Introduction
Free-floating cancer cells (FFCCs) are a population
of cells that are completely detached from the primary lesions and
float freely inside the lymph node sinuses. FFCCs are so small that
they are hard to detect using hematoxylin and eosin (H&E)
staining, but they can be easily observed by staining for
cytokeratin. Previous studies demonstrated that FFCCs in the lymph
node sinuses were of prognostic significance for colorectal and
gastric cancers (1–3). Mukai et al also reported that
FFCCs in the lymph node sinuses were prognostic markers for lung
cancer (4,5). However, these studies had poor
patient selection and inadequate analyses; the authors did not
include all patients who underwent resection for primary lung
tumors, and analyzed findings from lung, breast, and gastric cancer
patients collectively. Thus, the clinical significance of FFCCs in
the lymph node sinuses of non-small cell lung cancer (NSCLC)
patients currently remains unclear. In addition, some scientists
suspect that FFCCs may be the same as lymph node metastases. So, in
this study, we investigated whether there were prognostic
differences between FFCCs positive and negative groups in hilar
lymph node positive patients who underwent resection for primary
lung cancer.
In contrast to lymph nodes in the colon or stomach,
many of the lymph nodes in the lungs contain coal dust. Since
conventional cytokeratin immunostaining uses 3,3′-diaminobenzidine
(DAB), a brown stain, as a substrate to detect peroxidase (e.g.,
iVIEW DAB Detection kit; Roche Diagnostics K.K, Tokyo, Japan), the
detection of FFCCs in lymph node sinuses covered with black coal
dust is time-consuming. Therefore, we have used an alkaline
phosphatase-conjugated secondary antibody and Fast Red/naphthol,
which produces a red color in cytokeratin-positive cells, in order
to enable the distinction of cytokeratin-positive cells from coal
dust (6).
In the present study, we used Fast Red staining to
detect FFCCs in the lymph node sinuses of patients with both stage
I/II and hilar lymph node positive NSCLC patients. We investigated
various clinicopathological factors to assess the significance of
FFCCs in the lymph node sinuses.
Patients and methods
Patients
Stage I/II (the seventh edition of the TNM
classification) lung cancer patients (n=168) who underwent
lobectomy (pneumonectomy when required) and hilar-mediastinal lymph
node dissection between September 2002 and December 2011 at Tokai
University Hachioji Hospital were enrolled in the present study.
Three patients were excluded because of small cell lung cancers.
One patient was excluded because his primary lesion was not stained
by cytokeratin immunostaining using AE1/AE3 anti-body. So, 164
patients were investigated finally. Among stage I (n=132) and stage
II (n=32) patients, 122 had adenocarcinoma, 30 squamous cell
carcinoma, 6 large cell carcinoma, and 6 tumors with other
histological types (1 typical cartinoid, 2 adenosquamous carcinoma,
1 pleomorphic carcinoma, 1 mucoepidermoid carcinoma, and 1
unclassified non-small cell carcinoma). Of the 164 patients, 22 had
hilar lymph node metastases diagnosed by H&E staining. Of the
164 patients, 36 had recurrent diseases (n=18 and 18 for stages I
and II, respectively) and 128 were relapse-free (n=114 and 14 for
stages I and II, respectively). Resected lymph nodes were stained
for cytokeratin using Fast Red to detect FFCCs in the lymph node
sinuses, and clinicopathological features were investigated in
FFCCs+ and FFCCs-patients. Relapse-free survival (RFS) and overall
survival (OS) were calculated based on pathology reports and
electronic medical records stored at Tokai University Hachioji
Hospital. In patients with recurrent tumors, RFS and OS were
measured from the date of surgery to the date that recurrent tumors
were identified using CT, brain-MRI, bone-scan, FDG-PET, or to the
date of death; similarly, RFS and OS were measured in non-recurrent
patients from the date of surgery to December 31, 2016. All
patients were followed up for the duration of the study, including
those who were transferred to another hospital during the
observation period. At the end of the 5-year period, 130 patients
were alive, 25 were deceased, and 9 were lost to the follow-up
(94.5% follow-up rate). The present study was approved by the Tokai
University Institutional Review Board for Clinical Research (IRB
no. 14R-225; Isehara, Japan) and the patients' samples were
examined after receiving informed consent from the patients.
