Introduction
Ischemic heart disease (IHD) is a major threat to
human health, which increases the burden on the heart muscle
(1). It is estimated that >7
million succumbed to heart diseases in 2010 (2). Cardiac hypertrophy is a critical
phenotype that occurs in response to long-term cardiac overload
(3,4), which is frequently coupled with
inflammation, myocardial fibrosis and cell death. The
characteristics of cardiac hypertrophy consist of cell surface
enlargement, increased protein synthesis and alteration of
expression level of associated genes. Therefore, preventing and
reversing myocardial hypertrophy has become the main target of
cardiovascular disease treatment. Accordingly, it is essential to
develop drugs that can protect against myocardial injury.
Actinidia chinensis planch contains
polyphenolic acid. Its main active compound is Actinidia
chinensis planch polysaccharide (ACP) (5). ACP exhibits antibacterial, antiviral
and anti-tumor effects (6,7). However, little is known about the
effects of ACP on cardiomyocytes, and the underlying mechanisms
have not yet been fully elucidated and require further exploration.
In addition, investigating the potential molecular mechanisms will
provide important theoretical and practical significance for the
treatment of IHD. During the progression of IHD, a number of
cellular responses are involved, including gene transcription,
protein translation and cellular signal transduction (8,9). It
has been reported that apoptosis may be the cellular basis for the
pathological progression of cardiac hypertrophy, induced by
repeated ischemic events, to heart failure (4,10,11).
Currently, there are two main pathways associated with apoptosis,
the caspase-dependent and non-caspase-dependent pathways (12). The former includes the death
receptor-mediated extrinsic pathway and the intrinsic mitochondrial
pathway, which is associated with caspase-8 and caspase-9 (4,13).
Apoptosis-inducing factor mitochondria associated 1 (AIF) is
associated with non-caspase-dependent pathways. It has been
demonstrated that caspase-3 serves a pivotal role in the majority
of apoptotic processes (14).
Furthermore, it is reported that the hypoxic conditions of
cardiomyocytes could induce cell apoptosis in vitro
(15,16), which mimic the process of ischemia
of myocardial cells in the body. Phosphoinositide 3-kinase (PI3K)
is a lipid kinase and the downstream target is serine/threonine
kinase protein kinase B (AKT), a conserved signal transduction
enzyme. The PI3K/AKT signaling pathway is critical to regulate
cellular activation, inflammatory responses and apoptosis (17). In addition, the extracellular
signal-regulated kinase (ERK1/2) signaling pathway serves a key
role in cell proliferation, survival, transformation and apoptosis
(18). It is well known that
ERK1/2 and PI3K/AKT can be activated by phosphorylation. The
protective effects of the ERK1/2 and PI3K/AKT signaling pathways in
cardiomyocytes have been studied extensively (19–21).
In the present study, the protective effect and
mechanisms of ACP on the cardiomyocytes of rats was investigated.
The results of the present study indicated that ACP could protect
against apoptosis induced by hypoxia in cardiomyocytes treated with
Angiotensin II (Ang II). The potential mechanisms may be associated
with inhibiting the activation of ERK1/2 and PI3K/AKT. The results
of the present study provide important information for the
theoretical study and clinical treatment of the IHD.
Materials and methods
Cell culture and treatment
H9c2 cells were purchased from American Type Culture
Collection (Manassas, VA, USA). Purified ACP was acquired from Xian
Tianrui Biological Technology Co., Ltd. (Xi'an, China). Ang II used
in this study was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Cells were maintained in Dulbecco's modified
Eagle media (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(Sangon Biotech Co., Ltd., Shanghai, China), 100 U/ml penicillin
and 10 µg/ml streptomycin (Takara Biotechnology Co., Ltd., Dalian,
China) in a humid atmosphere of 5% CO2 and 95% air at
37°C. Hypoxia treatment was used to mimic myocardial ischemia in
the body (22). At ~80%
confluence, H9c2 cells were cultured in serum-free medium overnight
at 37°C and five treatment groups were applied for the subsequent
experiments: i) Normal group, H9c2 cells were cultured with 0.1%
DMSO; ii) model group (MG), H9c2 cells were treated with 1 µM Ang
II for 48 h; iii) hypoxia treatment group (MGH), H9c2 cells were
treated 1 µM Ang II for 48 h, then the cells were incubated at 37°C
in a humidified atmosphere containing 95% N2 and 5%
CO2 for 8 h; iv) ACP pretreatment groups, Ang II-treated
(1 µM, 48 h) H9c2 cells were incubated in the medium containing ACP
at different doses (1.25 and 2.5 mg/ml) for 6 h prior to hypoxia
treatment for 8 h. For the specific activation of ERK1/2 and
PI3K/AKT, according to previous studies (23,24),
the cells were treated with recombinant human epidermal growth
factor (EGF; 100 ng/ml) and insulin-like growth factor 1 (IGF-I;
100 ng/ml) for 12 h, respectively. Then the cells were subjected to
treatment with ACP and hypoxia.
Cell viability measurement
Cells in a 96-well plate at a density of
1×104 cells per well were serum starved overnight prior
to detection and then maintained as described above. Cell viability
was analyzed by Cell Counting kit-8 (CCK-8; Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. The absorbance at 450 nm was measured on a
spectrophotometric plate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Each group was repeated in three different
wells.
Mitochondrial membrane potential (MMP)
analysis
Cells in a 96-well plate at a density of
1×104 cell/well were treated as aforementioned. Under
normal conditions, the MMP is high and the JC-1 probe exhibits a
predominantly red fluorescence. The MMP is reduced in an apoptotic
or necrotic state and JC-1 dye fluoresces green. Cells were
incubated with the JC-1 (BioVision, Inc., Milpitas, CA, USA)
working solution for 20 min at 37°C in the dark. The MMP was
detected using a flow cytometer and CellQuest software version 3.3
(BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocols.
Measurement of apoptosis
Following treatment as described above, H9c2 cells
(2×105/well) in a 6-well plate were stained by Annexin-V
and propidium iodide using the fluorescein isothiocyanate (FITC)
Annexin-V apoptosis detection kit (Invitrogen; Thermo Fisher
Scientific, Inc.) as described previously (25). Then, flow cytometry was carried out
using a flow cytometer and CellQuest software version 3.3 (BD
Biosciences) according to the manufacturer's protocols.
Leucine incorporation assay
The protein synthesis rate was examined as
previously described (26).
According to the manufacturer's protocols, the incorporation of
3H-Leu [counts per minute CPM)] was measured by liquid
scintillation counter (Perkin Elmer, Inc., Waltham, MA, USA).
