Introduction
Premature ovarian failure (POF) can result from
various factors, including iatrogenic chemotherapy and
radiotherapy, autoimmune diseases, genetic defects and idiopathy
(1). POF may lead to premature
failure of ovarian function, which is primarily characterized by
menopausal symptoms, including low estrogen levels and high
gonadotropin levels. POF causes infertility and may also lead to a
series of nervous system, cardiovascular and metabolic diseases,
including Alzheimer's disease, hypercholesterolemia and early-onset
osteoporosis, due to hormone deficiencies (2) that may severely affect women's
health. However, the pathogenesis of POF remains unclear.
Studies have reported that the occurrence of POF is
closely associated with depression, anxiety and other negative
emotions (3,4). Approximately 43% of patients with POF
in the USA report a history of depression, of whom 26% have
experienced depression (4) 5 years
prior to POF diagnosis. This clinical evidence demonstrates that
chronic psychological stress, including anxiety and depression, may
damage female ovarian function leading to disorders of female
reproductive endocrine function. Barra et al (5) demonstrated that chronic cold stress
may cause ovarian dysfunction or POF in female adult rats. This
phenomenon is characterized by a prolonged sexual cycle or a lack
of physiological regulation, and decreased secretion of estradiol
and progesterone. Chronic cold stress may cause ovarian dysfunction
in female adult rats in addition to reducing the number of
primordial, primary and secondary follicles in the ovary,
decreasing the expression of follicle-stimulating hormone (FSH)
receptor, and delaying sexual development in young rats (6). The above evidence suggests that
chronic stress may be a crucial factor in the pathogenesis of
POF.
Since the etiology of POF is diverse and complex,
the pathogenesis of this disease remains unclear. To explore the
mechanisms as to how POF may be caused by different disorders,
animal models are essential. At present, two animal models of POF
are available including, the chemotherapy (7) and 4-vinylcyclohexene diepoxide
(8) models. However, a POF model
induced by chronic stress has not been developed so far.
Chronic unpredictable mild stress (CUMS) is a widely
used method for establishing models of depression, as proposed by
Willner et al (9) in 1987,
and includes a variety of chronic mild stresses: Wet bedding,
behavior restriction, fasting, water deprivation, forced swimming,
ice water, noise, hanging upside down day and night, and tail and
foot shock. Compared with single factor model, the multifactor
complex chronic stress model exhibits the characteristics of
variability and an unpredictable stress source, which may induce a
psychological stress response. However, whether the CUMS model may
be used to assess POF remains unknown. In the present study, adult
female rats were treated with the CUMS method to analyze the
effects of chronic psychological stress on ovarian reserve, in
order to demonstrate the feasibility of establishing a POF model
via the CUMS method. This approach may provide a suitable animal
model for further assessment of the pathogenesis of chronic
stress-induced POF.
Materials and methods
Animals
A total of 60 specific pathogen-free healthy female
nulligravid Sprague-Dawley rats, (12 weeks old; 238±26 g), were
provided by the Zhejiang Provincial Animal Experimental Center
[animal certificate no. SXXK (Zhejiang) 2014-0001]. The housing
conditions were as follows: Room temperature 20–25°C, relative
humidity 45–60%, 12 h light-dark cycle, drinking distilled water
ad libitum throughout the experiment. The present study was
approved by the Medical Ethics Committee of Jinhua Polytechnic
(Jinhua, China).
Experimental grouping and POF model
establishment
After 7 days of adaptation to the above conditions,
the rats were randomly divided into three groups (n=20), including
the control, cyclophosphamide (CTX) and CUMS groups. The control
rats were grabbed once daily by gently catching the tail and skin
of the back, and fixed for 5 sec, without any specific treatment.
The CTX group received an intraperitoneal injection of CTX (Baxter
Oncology GmbH, Halle, Germany) at a dose of 50 mg/kg on the first
experimental day, followed by 8 mg/kg/day for 14 days and were fed
normally for 2 months (10). Rats
of the CUMS group were housed individually and exposed to a set of
CUMS procedures repeatedly as follows (9): Wet pad for 24h, behavioral
restriction for 2 h, fasting for 24 h, water deprivation for 24 h,
forced ice water swimming at 4°C for 5 min, reversal of day and
night for 24 h, noise interference for 12 h, tail suspension for 30
min, and plantar electric shock (30 V) for 5 sec. A stimulus was
randomly administered daily for ≤35 days.
Rat estrous cycle assessment. Following adaptive
feeding for approximately 7 days, the estrous cycles of the rats
were monitored continuously by vaginal smear for 10 days. Vaginal
cytology was performed at 8:30 a.m. First, the rats were held, and
cotton swabs were moistened with saline and inserted into the
vagina, gently rotating twice against the vaginal wall. The swab
was removed and rolled on the slide. The obtained smears were
air-dried and stained for 3–5 min with alkaline methylene blue (pH
8.4) at room temperature. Following washing, the smears were
air-dried and observed by optical microscopy (magnification, ×100),
to determine the estrous cycle stages, including the proestrus,
estrus, metestrus and diestrus. The criteria have been described
elsewhere (Table I) (11).
 | Table I.Basic classification of estrous cycle
stages based on cell types and their relative numbers in vaginal
smears. |
Table I.
Basic classification of estrous cycle
stages based on cell types and their relative numbers in vaginal
smears.
| Stage | Relative cell
density | Neutrophils | Small nucleated
epithelial cells | Large nucleated
epithelial cells | Anucleated
keratinized epithelial cells | Mucous |
|---|
| Proestrus | Low to moderate | 0 to + | ++ to +++ | 0 to + | 0 to + | 0 |
| Estrus | Moderate to high | 0 to + | 0 to ++ | 0 to ++ | ++ to +++ | 0 |
| Metestrus | Moderate to
high | + to +++ | + to ++ | + to ++ | + to +++ | 0 to + |
| Diestrus | Low to
moderate | ++ to +++ | + to ++ | + to ++ | 0 to + | ++ to ++ |
Open field test
Animal activity was observed prior to and following
modeling on days 7, 14, 21 and 28, respectively. The box used for
the open field test was 100×100×50 cm. The bottom of the box was
divided into 25 squares (20×20 cm each). Each rat was placed in the
central square for an individual test. Then the subsequent
spontaneous activity of each individual rat was recorded for 3 min
under dark and quiet conditions. The total distance traveled was
considered a measure of motor ability based on the frequency of the
rat crossing any of the 25 squares. The total duration of stay in
the central area was calculated and defined as a measure of
anxiety. The vertical frequency was used to assess the exploratory
ability of the rats based on the frequency of rearing (raising both
forelimbs >1 cm from the ground or clinging to the walls). Prior
to each test, the box was completely cleaned. The behavioral
assessment was conducted in a quiet room by two observers between
8:00 a.m. and 12:00 p.m. at 25°C under (10±1) kPa. The data from
the two observers were averaged as the final analysis (12).