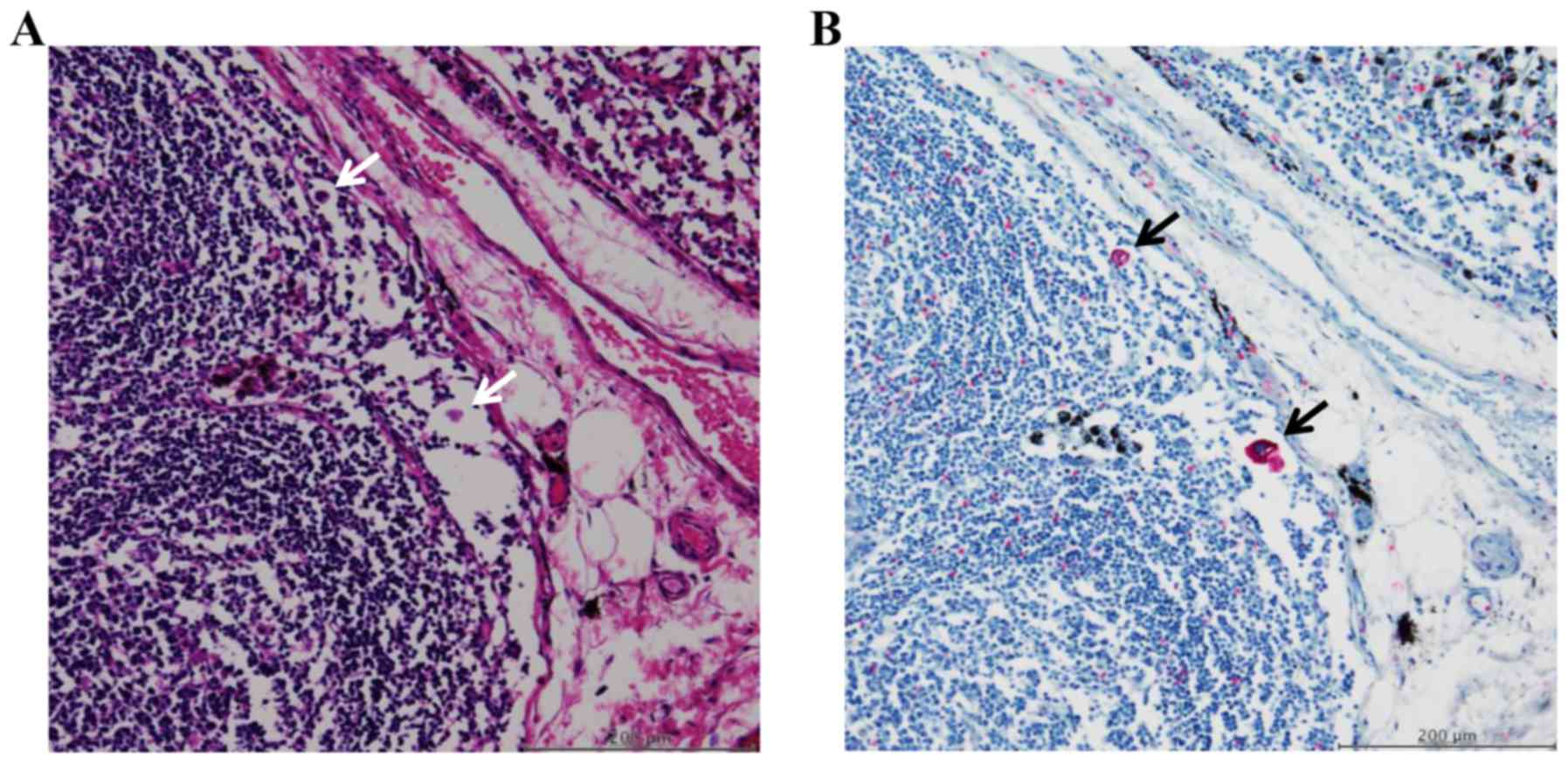
The concept and the definition of
FFCCs in lymph node sinuses
FFCCs are a population of cells that are completely
detached from the primary lesions and float freely inside the lymph
node sinuses. Its concept is different from that of lymph node
metastasis. In contrast to metastases in the lymph nodes detected
by H&E staining, FFCCs are difficult to detect by H&E
staining since they are very small in size; cytokeratin
immunostaining can be usually used as an alternative to identify
FFCCs in the lymph node sinuses. In the present study, FFCCs in the
lymph node sinuses were defined as those that i) it is difficult to
detect by H&E staining and can be detected by cytokeratin
immunostaining; ii) float freely in the lymph node sinuses and do
not invasive to and/or are not caught by the apparatus of the lymph
nodes such as cortex and paracortex area; and iii) have an intact
nucleus and are not damaged.
Immunohistochemistry
In order to achieve the clear distinction of
cytokeratin-positive cells from coal dust, lymph node tissues were
stained using a mouse monoclonal anti-cytokeratin antibody (Clone:
AE1, AE3, PCK26; Roche Diagnostics K.K.) and secondary antibody
conjugated with alkaline phosphatase, which was visualized by the
reaction with Fast Red/naphthol that produced a red color.
Regarding the preparation of tissues for staining, resected lymph
nodes were fixed in formalin, cut along the maximum dimension, and
then embedded in paraffin. Tissues were cut into 3-µm-thick
sections and processed using an automated system
(BenchMark®XT; Roche Diagnostics K.K.). Sections were
deparaffinized and treated with protease 1 (0.5 U/ml; Roche
Diagnostics K.K.) at 37°C for 4 min, followed by a mouse monoclonal
anti-cytokeratin antibody (Clone: AE1, AE3, PCK26; Roche
Diagnostics K.K.) at 37°C for 16 min. Following the reaction with
the primary antibody, sections were treated with the secondary
antibody conjugated with alkaline phosphatase, and were stained
using a detection kit (ultraView Universal Alkaline Phosphatase Red
Detection kit; Roche Diagnostics K.K.). Sections were then stained
with hematoxylin for the nucleus, dehydrated, and cleared, and
coverslips were placed to prepare the samples for analyses.
Tissues were sectioned serially for H&E and
cytokeratin staining. FFCCs were detected in the lymph node sinuses
based on the definition described above. Patients were categorized
as positive for FFCCs when one or more than one freely floating
cytokeratin-positive cells were detected in the lymph node sinuses
(FFCCs+), and were categorized as negative for FFCCs when none were
present (FFCCs-). 5-year RFS (5Y-RFS) and 5-year OS (5Y-OS) rates
were calculated in both groups of patients. In addition, FFCCs+ and
FFCCs-patients were categorized based on the disease stages (stage
I or II) as well as the histological types of their tumors,
including adenocarcinoma, squamous cell carcinoma, large cell
carcinoma, and others. The frequency of recurrence in FFCCs+ and
FFCCs-patients was analyzed to calculate the sensitivity,
specificity, false positive rate, false negative rate, positive
predictive value, negative predictive value, and accuracy of
detecting FFCCs. The first sites of recurrence were analyzed in
FFCCs+ and FFCCs-patients.
Statistical analysis
A Kaplan-Meyer survival analysis was used to
calculate 5Y-RFS and 5Y-OS rates (7), and the Log-rank test was used to
compare the two groups. Hazard ratios (HR) and 95% confidence
intervals (CIs) were calculated using Cox's proportional hazard
model (8). The chi-squared test
was used to compare FFCCs+ and FFCCs-patients for age, sex,
histological types of tumors, the presence of recurrent tumors.
