Introduction
Thyroid cancer is one of the most frequent types of
endocrine neoplasms worldwide, with ~300,000 new cases occurring
every year (1,2). Due to several environmental and
socioeconomic factors, the occurrence rate of thyroid cancer is
more increased among rural populations (3). Thyroid cancer has been identified as
one of the most common types of malignancy among Asian population
in 2015 (4). Based on the degree
of cell differentiation, thyroid cancer can be classified into
several types, including papillary thyroid carcinoma (PTC),
medullary thyroid carcinoma (MTC) and follicular thyroid carcinoma
(5). PTC accounts for the majority
of thyroid carcinomas, and is responsible for ~80% of global
thyroid cancer cases (6,7).
Although the 5-year survival rate of PTC exceeds
90%, the occurrence of lymph node metastasis is frequent and the
recurrence risk remains high (8,9). The
most common therapeutic strategy used for PTC treatment is
thyroidectomy, which is featured by a high success rate of ~85%
(10,11). However, previous studies have
reported that ~20% of patients with PTC who underwent thyroidectomy
exhibited regional recurrence during a mean follow-up of <5
years (10,12). In addition, thyroidectomy is
characterized by various limitations, and the application of
surgery for lymph node excision remains controversial (13,14).
Therefore, investigation of the cellular and molecular mechanisms
involved in PTC is essential for the development of more effective
therapeutic strategies to prevent the recurrence in patients.
MicroRNAs (miRNAs) are a class of small non-coding
RNA molecules with a length of 20–22 nucleotides, which regulate
gene expression at the post-transcriptional stage and participate
in numerous cellular processes, including cell proliferation,
differentiation and apoptosis (15,16).
miRNAs have been suggested to serve an important role in the
development of PTC (17). For
example, let-7 is associated with PTC, through the regulation of
high-mobility group AT-hook 2 (HMGA2) and solute carrier family 5
member 5 (SLC5A5) expression (18). miR-146 has been reported to
suppress the expression of the retinoic acid receptor β in PTC,
thus attenuating the efficiency of retinoic acid and radioactive
iodine treatment, which suggests that the deregulation of miRNA may
also influence the therapeutic outcome of PTC treatment (19). Previous studies have reported that
the metastasis and proliferation of thyroid carcinoma cells could
be regulated by several miRNAs (20), including miR-146b (21), miR-451a (22) and miR-205 (23). Sondermann et al (24) suggested that miR-9 may have
potential prognostic value for predicting the recurrence of PTC, as
miR-9 expression in patients with non-recurrent PTC was
significantly higher compared with patients with recurrent PTC.
Gundara et al (25)
reported that miR-9 directly targeted autophagy protein 5 (Atg5)
and could suppress the viability of MTC cells in vitro, thus
indicating an important role for miR-9 in the pathogenesis of
thyroid cancer. Furthermore, the aberrant expression of the
proto-oncogene BRAF has been associated with the development of
PTC, and the BRAFV600E mutation has been considered to
be implicated in the progression and lymph node metastasis of PTC
(26–28). Therefore, the present study aimed
to explore the putative relationship between miR-9 and BRAF
expression in PTC.
In the present study, the roles of miR-9 in the
development and progression of PTC were investigated in
vitro and in vivo. The present findings will help in the
understanding of the molecular processes involved in thyroid cancer
and in the development of novel therapeutic approaches for the
treatment of patients with PTC.
Materials and methods
Subjects
A total of 60 pairs of fresh frozen PTC tissue
samples and paired adjacent non-cancerous tissues were collected at
Sichuan Provincial People's Hospital (Chengdu, China) between March
2014 and November 2015. All samples were collected from patients
with PTC who had not received any previous adjuvant treatments,
including chemotherapy, radiotherapy or hormone therapy. All
patients were diagnosed with PTC based on histopathological
evaluation. The samples were immediately stored in liquid nitrogen
until further use. The present study was approved by the Ethics
Committee of Sichuan Provincial People's Hospital. Written informed
consent was obtained from all patients prior to enrollment in the
present study. The clinicopathological characteristics of the
patients are presented in Table
I.
 | Table I.Association between miR-9 expression
levels in PTC tissues and clinicopathological characteristics of
patients with PTC. |
Table I.
Association between miR-9 expression
levels in PTC tissues and clinicopathological characteristics of
patients with PTC.
|
|
| miR-9
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total number | Low (n=28) | High (n=32) | P-value |
|---|
| Age (years) |
|
|
|
|
|
<50 | 24 | 13 | 11 | 0.342 |
|
≥50 | 36 | 15 | 21 |
|
| Sex |
|
|
|
|
|
Male | 47 | 23 | 24 | 0.503 |
|
Female | 13 | 5 | 8 |
|
| Cervical lymph |
|
|
|
|
| node
metastasis |
|
|
|
|
|
Positive | 23 | 14 | 9 | 0.082 |
|
Negative | 37 | 14 | 23 |
|
| TNM stage |
|
|
|
|
|
I/II | 35 | 11 | 24 | 0.005 |
|
III/IV | 25 | 17 | 8 |
|
Cell culture
The human TPC-1 thyroid gland papillary carcinoma
cell line was purchased from Bena Culture Collection (Beijing,
China) and cultured in Dulbecco's modified Eagle's medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Cells were maintained in a humidified incubator in a 5%
CO2 atmosphere at 37°C.
Cell transfection
The miR-9 mimics, miR-9 inhibitor and scramble
oligonucleotides were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequence of the miR-9 mimics was
5′-UCUUUGGUUAUCUAGCUGUAUGA-3′; the sequence of the miR-9 inhibitor
was 5′-UCAUACAGCUAGAUAACCAAAGA-3′; the sequence of the scramble
oligonucleotides was 5′-CAGUACUUUUGUGUAGUACAA-3′. The
overexpression vector for BRAF (pcDNA3.1-BRAF) was constructed by
cloning the BRAF open reading frame sequence (range, 226–2,529,
without 3′-UTR) into the multiple cloning site of the pcDNA3.1
vector (Invitrogen; Thermo Fisher Scientific, Inc.).
TPC-1 cells (1×106) were cultured in
6-well culture plates, and transfection was performed using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol until
confluence reached 50–60%. Untransfected cells served as a control.