Sucrose consumption test
The animals were trained to adapt to sugary drinking
prior to the experiment in a quiet room at 25°C under (10±1) kPa in
single cages (54.5×39.5×20 cm). Each cage had two bottles of 1%
sucrose water placed simultaneously during the first 24 h, followed
by one bottle each of 1% sucrose water and pure water in the
subsequent 24 h. After 23 h of fasting, the animals underwent the
sucrose consumption test. Each rat was given two bottles of water
randomly placed at the same time: one contained 1% sucrose water
and the other having pure water. The two bottles were removed and
weighed after 1 h. Finally, sugar consumption was measured, as well
as sucrose preference (sucrose preference=sugar consumption/total
liquid consumption ×100) was calculated (12).
Ovarian morphology
Bilateral ovaries of the rats were removed and fixed
with 10% formaldehyde at 4°C for 24 h, dehydrated by an ethanol
gradient (70% for 8 h, 80% for 2 h, 90% for 1 h, 95% for 30 min and
twice with 100% for 15 min) at room temperature, paraffin embedded,
and sectioned in ~6 µm slices. The ovarian tissue sections were
stained with hematoxylin-eosin (hematoxylin for 5 min and eosin for
2 min) at room temperature. The stained ovarian tissue specimens
were observed by light microscopy (magnification, ×100) to assess
the structural alterations and enumerate follicles in all groups.
The follicular grading criteria by Yoshida et al (13) are described as follows: Small
follicles, including primordial and primary follicles, are those
with oocyte diameter <20 µm and <20 follicle cells; medium
follicles oocyte diameter in the range of 20–70 µm and a follicle
cell number of 21–200; large follicles, oocyte diameter >70 µm
and >201 follicle cells.
Serum levels of gonadotropin releasing
hormone (GnRH), FSH, estradiol (E2), and anti-Mullerian hormone
(AMH)
Blood samples (2–3 ml) were drawn from the posterior
venous plexus of rats, and placed at room temperature. Following
blood coagulation, serum was obtained by centrifugation (600 × g at
room temperature for 10 min). The levels of GnRH, FSH, E2 and AMH
in serum were detected using ELISA kits (CusaBio Biotech Co., Ltd.,
Wuhan, China; E2, cat. no. CSB-E05110r; AMH, cat. no. CSB-E11162r;
FSH, cat. no. CSB-E06869r; GnRH, cat. no. CSB-E08037r), according
to the manufacturer's instructions. Standard curves were plotted
using the Curve Expert 1.3 software (Hyams Development, Huntsville,
Alabama). The regression equation was fitted by the concentration
values of standard substance and its corresponding optical density
values (OD value). The OD value of each well was sequentially
measured with a microplate reader BIO-RAD Model 680, Bio-Rad
Laboratories Inc, Hercules, CA, USA) at a wavelength of 450 nm.
Analysis was performed within 10 min following termination of the
reaction. Then the optical density values of the detected sample
were substituted into the equation, then the sample concentration
was calculated.
Statistical methods
The data were assessed using SPSS 20.0 statistical
software (IMB Corp., Armonk, NY, USA). Measurement data are
expressed as the mean ± standard deviation. Single factor analysis
of variance was applied for multi-group comparisons, with post hoc
tests (Dunnett's tests) used to compare group pairs. Enumeration
data were analyzed by the nonparametric Kruskal-Wallis test, using
a nonparametric post hoc test (LSD test). P<0.05 was considered
statistically significant.
Results
Treatment with CUMS reduces the body
weight of rats
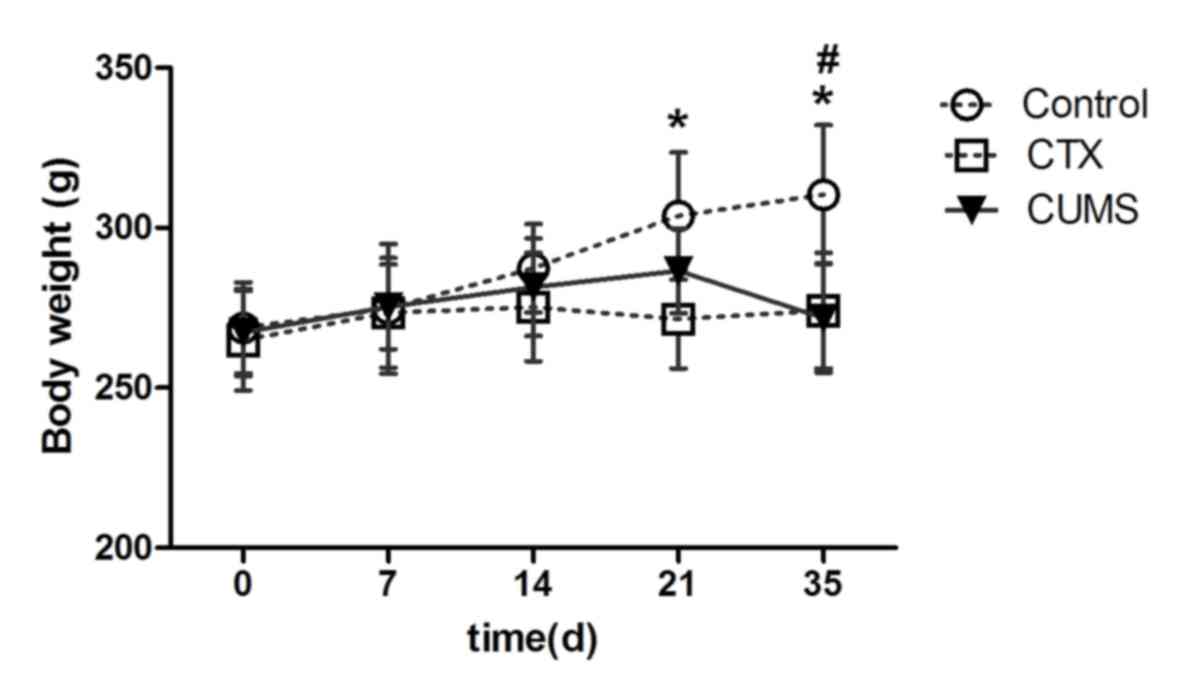
Body weights were recorded to assess the effects of
CUMS on rats. From day 21, the weight of rats of the control group
were significantly higher than those of the CTX group (P<0.05).
At day 35, the weight of the rats in the CUMS and CTX groups were
significantly reduced compared with in the control group
(P<0.05). However, at other time points, the CUMS group showed
no statistically significant difference (Fig. 1). This finding suggested that
similar to CTX, treatment with CUMS resulted in significant
reductions in body weight of rats at day 35.