Fisher's exact test was also used to compare both groups for the
primary sites of recurrence because the number is small. In
addition, the odds ratio and 95% CI were calculated in FFCCs+ and
FFCCs-patients with recurrent tumors. In all cases, P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using SPSS 22.0 statistical
software (IBM Corp., Armonk, NY, USA).
Results
Detection of FFCCs
FFCCs were detected in 11.0% of all patients (n=18),
with 4.5% in stage I patients (n=6) and 37.5% in stage II patients
(n=12), using Fast Red staining for cytokeratin. FFCCs were
observed as single cells and as clusters of several cells (Fig. 1A and B).
Clinical features and Histological
types of primary lesions in FFCCs+ and FFCCs-patients
There were no significant differences in age or sex
between the groups FFCCs+ and FFCCs-(Table I). Among 18 FFCCs+ patients, there
were 12 cases of adenocarcinoma (66.7%), 5 of squamous cell
carcinoma (27.8%), 0 of large cell carcinoma (0%), and 1 of tumors
with other histological types (5.6%, 1 mucoepidermoid carcinoma).
Among 146 FFCCs-patients, there were 110 cases of adenocarcinoma
(75.3%), 25 of squamous cell carcinoma (17.1%), 6 of large cell
carcinoma (4.1%), and 5 of tumors with other histological types
(3.4%, 1 typical cartinoid, 2 adenosquamous carcinoma, 1
pleomorphic carcinoma, and 1 unclassified non-small cell
carcinoma). No significant difference was observed between the two
groups in terms of the histological types of primary tumors
(Table I).
 | Table I.Clinical features and Histological
types of primary lesions. |
Table I.
Clinical features and Histological
types of primary lesions.
| Variable | Total cases
(n=164) | FFCC (−) (n=146) | FFCC (+) (n=18) | P-value |
|---|
| Age, median (range),
year | 66 (30–81) | 66 (36–81) | 64 (30–79) | P=0.749 |
| Sex, n (%) |
|
|
|
|
| Male | 107 (65.2) | 95 (65.1) | 12 (66.7) | P=0.759 |
|
Female | 57 (34.8) | 51 (34.9) | 6 (33.3) |
|
| Histological type, n
(%) |
|
|
|
|
|
Adenocarcinoma | 122 (74.4%) | 110 (75.3%) | 12 (66.7%) | P=0.302 |
| Squamous
cell carcinoma | 30 (18.3%) | 25 (17.1%) | 5 (27.8%) | P=0.342 |
| Large
cell carcinoma | 6 (3.7%) | 6 (4.1%) | 0 (0.0%) | P=1.000 |
|
Others | 6 (3.7%) | 5 (3.4%) | 1 (5.6%) | P=0.521 |
Recurrence in and prognosis of FFCCs+
and FFCCs- in all patients
Among 164 total patients, 18 belonged to the FFCCs+
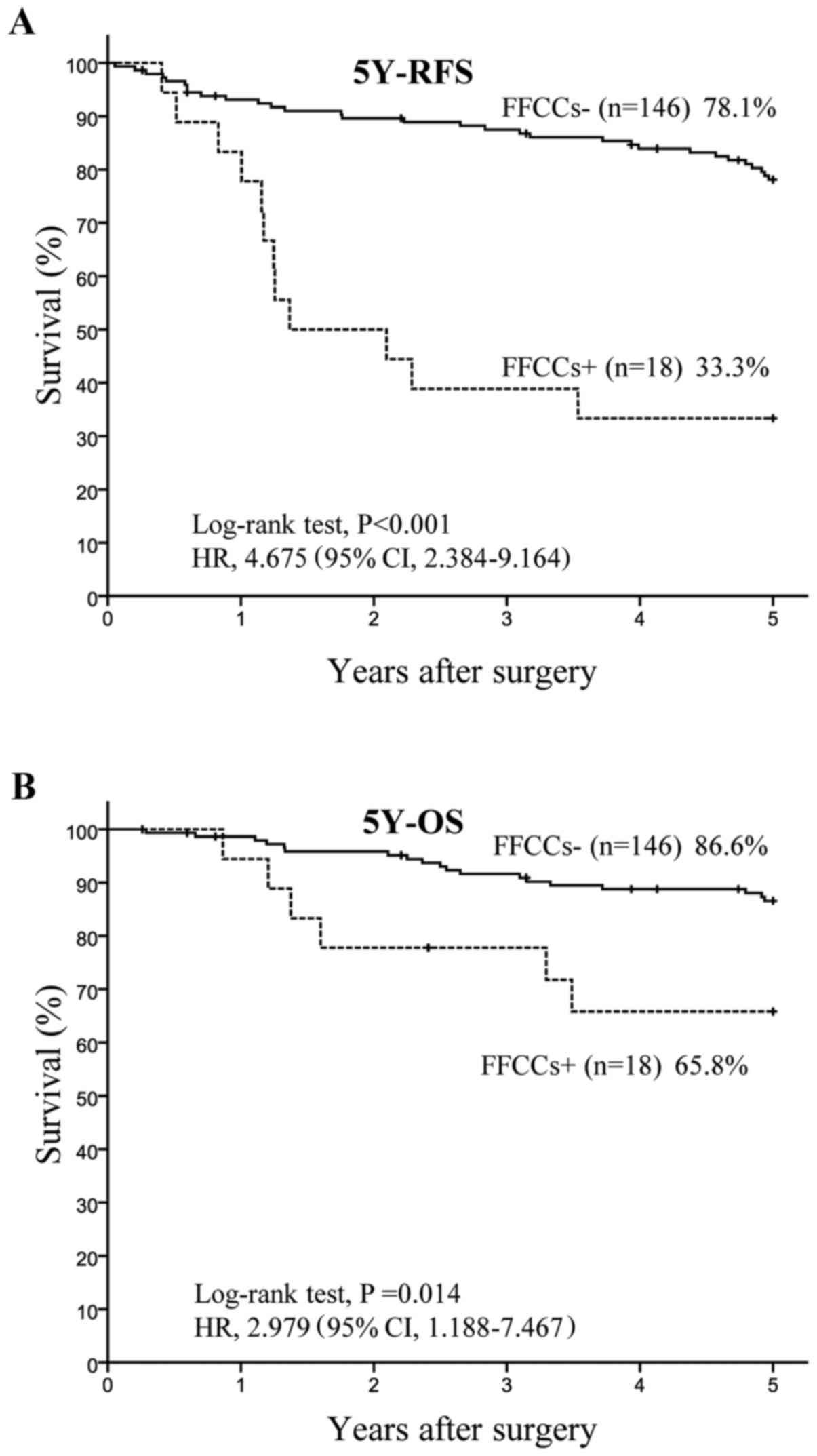
group and 146 belonged to the FFCCs-group. The 5Y-RFS rate was
significantly lower (P<0.001) in the FFCCs+ group (33.3%, n=18)
than in the FFCCs-group (76.9%, n=146), with HR of 4.675 and 95% CI
of 2.384–9.164 (Fig. 2A).
