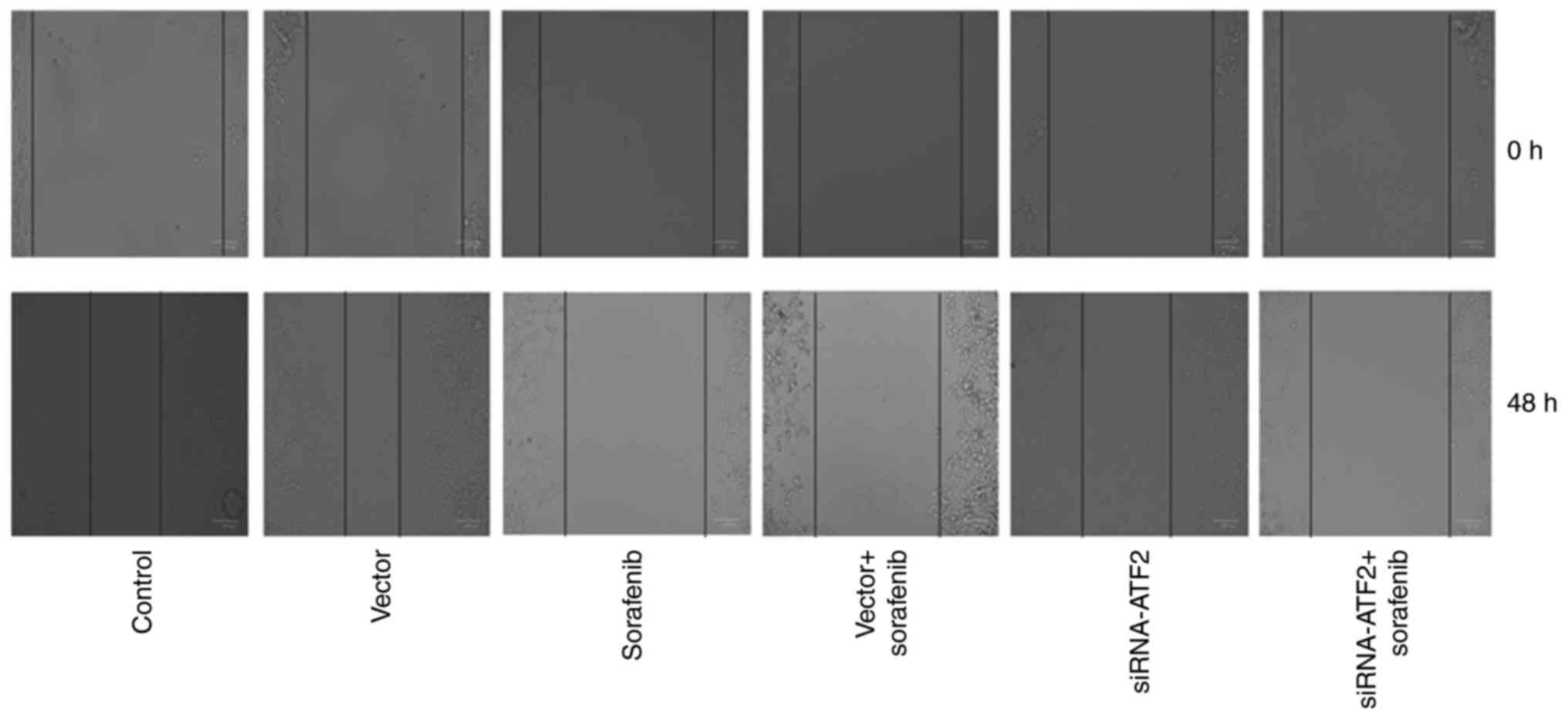
|
1
|
Bai DS, Zhang C, Chen P, Jin SJ and Jiang
GQ: The prognostic correlation of AFP level at diagnosis with
pathological grade, progression, and survival of patients with
hepatocellular carcinoma. Sci Rep. 7:128702017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thein HH, Qiao Y, Zaheen A, Jembere N,
Sapisochin G, Chan KKW, Yoshida EM and Earle CC: Cost-effectiveness
analysis of treatment with non-curative or palliative intent for
hepatocellular carcinoma in the real-world setting. PLoS One.
12:e01851982017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cainap C, Qin S, Huang WT, Chung IJ, Pan
H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, et al: Linifanib
versus Sorafenib in patients with advanced hepatocellular
carcinoma: Results of a randomized phase III trial. J Clin Oncol.
33:172–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervello M, Bachvarov D, Lampiasi N,
Cusimano A, Azzolina A, McCubrey JA and Montalto G: Molecular
mechanisms of sorafenib action in liver cancer cells. Cell Cycle.
11:2843–2855. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow AK, Ng L, Lam CS, Wong SK, Wan TM,
Cheng NS, Yau TC, Poon RT and Pang RW: The Enhanced metastatic
potential of hepatocellular carcinoma (HCC) cells with sorafenib
resistance. PLoS One. 8:e786752013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fouladi F, Jehn LB, Metzelder SK, Hub F,
Henkenius K, Burchert A, Brendel C, Stiewe T and Neubauer A:
Sorafenib induces paradoxical phosphorylation of the extracellular
signal-regulated kinase pathway in acute myeloid leukemia cells
lacking FLT3-ITD mutation. Leuk Lymphoma. 56:2690–2698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Endo M, Su L and Nielsen TO: Activating
transcription factor 2 in mesenchymal tumors. Hum Pathol.