Following 48 h of transfection, the cells were harvested and used
for further experiments.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from tissues and cells was isolated using
TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. For miR-9 detection, cDNA was synthesized from total RNA
using a One Step Prime script miRNA cDNA Synthesis kit (Qiagen,
Inc., Valencia, CA, USA). The relative expression levels of miR-9
were detected using the mirVana™ qRT-PCR miRNA Detection kit
(Invitrogen) on a preheated 7500 Real Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For BRAF mRNA
detection, cDNA was synthesized from total RNA using a PrimeScript
RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China).
qPCR was performed for BRAF mRNA detection using the SYBR Premix Ex
TaqII PCR kit (Takara Biotechnology Co., Ltd.). The relative
expression levels of miR-9 were normalized to U6, whereas BRAF mRNA
expression was normalized to β-actin. The data were analyzed
according to the 2−ΔΔCq method (29). The PCR cycling parameters were as
follows: 5 min denaturation at 95°C; then 30 cycles of 95°C for 15
sec, 55°C for 20 sec, 70°C for 30 sec; 5 min extension at 70°C. The
primers for miR-9 (30) were
designed by Applied Biosystems (Thermo Fisher Scientific, Inc.).
The remaining primers were as follows: BRAF forward,
5′-ACCACCCAATACACAGGAA-3′ and reverse, 5′-CATTGGGAGCTGATGAGGAT-3′;
U6 forward, 5′-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3′ and reverse,
5′-GCTTCACGAATTTGCGTGTCATCCTTGC-3′; and β-actin forward,
5′-AAACTGGAACGGTGAAGGTG-3′ and reverse,
5′-AGAGAAGTGGGGTGGCTTTT-3′.
Protein isolation and western blot
analysis
Cells were lysed using radioimmunoprecipitation
assay lysis buffer containing protease inhibitor cocktail (Beyotime
Institute of Biotechnology, Haimen, China). Protein concentration
was determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). Extracted protein samples (~30 µg) were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes
were blocked in TBS containing 0.1% Tween-20 (TBST) with 5% skimmed
milk for 1 h at room temperature, and were then incubated with
primary antibodies against BRAF (TA890103; 1:800; OriGene
Technologies, Inc., Beijing, China) at 4°C overnight. Following
washing, the membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (TA130023; 1:2,000;
OriGene Technologies, Inc.). β-actin was used as the endogenous
control (4970; 1:1,000 dilution; Cell Signaling Technology, Inc.,
Danvers, MA, USA). Protein bands were visualized using the enhanced
chemiluminescence (ECL) kit (Thermo Fisher Scientific, Inc.), and
blots were semi-quantified using ImageJ software version 1.46
(National Institutes of Health, Bethesda, MD, USA).
Luciferase reporter assay
Bioinformatics analysis was performed to predict
potential target genes for miR-9 using Targetscan (31) and miRanda (32). The results indicated that miR-9 may
directly interact with the 3′-untranslated region (UTR) of the BRAF
mRNA. To investigate the interaction in vitro, the 3′-UTR of
BRAF containing the putative binding site for miR-9 was cloned
downstream of the Renilla luciferase gene on a psiCHECK-2
reporter plasmid (Promega Corporation, Madison, WI, USA). BRAF
3′-UTR mutants were generated using the GeneTailor Site-Directed
Mutagenesis system (Invitrogen; Thermo Fisher Scientific, Inc.) and
were also ligated with the psiCHECK-2 vector. The constructed
luciferase vectors (0.2 µg) were co-transfected with miR-9 mimics,
miR-9 inhibitor or scramble oligonucleotides (100 nM) into TPC-1
cells using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The luciferase activities following 48 h
of transfection were measured by the Dual Luciferase Reporter assay
system (Promega Corporation).
Cellular apoptosis analysis
To assess cellular apoptosis, TPC-1 cells were
seeded into 6-well plates at a density of 1×105
cells/well. After 48 h, the cells were harvested and the apoptotic
rates were determined using an Annexin V-fluorescein
isothiocyanate/propidium iodide Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA) according to the manufacturer's
protocol. Cells were analyzed and the apoptotic rate was quantified
using a FACScan flow cytometer (BD Biosciences).
Cellular viability analysis
Cell viability was measured using an MTT assay.
TPC-1 cells were seeded in 96 well plates at a density of
5×103 cells/well. Following incubation for 24 h at 37°C,
the MTT Cell Proliferation and Cytotoxicity Assay kit (Beyotime
Institute of Biotechnology) was used, and 40 µl MTT stock solution
was added to each well for 4 h. The supernatants were discarded and
200 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to dissolve the
formazan crystals. The absorbance of each sample was measured at
570 nm using a microplate reader.
In vivo tumorigenesis
Male BALB/c athymic mice (n=30; age, 4–6 weeks;
weight, 20–22 g), purchased from Shanghai SLAC Laboratory Animal
Co., Ltd. (Shanghai, China), were housed in micro-isolator cages
(22–25°C, 50–60% humidity, 12-h light/dark cycle) with free access
to food and water. TPC-1 cells (2×106 cells in 100 µl
PBS) were subcutaneously inoculated into the right flank of the
mice. Then the mice were randomized into 5 groups (n=6 mice/group),
and miR-9 mimics, miR-9 inhibitor, pcDNA3.1-BRAF, or scramble
oligonucleotides (100 nM in 100 µl PBS) were injected into the
tumors directly twice a week. The mice in the control group
received vehicle (DMSO) injections. Tumor volumes were measured
every 4 days using a caliper according to the following formula:
Volume = length × width2/2. Mice were sacrificed 4 weeks
after the initial injection, and tumors were dissected. All
protocols of animal experiments were approved by the Animal Ethics
Committee of Sichuan Provincial People's Hospital (Sichuan,
China).
Statistical analysis
All statistical analyses were performed using SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad
Prism software 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
The statistical significance of the differences between two groups
was assessed using two-tailed unpaired Student's t-test.