Similarly, the 5Y-OS rate was significantly lower (P=0.014) in the
FFCCs+ group (65.8%, n=18) than in the FFCCs-group (86.6%, n=146),
with HR of 2.979 and 95% CI of 1.188–7.467 (Fig. 2B).
Recurrence in and prognosis of FFCCs+
and FFCCs- in n1 positive patients
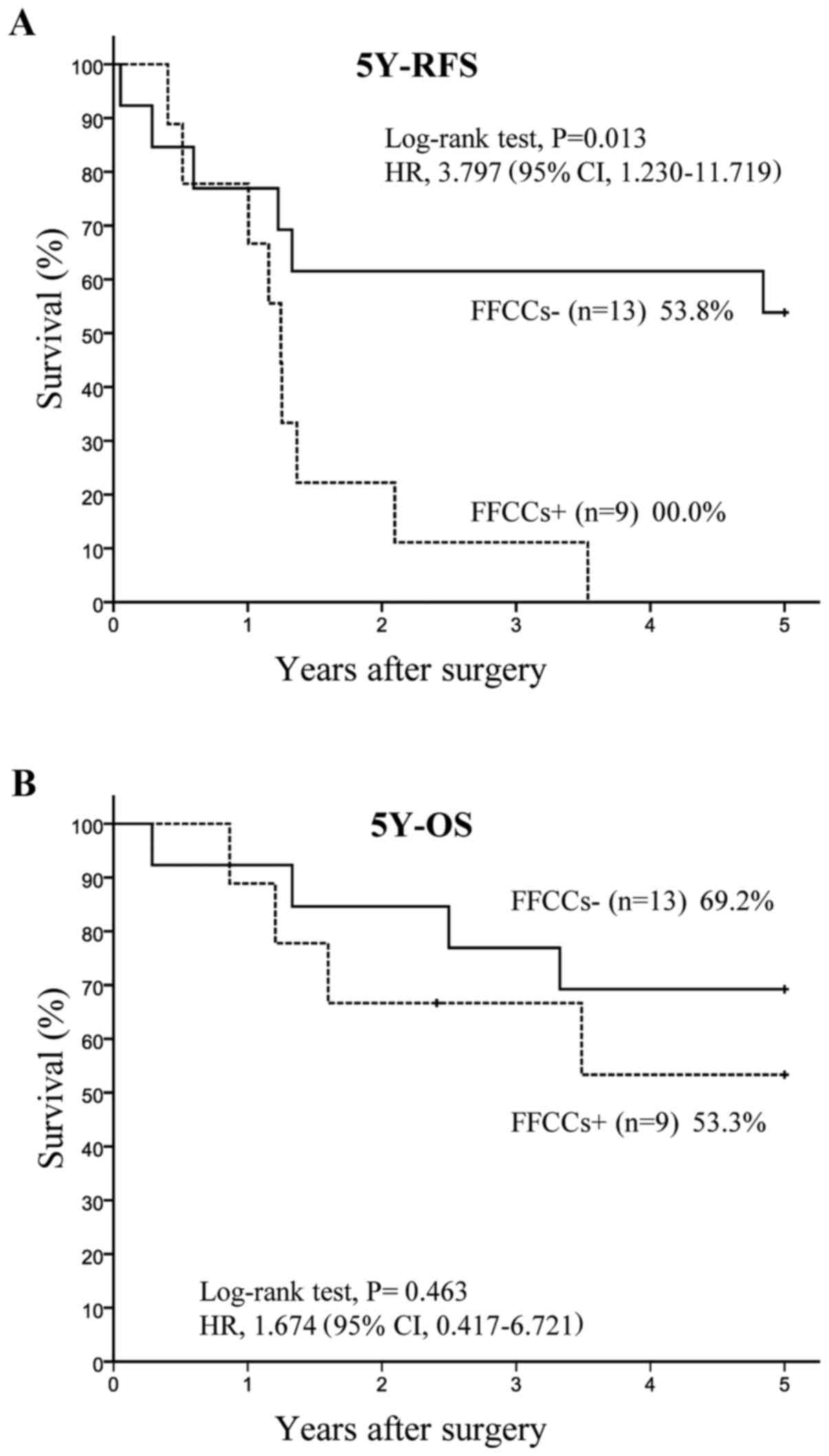
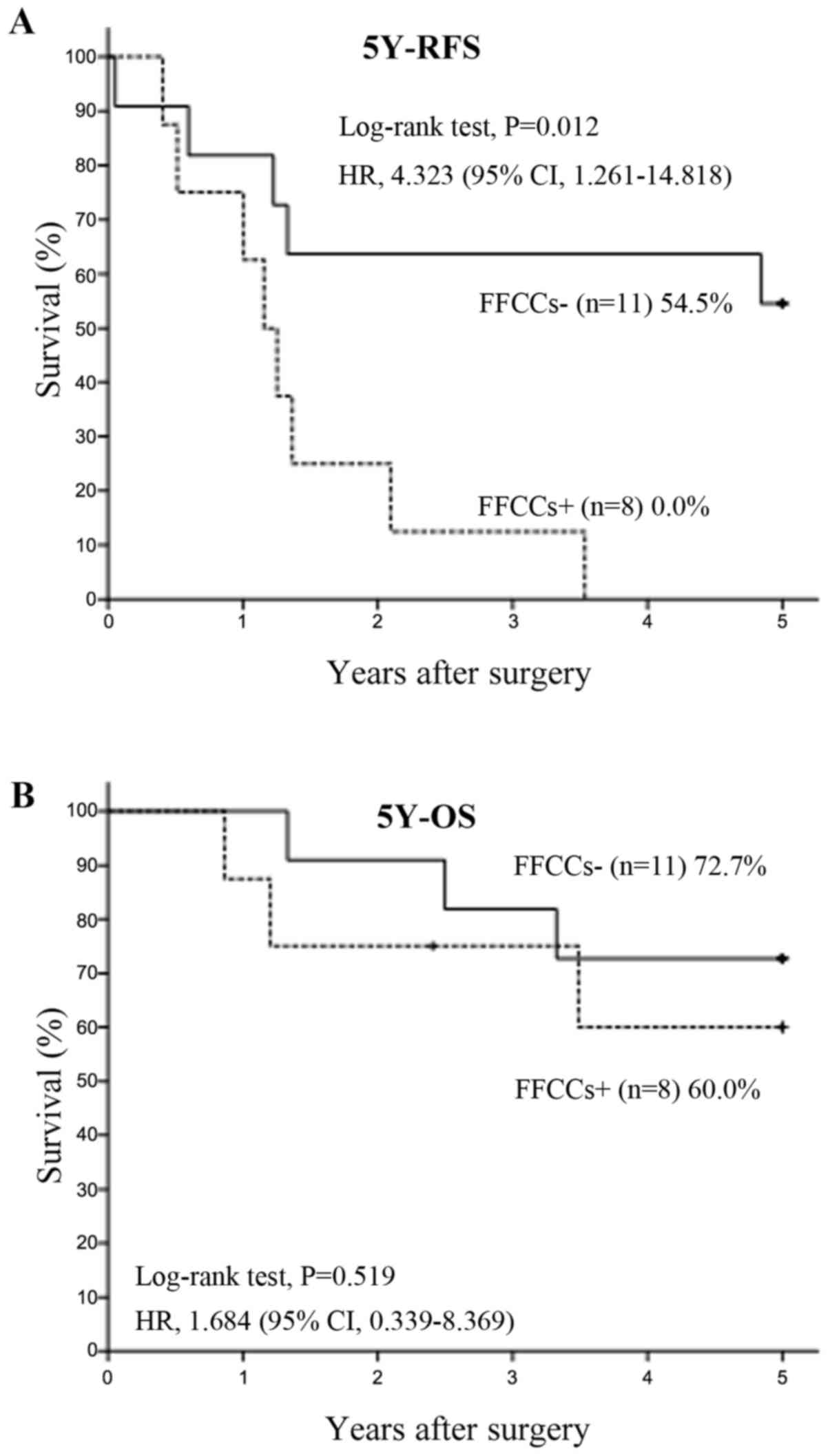
Twenty two patients had hilar lymph node metastases.
Among 22 n1 positive patients, 9 had FFCCs and 13 did not have any
FFCCs. The 5Y-RFS rate was significantly lower (P=0.006) in the
FFCCs+ group (0.0%, n=9) than in the FFCCs-group (53.8%, n=13),
with HR of 4.828 and 95% CI of 1.421–16.402 (Fig. 3A). The 5Y-OS rate tended to be
lower (P=0.463) in the FFCCs+ group (53.3%, n=9) than in the
FFCCs-group (69.2%, n=13), with HR of 1.674 and 95% CI of
0.417–6.721 (Fig. 3B).
Sensitivity, specificity, and accuracy
of the detection of FFCCs in recurrent and non-recurrent
patients
Among all study patients (n=164), FFCCs were
detected in recurrent and non-recurrent patients with 33.3%
sensitivity (12/36), 33.3% false positive rate (6/18), 95.3%
specificity (122/128), 16.4% false negative rate (24/146), 66.7%
positive predictive value (12/18), 83.6% negative predictive value
(122/146), and 81.7% accuracy (134/164), with an odds ratio of
10.167 (P<0.001; 95% CI: 3.537–29.225) (Table II).
 | Table II.Detection rates of FFCC in the
recurrence and the non-recurrence groups. |
Table II.
Detection rates of FFCC in the
recurrence and the non-recurrence groups.
| Total 164 cases
(predictive accuracy 81.7%) | Recurrence group
(n=36) | Non-recurrence group
(n=128) |
|---|
| FFCC(+) 18 cases (PPV
66.7%) | 12 casesa (Sensitivity 33.3%) | 6 cases (FP rate
33.3%) |
| FFCC(−) 146 cases
(NPV 83.6%) | 24 cases (FN rate
16.4%) | 122 cases
(specificity 95.3%) |
First sites of recurrence
Among FFCCs+ patients (n=18), 12 had recurrent
disease during the 5-year observational period. Tumor recurrence
was identified at 14 sites, including multiple simultaneous tumors.