Treatment with CUMS induces chronic
psychological stress in rats
In the present study, two behavioral evaluation
assays were used, including the open field test and sugar syrup
preference tests, to assess whether treatment with CUMS induces
chronic psychological stress in rats (Fig. 2). On day 14 (Fig. 2B) following model establishment,
vertical movement was significantly decreased in the CUMS group
compared with the control group (P<0.05). On day 35 after
following model establishment, vertical movement was significantly
decreased in the CUMS group compared with in the control group
(P<0.01). Horizontal movement (Fig.
2A) and central time (Fig. 2C)
in the CUMS group were significantly decreased compared with the
control (P<0.05) on day 35. Consistently, sugar consumption
(Fig. 2D) and sugar preference
(Fig. 2E) were significantly
decreased in the CUMS and CTX groups compared with control rats
(P<0.05), again only on day 35. In addition, the CTX and CUMS
groups did not exhibit statistically significant differences at any
time point (P>0.05) for all factors (Fig. 2). The open field and sucrose
preference tests demonstrated that the CUMS group exhibited
depressive-like behaviors at 35 days post-treatment with CUMS.
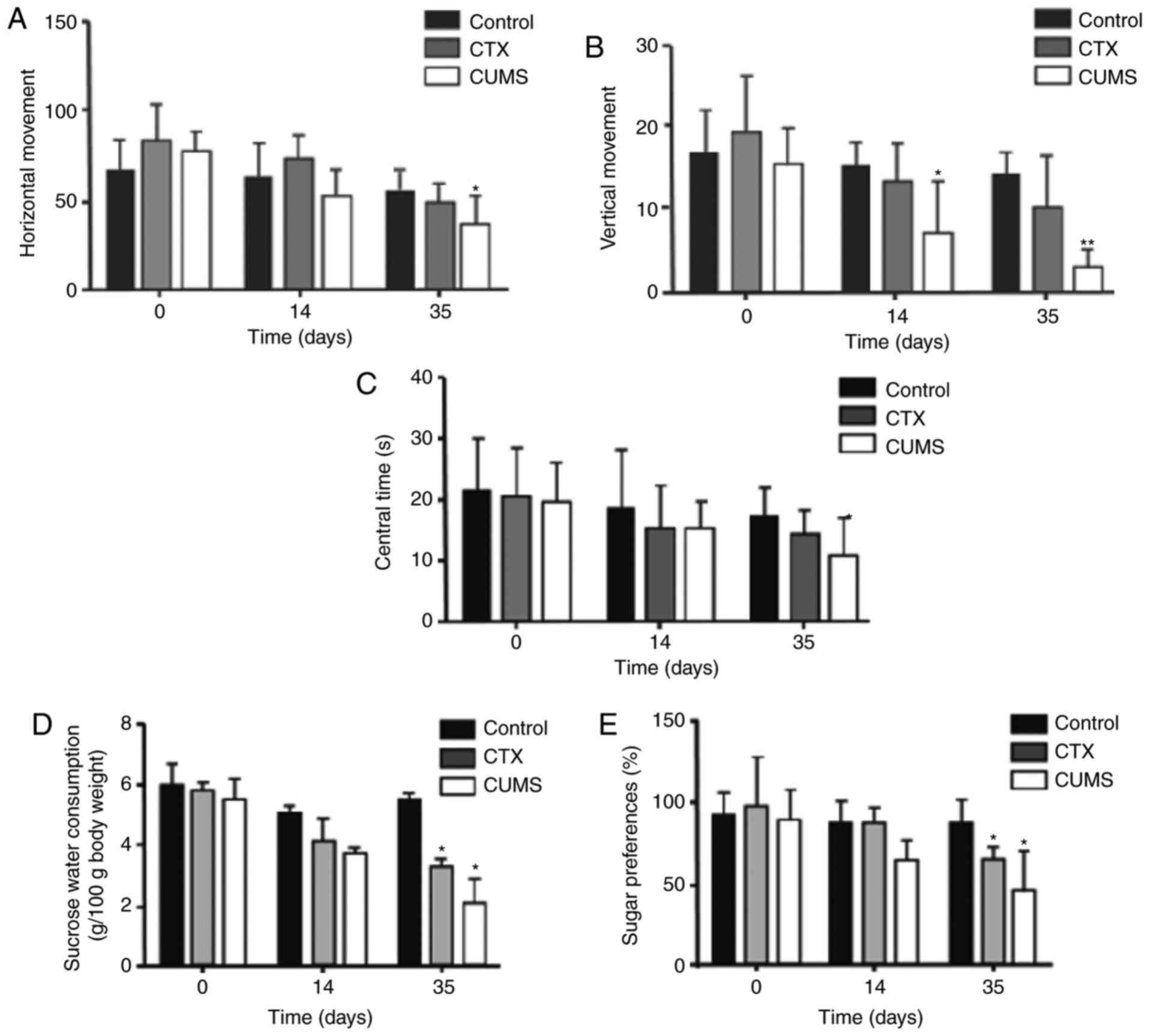 | Figure 2.Treatment with CUMS induces chronic
mental stress in rats. (A) Horizontal movement in the open field
test in rats after 0, 14, and 35 days of treatment with CUMS. (B)
Vertical movement in the open field test in rats after 0, 14, and
35 days of treatment with CUMS. (C) Time spent in the center in the
open field test in rats after 0, 14, and 35 days of treatment with
CUMS. (D) Sucrose water consumption of rats after 0, 14, and 35
days of treatment with CUMS. (E) Sucrose preference in rats after
0, 14, and 35 days of treatment with CUMS. Data are mean ± standard
deviation (n=20). *P<0.05 and **P<0.01 vs. control group.