Categorical data was compared using the Chi-square test. Data were
presented as the mean ± standard deviation from at least three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-9 expression is downregulated in
PTC tissues
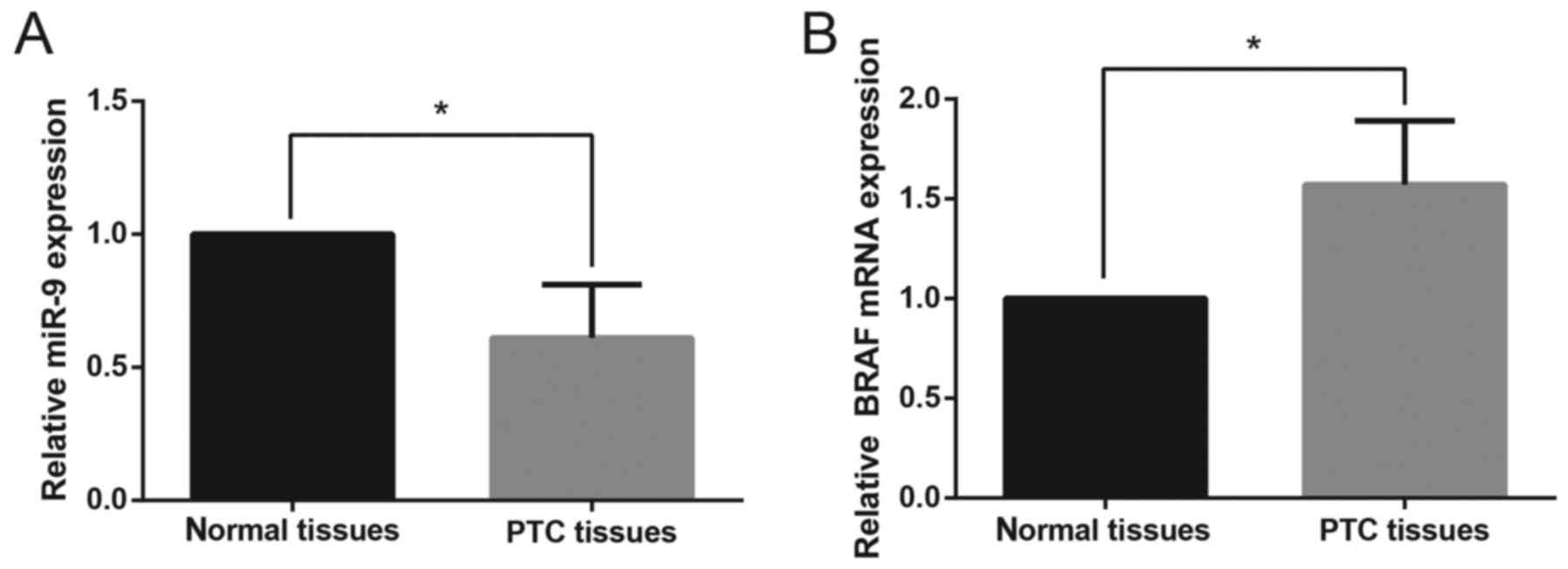
The relative expression levels of miR-9 and BRAF
mRNA in PTC tissues and paired adjacent non-cancerous tissues were
assessed using RT-qPCR. The relative expression levels of miR-9 in
PTC tissues were significantly downregulated compared with in
paired non-cancerous tissues (Fig.
1A). In addition, the mRNA expression levels of BRAF were
significantly upregulated in PTC tissues compared with normal
tissue samples (Fig. 1B). As
presented in Table I, the
downregulation of miR-9 expression was significantly associated
with the advanced TNM stage (P=0.005), whereas no significant
correlation was found between miR-9 expression and age (P=0.342),
sex (P=0.503) or cervical lymph node metastasis (P=0.082).
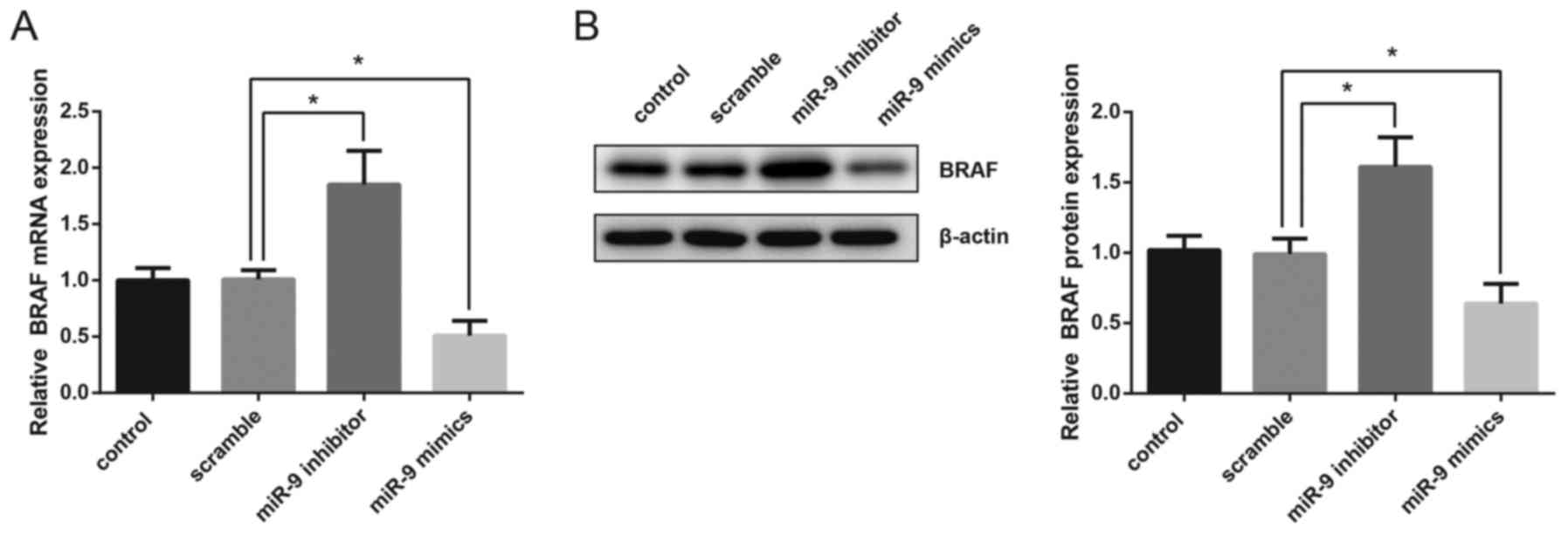
BRAF expression is suppressed by miR-9
in TPC-1 cells
In the present study, human TPC-1 thyroid gland
papillary carcinoma cells were transfected with miR-9 mimics or
miR-9 inhibitor and the mRNA and protein expression levels of BRAF
were then detected. The results of RT-qPCR demonstrated that
overexpression of miR-9 significantly suppressed the mRNA
expression of BRAF, whereas inhibition of miR-9 resulted in a
significant upregulation in BRAF mRNA levels (Fig. 2A). Western blot analysis also
revealed that the protein expression levels of BRAF were
significantly decreased following miR-9 overexpression, whereas
they were increased following miR-9 inhibition (Fig. 2B).