Bradford assay
H9c2 cells were treated as aforementioned. Bradford
reagent (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) was added to each sample according to the
manufacturer's protocol. Then the samples were analyzed to
determine their absorbance at 595 nm. A standard curve of bovine
serum albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.) was used
as a control for all experiments. The protein content was detected
by spectrophotometry (Nanodrop; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA).
Immunofluorescence staining assay
The cells in control, Ang II and ACP+Ang II groups
were prepared on slides for the subsequent experiments. The slides
were blocked by 5% BSA for 30 min at room temperature prior to the
cardiac troponin-T specific monoclonal antibody (cat. no. ab8259;
1:200; Abcam, Cambridge, UK) being added at 4°C, overnight. Then
the slides were incubated with the anti-immunoglobulin G/FITC (cat.
no. ab150117; 1 µg/ml; Abcam, Cambridge, UK) secondary antibody at
room temperature for 1 h; nuclei were stained with DAPI for 15 min
at 37°C. The slides were mounted, a coverslip was added and then
the slides were observed with a fluorescence microscope (Olympus
Corporation, Tokyo, Japan). Then, 4 fields in each group were
randomly selected for analysis. The cell surface area was analyzed
by Image Pro-Plus software version 6.1 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with the TRIzol reagent,
according to the manufacturer's instructions (Life Technologies;
Thermo Fisher Scientific, Inc.). The RNA (2 µg) was reverse
transcribed using oligo (dT) primer (Takara Biotechnology Co.,
Ltd.) and the M-MLV reverse transcriptase (Promega Corporation,
Madison, WI, USA). The protocol was 65°C for 5 min; 25°C for 10
min; 42°C for 50 min; 70°C for 15 min; and holding at 4°C. The mRNA
expression was quantified using ABI 7500 Real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and SYBR-Green
method (27,28) SYBR Premix Taq™ II kit (Takara Bio,
Inc., Otsu, Japan) was adopted for the amplification. Relative
expression levels were calculated using the 2−∆∆Cq
method, according to the previous description (29,30).
The amplification cycling conditions were as follows: 10 min at
95°C, then 40 cycles of 15 sec at 95°C, 30 sec at 60°C and then a
final extension step of 7 min at 72°C. Primer sequences for RT-qPCR
were: AIF forward, 5′-CCGGGTAAATGCAGAGCTTC-3′ and reverse,
5′-GCTCTGCATTTACCCGGAAG-3′; caspase-3 forward,
5′-AGAGCTGGACTGCGGTATTGAG-3′ and reverse,
5′-GAACCATGACCCGTCCCTTG-3′; caspase-8 forward,
5′-GAGGAAATGGTGAGGGAGCT-3′ and reverse, 5′-GCTCGAGTTGTCTTGCAGTT-3′;
caspase-9 forward, 5′-CATTGGTTCTGGCAGAGCTC-3′ and reverse,
5′-TCAGGTCGTTCTTCACCTCC-3′; GAPDH forward,
5′-GGCACAGTCAAGGCTGAGAATG-3′ and reverse,
5′-ATGGTGGTGAAGACGCCAGTA-3′.
Western blot analysis
Total proteins were extracted using the Total
protein extraction kit (Beijing Solarbio Science & Technology
Co., Ltd.). The concentration of proteins was determined by BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Equivalent amounts (15 µg) of protein
were separated by 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
For non-specific blocking, 5% non-fat milk was used. The blots were
then incubated with primary antibodies at 4°C overnight. The next
day, the blots were incubated with a horseradish
peroxidase-conjugated secondary antibody at room temperature for 1
h. Blots were developed using the enhanced chemiluminescence
Western Blotting Substrate (Pierce; Thermo Fisher Scientific,
Inc.). The antibodies used were anti-cleaved caspase-3 (cat. no.
9664; 1:1,000), anti-cleaved caspase-8 (cat. no. 9496; 1:1,000),
anti-cleaved caspase-9 (cat. no. 9505; 1:1,000), anti-AIF (cat. no.
5318; 1:1,000), anti-phosphorylated (p)-ERK1/2 (cat. no. 4370;
1:2,000), anti-p-AKT (cat. no. 4060; 1:2,000), anti-p-PIK3 (cat.
no. 4228; 1:1,000), anti-ERK1/2 (cat. no. 4695; 1:1,000), anti-AKT
(cat. no. 4685; 1:1,000), anti-PIK3 (cat. no. 3358; 1:1,000) and
anti-GAPDH (cat. no. 5174; 1:1,000), all of which were from Cell
Signaling Technology, Inc. (Danvers, MA, USA). The horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2004;
1:5,000) was from Santa Cruz Biotechnology, Inc., (Dallas, TX,
USA). Signals were visualized using BeyoECL Plus (Beyotime
Institute of Biotechnology, Haimen, China). The density of the
protein band was quantified with Quantity One Basic software
version 4.4.0 (Bio-Rad).
Statistical analysis
All experiments in the present study were repeated
independently ≥3 times. All the data were analyzed using GraphPad
software version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA)
by one-way analysis of variance followed by Tukey's multiple
comparison test. Data was expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Analysis of the cytotoxicity of ACP in
cardiomyocytes
To optimize the concentration used in the present
study, the cytotoxicity of ACP at different doses on cardiomyocytes
was examined, as described by a previous study (31). The CCK-8 assay revealed that cell
viability began to be inhibited at 5 mg/ml, although there was no
significant difference compared with the control group until 10
mg/ml. Thus, the viability of cardiomyocytes was suppressed by ACP
at 10 mg/ml (Fig. 1). Therefore,
the concentrations of 1.25 and 2.5 mg/ml were used for the
subsequent experiments.