CTX, cyclophosphamide; CUMS, chronic unpredictable mild stress. |
Treatment with CUMS prolongs the
estrous cycle in rats
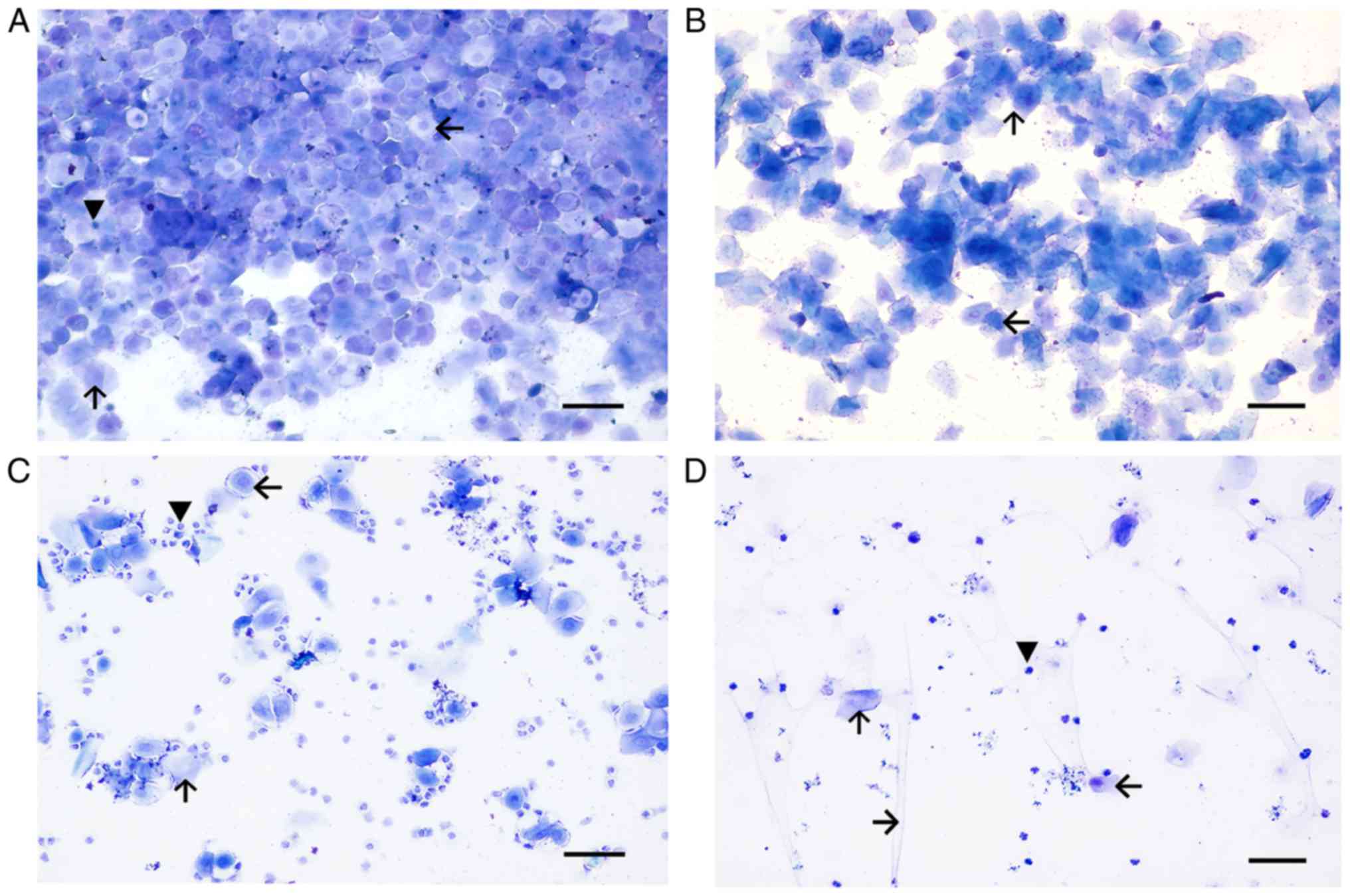
Prior to establishment, all three groups presented a
regular estrous cycle (Fig. 3).
The duration of the normal estrous cycle was 4–5 days, constituting
the proestrus (Fig. 3A), estrus
(Fig. 3B), metestrus (Fig. 3C) and diestrus (Fig. 3D) of 1, 0.5, 1, 1.5–2 days,
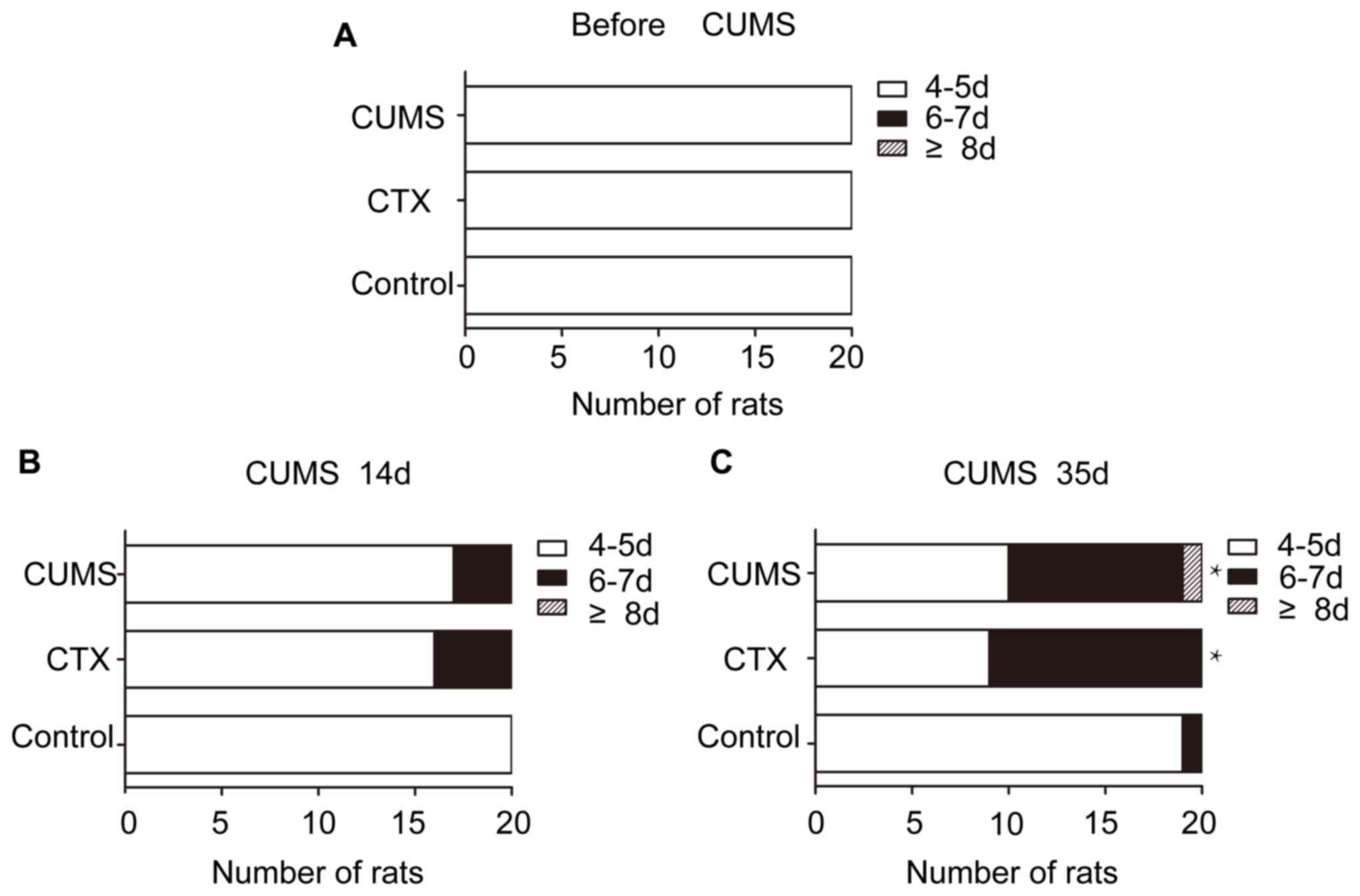
respectively. At the end of modeling, 12 rats in the CTX group and
9 rat in the CUMS group exhibited an estrous cycle of 6–7 days.