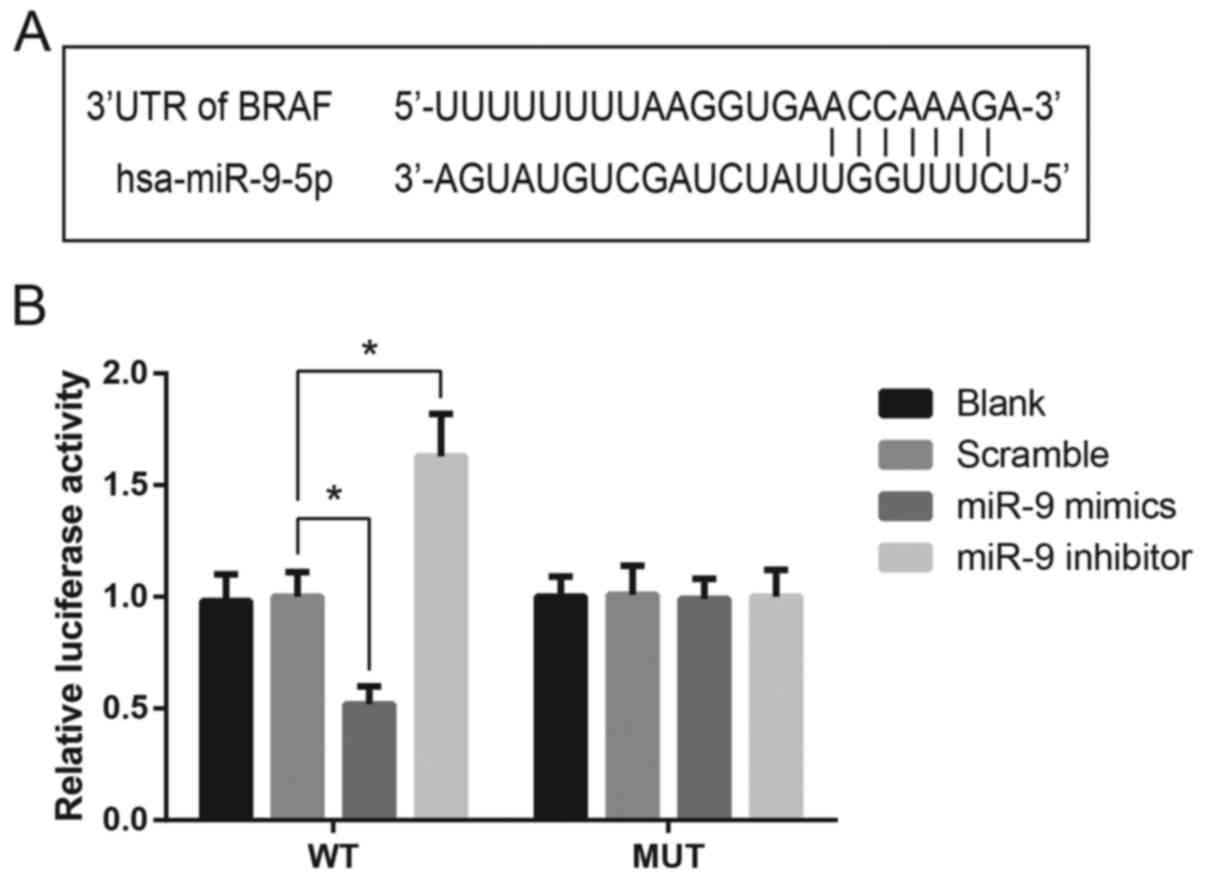
BRAF is a direct target gene of miR-9
in TSC-1 cells
Bioinformatics analysis identified a potential
binding site for miR-9 in the 3′-UTR of the BRAF mRNA (Fig. 3A). A luciferase reporter assay was
performed to confirm the putative interaction in vitro. As
presented in Fig. 3B, the
luciferase activity in TSC-1 cells transfected with a luciferase
vector encoding the wild-type BRAF 3′-UTR was significantly
suppressed following transfection with miR-9 mimics. Conversely,
TSC-1 cells transfected with the mutated BRAF 3′-UTR sequence were
not markedly affected by miR-9 mimics or inhibitor, indicating that
miR-9 may directly target BRAF expression in TSC-1 cells (Fig. 3B).
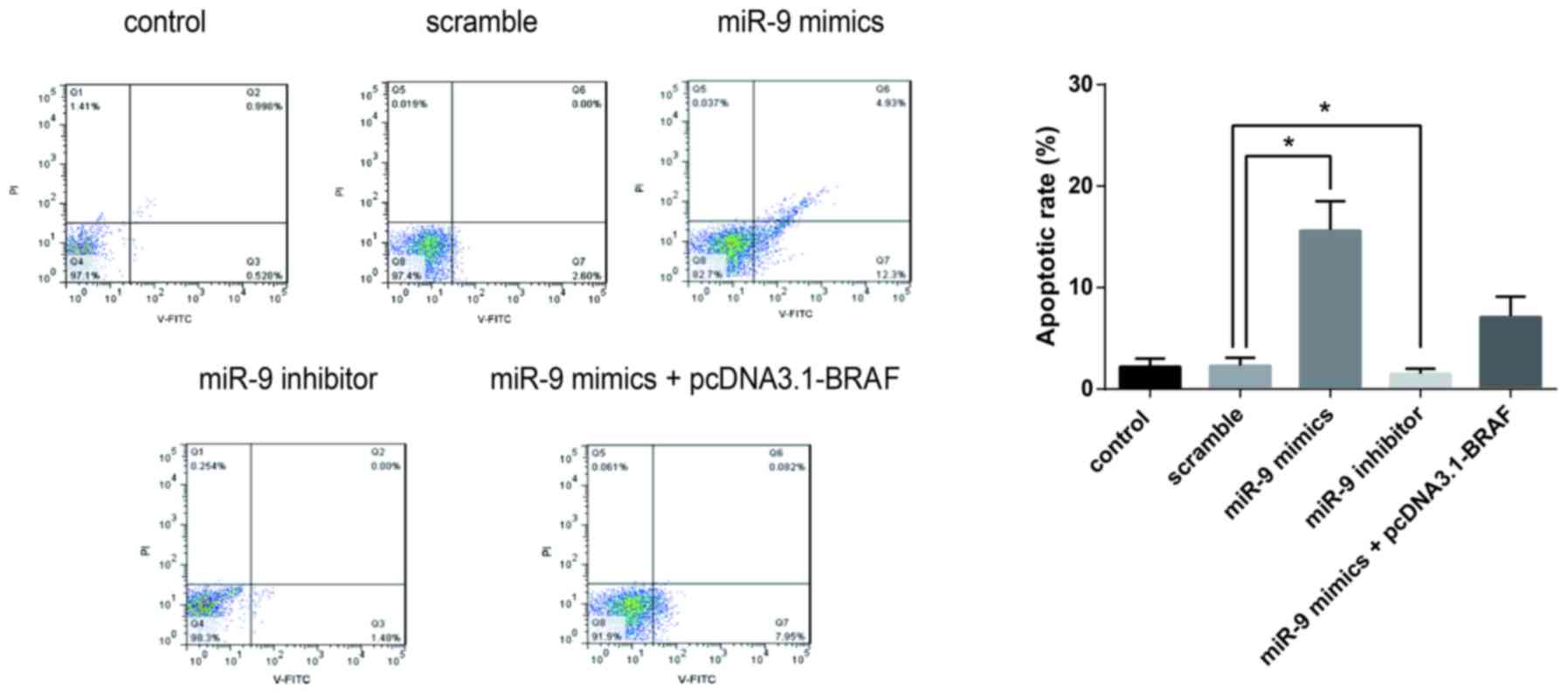
miR-9 inhibits the proliferation and
promotes the apoptosis of TSC-1 cells
Flow cytometric analysis was performed to evaluate
the effects of miR-9 on cancer cell apoptosis. The apoptotic rates
of TSC-1 cells were significantly elevated following transfection
with miR-9 mimics. By contrast, co-transfection with pcDNA3.1-BRAF
suppressed the increase of apoptotic rates. These findings
suggested that miR-9 may be involved in the regulation of PTC cell
apoptosis, through the modulation of BRAF expression (Fig. 4).