ACP alleviates Ang II induced cardiac
hypertrophy
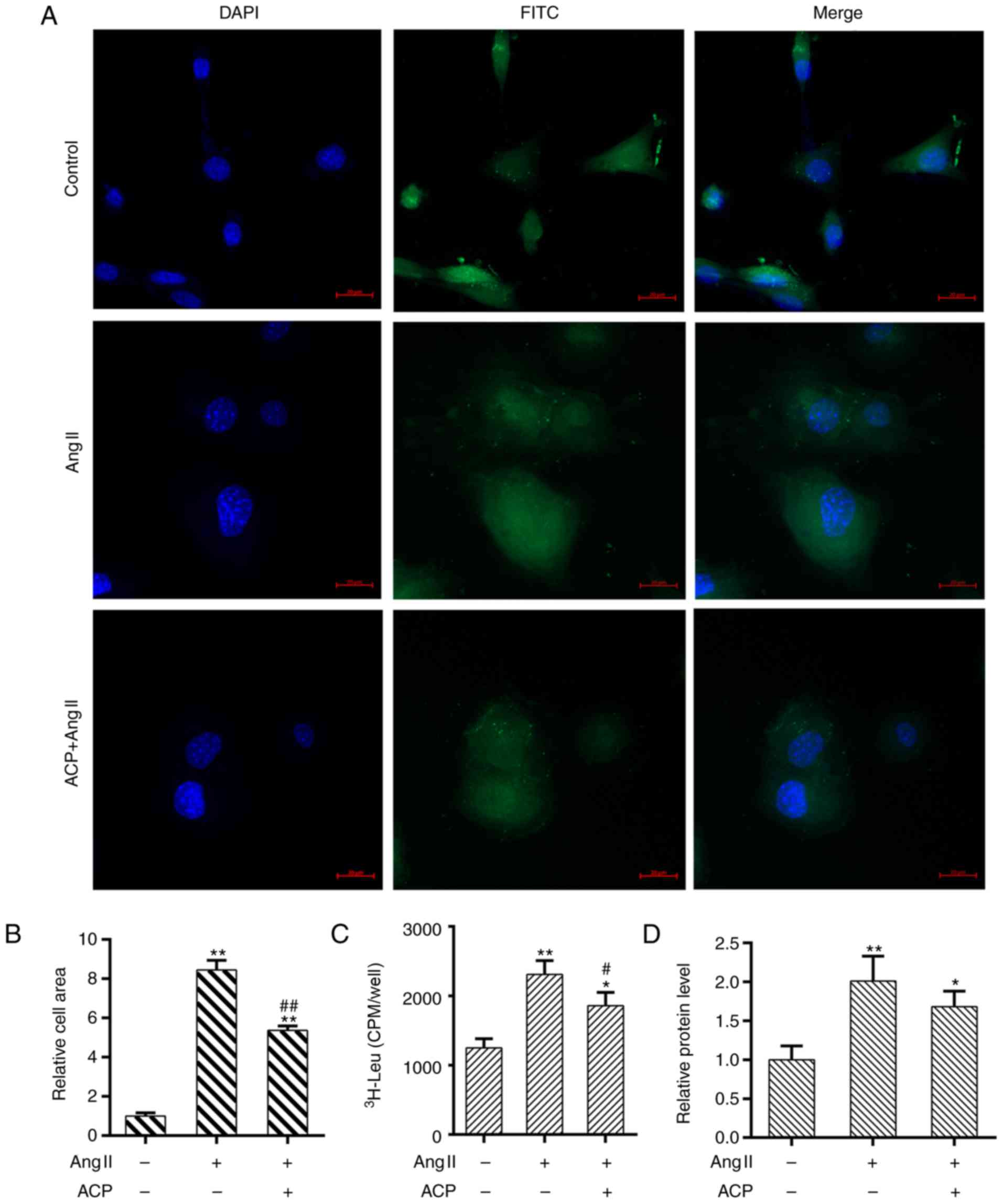
As shown in Fig.
2A, the cardiac phenotype of the obtained cells was identified.
The surface area of the cell was enlarged in cardiomyocytes
stimulated with Ang II, while ACP significantly reduced the cardiac
hypertrophy compared with the Ang II treated group (P<0.01;
Fig. 2A and B). Additionally, the
leucine incorporation assay demonstrated that ACP significantly
decreased the elevation in protein synthesis rate induced by
treatment with Ang II. (P<0.05; Fig. 2C). In addition, the Bradford assay
demonstrated that the protein content was significantly decreased
by ACP treatment compared with Ang II alone (P<0.05; Fig. 2D).
ACP improves the survival of
cardiomyocytes
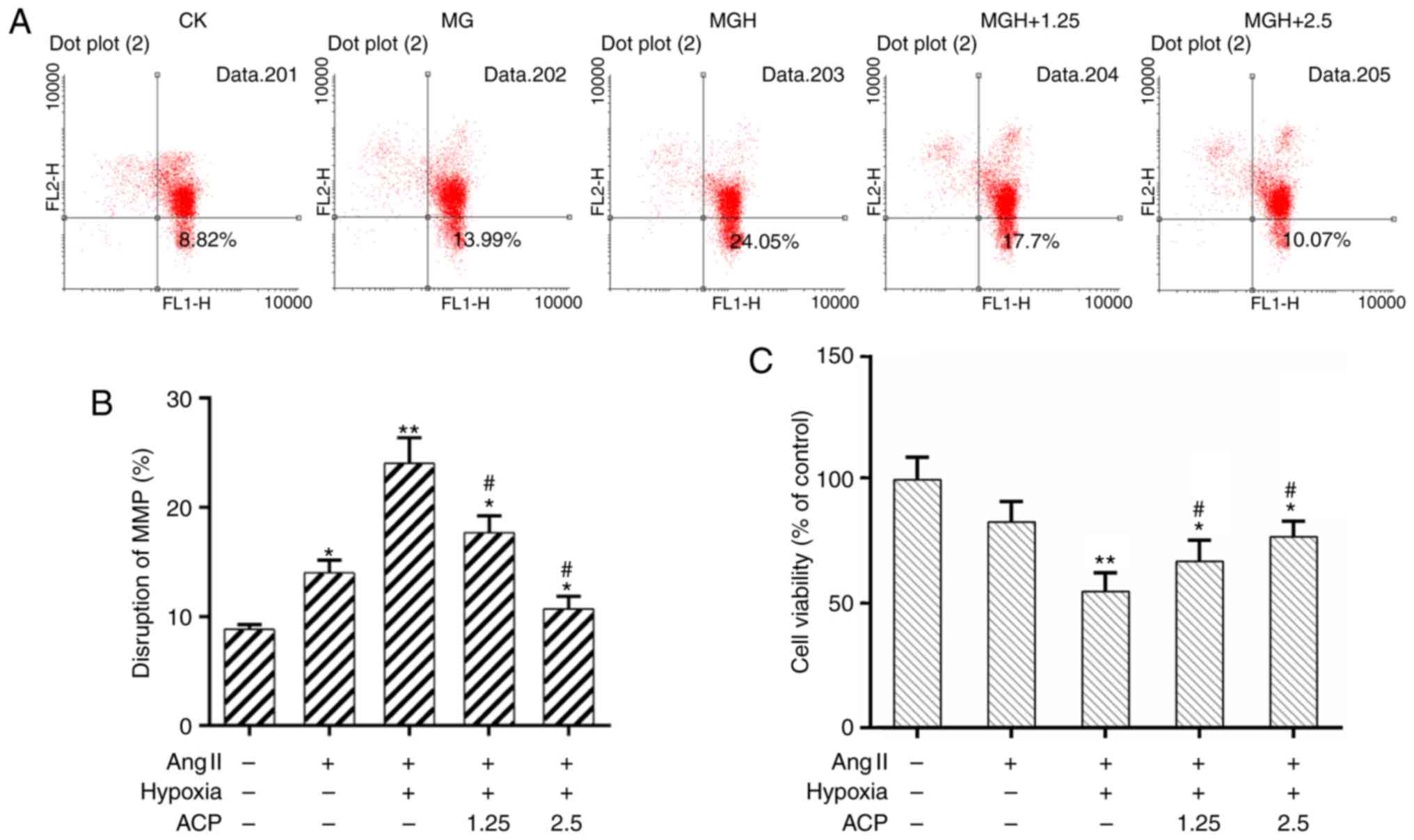
It is known that mitochondrial dysfunction will
trigger a cellular crisis (32,33).