There was 1 case of local recurrence (7.1%), 3 of pleural
dissemination or carcinomatous pleuritis (21.4%), 5 of lymph node
metastasis (35.7%), 0 of liver metastasis (0.0%), 0 of lung
metastasis (0.0%), 1 of brain metastasis (7.1%), 4 of bone
metastasis (28.6%), 0 of adrenal metastasis (0.0%), and 0 of
metastasis in other/unknown sites (0.0%) (Table III). Among FFCCs-patients
(n=146), 24 had recurrent disease during the 5-year observational
period. Tumor recurrence was identified at 29 sites, including
multiple simultaneous tumors. There was 1 case of local recurrence
(3.4%), 4 of pleural dissemination or carcinomatous pleuritis
(13.8%), 3 of lymph node metastasis (10.3%), 0 of liver metastasis
(0.0%), 15 of lung metastasis (51.7%), 3 of brain metastasis
(10.3%), 2 of bone metastasis (6.9%), 0 of adrenal metastasis
(0.0%), and 1 of metastasis in other/unknown sites (3.4%) (Table III). In this study, lung
metastases were detected at a higher frequency in the FFCCs-group
than the FFCCs+ group (P<0.001; Table III).
 | Table III.Pattern and site of
recurrence/metastasis (N=47). |
Table III.
Pattern and site of
recurrence/metastasis (N=47).
| Variable (number of
recurrences) | Total cases
(n=43) | FFCC (−) (n=29) | FFCC (+) (n=14) | P-value |
|---|
| Site of
recurrence/metastasis, n |
|
|
|
|
|
Local | 2 | 1 | 1 | P=1.000 |
| Pleural
dissemination/Carcinomatous pleuritis | 7 | 4 | 3 | P=0.666 |
| Lymph
node | 7 | 3 | 5 | P=0.253 |
| Lung | 15 | 15 | 0 | P<0.001 |
|
Brain | 5 | 3 | 1 | P=1.000 |
| Bone | 6 | 2 | 4 | P=0.153 |
|
Liver | 0 | 0 | 0 | P=1.000 |
|
Others/unknown | 1 | 1 | 0 | P=1.000 |
Discussion
The present study investigated FFCCs and
recurrence/prognosis of stage I/II NSCLC, and demonstrated that
FFCCs are of prognostic significance in stage I/II NSCLC (Fig. 2A and B). However, some scientists
suspect that FFCCs may be the same as lymph node metastases. Thus,
this study investigated whether there were prognostic differences
between FFCCs positive and negative groups in hilar lymph node
positive patients who underwent resection for primary lung cancer,
and demonstrated that the 5Y-RFS rate was significantly lower and
the 5Y-OS rate tended to be lower in FFCCs+ patients among n1
positive patients (Fig. 3A and
B).
FFCCs are a population of cells that are completely
detached from the primary lesions and float freely inside the lymph
node sinuses. We estimate that FFCCs progress three pathway as
follows: i) are caught by the immune systems and die; ii) are
settled in lymph nodes and develop into lymph node metastases; and,
iii) pass through lymph nodes and the immune systems and circulate
into the whole bodies. In this study, the 5Y-RFS rate was
significantly lower and the 5Y-OS rate tended to be lower in FFCCs+
patients among n1 positive patients. We think the reason is that
some FFCCs progress towards the iii) pathway as stated above.
We think that FFCCs which progress towards the iii)
pathway as stated above, may be similar to circulating tumor cells
(CTCs). Rack et al identified tumor cells that were
circulating in blood using a cell search system (CellTrack
Analyzer), and demonstrated that CTCs predict metastasis and
survival in breast cancer patients (9). Furthermore, previous studies
demonstrated that CTCs are of prognostic significance in lung
cancer patients (10–14). Thus, CTCs and FFCCs may have
similar properties despite being present in different locations
(blood and lymph node sinuses, respectively). However, that is a
mere conjecture and requires more investigations.
The detection of FFCCs using DAB, which stains
cytokeratin brown, is complicated by the presence of black coal
dust inside the lymph nodes. In the present study, we used Fast Red
staining to enable the distinction from coal dust, and so we could
find FFCCs more quickly and were not tired relatively.
It seems to be difficult to count the exact number
of dissected lymph nodes in the chest, different from the colon and
stomach because the lymph nodes in the chest often are not separate
but seem to be a lump. However, in this time, we analyzed the
number of dissected lymph nodes between the FFCCs+ group and
FFCCs-group as exact as possible. Then, there were no significant
differences in the number of dissected lymph nodes between the
groups FFCCs+ and FFCCs-among both all patients and n1 positive
patients (Table IV).
 | Table IV.The number of dissected lymph
nodes. |
Table IV.
The number of dissected lymph
nodes.
|
| Total cases | FFCCs (−) | FFCCs (+) | P-value |
|---|
| All patients | (n=164) | (n=146) | (n=18) |
|
| Lymph nodes, median
(range) | 14 (45) | 13 (45) | 22 (34) | P=0.156a |
| N1 positive
patients | (n=22) | (n=13) | (n=9) |
|
| Lymph nodes, mean
(±SD) | 17 (±8) | 16 (±6) | 18
(±10) | P=0.101b |
In a previous study on gastric cancer patients,
FFCCs were identified in poorly differentiated tissues such as
signet ring cell carcinoma and poorly differentiated adenocarcinoma
(3). In the present study, we
detected FFCCs in all histological types of lung cancers, including
adenocarcinoma, squamous cell carcinoma, large cell carcinoma, and
others, with no significant differences (Table I). This result indicates that FFCCs
originate from any histological type of NSCLC.
Recurrence in lung cancer patients takes various
forms, such as metastasis in the lungs, brain, bone, liver, adrenal
glands, and lymph nodes as well as pleural dissemination. And, in
this study, lung metastases were lower in FFCCs+ patients than
FFCCs-patients (Table III). In
addition, FFCCs were not detected in stage II (T3N0M0) patients, in
which T3 was restricted to metastasis in the same lobe as the
location of the primary tumor (data not shown). Thus, lung
metastases might be occurred through the respiratory tract which
has been found in patients with invasive mucinous adenocarcinoma or
papillary adenocarcinoma (15,16),
rather than via the lymphatic and vascular systems associated with
FFCCs. But, that is a mere conjecture and the evidence as above
requires more minute investigations including prospective cohort
studies with a large number of patients.