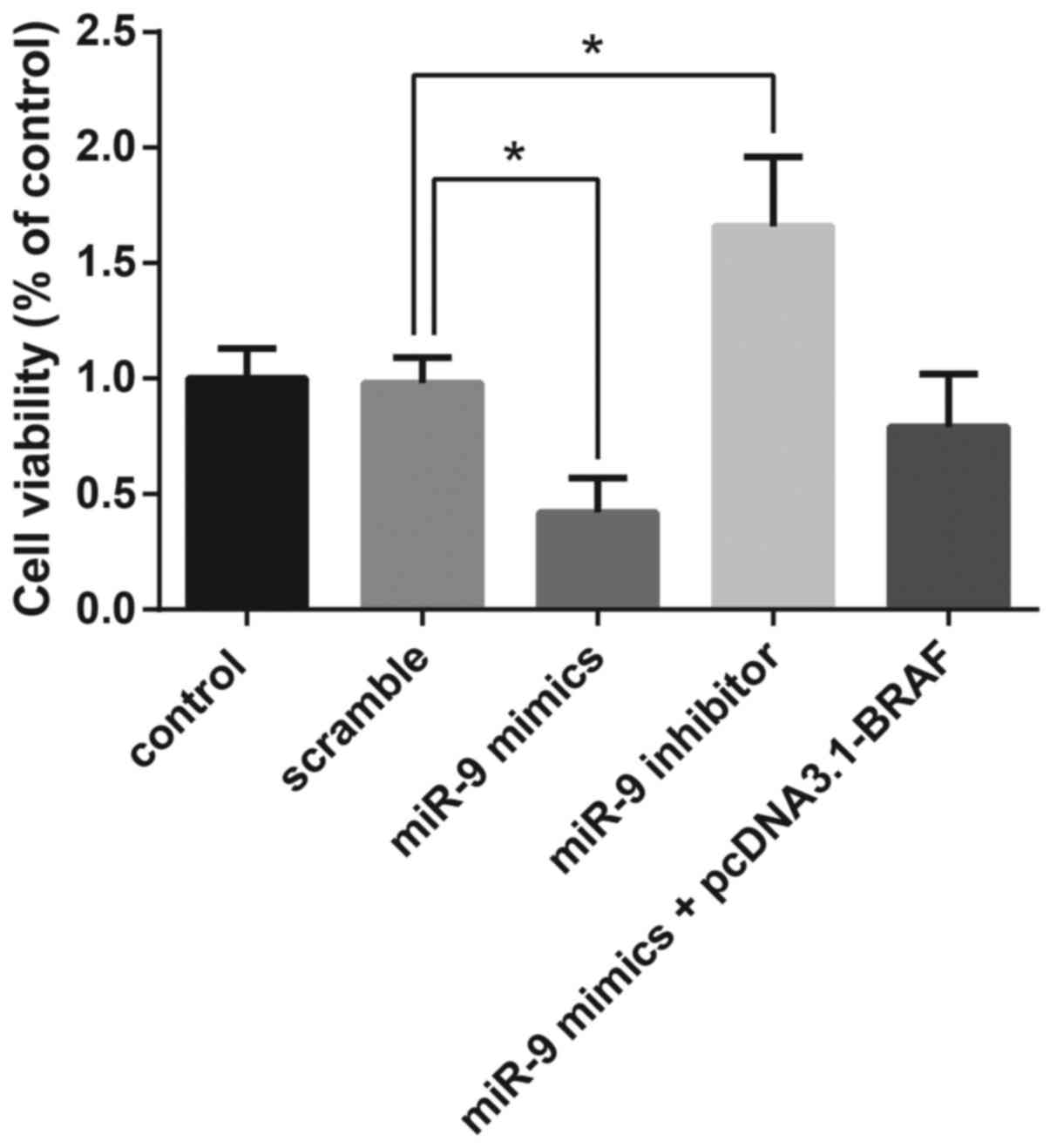
MTT assay demonstrated that overexpression of miR-9
resulted in the significant suppression of TPC-1 cell
proliferation, whereas co-transfection with pcDNA3.1-BRAF restored
the miR-9-induced inhibition of cell viability (Fig. 5).