It was demonstrated using flow cytometric analysis that the
disruption of the MMP was increased in the MGH group when compared
with the model group. The MMP was recovered effectively by
pretreatment with ACP (Fig. 3A and
B). In addition, the CCK-8 assay demonstrated that cell
viability was significantly improved in the ACP pretreatment groups
compared with the MGH group (P<0.05; Fig. 3C). These results suggested that ACP
could improve the survival of cardiomyocytes.
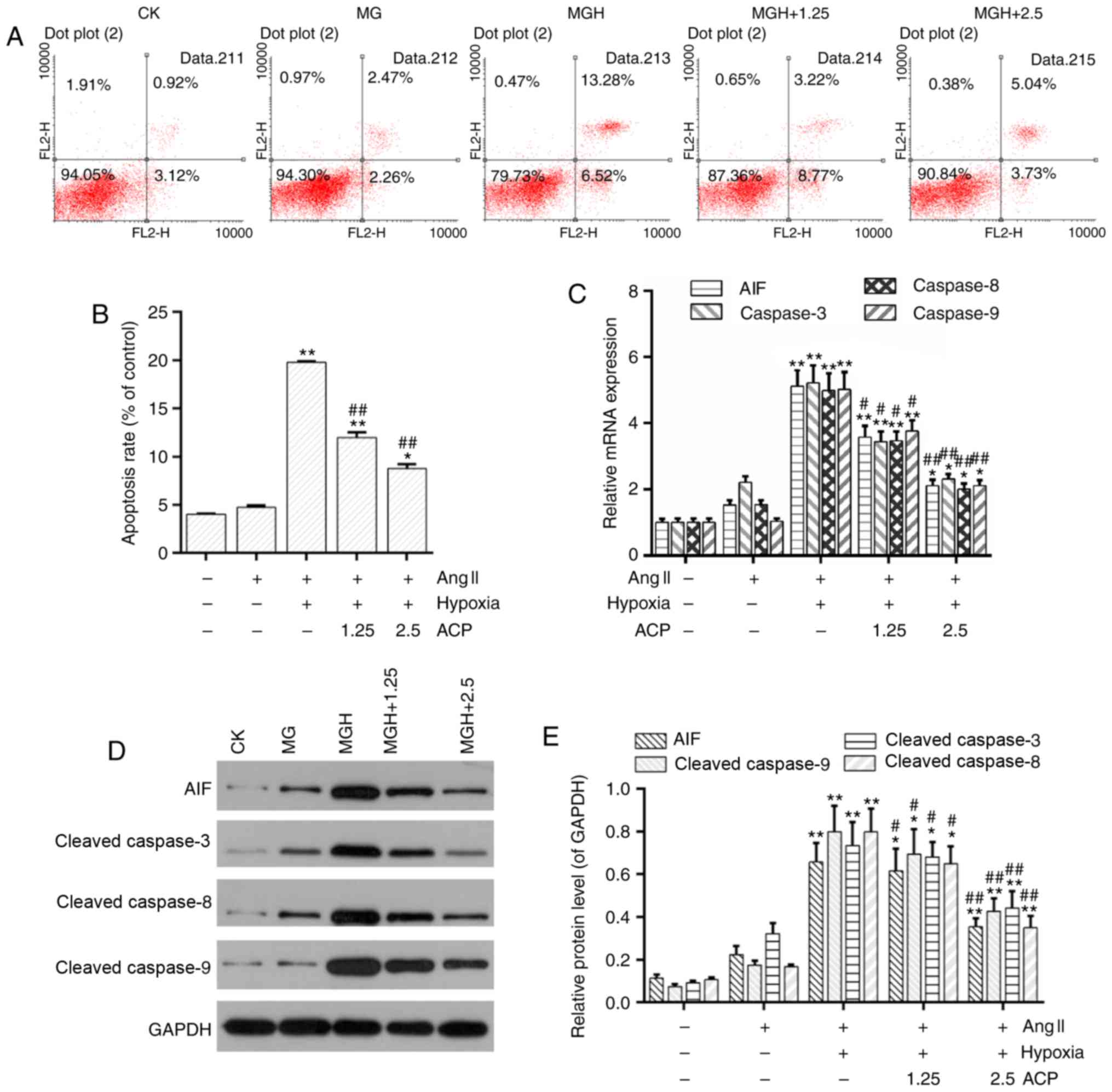
ACP mitigates apoptosis induced by
hypoxia in hypertrophic cardiomyocytes
Disruption of the MMP is recognized as an important
event during the early stage of apoptosis (34,35).
The flow cytometric analysis demonstrated that cell apoptosis was
low in the model group whereas it was significantly increased in
the MGH group in comparison (P<0.01; Fig. 4A and B). However, cell apoptosis
was significantly decreased in the ACP pretreatment groups compared
with the MGH group (P<0.01; Fig. 4A
and B). Furthermore, the mRNA expression of
apoptosis-associated genes including AIF and caspase-3/8/9 were
downregulated in the ACP pretreatment groups compared with the MGH
group (Fig. 4C). Western blot
analysis demonstrated the protein levels of AIF and cleaved
caspase-3/8/9 were decreased in the ACP pretreatment groups
compared with the MGH group (Fig. 4D
and E).