Lung cancer has the highest incidence and mortality
rates among all malignant tumors worldwide (17). Stage I/II lung cancer patients
sometimes have a poor prognosis, with 5-year survival rates varying
between 53 and 92% (18).
Recently, the TNM classification became eighth edition, thus we
reclassified this study patients according the latest 8th TNM
staging system and reanalyzed the 5Y-RFS rates and the 5Y-OS rates
on the 8th TNM stage. Then, two patients of FFCCs+ group and four
patients of FFCCs- group became stage IIIA (among n1 positive
patients, one patients of FFCCs+ group and two patients of FFCCs-
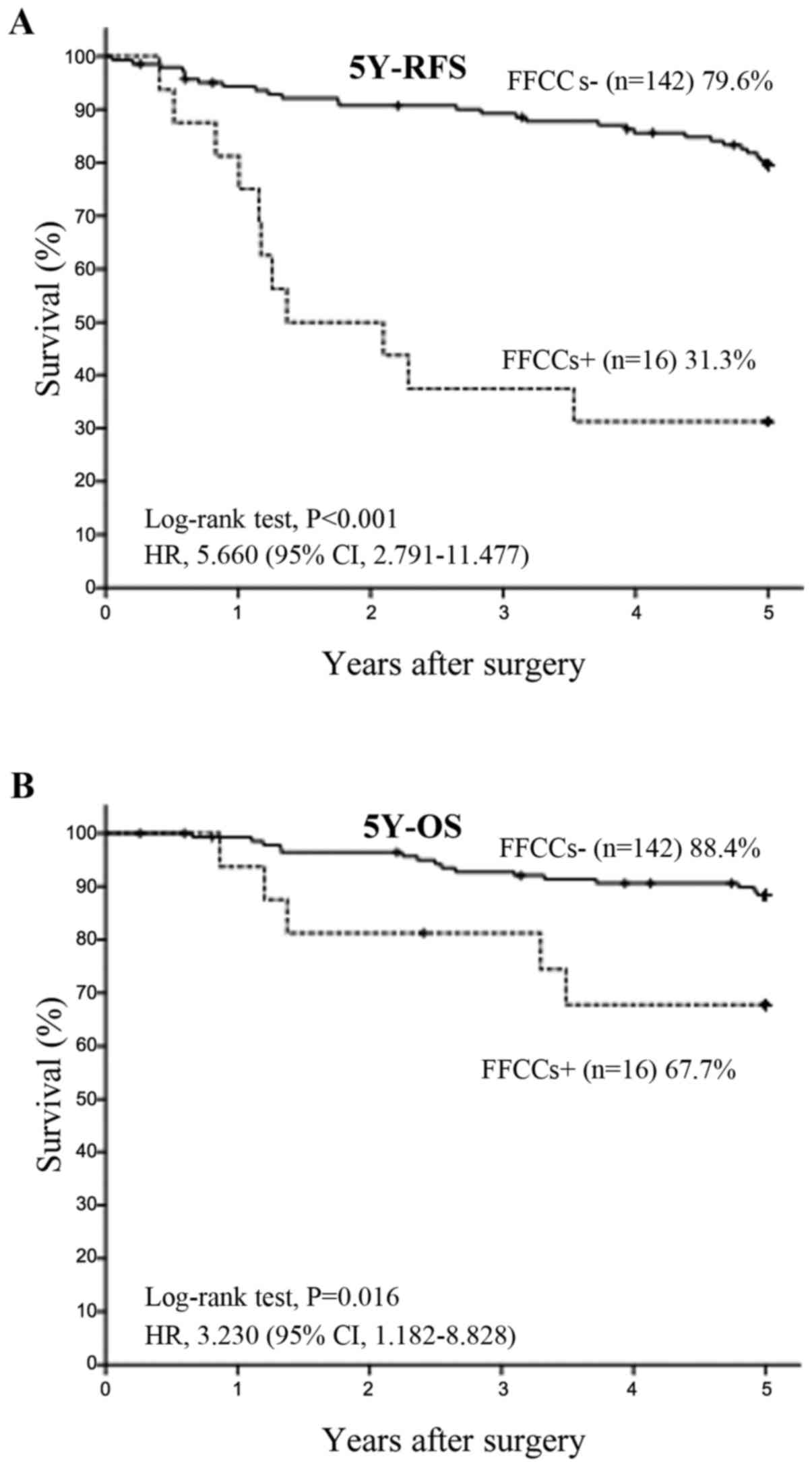
group became stage IIIA). 5Y-RFS rates of FFCCs+ groups in all
patients (Fig. 4A) and n1 positive
patients (Fig. 5A) were also
significant lower than FFCCs- groups. The 5Y-OS rate of FFCCs+
group in all patients was also significant lower than FFCCs- group
(Fig. 4B). The 5Y-OS rate of
FFCCs+ group in n1 positive patients also tended to be lower than
FFCCs- group (Fig. 5B).
In the present study, FFCCs were detected in 11.0%
of stage I/II lung cancer patients (18/164), and their presence was
associated with a poor prognosis (Fig.
2A and B). Our results indicate that FFCCs have potential as
useful markers for identifying patients at high risk of
postoperative recurrence. In addition, the lack of FFCCs may be
associated with patients who are at low risk of recurrence based on
the high detection specificity and negative predictive value.
Therefore, future clinical practice may consider additional
postoperative adjuvant chemotherapy and more frequent follow-ups
for FFCCs+ patients, and the omission of postoperative adjuvant
chemotherapy and less frequent follow-ups for FFCCs- patients.
In conclusion, the presence of FFCCs in stage I/II
lung cancer patients was associated with a poor prognosis. In
addition, FFCCs in hilar lymph node positive patients also have
potential as a useful marker foreseeing the recurrence.