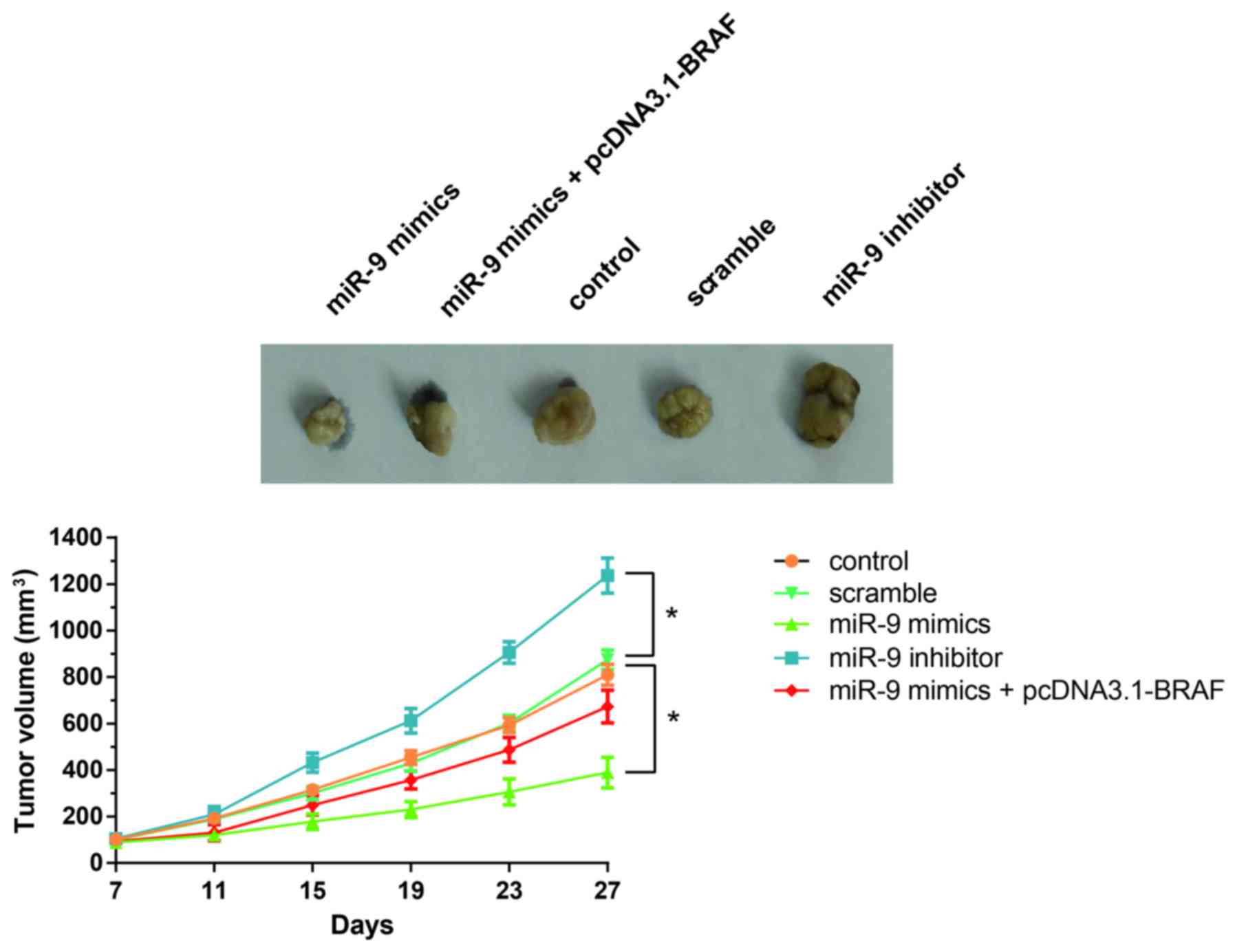
miR-9 suppresses in vivo PTC
tumorigenesis in xenografted mice
As shown in Fig. 6,
tumor growth in mice treated with the miR-9 inhibitor was
significantly increased compared with the control or
scramble-treated mice. Notably, miR-9 overexpression significantly
suppressed tumor growth in vivo, whereas this effect was
restored following the co-administration of pcDNA3.1-BRAF (Fig. 6). These results indicated that
miR-9/BRAF may be implicated in the regulation of in vivo
PTC tumorigenesis.
Discussion
PTC is one of the most common types of thyroid
cancer, and its incidence rate has increased by >240%, with
~62,980 expected new cases in 2014, thus making PTC a major issue
for human health worldwide (33).
Currently, miRNAs have been suggested as potential prognostic and
diagnostic biomarkers in various types of human cancer. Previous
studies have demonstrated that dysregulation of the expression of
several miRNAs was involved in the pathogenesis of thyroid cancer
(34–36). The recurrence of PTC still remains
high following thyroidectomy, thus stressing the need for the
elucidation of the exact molecular mechanisms implicated in PTC
pathogenesis.
The results of the present study demonstrated that
miR-9 was significantly downregulated in PTC tissues compared with
adjacent non-cancerous tissue samples. In addition, luciferase
activity assay revealed that miR-9 directly targeted BRAF and
suppressed its expression, thereby suppressing the viability and
enhancing the apoptosis of human TSC-1 thyroid gland papillary
carcinoma cells. Furthermore, the results of a mouse thyroid tumor
xenograft model suggested that miR-9 may suppress tumor growth
in vivo.
The present results demonstrating the suppression of
miR-9 expression in PTC tissues were consistent with previous
studies (20,24) investigating the roles of miR-9 in
thyroid cancer. Gundara et al (25) reported that miR-9 inhibited the
autophagic flux and increased the apoptosis of cancer cells in MTC;
autophagy has been suggested as one of the survival mechanisms in
MTC cells. However, the association between miR-9 and autophagy has
yet to be further explored.
In recent years, studies of signal transduction
pathways have made a great contribution to the understanding of the
molecular mechanisms implicated in tumorigenesis. At present, four
mitogen-activated protein kinase (MAPK) signal transduction
pathways have been identified in eukaryotic cells, including the
extracellular signal-regulated kinase (ERK) pathway, the c-Jun
N-terminal kinase/stress activated protein kinase pathway, the p38
pathway and the ERK5 pathway. The MAPK pathway, which includes the
kinases Ras, Raf, MAPK/ERK kinase (MEK) and ERK, is implicated in
the regulation of several cellular processes, including
proliferation, differentiation, apoptosis and survival (37). In patients with PTC, ~80% of
gain-of-function mutations have been identified in genes encoding
signaling molecules participating in the MAPK pathways, thus
suggesting that MAPK pathways may serve a critical role in the
pathogenesis of PTC (38).
Zawistowski et al (39)
reported that miR-9 inhibited the MEK/ERK signaling pathway via
targeting integrinβ-1 in breast cancer. However, the exact
molecular mechanisms underlying the involvement of miR-9 in the
development of PTC have yet to be elucidated. To the best of our
knowledge, the present study is the first to demonstrate that miR-9
could directly target BRAF to suppress the proliferation and growth
of thyroid cancer cells in vitro.
BRAF is implicated in the activation of the MAPK
pathway and belongs to the Raf family of kinases. BRAF is a
critical factor participating in the pathogenesis of thyroid
cancer, and a high frequency of BRAF mutations has been detected
among patients with PTC (40).
Accumulating evidence suggests that a mutation in BRAF at the amino
acid residue 600 (V600E) may be associated with the prognosis of
patients with PTC (41–43). BRAF may be implicated in PTC
progression through several signaling pathways, including the
MEK/ERK and the phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt
pathway (44–46). Notably, Zhang et al
(47) demonstrated that inhibition
of miR-9 reversed the effects of BRAF-activated non-coding RNA on
gastric cancer cell growth and apoptosis, via targeting nuclear
factor-κB1.