45:276–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goetz J, Chatton B, Mattei MG and Kedinger
C: Structure and expression of the ATFa gene. J Biol Chem.
271:29589–29598. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lo Iacono M, Monica V, Vavala T, Gisabella
M, Saviozzi S, Bracco E, Novello S, Papotti M and Scagliotti GV:
ATF2 contributes to cisplatin resistance in non-small cell lung
cancer and celastrol induces cisplatin resensitization through
inhibition of JNK/ATF2 pathway. Int J Cancer. 136:2598–2609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shatanawi A, Lemtalsi T, Yao L, Patel C,
Caldwell RB and Caldwell RW: Angiotensin II limits NO production by
upregulating arginase through a p38 MAPK-ATF-2 pathway. Eur J
Pharmacol. 746:106–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sreekanth GP, Chuncharunee A,
Sirimontaporn A, Panaampon J, Noisakran S, Yenchitsomanus PT and
Limjindaporn T: SB203580 modulates p38 MAPK signaling and dengue
virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2
phosphorylation. PLoS One. 11:e01494862016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Z, Duan X, Cai H, Wang L, Li M, Qu J,
Li W, Wang Y and Wang J: Curcumin inhibits the invasion of lung
cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling
pathway. Oncol Rep. 34:691–698. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song S, Fajol A, Tu X, Ren B and Shi S:
miR-204 suppresses the development and progression of human
glioblastoma by targeting ATF2. Oncotarget. 7:70058–70065. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HS, Choi ES, Shin JA, Jang YK and Park
SD: Regulation of Swi6/HP1-dependent heterochromatin assembly by
cooperation of components of the mitogen-activated protein kinase
pathway and a histone deacetylase Clr6. J Biol Chem.
279:42850–42859. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shah M, Bhoumik A, Goel V, Dewing A,
Breitwieser W, Kluger H, Krajewski S, Krajewska M, Dehart J, Lau E,
et al: A role for ATF2 in regulating MITF and melanoma development.
PLoS Genet. 6:e10012582010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You Z, Zhou Y, Guo Y, Chen W, Chen S and
Wang X: Activating transcription factor 2 expression mediates cell
proliferation and is associated with poor prognosis in human
non-small cell lung carcinoma. Oncol Lett. 11:760–766. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maekawa T, Shinagawa T, Sano Y, Sakuma T,
Nomura S, Nagasaki K, Miki Y, Saito-Ohara F, Inazawa J and Kohno T:
Reduced levels of ATF-2 predispose mice to mammary tumors. Mol Cell
Biol. 27:1730–1744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhoumik A, Fichtman B, Derossi C,
Breitwieser W, Kluger HM, Davis S, Subtil A, Meltzer P, Krajewski
S, Jones N and Ronai Z: Suppressor role of activating transcription
factor 2 (ATF2) in skin cancer. Proc Natl Acad Sci USA.
105:1674–1679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WL, Hsieh CL, Chen JH, Huang CS, Chen
WT, Kuo YC, Chen CY and Hsu FT: Amentoflavone enhances
sorafenib-induced apoptosis through extrinsic and intrinsic
pathways in sorafenib-resistant hepatocellular carcinoma SK-Hep1
cells in vitro. Oncol Lett. 14:3229–3234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li S, Dai W, Mo W, Li J, Feng J, Wu L, Liu
T, Yu Q, Xu S, Wang W, et al: By inhibiting PFKFB3, aspirin
overcomes sorafenib resistance in hepatocellular carcinoma. Int J
Cancer. 141:2571–2584. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quintavalle C, Hindupur SK, Quagliata L,
Pallante P, Nigro C, Condorelli G, Andersen JB, Tagscherer KE, Roth
W, Beguinot F, et al: Phosphoprotein enriched in diabetes
(PED/PEA15) promotes migration in hepatocellular carcinoma and
confers resistance to sorafenib. Cell Death Dis. 8:e31382017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shivashankar R and Pardi DS: Use of
anti-tumor necrosis factors and anti-integrins in the treatment of
crohn's disease. Gastroenterol Clin North Am. 46:589–601. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sedger LM and McDermott MF: TNF and
TNF-receptors: From mediators of cell death and inflammation to
therapeutic giants-past, present and future. Cytokine Growth Factor
Rev. 25:453–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X and Lin Y: Tumor necrosis factor
and cancer, buddies or foes? Acta Pharmacol Sin. 29:1275–1288.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Xia Y, Liu T, Wang J, Dai W, Wang F,
Zheng Y, Chen K, Li S, Abudumijiti H, et al: Protective effects of
astaxanthin on ConA-induced autoimmune hepatitis by the JNK/p-JNK
pathway-mediated inhibition of autophagy and apoptosis. PLoS One.
10:e01204402015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251, 0000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi J, Yang X, Zheng L, Yang G, Sun L, Bao
Y, Wu Y, Huang Y, Yu C, Yang SN and Li Y: Photoactivation of
hypericin decreases the viability of RINm5F insulinoma cells
through reduction in JNK/ERK phosphorylation and elevation of
caspase-9/caspase-3 cleavage and Bax-to-Bcl-2 ratio. Biosci Rep.
35:pii: e00195. 352015
|
|
30
|
Deng Y, Ren X, Yang L, Lin Y and Wu X: A
JNK-dependent pathway is required for TNFalpha-induced apoptosis.
Cell. 115:61–70. 2003. View Article : Google Scholar : PubMed/NCBI
|