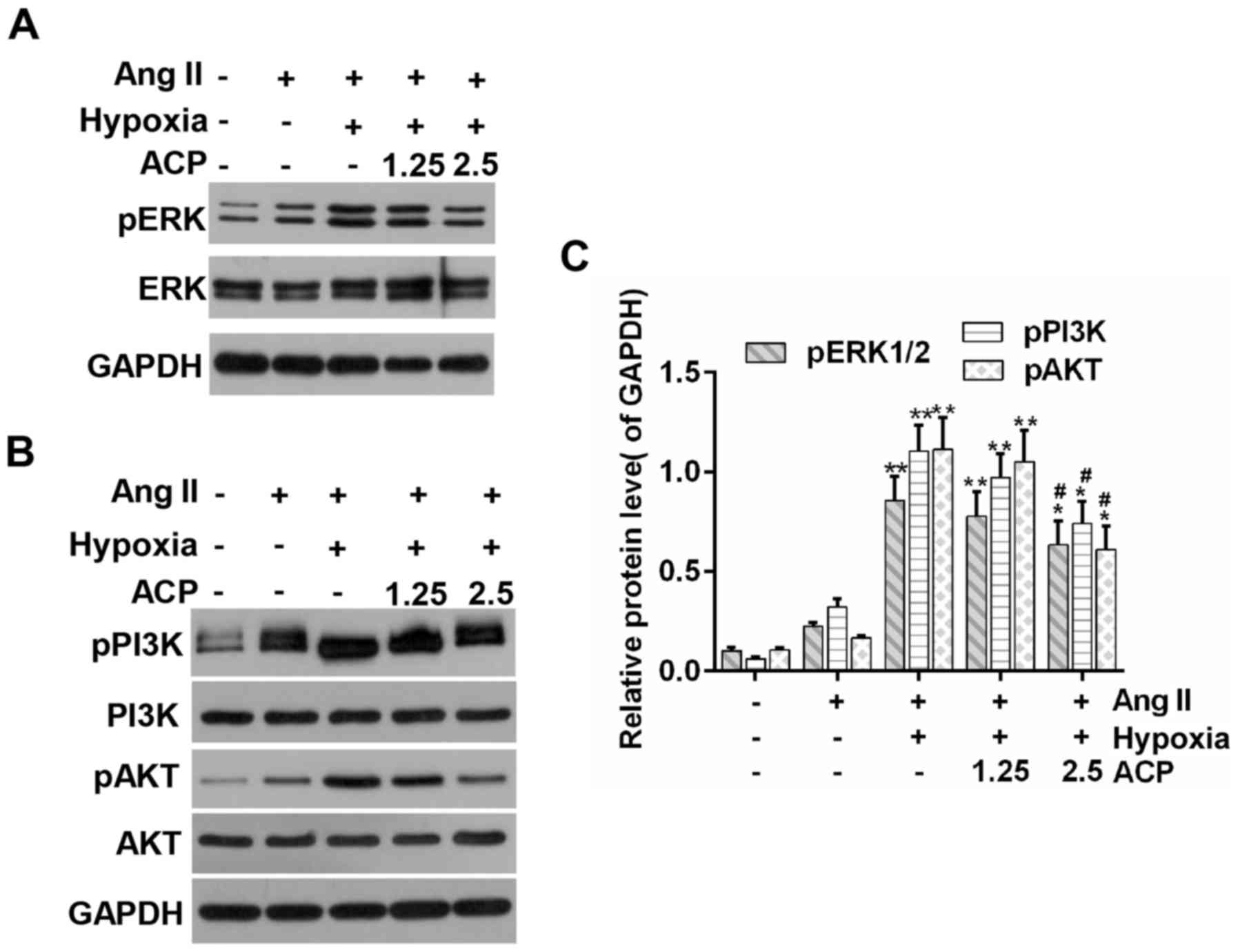
ACP inhibits the phosphorylation of
ERK1/2 and PI3K/AKT
It has been reported that the ERK1/2 and PI3K/AKT
signaling pathways contribute to the maintenance of myocardial
morphology and function (36).
Western blot analysis indicated that the phosphorylation levels of
ERK1/2 and PI3K/AKT were increased in the model group compared to
control group; while it was further increased in the MGH groups.
The expression of p-ERK1/2, p-PI3K and p-AKT were depressed in the
ACP pretreatment groups when compared with the MGH group (Fig. 5).
Specific activation of ERK1/2 and
PI3K/AKT reverses the inhibitory effect of ACP on apoptosis
Furthermore, specific activators were used to
determine the involvement of ERK1/2 and PI3K/AKT in the protective
role of ACP. Recombinant human EGF and IGF-I are activators for
ERK1/2 and PI3K/AKT, respectively. Results from flow cytometry
analysis demonstrated that the activation of ERK1/2 and PI3K/AKT
did not inhibit hypoxia-induced apoptosis even in the presence of
ACP (Fig. 6). It was suggested
that the protective effects of are ACP likely dependent on
inhibiting the activation of the ERK1/2 and PI3K/AKT signaling
pathways.