Acknowledgements
The authors would like to thank laboratory
technicians Mr. Machida Tomohisa, Mrs. Nomura Nozomi and Miss
Hagiwara Noriko from the Department of Pathology, Tokai University
Hachioji Hospital (Hachioji-shi, Japan), and Dr. Suga Atushi
(Department of General Thoracic Surgery, Yamato Manicipal Hospital,
Yamato-shi, Japan) and Dr. Hamanaka Rurika (Department of General
Thoracic Surgery, Ebina General Hospital, Ebina-shi, Japan) who
collected the data from patients that were transferred to another
hospital during the observation period.
Funding
This study was supported by grants from AstraZeneca
K.K. and Eli Lilly Japan K.K.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YN acquired, analyzed and interpreted all of the
data, and was a major contributor in writing the manuscript. MM
made substantial contributions to conception and design. SH, TS and
TT performed the histological examination of FFCCs. KK, SY and MI
acquired, analyzed and interpreted all of the data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Tokai
University Institutional Review Board for Clinical Research (IRB
no. 14R-225, Isehara, Japan). This study is a retrospective study
using the sample which had already been gotten in the past. It was
difficult to obtain consent from some research subjects, because
they were deceased. Thus, in accordance with ‘Ethical Guidelines
for Medical and Health Research Involving Human Subjects’ as
indicated by the Japanese Ministry of Health, Labour and Welfare
and the Japanese Ministry of Education, Culture, Sports, Science
and Technology, we made information public including the purpose of
utilization of the sample with respect to implementing the research
and ensured the opportunities to refuse that the research be
implemented for the research subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with Dukes' C colorectal cancer. Oncol Rep. 10:1165–1169.
2003.PubMed/NCBI
|
|
2
|
Mukai M, Sato S, Komatsu N, Nishida T,
Shiba K, Ito I, Nakasaki H and Makuuchi H: Correlation between
occult neoplastic cells in the lymph node sinuses and recurrence in
patients with curatively resected Dukes' B colorectal cancer. Oncol
Rep. 10:1177–1181. 2003.PubMed/NCBI
|
|
3
|
Sekido Y, Mukai M, Yamazaki M, Tajima T,
Yamamoto S, Hasegawa S, Kishima K, Tajiri T and Nakamura N: Occult
neoplastic cells in lymph node sinuses and recurrence/metastasis of
stage II/III gastric cancer. Oncol Let. 7:53–58. 2014. View Article : Google Scholar
|
|
4
|
Mukai M, Sato S, Nakasaki H, Tajiri T,
Saito Y, Nishiumi N, Iwasaki M, Tokuda Y, Ogoshi K, Inoue H and
Makuuchi H: Occult neoplastic cells in the lymph node sinuses and
recurrence of primary breast, lung, esophageal and gastric cancer.
Oncol Rep. 11:81–84. 2004.PubMed/NCBI
|
|
5
|
Mukai M, Sato S, Tajima T, Ninomiya H,
Wakui K, Komatsu N, Tsuchiya K, Nakasaki H and Makuuchi H:
Recurrence and 5-FU sensitivity of stage I/II node-negative breast,
lung, or gastric cancer with occult neoplastic cells in lymph node
sinuses. Oncol Rep. 15:815–820. 2006.PubMed/NCBI
|
|
6
|
Conner JR, Cibas ES, Hornick JL and Qian
X: Wilms Tumor 1/Cytokeratin Dual-Color Immunostaining reveals
distinctive staining patterns in metastatic melanoma, metastatic
carcinoma, and mesothelial cells in pleural fluids: An effective
first-line test for the workup of malignant effusions. Cancer
Cytopathol. 122:586–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
8
|
Cox DR: Regression models and life-tables.
J R Stat Soc B. 34:187–220. 1972.
|
|
9
|
Rack B, Schindlbeck C, Jückstock J,
Andergassen U, Hepp P, Zwingers T, Friedl TW, Lorenz R, Tesch H,
Fasching PA, et al: Circulating tumor cells predict survival in
early average-to-high risk breast cancer patients. J Natl Cancer
Inst. 106:pii: dju066. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Igawa S, Gohda K, Fukui T, Ryuge S, Otani
S, Masago A, Sato J, Murakami K, Maki S, Katono K, et al:
Circulating tumor cells as a prognostic factor in patients with
small cell lung cancer. Oncol Let. 7:1469–1473. 2014. View Article : Google Scholar
|
|
11
|
Naito T, Tanaka F, Ono A, Yoneda K,
Takahashi T, Murakami H, Nakamura Y, Tsuya A, Kenmotsu H, Shukuya
T, et al: Prognostic impact of circulating tumor cells in patients
with small cell lung cancer. J Thorac Oncol. 7:512–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou JM, Krebs MG, Lancashire L, Sloane R,
Backen A, Swain RK, Priest LJ, Greystoke A, Zhou C, Morris K, et
al: Clinical significance and molecular characteristics of
circulating tumor cells and circulating tumor microemboli in
patients with small-cell lung cancer. J Clin Oncol. 30:525–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Wang K, Xu J, Huang J and Zhang
T: Prognostic significance of circulating tumor cells in
non-small-cell lung cancer patients: A meta-analysis. PLoS One.
8:e780702013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aokage K, Ishii G, Nagai K, Kawai O, Naito
Y, Hasebe T, Nishimura M, Yoshida J and Ochiai A: Intrapulmonary
metastasis in resected pathological stage IIIB non-small cell lung
cancer: Possible contribution of aerogenous metastasis to the
favorable outcome. J Thorac Cardiovasc Surg. 134:386–391. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaeta M, Blandino A, Pergolizzi S,
Mazziotti S, Caruso R, Barone M and Cascinu S: Patterns of
recurrence of bronchioloalveolar cell carcinoma after surgical
resection: A radiological, histological, and immunohistochemical
study. Lung Cancer. 42:319–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) Edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|