Abnormal estrous cycles in the CTX (P<0.05) and CUMS groups
(P<0.05) were significantly different compared with control
after 35 days of treatment with CUMS (Fig. 4). The estrous cycle was primarily
manifested as continuous diestrus. The CTX and CUMS groups appeared
to show estrous cycle extension; however, no rat exhibited
disappearance of the estrous cycle.
Treatment with CUMS results in
morphological alterations and decreased follicular counts in the
ovaries of rats
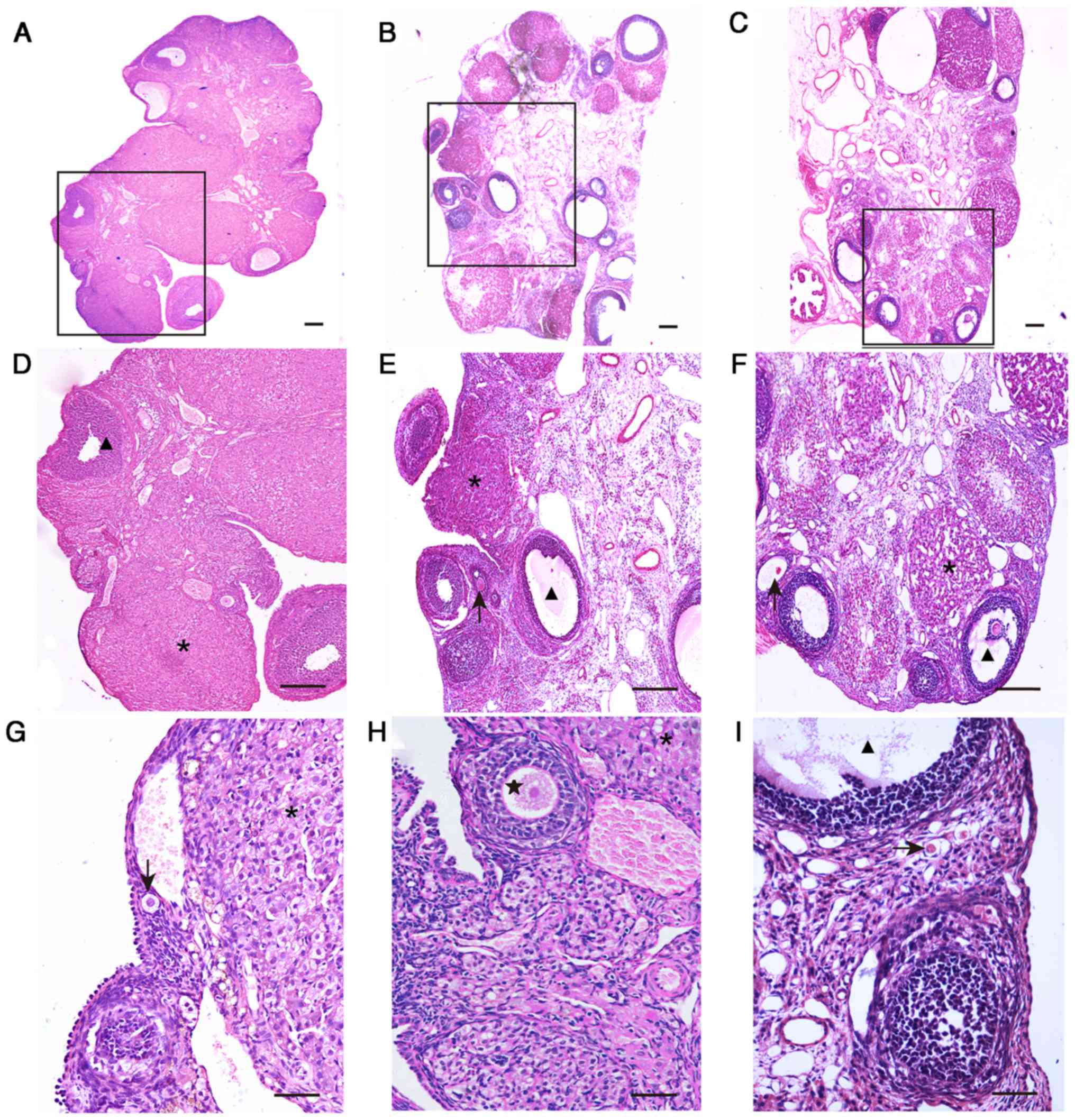
On day 35 following model establishment, ovaries of
the CTX and CUMS group rats exhibited notable atrophy compared with
the control group. The primary histological alterations were as
follows. First, analysis of the ovarian stroma revealed severe
fibrosis, cortical thickening and a disorganized structure.
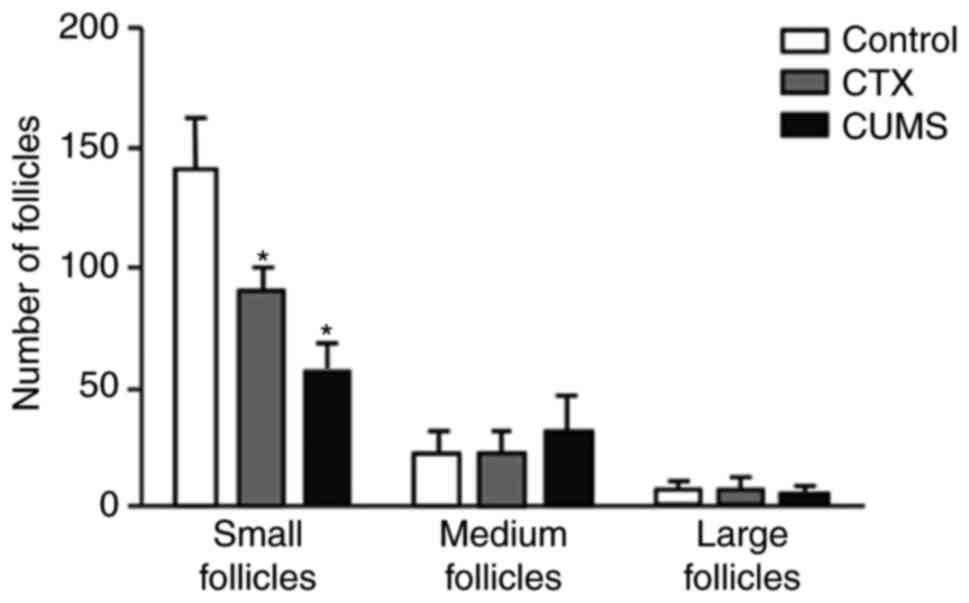
Secondly, the number of follicles at each stage of ovarian
development were reduced, while atretic follicles were markedly
increased. In addition, the corpus luteum exhibited fibrosis and
increased in number (Fig. 5). The
number of small follicles were obtained in serial sections, and was
observed to be 85.50±37.57 and 58.90±26.25 in the CTX and CUMS
groups, respectively. These values were significantly decreased
compared with the control amounts of 137.13±48.55 (P<0.05).