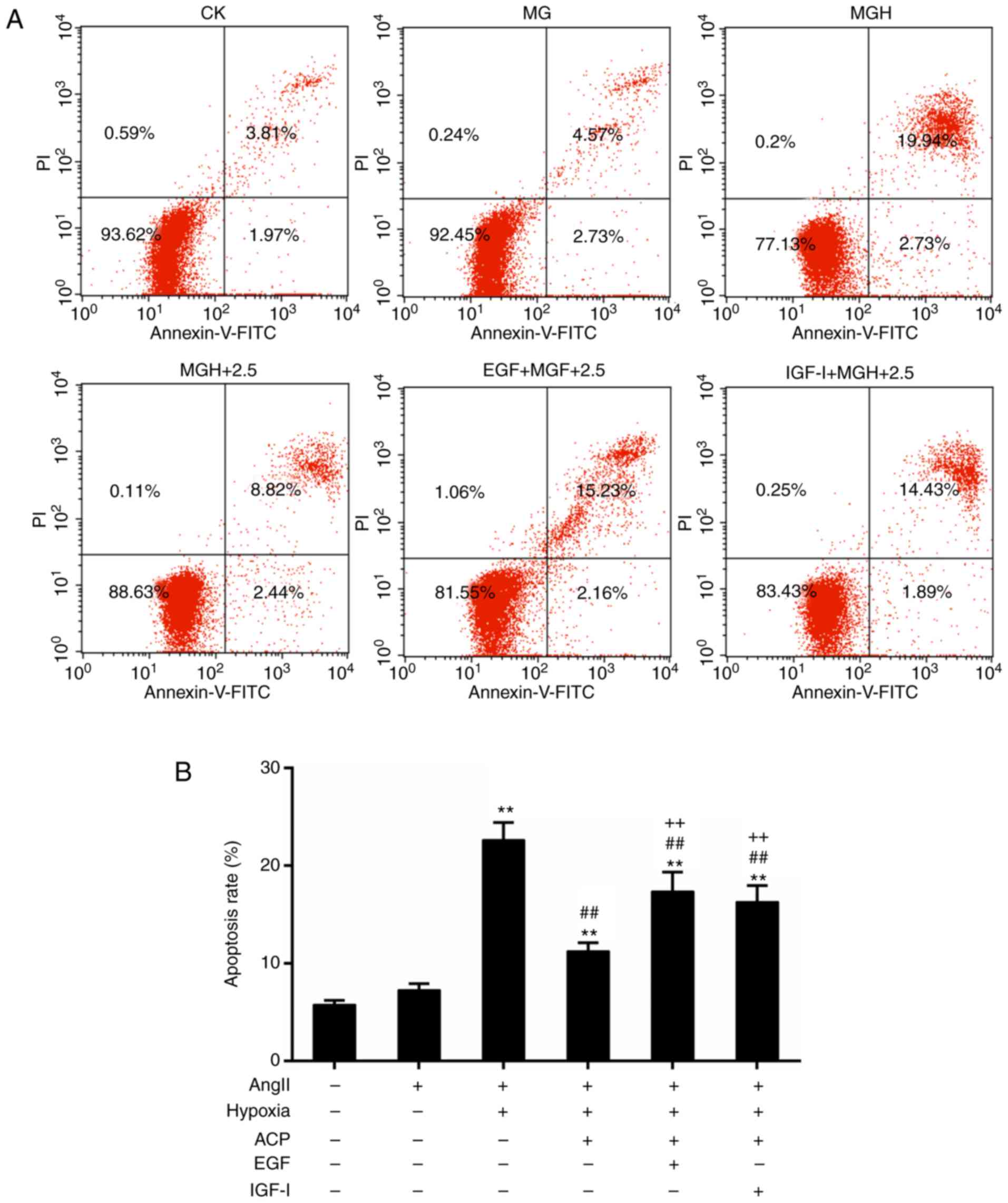 | Figure 6.Activation of ERK1/2 and PI3K/AKT
reversed the apoptotic effect of ACP. (A) Flow cytometric analysis
for the detection of apoptosis. (B) Quantitative analysis of the
rate of apoptosis. The ACP (2.5 mg/ml) pretreatment groups included
the following: (MGH+2.5), EGF (100 ng/ml) and ACP (2.5 mg/ml)
treatment group (EGF+ MGH+2.5), and the IGF-I (100 ng/ml) and ACP
(2.5 mg/ml) treatment group (IGF-I + MGH+2.5). *P<0.05 and
**P<0.01 vs. CK; ##P<0.01 vs. MGH;
++P<0.01 vs. MGH+ACP. ACP, Actinidia chinensis
planch polysaccharide; CK, control mock; MG, model group; MGH,
hypoxia treatment group; EGF, epidermal growth factor; IGF-I,
insulin-like growth factor 1; Ang II, angiotensin II; PI, propidium
iodide; FITC, fluorescein isothiocyanate. |
Discussion
IHD is a major threat to human health. Cardiac
hypertrophy is a common complication during the progression of IHD.
It is recognized that apoptosis serves an important role during the
progression from cardiac hypertrophy to heart failure (37,38).
It is hypothesized that cell apoptosis possesses a close connection
with the development of the heart diseases. As the center of
cellular energy and metabolism, the mitochondria serve a critical
role in the progression of cellular apoptosis (39). It has been reported that ACP
possesses multiple bioactivities (6,7). In
the present study, the potential effects of ACP on cardiomyocytes
of rats were investigated in vitro.
In the present study, Ang II was used to induce
cardiac hypertrophy. In addition, pretreatment with ACP reduced
cardiac hypertrophy. The collapse of the MMP leads to release of
pro-apoptotic molecules into the cytoplasm (40). Pretreatment with ACP rescued the
disruption of the MMP and improved the cell viability of
cardiomyocytes. It was indicated that the prevention of
mitochondrial dysfunction could protect against myocardial injury
(41). Furthermore, the rate of
apoptosis was decreased in the ACP pretreatment groups compared
with the MGH group. Several important molecules are involved in
apoptosis signals including AIF (42) and caspases-3/8/9 (43). The results of the present study
demonstrated that the expression of AIF and caspase-3/8/9 was
downregulated in the ACP pretreatment groups compared with those of
the MGH group at the transcriptional and translational levels.
These results suggested that ACP could protect against
cardiomyocyte apoptosis by regulating the apoptosis-associated
genes (AIF and caspase-3/8/9), which is in agreement with the
published anti-apoptotic effect of ACP in Neuro-2A cells (39). However, a previous study
demonstrated that ACP inhibited growth and induced apoptosis in
human gastric cancer cells (31).
These results may be attributed to several factors, including the
difference in the dosage of ACP, cell types and pathological
processes. A previous study demonstrated that the endoplasmic
reticulum stress-mediated pathway is involved in apoptosis
(44). However, the role of
endoplasmic reticulum-mediated apoptosis in the present study model
is not clear. Additionally, it is known that the ERK1/2 and
PI3K/AKT signaling pathways are involved in cardio-protection
(45,46). Notably, the results of the present
study demonstrated that pretreatment with ACP inhibited the
expression of p-ERK1/2 and p-PI3K/AKT. In addition, the activation
of ERK1/2 and PI3K/AKT reversed the inhibitory effect of ACP on
apoptosis. It is possible that ACP inhibited the phosphorylation of
ERK1/2 and PI3K/AKT thereby decreasing myocardial apoptosis induced
by hypoxia. However, it was reported that ERK1/2 and PI3K/AKT were
activated in multiple types of cancer, promoting cell survival and
inhibiting apoptosis (47–49). Nevertheless, previous studies have
demonstrated that instead of inhibiting cell death, the sustained
activation of ERK1/2 and PI3K/AKT rendered cells more sensitive to
metabolic stress (50–53). This implies dual roles for ERK1/2
and PI3K/AKT in tumor progression and the stress response (50). Currently, it has been reported that
several downstream targets were regulated by PI3K/AKT, including
Forkhead box protein O1, mammalian target of rapamycin and
endogenous nitric oxide synthase (54). However, the downstream targets of
ERK1/2 and PI3K/AKT are not clear in the present study.