The results of the present study suggested that
miR-9 may suppress the activation of the MEK/ERK signaling pathway
via binding to the 3′-UTR of BRAF and suppressing its expression;
low expression levels of BRAF may inhibit the activation of MEK/ERK
signaling. Inhibition of the MEK/ERK pathway using synthetic
pharmacological agents has been demonstrated to inhibit the
progression of various types of cancer (48). In addition, BRAF has been
associated with cancer development and it may serve an important
role in signal transduction through the phosphorylation of MEK1/2
and ERK1/2 (49).
In conclusion, the present study revealed that miR-9
expression was downregulated in PTC tissues, whereas miR-9
overexpression inhibited BRAF expression, suppressed PTC cell
proliferation and promoted PTC cell apoptosis. Further studies are
required to investigate the molecular mechanisms underlying the
pathogenesis of PTC, and identify novel therapeutic approaches for
the treatment of patients with PTC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG designed the research, analyzed data and wrote
the manuscript. NY, LY, CF and TL performed the research and
analyzed data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Sichuan Provincial People's Hospital (Sichuan, China).
Written informed consent was obtained from all patients prior to
enrollment in the present study. All protocols of animal
experiments were approved by the Animal Ethics Committee of Sichuan
Provincial People's Hospital.
Consent for publication
Written informed consent was obtained from all
patients prior to enrollment in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pacini F: Thyroid microcarcinoma. Best
Pract Res Clin Endocrinol Metab. 26:381–389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanley JP, Jackson E, Morrissey LA, Rizzo
DM, Sprague BL, Sarkar IN and Carr FE: Geospatial and temporal
analysis of thyroid cancer incidence in a rural population.
Thyroid. 25:812–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Magreni A, Bann DV, Schubart JR and
Goldenberg D: The effects of race and ethnicity on thyroid cancer
incidence. JAMA Otolaryngol Head Neck Surg. 141:319–323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zolotov S: Genetic testing in
differentiated thyroid carcinoma: Indications and clinical
implications. Rambam Maimonides Med J. 7:28–Jan;2016.doi:
10.5041/RMMJ.10236. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griniatsos J, Tsigris C, Kanakis M,
Kaltsas G, Michail O, Dimitriou N, Argyrakopoulou G, Delladetsima
I, Kyriakou V, Syriou V, et al: Increased incidence of papillary
thyroid cancer detection among thyroidectomies in Greece between
1991 and 2006. Anticancer Res. 29:5163–5169. 2009.PubMed/NCBI
|
|
7
|
Hakala T, Kellokumpu-Lehtinen P, Kholová
I, Holli K, Huhtala H and Sand J: Rising incidence of small size
papillary thyroid cancers with no change in disease-specific
survival in finnish thyroid cancer patients. Scand J Surg.
101:301–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuo EJ, Goffredo P, Sosa JA and Roman SA:
Aggressive variants of papillary thyroid microcarcinoma are
associated with extrathyroidal spread and lymph-node metastases: A
population-level analysis. Thyroid. 23:1305–1311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghossein R, Ganly I, Biagini A, Robenshtok
E, Rivera M and Tuttle RM: Prognostic factors in papillary
microcarcinoma with emphasis on histologic subtyping: A
clinicopathologic study of 148 cases. Thyroid. 24:245–253. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grant CS: Recurrence of papillary thyroid
cancer after optimized surgery. Gland Surg. 4:52–62.
2015.PubMed/NCBI
|
|
11
|
Byeon HK, Ban MJ, Lee JM, Ha JG, Kim ES,
Koh YW and Choi EC: Robot-assisted Sistrunk's operation, total
thyroidectomy, and neck dissection via a transaxillary and
retroauricular (TARA) approach in papillary carcinoma arising in
thyroglossal duct cyst and thyroid gland. Ann Surg Oncol.
19:4259–4261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang BH, Ng SH, Lau LL, Cowling BJ, Wong
KP and Wan KY: A systematic review and meta-analysis of
prophylactic central neck dissection on short-term locoregional
recurrence in papillary thyroid carcinoma after total
thyroidectomy. Thyroid. 23:1087–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Macedo FI and Mittal VK: Total
thyroidectomy versus lobectomy as initial operation for small
unilateral papillary thyroid carcinoma: A meta-analysis. Surg
Oncol. 24:117–122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsuzu K, Sugino K, Masudo K, Nagahama M,
Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Suzuki A, Magoshi S, et
al: Thyroid lobectomy for papillary thyroid cancer: Long-term
follow-up study of 1,088 cases. World J Surg. 38:68–79. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He Y, Lin J, Kong D, Huang M, Xu C, Kim
TK, Etheridge A, Luo Y, Ding Y and Wang K: Current state of
circulating MicroRNAs as cancer biomarkers. Clin Chem.
61:1138–1155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Du YL, Jin P and Ma F:
Bioinformatic analysis of cancer-related microRNAs and their target
genes. Yi Chuan. 37:855–864. 2015.PubMed/NCBI
|
|
17
|
Yoruker EE, Terzioglu D, Teksoz S, Uslu
FE, Gezer U and Dalay N: MicroRNA expression profiles in papillary
thyroid carcinoma, benign thyroid nodules and healthy Controls. J
Cancer. 7:803–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Damanakis AI, Eckhardt S, Wunderlich A,
Roth S, Wissniowski TT, Bartsch DK and Di Fazio P: MicroRNAs let7
expression in thyroid cancer: Correlation with their deputed
targets HMGA2 and SLC5A5. J Cancer Res Clin Oncol. 142:1213–1220.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czajka AA, Wójcicka A, Kubiak A, Kotlarek
M, Bakuła-Zalewska E, Koperski Ł, Wiechno W and Jażdżewski K:
Family of microRNA-146 regulates RARβ in papillary thyroid
carcinoma. PLoS One. 11:e01519682016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cong D, He M, Chen S, Liu X and Sun H:
Expression profiles of pivotal microRNAs and targets in thyroid
papillary carcinoma: An analysis of the cancer genome atlas. Onco
Targets Ther. 8:2271–2277. 2015.PubMed/NCBI
|
|
21
|
Lima CR, Geraldo MV, Fuziwara CS, Kimura
ET and Santos MF: MiRNA-146b-5p upregulates migration and invasion
of different papillary thyroid carcinoma cells. BMC Cancer.
16:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minna E, Romeo P, Dugo M, De Cecco L,
Todoerti K, Pilotti S, Perrone F, Seregni E, Agnelli L, Neri A, et
al: miR-451a is underexpressed and targets AKT/mTOR pathway in
papillary thyroid carcinoma. Oncotarget. 7:12731–12747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salajegheh A, Vosgha H, Rahman Md A, Amin
M, Smith RA and Lam AK: Modulatory role of miR-205 in angiogenesis
and progression of thyroid cancer. J Mol Endocrinol. 55:183–196.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sondermann A, Andreghetto FM, Moulatlet
AC, da Silva Victor E, de Castro MG, Nunes FD, Brandão LG and
Severino P: MiR-9 and miR-21 as prognostic biomarkers for
recurrence in papillary thyroid cancer. Clin Exp Metastasis.
32:521–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gundara JS, Zhao J, Gill AJ, Lee JC,
Delbridge L, Robinson BG, McLean C, Serpell J and Sidhu SB:
Noncoding RNA blockade of autophagy is therapeutic in medullary
thyroid cancer. Cancer Med. 4:174–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma B, Shi R, Yang S, Zhou L, Qu N, Liao T,
Wang Y, Wang Y and Ji Q: DUSP4/MKP2 overexpression is associated
with BRAF(V600E) mutation and aggressive behavior of papillary
thyroid cancer. Onco Targets Ther. 9:2255–2263. 2016.PubMed/NCBI
|
|
27
|
Gao Q, Zhang W, Wang N, Duan H, Zhou Y,
Zhang W and Zhao D: Study on the correlation between BRAF(V600E)
mutation and lymphatic metastases in papillary thyroid cancer
staged preoperativelv as N0. Lin Chung Er Bi Yan Hou Tou Jing Wai
Ke Za Zhi. 29:2048–2052. 2015.(In Chinese). PubMed/NCBI
|
|
28
|
Cordioli MI, Moraes L, Carvalheira G,
Sisdelli L, Alves MT, Delcelo R, Monte O, Longui CA, Cury AN and
Cerutti JM: AGK-BRAF gene fusion is a recurrent event in sporadic
pediatric thyroid carcinoma. Cancer Med. 5:1535–1541. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhuang G, Wu X, Jiang Z, Kasman I, Yao J,
Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D and Ferrara N:
Tumour-secreted miR-9 promotes endothelial cell migration and
angiogenesis by activating the JAK-STAT pathway. EMBO J.
31:3513–3523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:12–Aug;2015.doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
32
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conzo G, Tartaglia E, Avenia N, Calò PG,
de Bellis A, Esposito K, Gambardella C, Iorio S, Pasquali D,
Santini L, et al: Role of prophylactic central compartment lymph
node dissection in clinically N0 differentiated thyroid cancer
patients: Analysis of risk factors and review of modern trends.
World J Surg Oncol. 14:1492016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chruścik A and Lam AK: Clinical
pathological impacts of microRNAs in papillary thyroid carcinoma: A
crucial review. Exp Mol Pathol. 99:393–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Min XS, Huang P, Liu X, Dong C, Jiang XL,
Yuan ZT, Mao LF and Chang S: Bioinformatics analyses of significant
prognostic risk markers for thyroid papillary carcinoma. Tumour
Biol. 36:7457–7463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forte S, La Rosa C, Pecce V, Rosignolo F
and Memeo L: The role of microRNAs in thyroid carcinomas.
Anticancer Res. 35:2037–2047. 2015.PubMed/NCBI
|
|
37
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schlumberger M and Sherman SI: Approach to
the patient with advanced differentiated thyroid cancer. Eur J
Endocrinol. 166:5–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zawistowski JS, Nakamura K, Parker JS,
Granger DA, Golitz BT and Johnson GL: MicroRNA 9-3p targets β1
integrin to sensitize claudin-low breast cancer cells to MEK
inhibition. Mol Cell Biol. 33:2260–2274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ito T, Seyama T, Hayashi Y, Dohi K and
Akiyama M: Unique association of p53 mutations with
undifferentiated carcinoma of the thyroid. Nihon Rinsho.
52:1069–1074. 1994.(In Japanese). PubMed/NCBI
|
|
41
|
Falchook GS, Millward M, Hong D, Naing A,
Piha-Paul S, Waguespack SG, Cabanillas ME, Sherman SI, Ma B, Curtis
M, et al: BRAF inhibitor dabrafenib in patients with metastatic
BRAF-mutant thyroid cancer. Thyroid. 25:71–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rothenberg SM, McFadden DG, Palmer EL,
Daniels GH and Wirth LJ: Redifferentiation of iodine-refractory
BRAF V600E-mutant metastatic papillary thyroid cancer with
dabrafenib. Clin Cancer Res. 21:1028–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fnais N, Soobiah C, Al-Qahtani K, Hamid
JS, Perrier L, Straus SE and Tricco AC: Diagnostic value of fine
needle aspiration BRAF(V600E) mutation analysis in papillary
thyroid cancer: A systematic review and meta-analysis. Hum Pathol.
46:1443–1454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yi W, Zhong D and Zou Q: Expression of
BRAF and its extracellular signal-regulated kinase 1/2 signal
pathway in papillary thyroid cancer. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 37:889–894. 2012.(In Chinese). PubMed/NCBI
|
|
45
|
Kandil E, Tsumagari K, Ma J, Elmageed Abd
ZY, Li X, Slakey D, Mondal D and Abdel-Mageed AB: Synergistic
inhibition of thyroid cancer by suppressing MAPK/PI3K/AKT pathways.
J Surg Res. 184:898–906. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McCarty SK, Saji M, Zhang X, Knippler CM,
Kirschner LS, Fernandez S and Ringel MD: BRAF activates and
physically interacts with PAK to regulate cell motility. Endocr
Relat Cancer. 21:865–877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang ZX, Liu ZQ, Jiang B, Lu XY, Ning XF,
Yuan CT and Wang AL: BRAF activated non-coding RNA (BANCR)
promoting gastric cancer cells proliferation via regulation of
NF-κB1. Biochem Biophys Res Commun. 465:225–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hu Y, Mintz A, Shah SR, Quinones-Hinojosa
A and Hsu W: The FGFR/MEK/ERK/brachyury pathway is critical for
chordoma cell growth and survival. Carcinogenesis. 35:1491–1499.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wellbrock C and Arozarena I: The
Complexity of the ERK/MAP-kinase pathway and the treatment of
melanoma skin cancer. Front Cell Dev Biol. 4:332016. View Article : Google Scholar : PubMed/NCBI
|