However, no significant differences were observed in the amounts
medium and large follicles in the CTX and CUMS groups compared with
their respective control groups (Fig.
6). Morphological assessment of ovaries in the CTX and CUMS
groups demonstrated abnormal alterations, with the number of small
follicles decreasing significantly.
Treatment with CUMS reduces the levels
of serum E2, AMH and GnRH, and elevates FSH levels in rats
Prior to modeling, no significant differences were
observed in E2, AMH, GnRH, and FSH levels among the three groups.
On day 14, serum E2, AMH, and GnRH levels in the CTX and CUMS
groups were decreased compared with control values; with the
difference of AMH being statistically significant (P<0.05).
However, FSH levels were higher in the CTX and CUMS groups compared
with control group, without significant differences. In addition,
serum E2, AMH, and in the GnRH levels were significantly decreased
on day 35 in the CTX and CUMS groups compared with in the control
group (P<0.05), while FSH levels in the CTX (P<0.05) and CUMS
(P<0.01) groups were significantly higher than the control
group. Furthermore, E2, AMH, and GnRH levels on day 35 in the CTX
and CUMS groups were lower than those obtained before modeling
(P<0.05), while FSH levels were significantly higher compared
with in the respective control groups (CTX, P<0.05; CUMS,
P<0.01) (Fig. 7). These
findings indicated that the serum levels of the serum hormones E2,
AMH, GnRH and FSH were altered in the CTX and CUMS groups.
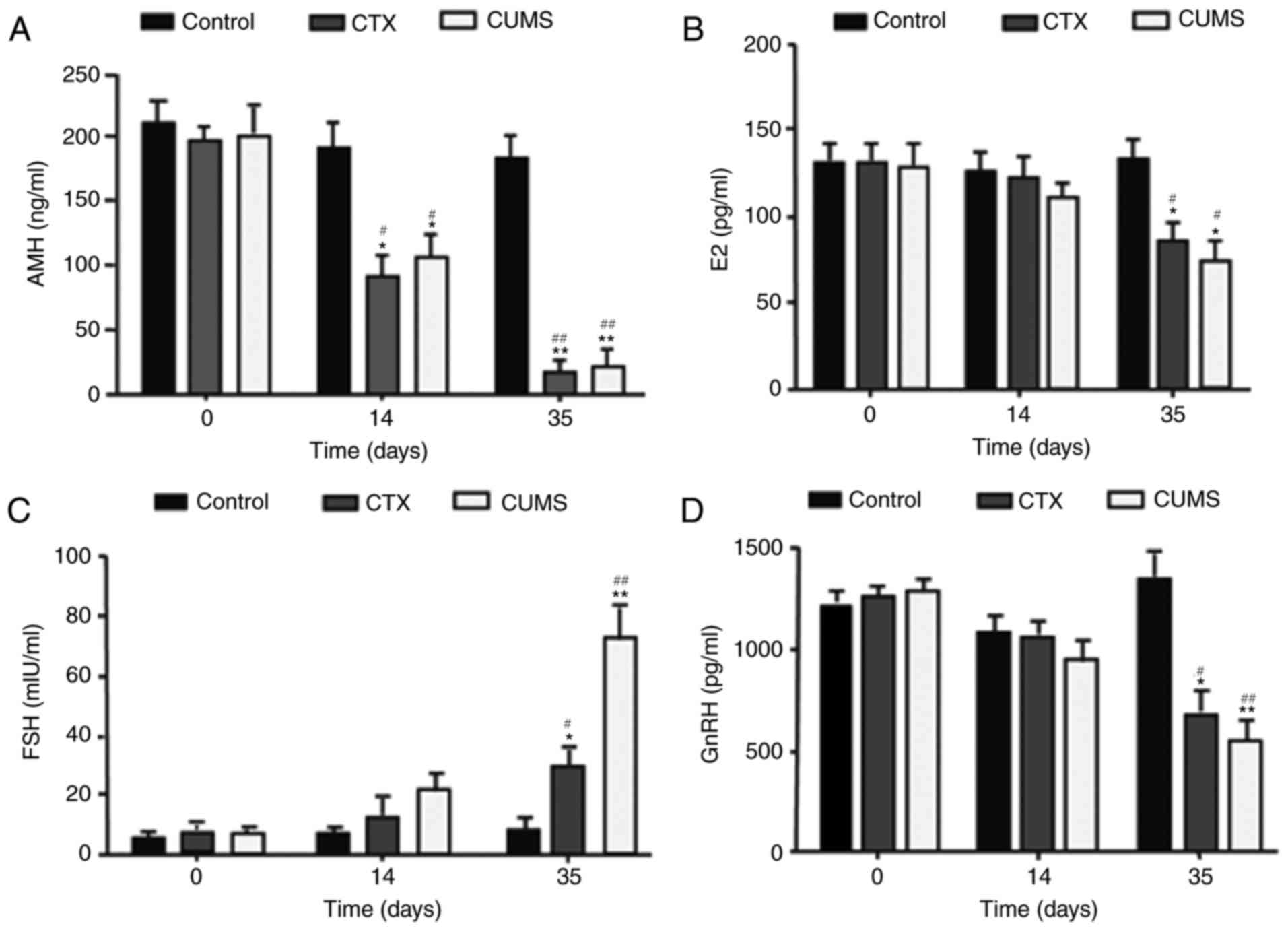 | Figure 7.Effects of CUMS on serum levels of
AMH, E2, GnRH, and FSH in rats. (A) AMH, (B) E2, (C) FSH, (D) GnRH.