Furthermore, the mechanisms of cardiac protection involve a complex
signaling cascade, and how ERK1/2 and PI3K/AKT signals crosstalk
with other signaling pathways requires further investigation.
In conclusion, the present study identified that ACP
decreased mitochondrial dysfunction and improved cell viability of
cardiomyocytes treated with Ang II. In addition, ACP decreased
hypoxia-induced apoptosis in cardiomyocytes treated with Ang II.
Furthermore, ACP inhibited the activation of ERK1/2 and PI3K/AKT
signaling pathways. It can be hypothesized that the protective
effects of ACP on hypoxia-induced apoptosis may depend on
inactivating the ERK1/2 and PI3K/AKT signaling pathways. The
results of the present study demonstrated the potential application
of ACP and provide important molecular evidence for the clinical
treatment of cardiac diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
QiaW wrote the main manuscript. YX and YG performed
the experiments. QiaW and QW designed the study. YX performed data
analysis. QiaW and QW contributed to manuscript revisions and all
authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morrone D, Marzilli M, Kolm P and
Weintraub WS: Do clinical trials in ischemic heart disease meet the
needs of those with ischemia? J Am Coll Cardiol. 65:1596–1598.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lozano R, Naghavi M, Foreman K, Lim S,
Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et
al: Global and regional mortality from 235 causes of death for 20
age groups in 1990 and 2010: A systematic analysis for the global
burden of disease study 2010. Lancet. 380:2095–2128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sag CM, Santos CX and Shah AM: Redox
regulation of cardiac hypertrophy. J Mol Cell Cardiol. 73:103–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fumarola C and Guidotti GG: Stress-induced
apoptosis: Toward a symmetry with receptor-mediated cell death.
Apoptosis. 9:77–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou XF, Zhang P, Pi HF, Zhang YH, Ruan
HL, Wang H and Wu JZ: Triterpenoids from the roots of actinidia
chinensis. Chem Biodivers. 6:1202–1207. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim S, Han SH, Kim J, Lee HJ, Lee JG and
Lee EJ: Inhibition of hardy kiwifruit (Actinidia aruguta) ripening
by 1-methylcyclopropene during cold storage and anticancer
properties of the fruit extract. Food Chem. 190:150–157. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Concha-Meyer AA, D'Ignoti V, Saez B, Diaz
RI and Torres CA: Effect of storage on the physico-chemical and
antioxidant properties of strawberry and kiwi leathers. J Food Sci.
81:C569–C577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lyon RC, Zanella F, Omens JH and Sheikh F:
Mechanotransduction in cardiac hypertrophy and failure. Circ Res.
116:1462–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maillet M, van Berlo JH and Molkentin JD:
Molecular basis of physiological heart growth: Fundamental concepts
and new players. Nat Rev Mol Cell Biol. 14:38–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Wang F, Zhang YM, Zhou JJ and Zhang
Y: Activation of cannabinoid type 2 receptor by JWH133 protects
heart against ischemia/reperfusion-induced apoptosis. Cell Physiol
Biochem. 31:693–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitrega KA, Nozynski J, Porc M, Spalek AM
and Krzeminski TF: Dihydropyridines' metabolites-induced early
apoptosis after myocardial infarction in rats; new outlook on
preclinical study with M-2 and M-3. Apoptosis. 21:195–208. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu Q, Yang C, Xiong W, Tahiri H, Payeur
M, Superstein R, Carret AS, Hamel P, Ellezam B, Martin B, et al:
SYK is a target of lymphocyte-derived microparticles in the
induction of apoptosis of human retinoblastoma cells. Apoptosis.
20:1613–1622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santos N, Silva RF, Pinto M, Silva EBD,
Tasat DR and Amaral A: Active caspase-3 expression levels as
bioindicator of individual radiosensitivity. An Acad Bras Cienc. 89
1 Suppl 0:S649–S659. 2017. View Article : Google Scholar
|
|
15
|
McDougal AD and Dewey CF Jr: Modeling
oxygen requirements in ischemic cardiomyocytes. J Biol Chem.
292:11760–11776. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Ding S, Xu G, Chen F and Ding F:
MicroRNA-15a inhibition protects against
hypoxia/reoxygenation-induced apoptosis of cardiomyocytes by
targeting mothers against decapentaplegic homolog 7. Mol Med Rep.
15:3699–3705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dorn GW II and Force T: Protein kinase
cascades in the regulation of cardiac hypertrophy. J Clin Invest.
115:527–537. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Sun G, Meng X, Wang H, Luo Y, Qin
M, Ma B, Wang M, Cai D, Guo P, et al: Isorhamnetin protects against
doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS One.
8:e645262013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chanda D, Luiken JJ and Glatz JF:
Signaling pathways involved in cardiac energy metabolism. FEBS
Lett. 590:2364–2374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daskalopoulos EP, Dufeys C, Bertrand L,
Beauloye C and Horman S: AMPK in cardiac fibrosis and repair:
Actions beyond metabolic regulation. J Mol Cell Cardiol.
91:188–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena A, Shinde AV, Haque Z, Wu YJ, Chen
W, Su Y and Frangogiannis NG: The role of Interleukin Receptor
Associated Kinase (IRAK)-M in regulation of myofibroblast phenotype
in vitro and in an experimental model of non-reperfused myocardial
infarction. J Mol Cell Cardiol. 89:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sepuri NBV, Angireddy R, Srinivasan S,
Guha M, Spear J, Lu B, Anandatheerthavarada HK, Suzuki CK and
Avadhani NG: Mitochondrial LON protease-dependent degradation of
cytochrome c oxidase subunits under hypoxia and myocardial
ischemia. Biochimica Biophys Acta. 1858:519–528. 2017. View Article : Google Scholar
|
|
23
|
Huang P, Xu X, Wang L, Zhu B, Wang X and
Xia J: The role of EGF-EGFR signalling pathway in hepatocellular
carcinoma inflammatory microenvironment. J Cell Mol Med.
18:218–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD,
Tang D, Sheu TJ, Li TF, Shi Q and Wang YJ: IGF-1 regulation of type
II collagen and MMP-13 expression in rat endplate chondrocytes via
distinct signaling pathways. Osteoarthritis Cartilage. 17:100–106.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai XY, Xia Y, Yang SH, Liu XZ, Shao ZW,
Liu YL, Yang W and Xiong LM: Ropivacaine- and bupivacaine-induced
death of rabbit annulus fibrosus cells in vitro: Involvement of the
mitochondrial apoptotic pathway. Osteoarthritis Cartilage.