Data are mean ± standard deviation (SD) (n=20). *P<0.05,
**P<0.01 vs. control group of corresponding day.
#P<0.05, ##P<0.01 vs. 0 day. CTX,
cyclophosphamide; CUMS, chronic unpredictable mild stress; AMH,
anti-Mullerian hormone; E2, estradiol; FSH, follicle-stimulating
hormone; GnRH, gonadotropin releasing hormone. |
Discussion
Establishing an animal model is essential for
assessing the association of chronic stress with POF. The present
study examined the feasibility of the CUMS method in developing a
rat model of POF. CUMS has been used to establish a model of
depression, and may cause chronic psychological stress in rats,
therefore, it was used in the present study (9). CUMS may cause behavioral effects,
including anxiety and depression-like behaviors in rats. In
addition, chronic psychological stress may alter reproductive
endocrine function in rats (5,6),
which ultimately leads to ovarian dysfunction and premature ovarian
failure (14). The present study
performed open field and sugar consumption tests to evaluate
anxiety and depression-like behaviors in rats, in order to
determine whether the animals have developed psychological stress.
As aforementioned, body weight, time spent in the center,
horizontal movement and vertical frequency were all significantly
decreased. In addition, sucrose consumption and preference were
significantly reduced. In addition, the estrous cycle was prolonged
significantly. Furthermore, serum E2, AMH and GnRH levels decreased
significantly, while FSH levels were significantly increased.
Furthermore, significantly fewer small follicles were detected in
the CUMS group compared with the control following model
establishment.
In the open field test, horizontal movement,
vertical movement and central residence time were significantly
decreased in CUMS-treated rats compared with the control group,
supporting the findings of Strekalova et al (15), which demonstrated that chronic
stress may cause horizontal movement in model animals. The rats
that experienced chronic stress were predisposed to avoid the
central grid. Reduction in central residence in the open box was a
manifestation of fear of danger, attributed to autonomic nervous
system dysfunction, or may reflect a lack of curiosity about the
environment (16). Vertical
movement reflects the inquiring behavior of rats and confidence in
an unfamiliar environment (17).
The aforementioned results revealed a decreased interest in the
unfamiliar environment in rats under chronic stress. The sugar
preference test may be a crucial method for the detection of
anhedonia (18,19), which is a core symptom of clinical
depression. The sugar preference test revealed that sugary water
consumption and sucrose preference were decreased significantly in
the CUMS group compared with the control group, indicating
anhedonia in these animals. Behavioral evidence uncovered by tests
conducted in the present study demonstrated that the rats
demonstrated a degree of depressive symptoms following modeling.
These findings suggested that the CUMS program may induce chronic
psychological stress in rats.
The animals experiencing chronic stress exhibit
ovarian dysfunction in addition to behavioral alterations. Vaginal
cytology is an essentially noninvasive procedure for evaluating the
estrous cycle in laboratory rodents and may serve as a useful
measure of the integrity of the hypothalamic-pituitary-ovarian
reproductive axis (20). The
aforementioned experimental results revealed that the estrous cycle
of rats of the CTX and CUMS groups was prolonged significantly,
particularly due to prolonged diestrus. This finding suggested that
treatment with CUMS, similar to CTX, may cause dysfunction of the
hypothalamic-pituitary-ovarian axis in rats. In addition to the
estrous cycle, serum hormones, including E2 and FSH, are essential
for the evaluation of POF models. POF is biochemically
characterized by low levels of gonadal hormones (estrogens) and
high levels of gonadotropins (FSH) (21). AMH is a crucial index in evaluating
the ovarian reserve (22). The
present study revealed that the serum levels of E2 and AMH were
decreased significantly, while FSH amounts were significantly
increased in CUMS-treated rats, similar to those of the CTX group.
These findings were in accordance with those of Lu et al
(23), who reported E2 and AMH
levels in CUMS rats with stress resulted in disordered estrous
cycles. Thus, these characteristics were consistent with those
reported in POF models induced by chemotherapeutics (24,25).
A marked decrease in AMH hormone suggested a declined ovarian
reserve. Taken together, the alterations in hormonal levels and
prolonged estrous cycles demonstrated that CUMS-treated rats
exhibited ovarian dysfunction similar to CTX-treated rats. Thus,
treatment with CUMS may affect the hypothalamus-pituitary-gonadal
axis, resulting in ovary dysfunction.
Morphological assessment demonstrated that, compared
with control rats, ovarian tissues of the CTX and CUMS groups
exhibited interstitial fibrosis, cortex thickening and disorganized
tissue structure; alterations in the CTX group were more pronounced
compared with those of the CUMS group. These fibrotic alterations
were consistent with previous findings evaluating ovarian tissues
in patients with POF (26).
Additionally, significantly fewer follicles, particularly primitive
follicles, were observed following model establishment. The
aforementioned morphological alterations were consistent with those
of ovarian tissue samples in the chemotherapeutic CTX model
(27). Furthermore, the
morphological evidence suggested that chronic stress may modify
ovarian structure, similar to treatment with CTX.
An ideal animal model should have the following
characteristics: First, pathogenic pathways and processes similar
to those observed in humans; secondly, pathological alterations in
the model may be reversed by drugs; and thirdly, reproducibility of
results. The present model has the following advantages, according
to the aforementioned criteria. The modeling method is consistent
with known primary pathogenic factors of human POF, with the
pathogenic pathway and pathological alterations being similar to
clinical observations. In addition, the modeling method of CUMS is
simple and feasible; it may induce chronic psychological stress and
cause depression or anxiety in rats. Finally, it may induce a
number of POF features in experimental animals, including
alterations in serum hormone levels and ovarian morphology, and
prolongation estrous cycle. However, the model has a limitation in
that the estrous cycle the experimental animals did not
disappear.
Due to different animal strain, various modeling
methods and evaluation indexes have been adopted by researchers;
thus, a POF animal model has not been established as a unified
evaluation standard. The majority of these models have been tested
for serological hormones, particularly E2 and FSH. However, these
hormones do not constitute a precise standard for assessment,
unlike the diagnostic criteria for human POF. A simple and
efficient estrous cycle, as an evaluation index of the rodent
reproductive function, may be combined with serum E2, FSH and AMH
levels to construct an ideal and accurate evaluation system for the
POF animal model. The estrous cycle of most experimental animals
did not disappear in the present study. The CUMS model was able to
cause psychological stress and induce ovarian dysfunction in rats.
However, whether the CUMS model induces POF efficiently in
experimental animals requires further investigation.
The aforementioned CUMS POF animal model revealed
that CUMS may lead to ovarian dysfunction. The results of the
present study suggested that the CUMS model may provide possible
directions and research approaches for ovarian dysfunction and POF,
caused by psychological stress. Further studies are required to
evaluate the interaction between psychological stress and ovarian
function in order to define the pathogenesis of psychological
stress-associated human ovarian dysfunction and POF.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Experimental Animal Science and Technology Project of Zhejiang
Province, China (grant no. 2015C37104).
Availability of data and materials
Not applicable.
Authors' contributions
XYF and HHC made substantial contributions to the
concept and design of the present study. YEQ, YSF conducted animal
feeding and model establishment. XYF, HHC, NZ conducted open field
test, sucrose consumption test and ELISA. XMP conducted
morphological evaluation of ovarian tissue samples. YPL and QZ
conducted ELISA. WQW and MXD performed statistical analysis; XYF
drafted the manuscript. XYF, HHC and MXD critically revised the
manuscript for important intellectual content. All authors gave
final approval, and agreed to be accountable for all aspects of
work in ensuring that questions regarding the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Jinhua Polytechnic (Jinhua, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMH
|
anti-Mullerian hormone
|
|
CTX
|
cyclophosphamide
|
|
CUMS
|
chronic unpredictable mild stress
|
|
E2
|
estradiol
|
|
FSH
|
follicle-stimulating hormone
|
|
GnRH
|
gonadotropin releasing hormone
|
|
POF
|
premature ovarian failure
|
References
|
1
|
Shelling AN: Premature ovarian failure.
Reproduction. 140:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shuster LT, Rhodes DJ, Gostout BS,
Grossardt BR and Rocca WA: Premature menopause or early menopause:
long-term health consequences. Maturitas. 65:161–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tseng LA, El Khoudary SR, Young EA, Farhat
GN, Sowers M, Sutton-Tyrrell K and Newman AB: The association of
menopause status with physical function: The Study of Women's
Health Across the Nation. Menopause. 19:1186–1192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allshouse AA, Semple AL and Santoro NF:
Evidence for prolonged and unique amenorrhea-related symptoms in
women with premature ovarian failure/primary ovarian insufficiency.
Menopause. 22:166–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barra R, Cruz G, Mayerhofer A, Paredes A
and Lara HE: Maternal sympathetic stress impairs follicular
development and puberty of the offspring. Reproduction.
148:137–145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dorfman M, Arancibia S, Fiedler JL and
Lara HE: Chronic intermittent cold stress activates ovarian
sympathetic nerves and modifies ovarian follicular development in
the rat. Biol Reprod. 68:2038–2043. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu TE, Wang S, Zhang L, Guo L, Yu Z, Chen
C and Zheng J: Growth hormone treatment of premature ovarian
failure in a mouse model via stimulation of the Notch-1 signaling
pathway. Exp Ther Med. 12:215–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Fan S, Han D, Xie J, Kuang H and Ge
P: Microarray gene expression profiling and bioinformatics analysis
of premature ovarian failure in a rat model. Exp Mol Pathol.
97:535–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willner P, Towell A, Sampson D,
Sophokleous S and Muscat R: Reduction of sucrose preference by
chronic unpredictable mild stress, and its restoration by a
tricyclic antidepressant. Psychopharmacology (Berl). 93:358–364.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun M, Wang S, Li Y, Yu L, Gu F, Wang C
and Yao Y: Adipose-derived stem cells improved mouse ovary function
after chemotherapy-induced ovary failure. Stem Cell Res Ther.
4:802013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cora MC, Kooistra L and Travlos G: Vaginal
cytology of the laboratory rat and mouse: Review and criteria for
the staging of the estrous cycle using stained vaginal smears.
Toxicol Pathol. 43:776–793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen HH, Zhang N, Li WY, Fang MR, Zhang H,
Fang YS, Ding MX and Fu XY: Overexpression of brain-derived
neurotrophic factor in the hippocampus protects against post-stroke
depression. Neural Regen Res. 10:1427–1432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshida M, Sanbuissyo A, Hisada S,
Takahashi M, Ohno Y and Nishikawa A: Morphological characterization
of the ovary under normal cycling in rats and its viewpoints of
ovarian toxicity detection. J Toxicol Sci. 34 Suppl 1:SP189–SP197.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Omu FE, Biaa AAME, Ghafour AA, Gadalla IT
and Omu AE: Emotional impacts of premature ovarian failure in
Kuwait. Health. 8:262–278. 2016. View Article : Google Scholar
|
|
15
|
Strekalova T, Evans M, Chernopiatko A,
Couch Y, Costa-Nunes J, Cespuglio R, Chesson L, Vignisse J,
Steinbusch HW, Anthony DC, et al: Deuterium content of water
increases depression susceptibility: the potential role of a
serotonin-related mechanism. Behav Brain Res. 277:237–244. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prut L and Belzung C: The open field as a
paradigm to measure the effects of drugs on anxiety-like behaviors:
A review. Eur J Pharmacol. 463:3–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haloui M, Tahraoui A, Bououza F, Bairi A,
Boukhris N, Boulaakoud M and Ouakid M: Effects of chronic restraint
stress on energetic metabolism and the evolution of depression,
evaluated in the Open Field test in female wistar rat. Ann BiolRes.
5:1–7. 2014.
|
|
18
|
Baker SL, Kentner AC, Konkle AT,
Barbagallo Santa-Maria L and Bielajew C: Behavioral and
physiological effects of chronic mild stress in female rats.
Physiol Behav. 87:314–322. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schweizer MC, Henniger MS and Sillaber I:
Chronic mild stress (CMS) in mice: of anhedonia, ‘anomalous
anxiolysis’ and activity. PLoS One. 4:e43262009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldman JM, Murr AS and Cooper RL: The
rodent estrous cycle: characterization of vaginal cytology and its
utility in toxicological studies. Birth Defects Res B Dev Reprod
Toxicol. 80:84–97. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beck-Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Helden JV and Weiskirchen R:
Age-independent anti-Mullerian hormone (AMH) standard deviation
scores to estimate ovarian function. Eur J Obstet Gynecol Reprod
Biol. 213:64–70. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Wu XY, Zhu QB, Li J, Shi LG, Wu JL,
Zhang QJ, Huang ML and Bao AM: Sex differences in the stress
response in SD rats. Behav Brain Res. 284:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elfayomy AK, Almasry SM, El-Tarhouny SA
and Eldomiaty MA: Human umbilical cord blood-mesenchymal stem cells
transplantation renovates the ovarian surface epithelium in a rat
model of premature ovarian failure: Possible direct and indirect
effects. Tissue Cell. 48:370–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi
Y, Gao L, Wang G, Liu Z, Li H, et al: Human umbilical cord
mesenchymal stem cells therapy in cyclophosphamide-induced
premature ovarian failure rat model. Biomed Res Int.
2016:25175142016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haidar MA, Baracat EC, Simoes MJ, Focchi
GR, Neto Evencio J and de Lima GR: Premature ovarian failure:
Morphological and ultrastructural aspects. Sao Paulo Med J.
112:534–538. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Wang S, Li Q, Huang Y, Chen C and
Zheng J: Telocytes as potential targets in a
cyclophosphamide-induced animal model of premature ovarian failure.
Mol Med Rep. 14:2415–2422. 2016. View Article : Google Scholar : PubMed/NCBI
|