23:1763–1775. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirchman D, K'Nees E and Hodson R: Leucine
incorporation and its potential as a measure of protein synthesis
by bacteria in natural aquatic systems. Appl Environ Microbiol.
49:599–607. 1985.PubMed/NCBI
|
|
27
|
Richards GP, Watson MA and Kingsley DH: A
SYBR green, real-time RT-PCR method to detect and quantitate
Norwalk virus in stools. J Virol Methods. 116:63–70. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du Z, Li S, Liu L, Yang Q, Zhang H and Gao
C: NADPH oxidase 3associated oxidative stress and caspase
3-dependent apoptosis in the cochleae of D-galactose induced aged
rats. Mol Med Rep. 12:7883–7890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song WY, Xu GH and Zhang GJ: Effect of
Actinidia chinensis planch polysaccharide on the growth and
apoptosis and p-p38 expression in human gastric cancer SGC-7901
cells. Zhongguo Zhong xi yi jie he za zhi. 34:329–333. 2014.(In
Chinese). PubMed/NCBI
|
|
32
|
Verma P, Singh A, Nthenge-Ngumbau DN,
Rajamma U, Sinha S, Mukhopadhyay K and Mohanakumar KP: Attention
deficit-hyperactivity disorder suffers from mitochondrial
dysfunction. BBA Clin. 6:153–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jamshidzadeh A, Heidari R, Abasvali M,
Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei
A, et al: Taurine treatment preserves brain and liver mitochondrial
function in a rat model of fulminant hepatic failure and
hyperammonemia. Biomedicine Pharmacother. 86:514–520. 2017.
View Article : Google Scholar
|
|
34
|
Yan X, Wang L, Yang X, Qiu Y, Tian X, Lv
Y, Tian F, Song G and Wang T: Fluoride induces apoptosis in H9c2
cardiomyocytes via the mitochondrial pathway. Chemosphere.
182:159–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang W, Liao Y, Zhang J, Huang Q, Luo W,
Yu J, Gong J, Zhou Y, Li X, Tang B, et al: Heat shock factor 1
inhibits the mitochondrial apoptosis pathway by regulating second
mitochondria-derived activator of caspase to promote pancreatic
tumorigenesis. J Exp Clin Cancer Res. 36:642017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jeong JJ, Ha YM, Jin YC, Lee EJ, Kim JS,
Kim HJ, Seo HG, Lee JH, Kang SS, Kim YS and Chang KC: Rutin from
Lonicera japonica inhibits myocardial
ischemia/reperfusion-induced apoptosis in vivo and protects H9c2
cells against hydrogen peroxide-mediated injury via ERK1/2 and
PI3K/Akt signals in vitro. Food Chem Toxicol. 47:1569–1576. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fazal L, Laudette M, Paula-Gomes S, Pons
S, Conte C, Tortosa F, Sicard P, Sainte-Marie Y, Bisserier M,
Lairez O, et al: Multifunctional mitochondrial epac1 controls
myocardial cell death. Circ Res. 120:645–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krech J, Tong G, Wowro S, Walker C,
Rosenthal LM, Berger F and Schmitt KRL: Moderate therapeutic
hypothermia induces multimodal protective effects in oxygen-glucose
deprivation/reperfusion injured cardiomyocytes. Mitochondrion.
35:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roberts ER and Thomas KJ: The role of
mitochondria in the development and progression of lung cancer.
Comput Struct Biotechnol J. 6:e2013030192013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gharanei M, Hussain A, Janneh O and
Maddock HL: Doxorubicin induced myocardial injury is exacerbated
following ischaemic stress via opening of the mitochondrial
permeability transition pore. Toxicol Appl Pharmacol. 268:149–156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahmed LA and El-Maraghy SA: Nicorandil
ameliorates mitochondrial dysfunction in doxorubicin-induced heart
failure in rats: Possible mechanism of cardioprotection. Biochem
Pharmacol. 86:1301–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grutter MG: Caspases: Key players in
programmed cell death. Curr Opi Struct Biol. 10:649–655. 2000.
View Article : Google Scholar
|
|
44
|
Nasu K, Nishida M, Kawano Y, Tsuno A, Abe
W, Yuge A, Takai N and Narahara H: Aberrant expression of
apoptosis-related molecules in endometriosis: A possible mechanism
underlying the pathogenesis of endometriosis. Reprod Sci.
18:206–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu Y, Li L, Yin W, Shen L, You B and Gao
H: Protective effect of proanthocyanidins on anoxia-reoxygenation
injury of myocardial cells mediated by the PI3K/Akt/GSK-3beta
pathway and mitochondrial ATP-sensitive potassium channel. Mol Med
Rep. 10:2051–2058. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Z, Zhang H, Xu X, Shi H, Yu X, Wang
X, Yan Y, Fu X, Hu H, Li X and Xiao J: bFGF inhibits ER stress
induced by ischemic oxidative injury via activation of the PI3K/Akt
and ERK1/2 pathways. Toxicol Lett. 212:137–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramalingam M, Kwon YD and Kim SJ: Insulin
as a potent stimulator of Akt, ERK and inhibin-βE signaling in
osteoblast-like UMR-106 cells. Biomol Ther (Seoul). 24:589–594.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pillai VB, Kanwal A, Fang YH, Sharp WW,
Samant S, Arbiser J and Gupta MP: Honokiol, an activator of
Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart
from doxorubicin-induced cardiomyopathy in mice. Oncotarget.
8:34082–34098. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Los M, Maddika S, Erb B and
Schulze-Osthoff K: Switching Akt: From survival signaling to deadly
response. Bioessays. 31:492–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Coloff JL, Mason EF, Altman BJ, Gerriets
VA, Liu T, Nichols AN, Zhao Y, Wofford JA, Jacobs SR, Ilkayeva O,
et al: Akt requires glucose metabolism to suppress puma expression
and prevent apoptosis of leukemic T cells. J Biol Chem.
286:5921–5933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM and
Thompson CB: Akt stimulates aerobic glycolysis in cancer cells.
Cancer Res. 64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nogueira V, Park Y, Chen CC, Xu PZ, Chen
ML, Tonic I, Unterman T and Hay N: Akt determines replicative
senescence and oxidative or oncogenic premature senescence and
sensitizes cells to oxidative apoptosis. Cancer cell. 14:458–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Matsui T and Rosenzweig A: Convergent
signal transduction pathways controlling cardiomyocyte survival and
function: The role of PI 3-kinase and Akt. J Mol Cell Cardiol.
38:63–71. 2005. View Article : Google Scholar : PubMed/NCBI
|